Continentalic Acid Rather Than Kaurenoic Acid Is Responsible for the Anti-Arthritic Activity of Manchurian Spikenard In Vitro and In Vivo
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Ethanol Content of the Extraction Solvents in RAW264.7 Cells
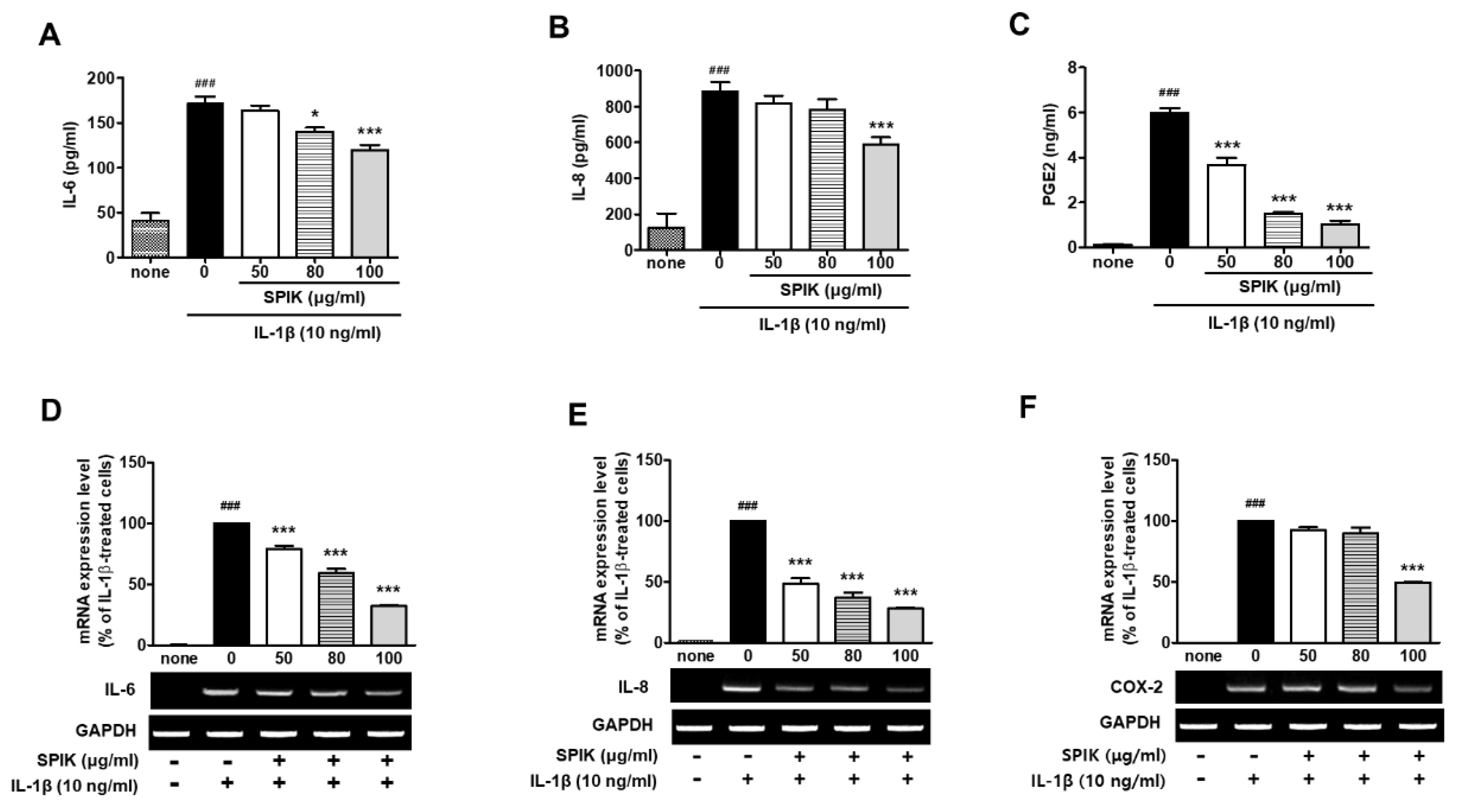
2.2. The 50% Ethanolic Extract of Manchurian Spikenard Inhibits Interleukin (IL)-1β-Stimulated Production of Inflammatory Mediators in Human Chondrocytes
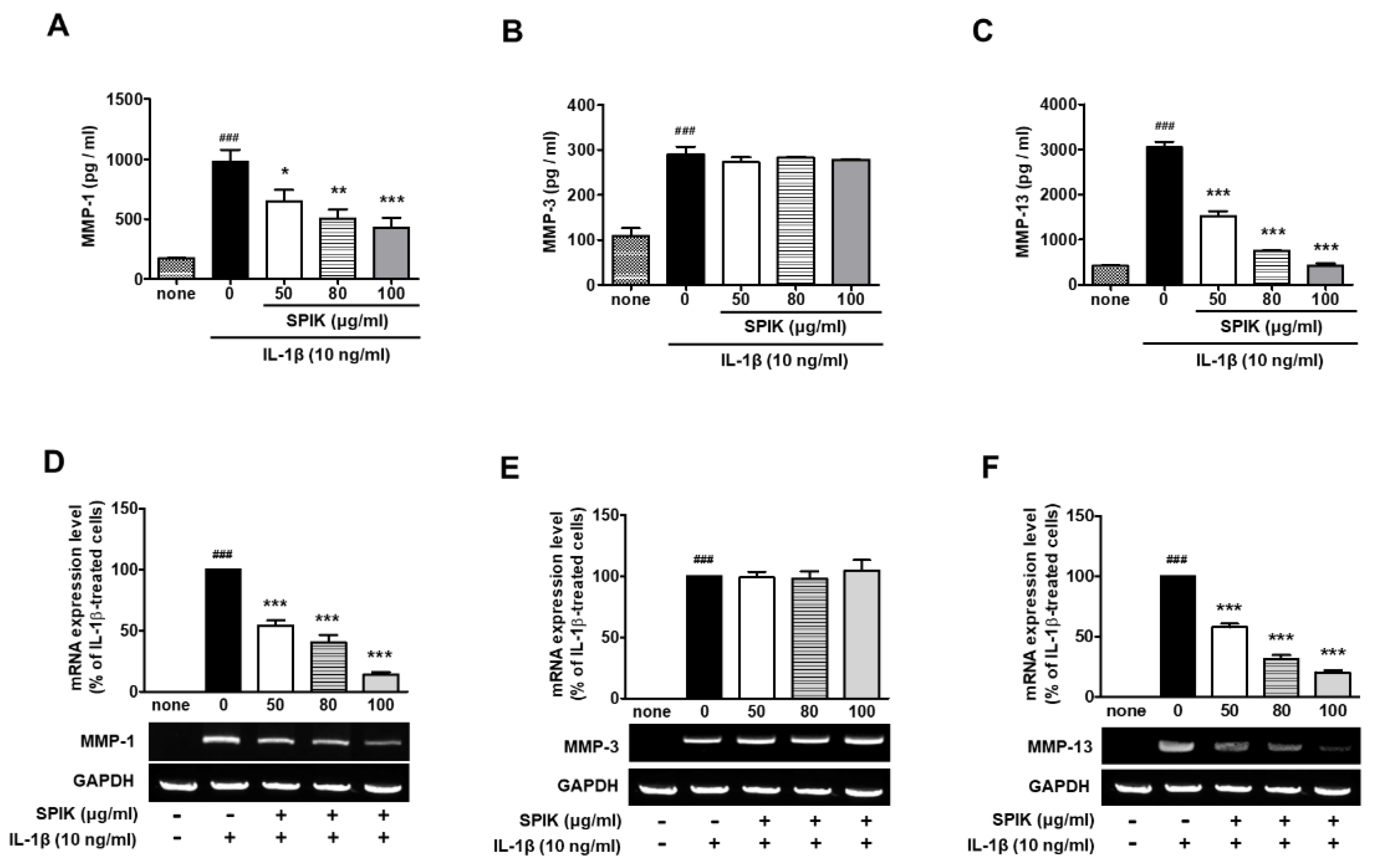
2.3. The 50% Ethanolic Extract of Manchurian Spikenard Inhibits IL-1β-Stimulated Expression of Matrix Metalloproteinases (MMPs) in Human Chondrocytes
2.4. Continentalic Acid, Not Kaurenoic Acid, is Responsible for the In Vitro Anti-Arthritic Activity of Manchurian Spikenard
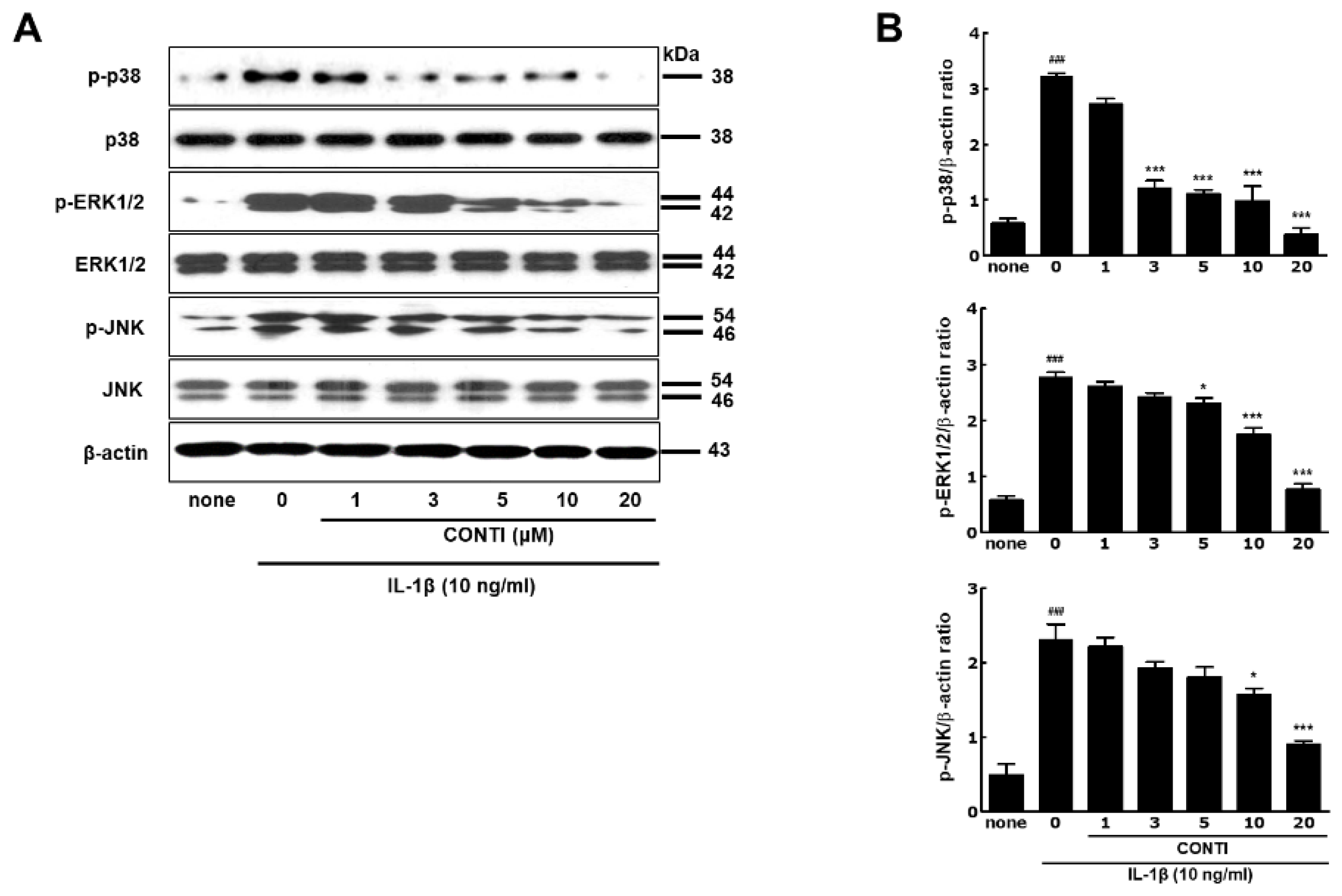
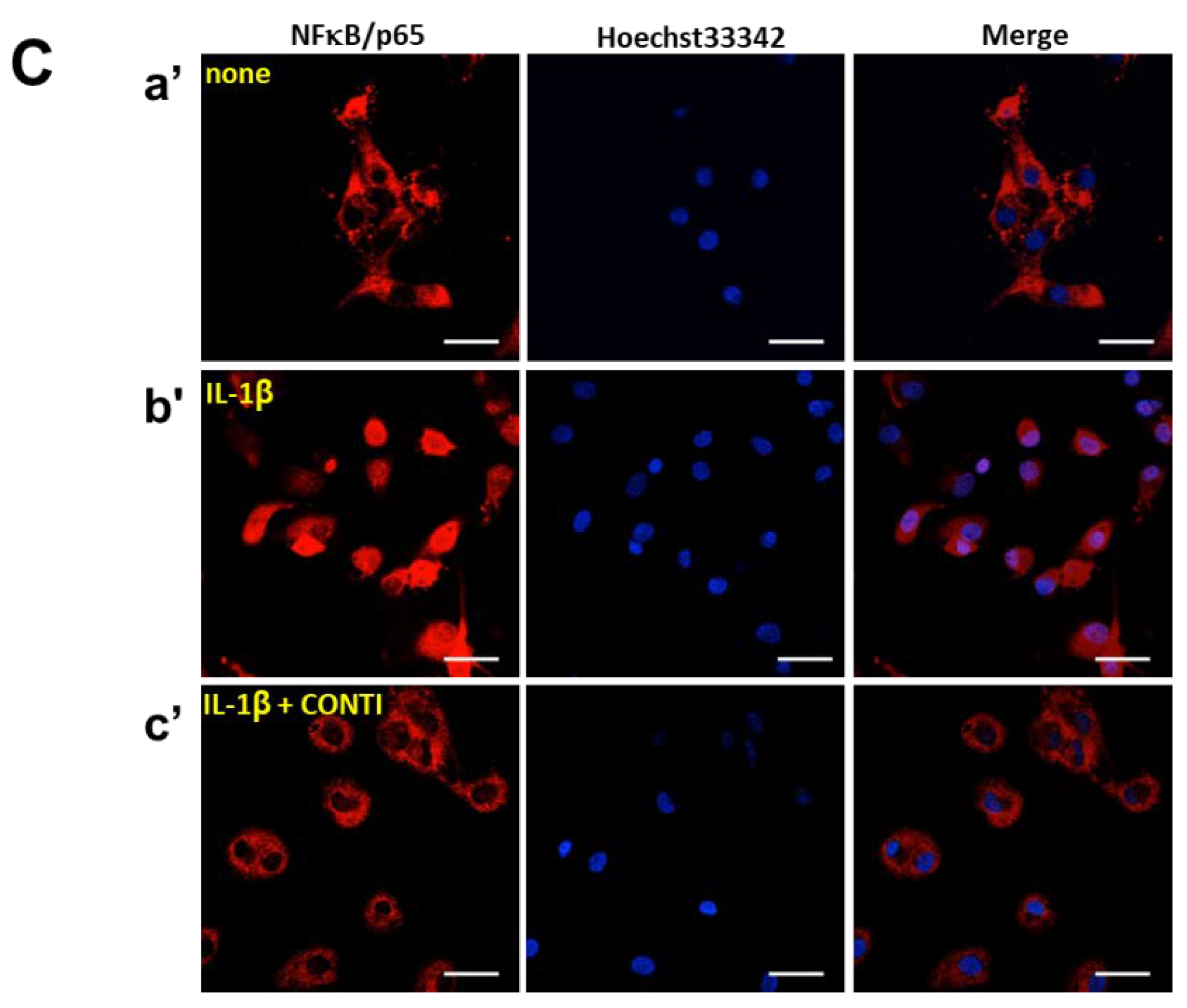
2.5. Continentalic Acid in the Spikenard Extract Inhibits IL-1β-Induced Phosphorylation of Extracellular Signal-Regulated Kinase/ Jun Amino-Terminal Kinase/p38 Mitogen-Activated Protein (ERK/JNK/p38 MAP) Kinases and Nuclear Translocation of the NF-κB/p65 Subunit
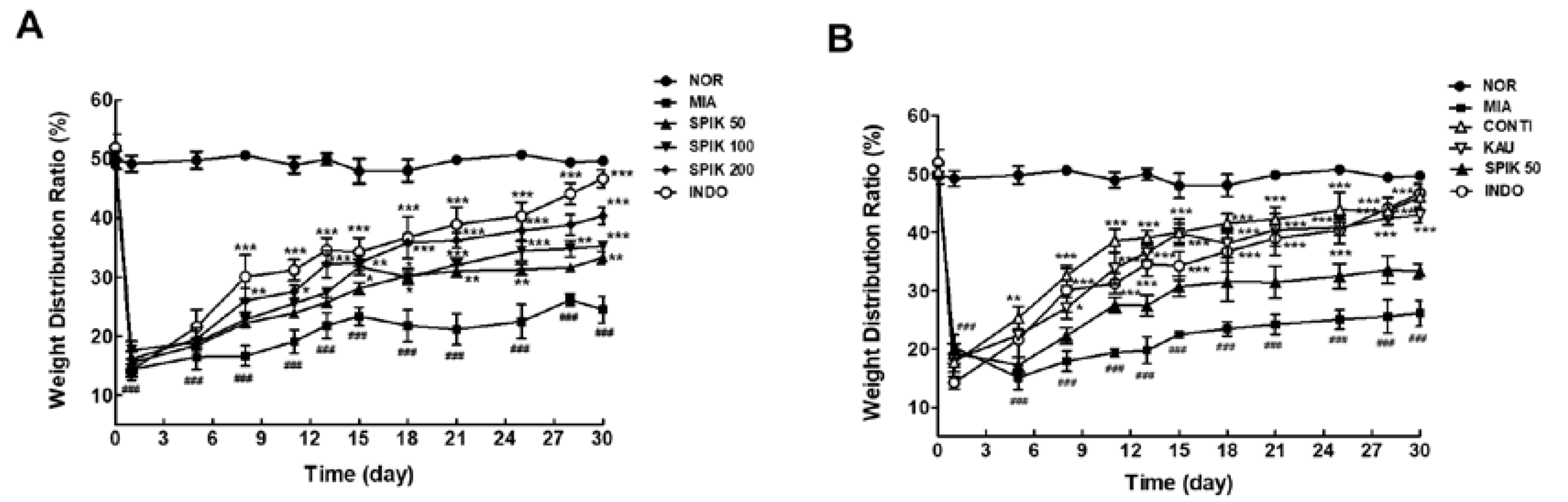
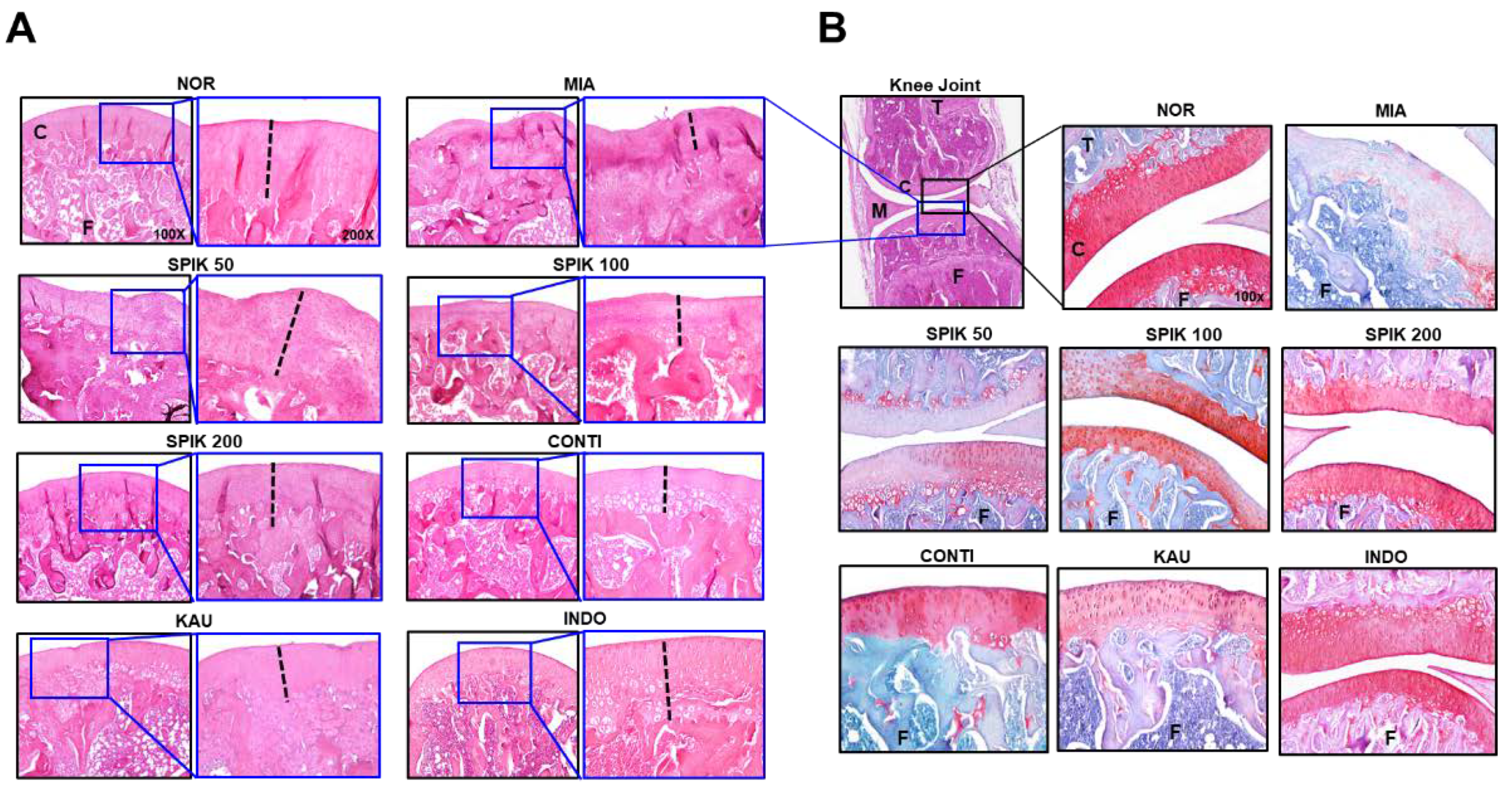
2.6. The Spikenard Extract and Continentalic Acid Alleviates Monoiodoacetate (MIA)-Induced Osteoarthritis in Rats
3. Materials and Methods
3.1. Cell Experiments
3.1.1. Plant Material and Chemicals
3.1.2. Cell Culture
3.1.3. Reverse Transcription Polymerase Chain Reaction (RT−PCR) and Enzyme-Linked Immunosorbent Assay (ELISA)
3.1.4. Western Hybridization
3.1.5. Immunofluorescence Microscopy
3.2. Animal Experiments
3.2.1. Animals and Groups
3.2.2. Monoiodoacetate(MIA)-Induced Knee Arthritis
3.2.3. Evaluation of Arthritic Symptoms
3.2.4. Histology
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldring:, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Herbalogy; Young Lim Sa: Seoul, Korea, 2000. [Google Scholar]
- Huh, J. Dong Ui Bo Gam: The precious mirror of Oriental Medicine, 2nd ed.; Beob In Mun Hwa Sa: Seoul, Korea, 2012. [Google Scholar]
- Okuyama, E.; Nishimura, S.; Yamazaki, M. Analgesic principles from Aralia cordata Thunb. Chem. Pharm. Bull. 1991, 39, 405–407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, H.; Jung, H.A.; Choi, J.S.; Kim, Y.S.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of the constituents of the roots of Aralia continentalis. Arch. Pharm. Res. 2009, 32, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Hung, T.M.; Min, B.S.; Lee, I.S.; Na, M.K.; Woo, M.H.; Son, J.K.; Kim, Y.H.; Choi, J.S.; Bae, K.H. Quantitative determination of diterpenoids from the roots of Aralia cordata. Nat. Prod. Sci. 2009, 15, 50–54. [Google Scholar]
- Hong, R.; Sur, B.; Yeom, M.; Lee, B.; Kim, K.S.; Rodriguez, J.P.; Lee, S.; Kang, K.S.; Huh, C.K.; Lee, S.C.; et al. Anti-inflammatory and anti-arthritic effects of the ethanolic extract of Aralia continentalis Kitag. in IL-1β-stimulated human fibroblast-like synoviocytes and rodent models of polyarthritis and nociception. Phytomedicine 2018, 38, 45–56. [Google Scholar] [PubMed]
- Dunham, J.; Hoedt-Schmidt, S.; Kalbhen, D.A. Prolonged effect of iodoacetate on articular cartilage and its modification by an anti-rheumatic drug. Int. J. Exp. Pathol. 1993, 74, 283–289. [Google Scholar]
- Murphy, G.; Knäuper, V.; Atkinson, S.; Butler, G.; English, W.; Hutton, M.; Stracke, J.; Clark, I. Matrix metalloproteinases in arthritic disease. Arthritis Res. Ther. 2002, 4, S39–S49. [Google Scholar] [CrossRef]
- Jabłonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157–164. [Google Scholar] [CrossRef]
- Bang, J.S.; Oh, D.H.; Choi, H.M.; Sur, B.J.; Lim, S.J.; Kim, J.Y.; Yang, H.I.; Yoo, M.C.; Hahm, D.H.; et al. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009, 11, R49. [Google Scholar] [CrossRef]
- Kim, K.S.; Choi, H.M.; Oh, D.H.; Kim, C.; Jeong, J.S.; Yoo, M.C.; Yang, H.I. Effect of taurine chloramine on the production of matrix metalloproteinases (MMPs) in adiponectin- or IL-1β-stimulated fibroblast-like synoviocytes. J. Biomed. Sci. 2010, 17, S27. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Huang, J.F.; Du, W.X.; Tong, P.J. Expression and significance of MMP3 in synovium of knee joint at different stage in osteoarthritis patients. Asian Pac. J. Trop. Med. 2014, 7, 297–300. [Google Scholar] [CrossRef]
- Uemura, Y.; Hayashi, H.; Takahashi, T.; Saitho, T.; Umeda, R.; Ichise, Y.; Sendo, S.; Tsuji, G.; Kumagai, S. MMP-3 as a biomarker of disease activity of rheumatoid arthritis. Rinsho Byori. 2015, 63, 1357–1364. [Google Scholar] [PubMed]
- Green, M.J.; Gough, A.K.; Devlin, J.; Smith, J.; Astin, P.; Taylor, D.; Emery, P. Serum MMP-3 and MMP-1 and progression of joint damage in early rheumatoid arthritis. Rheumatology 2003, 42, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Peeters-Joris, C.; Hammani, K.; Singer, C.F. Differential regulation of MMP-13 (collagenase-3) and MMP-3 (stromelysin-1) in mouse calvariae. Biochim. Biophys. Acta. 1998, 1405, 14–28. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, E.B.; Kwon, Y.E.; Lee, J.J.; Cho, W.S.; Kim, H.A.; Song, Y.W. Effect of estrogen on the expression of matrix metalloproteinase (MMP)-1, MMP-3, and MMP-13 and tissue inhibitor of metalloproternase-1 in osteoarthritis chondrocytes. Rheumatol. Int. 2003, 23, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.H.; Huh, J.E.; Lee, J.D.; Choi, D.Y.; Park, D.S. Effect of Aralia cordata extracts on cartilage protection and apoptosis inhibition. Biol. Pharm. Bull. 2006, 29, 1423–1430. [Google Scholar] [CrossRef]
- Park, D.S.; Huh, J.E.; Baek, Y.H. Therapeutic effect of Aralia cordata extracts on cartilage protection in collagenase-induced inflammatory arthritis rabbit model. J. Ethnopharmacol. 2009, 125, 207–217. [Google Scholar] [CrossRef]
- Tsakiri, N.; Kimber, I.; Rothwell, N.J.; Pinteaux, E. Differential effects of interleukin-1α and β on interleukin-6 and chemokine synthesis in neurones. Mol. Cell Neurosci. 2008, 38, 259–265. [Google Scholar] [CrossRef]
- Perry, L.M. Medicinal plants of East and Southeast Asia: Attributed Properties and Uses; MIT Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Kim, K.H.; Sadikot, R.T.; Joo, M. Therapeutic effect of ent-kaur-16-en-19-oic acid on neutrophilic lung inflammation and sepsis is mediated by Nrf2. Biochem. Biophys. Res. Commun. 2016, 474, 534–540. [Google Scholar] [CrossRef]
- Lyu, J.H.; Lee, G.S.; Kim, K.H.; Kim, H.W.; Cho, S.I.; Jeong, S.I.; Kim, H.J.; Ju, Y.S.; Kim, H.K.; Sadikot, R.T.; et al. ent-kaur-16-en-19-oic Acid, isolated from the roots of Aralia continentalis, induces activation of Nrf2. J. Ethnopharmacol. 2011, 137, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Han, W.S.; Yun, Y.H.; Kim, K.J. Continentalic acid from Aralia continentalis shows activity against methicillin-resistant Staphylococcus aureus. Phytother. Res. 2006, 20, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.O.; Jeong, S.I.; Kwon, J.W.; Kim, Y.C.; Jang, S.I. Continentalic acid from Aralia continentalis induces growth inhibition and apoptosis in HepG2 cells. Arch. Pharm. Res. 2008, 31, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; Arora, B.S.; Khajuria, A.; Sidiq, T.; Kishore, D.; Vishwakarma, R.A. Isolation of continentalic acid from Aralia cachemirica and its immune-biological evaluation. Int. J. Pharm. Sci. Res. 2011, 2, 2183–2189. [Google Scholar]
- Villa-Ruano, N.; Lozoya-Gloria, E.; Pacheco-Hernández, Y. Chapter 3—Kaurenoic acid: A diterpene with a wide range of biological activities. Stud. Nat. Products Chem. 2016, 51, 151–174. [Google Scholar]
- Loesera, R.F.; Ericksona, E.A.; Longa, D.L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 581–586. [Google Scholar] [CrossRef]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Monoiodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef]

| Gene (bp) | Nucleotide Sequence | Annealing Temp. (°C)/Cycles | ||
|---|---|---|---|---|
| human | ||||
| GAPDH (579) | sense | 5′-ATC CCA TCA CCA TCT TCC AG-3′ | 58/30 | |
| antisense | 5′-CCT GCT TCA CCA CCT TCT TG-3′ | |||
| IL-6 (150) | sense | 5′-AGT TGC CTT CTT GGG ACT GA -3′ | 55/31 | |
| antisense | 5′-TCC ACG ATT TCC CAG AGA AC -3′ | |||
| IL-8 (166) | sense | 5′-GTT TTG CCA AGG AGT GCT AA -3′ | 55/31 | |
| antisense | 5′-CCA GAC AGA GCT CTC TTC CA -3′ | |||
| COX-2 (158) | sense | 5′-TGA GCA TCT ACG GTT TGC TG -3′ | 55/31 | |
| antisense | 5′-TGC TTG TCT GGA ACA ACT GC -3′ | |||
| MMP-1 (388) | sense | 5′-CCT AGC TAC ACC TTC AGT GG-3′ | 57/29 | |
| antisense | 5′-GCC CAG TAC TTA TTC CCT TT-3′ | |||
| MMP-3 (365) | sense | 5′-TCC CCC TGA CTC CCC TGA-3′ | 57/29 | |
| antisense | 5′-TCC TCA CGG TTG GAG GGA AA-3′ ′ | |||
| MMP-13 (150) | sense | 5′-TGA CCC TTC CTT ATC CCT TG-3′ | 57/29 | |
| antisense | 5′-ATA CGG TTG GGA AGT TCT GG-3′ | |||
| iNOS (320) | sense | 5′-GCA TGT ACC CTC GGT TCT GT-3′ | 58/30 | |
| antisense | 5′-CAT GGT GAA CAC GTT CTT GG-3′ | |||
| mouse | ||||
| GAPDH (223) | sense | 5′-AAC TTT GGC ATT GTG GAA GG-3′ | 58/30 | |
| antisense | 5′-ACA CAT TGG GGG TAG GAA CA-3′ | |||
| IL-6 (159) | sense | 5′-AGT TGC CTT CTT GGG ACT GA-3′ | 52/27 | |
| antisense | 5′-TCC ACG ATT TCC CAG AGA AC-3′ | |||
| COX-2 (194) | sense | 5′-AGA AGG AAA TGG CTG CAGAA 3′ | 55/28 | |
| antisense | 5′-GCT CGG CTT CCA GTA TTG AG -3′ | |||
| iNOS (199) | sense | 5′-CCT CCT CCA CCC TAC CAA GT-3′ | 57/28 | |
| antisense | 5′-CAC CCA AAG TGC TTC AGT CA-3′ | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, R.; Kim, K.S.; Choi, G.M.; Yeom, M.; Lee, B.; Lee, S.; Kang, K.S.; Lee, H.S.; Park, H.-J.; Hahm, D.-H. Continentalic Acid Rather Than Kaurenoic Acid Is Responsible for the Anti-Arthritic Activity of Manchurian Spikenard In Vitro and In Vivo. Int. J. Mol. Sci. 2019, 20, 5488. https://doi.org/10.3390/ijms20215488
Hong R, Kim KS, Choi GM, Yeom M, Lee B, Lee S, Kang KS, Lee HS, Park H-J, Hahm D-H. Continentalic Acid Rather Than Kaurenoic Acid Is Responsible for the Anti-Arthritic Activity of Manchurian Spikenard In Vitro and In Vivo. International Journal of Molecular Sciences. 2019; 20(21):5488. https://doi.org/10.3390/ijms20215488
Chicago/Turabian StyleHong, Riwon, Kyoung Soo Kim, Gwang Muk Choi, Mijung Yeom, Bombi Lee, Sanghyun Lee, Ki Sung Kang, Hyang Sook Lee, Hi-Joon Park, and Dae-Hyun Hahm. 2019. "Continentalic Acid Rather Than Kaurenoic Acid Is Responsible for the Anti-Arthritic Activity of Manchurian Spikenard In Vitro and In Vivo" International Journal of Molecular Sciences 20, no. 21: 5488. https://doi.org/10.3390/ijms20215488
APA StyleHong, R., Kim, K. S., Choi, G. M., Yeom, M., Lee, B., Lee, S., Kang, K. S., Lee, H. S., Park, H.-J., & Hahm, D.-H. (2019). Continentalic Acid Rather Than Kaurenoic Acid Is Responsible for the Anti-Arthritic Activity of Manchurian Spikenard In Vitro and In Vivo. International Journal of Molecular Sciences, 20(21), 5488. https://doi.org/10.3390/ijms20215488








