Problems Associated with Deprescribing of Proton Pump Inhibitors
Abstract
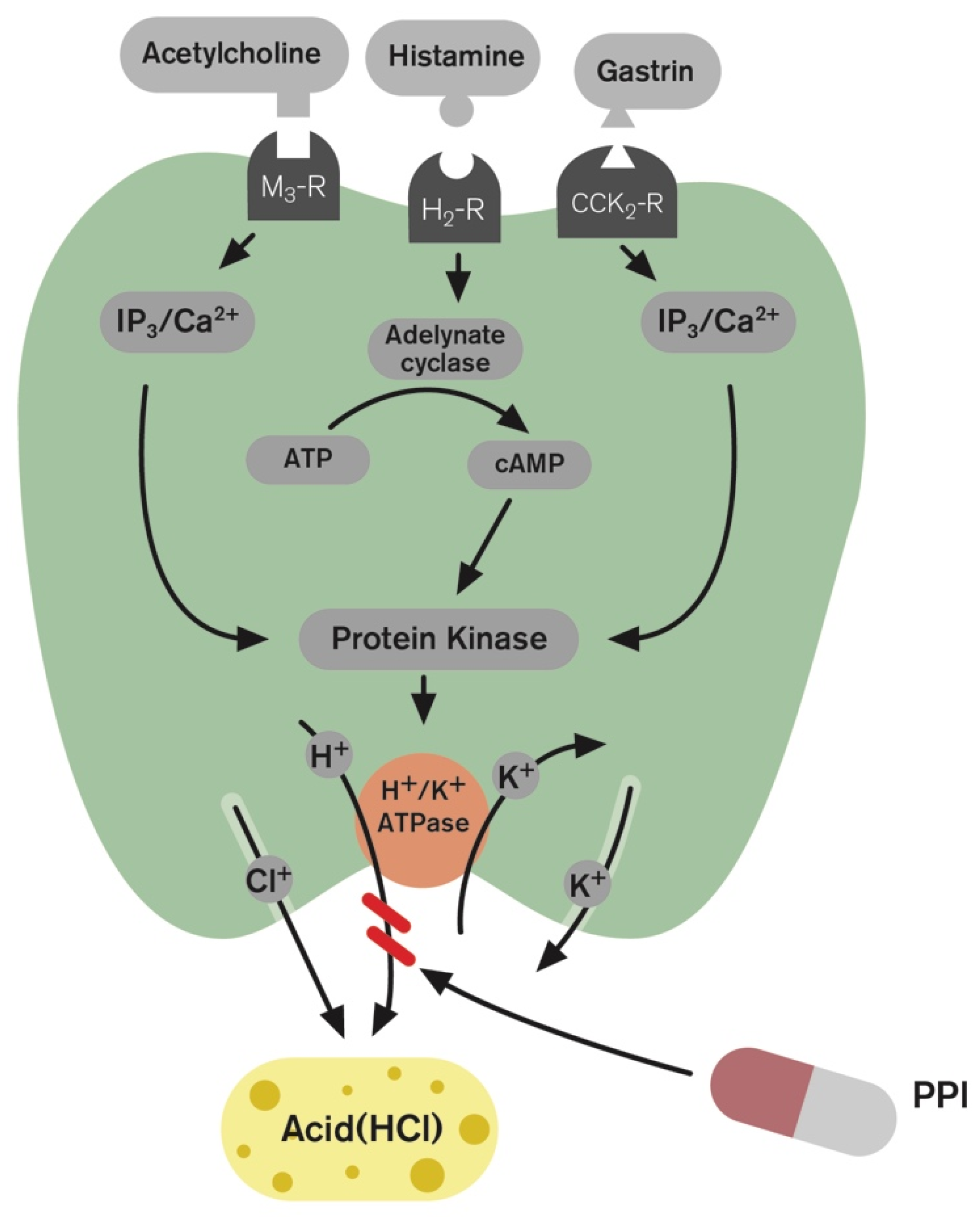
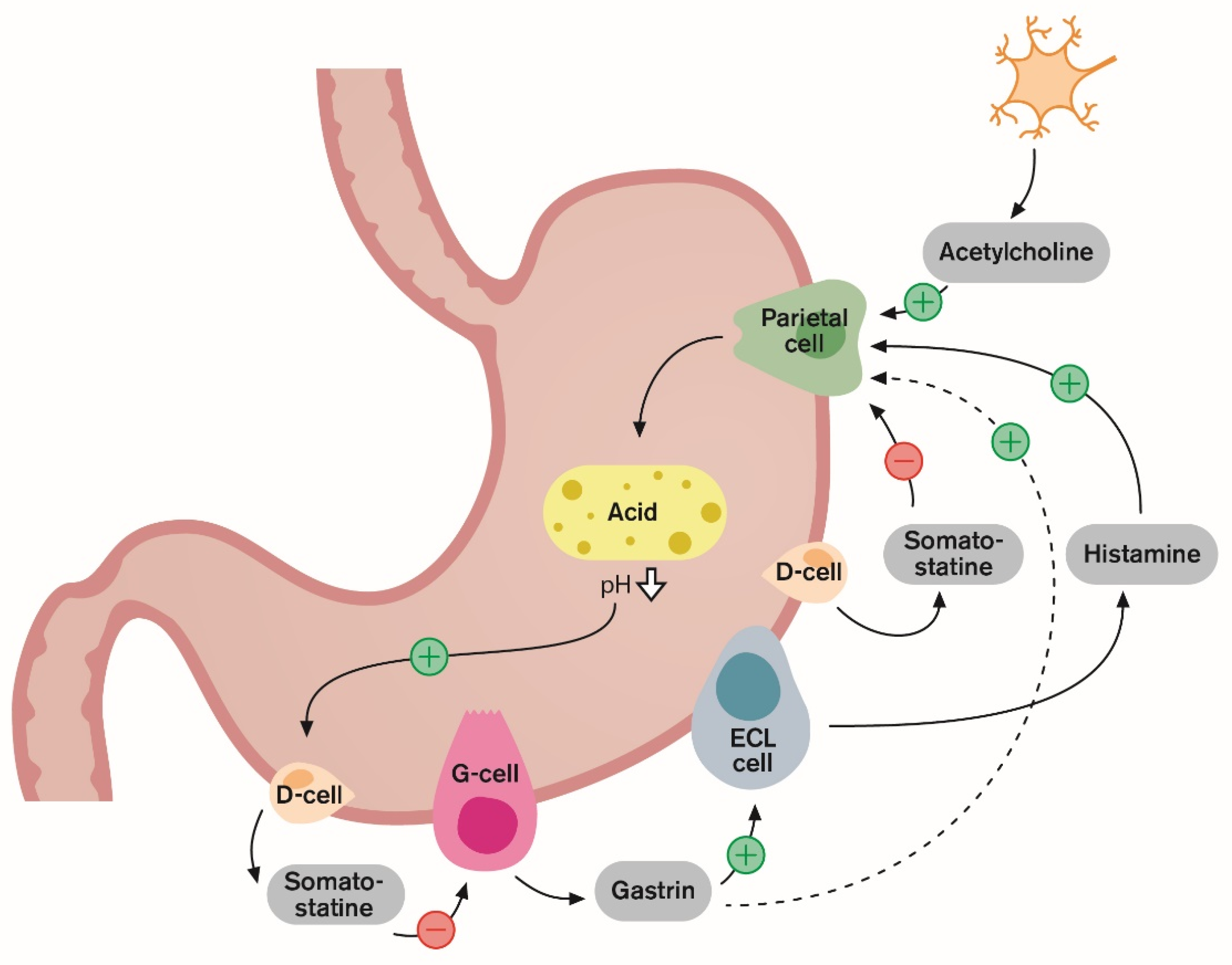
1. Introduction and Gastric Acid Regulation
2. Safety of Long-Term PPI Use
3. Definition of PPI Deprescribing
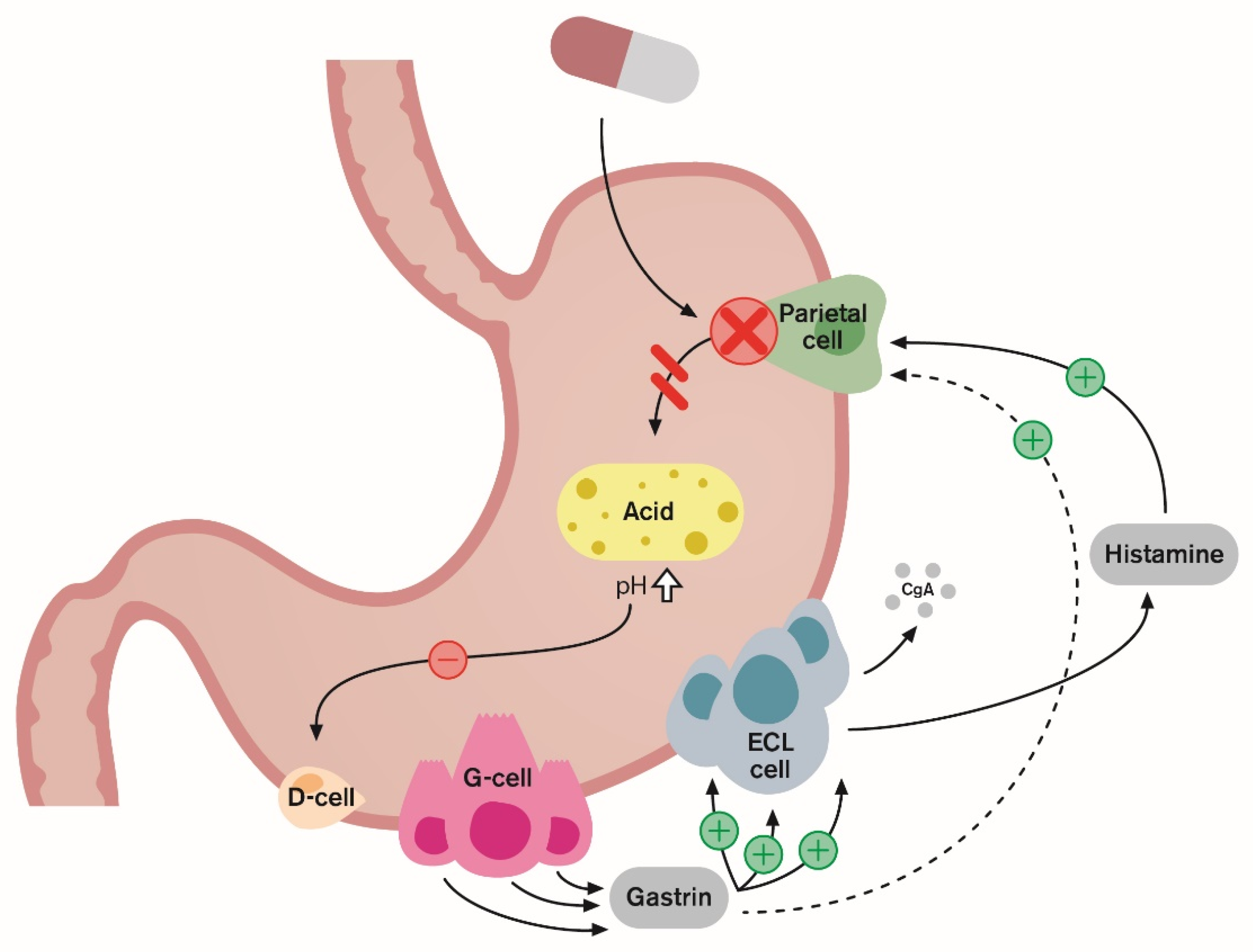
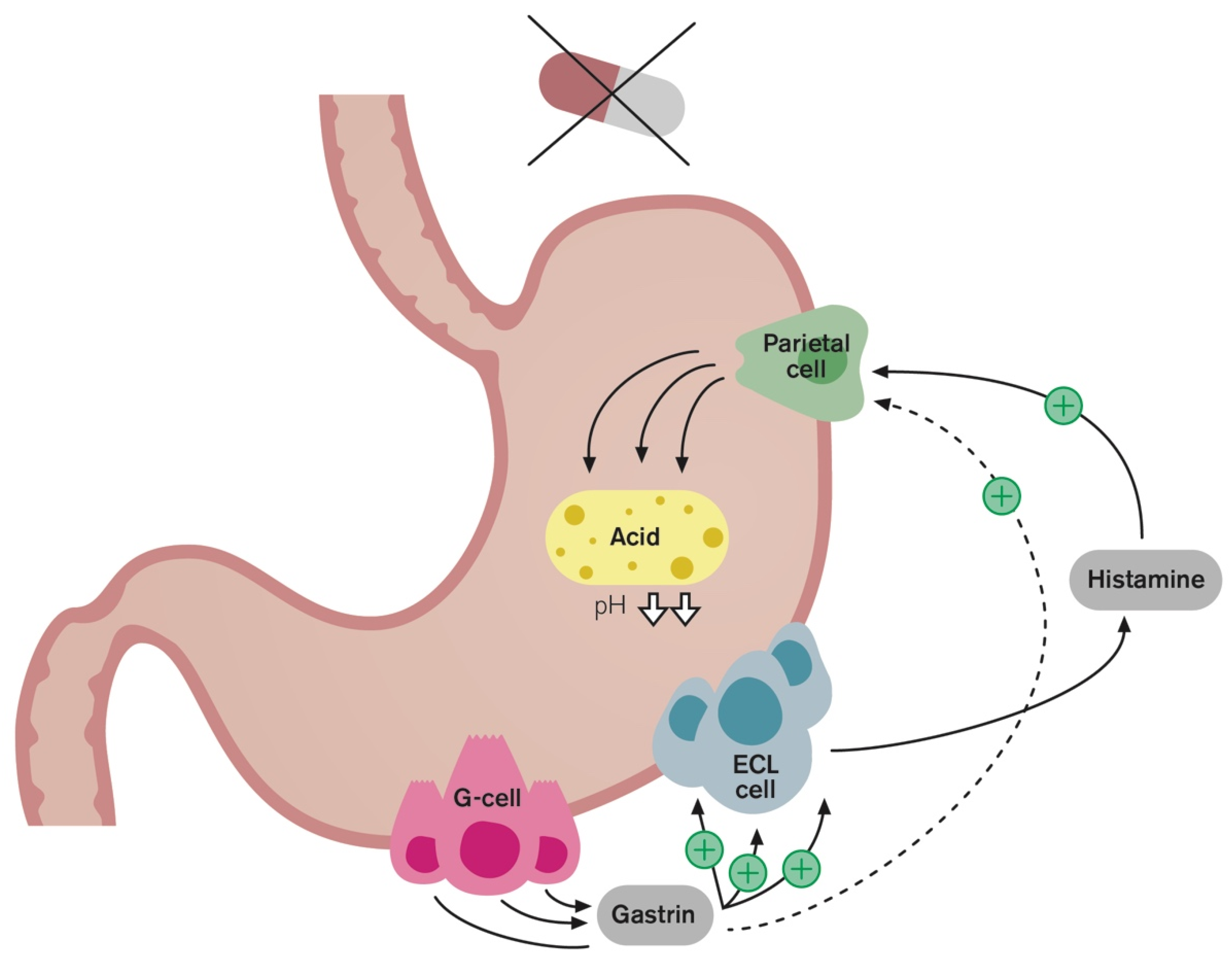
4. Rebound Acid Hypersecretion
5. PPI Desprescriping Trials
6. Deprescribing Guidelines
7. Large Gap in the Knowledge of What Should Be Recommended
8. Conclusions
Funding
Conflicts of Interest
References
- Chu, S.; Schubert, M.L. Gastric secretion. Curr. Opin. Gastroenterol. 2013, 29, 636–641. [Google Scholar] [CrossRef]
- Lindberg, P.; Brandstrom, A.; Wallmark, B.; Mattsson, H.; Rikner, L.; Hoffmann, K.J. Omeprazole: The first proton pump inhibitor. Med. Res. Rev. 1990, 10, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Kim, N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J. Neurogastroenterol. Motil. 2013, 19, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Savarino, V.; Di Mario, F.; Scarpignato, C. Proton pump inhibitors in GORD An overview of their pharmacology, efficacy and safety. Pharmacol. Res. 2009, 59, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Waldum, H.L. Physiological and clinical significance of enterochromaffin-like cell activation in the regulation of gastric acid secretion. World J. Gastroenterol. 2007, 13, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Bakke, I.; Qvigstad, G.; Sandvik, A.K.; Waldum, H.L. The CCK-2 receptor is located on the ECL cell, but not on the parietal cell. Scand. J. Gastroenterol. 2001, 36, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Bloom, S.R.; Mortimer, C.H.; Thorner, M.O.; Besser, G.M.; Hall, R.; Gomez-Pan, A.; Roy, V.M.; Russell, R.C.; Coy, D.H.; Kastin, A.J.; et al. Inhibition of gastrin and gastric-acid secretion by growth-hormone release-inhibiting hormone. Lancet 1974, 2, 1106–1109. [Google Scholar] [CrossRef]
- Gedda, K.; Scott, D.; Besancon, M.; Lorentzon, P.; Sachs, G. Turnover of the gastric H+,K(+)-adenosine triphosphatase alpha subunit and its effect on inhibition of rat gastric acid secretion. Gastroenterology 1995, 109, 1134–1141. [Google Scholar] [CrossRef]
- Hatlebakk, J.G.; Katz, P.O.; Camacho-Lobato, L.; Castell, D.O. Proton pump inhibitors: Better acid suppression when taken before a meal than without a meal. Aliment. Pharmacol. Ther. 2000, 14, 1267–1272. [Google Scholar] [CrossRef]
- Walsh, J.H. Role of gastrin as a trophic hormone. Digestion 1990, 47, 11–16. [Google Scholar] [CrossRef]
- Bjornsson, E.; Abrahamsson, H.; Simren, M.; Mattsson, N.; Jensen, C.; Agerforz, P.; Kilander, A. Discontinuation of proton pump inhibitors in patients on long-term therapy: A double-blind, placebo-controlled trial. Aliment. Pharmacol. Ther. 2006, 24, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Berna, M.J.; Hoffmann, K.M.; Serrano, J.; Gibril, F.; Jensen, R.T. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore) 2006, 85, 295–330. [Google Scholar] [CrossRef] [PubMed]
- Helgadottir, H.; Lund, S.H.; Gizurarson, S.; Metz, D.C.; Bjornsson, E.S. Predictors of Gastrin Elevation Following Proton Pump Inhibitor Therapy. J. Clin. Gastroenterol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Klotz, U. Proton pump inhibitors: An update of their clinical use and pharmacokinetics. Eur. J. Clin. Pharmacol. 2008, 64, 935–951. [Google Scholar] [CrossRef]
- Halfdanarson, O.O.; Pottegard, A.; Bjornsson, E.S.; Lund, S.H.; Ogmundsdottir, M.H.; Steingrimsson, E.; Ogmundsdottir, H.M.; Zoega, H. Proton-pump inhibitors among adults: A nationwide drug-utilization study. Therap. Adv. Gastroenterol. 2018, 11, 1756284818777943. [Google Scholar] [CrossRef]
- Pottegard, A.; Broe, A.; Hallas, J.; de Muckadell, O.B.; Lassen, A.T.; Lodrup, A.B. Use of proton-pump inhibitors among adults: A Danish nationwide drug utilization study. Therap. Adv. Gastroenterol. 2016, 9, 671–678. [Google Scholar] [CrossRef]
- Hollingworth, S.; Duncan, E.L.; Martin, J.H. Marked increase in proton pump inhibitors use in Australia. Pharmacoepidemiol. Drug Saf. 2010, 19, 1019–1024. [Google Scholar] [CrossRef]
- Katz, P.O.; Gerson, L.B.; Vela, M.F. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am. J. Gastroenterol. 2013, 108, 308–328. [Google Scholar] [CrossRef]
- Heidelbaugh, J.J.; Goldberg, K.L.; Inadomi, J.M. Overutilization of proton pump inhibitors: A review of cost-effectiveness and risk [corrected]. Am. J. Gastroenterol. 2009, 104 (Suppl. 2), S27–S32. [Google Scholar] [CrossRef]
- Ahrens, D.; Chenot, J.F.; Behrens, G.; Grimmsmann, T.; Kochen, M.M. Appropriateness of treatment recommendations for PPI in hospital discharge letters. Eur J. Clin. Pharmacol. 2010, 66, 1265–1271. [Google Scholar] [CrossRef]
- Molloy, D.; Molloy, A.; O’Loughlin, C.; Falconer, M.; Hennessy, M. Inappropriate use of proton pump inhibitors. Ir. J. Med. Sci. 2010, 179, 73–75. [Google Scholar] [CrossRef]
- Reimer, C.; Bytzer, P. Clinical trial: Long-term use of proton pump inhibitors in primary care patients—A cross sectional analysis of 901 patients. Aliment. Pharmacol. Ther. 2009, 30, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Metz, D.C. Safety of proton pump inhibitor exposure. Gastroenterology 2010, 139, 1115–1127. [Google Scholar] [CrossRef]
- Laheij, R.J.; Sturkenboom, M.C.; Hassing, R.J.; Dieleman, J.; Stricker, B.H.; Jansen, J.B. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004, 292, 1955–1960. [Google Scholar] [CrossRef]
- Yang, Y.X.; Lewis, J.D.; Epstein, S.; Metz, D.C. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006, 296, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Janarthanan, S.; Ditah, I.; Adler, D.G.; Ehrinpreis, M.N. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: A meta-analysis. Am. J. Gastroenterol. 2012, 107, 1001–1010. [Google Scholar] [CrossRef]
- Siple, J.F.; Morey, J.M.; Gutman, T.E.; Weinberg, K.L.; Collins, P.D. Proton pump inhibitor use and association with spontaneous bacterial peritonitis in patients with cirrhosis and ascites. Ann. Pharmacother. 2012, 46, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Eikelboom, J.W.; Bosch, J.; Connolly, S.J.; Dyal, L.; Shestakovska, O.; Leong, D.; Anand, S.S.; Stork, S.; Branch, K.R.H.; et al. Safety of Proton Pump Inhibitors Based on a Large, Multi-Year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin. Gastroenterology 2019, 157, 682–691.e2. [Google Scholar] [CrossRef]
- Myers, R.P.; McLaughlin, K.; Hollomby, D.J. Acute interstitial nephritis due to omeprazole. Am. J. Gastroenterol. 2001, 96, 3428–3431. [Google Scholar] [CrossRef] [PubMed]
- Shiraev, T.P.; Bullen, A. Proton Pump Inhibitors and Cardiovascular Events: A Systematic Review. Heart Lung Circ. 2018, 27, 443–450. [Google Scholar] [CrossRef]
- Gomm, W.; von Holt, K.; Thome, F.; Broich, K.; Maier, W.; Fink, A.; Doblhammer, G.; Haenisch, B. Association of Proton Pump Inhibitors With Risk of Dementia: A Pharmacoepidemiological Claims Data Analysis. JAMA Neurol. 2016, 73, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Morath, T.; Stegherr, J.; Braun, S.; Vogt, W.; Hadamitzky, M.; Schomig, A.; Kastrati, A.; von Beckerath, N. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb. Haemost. 2009, 101, 714–719. [Google Scholar] [PubMed]
- Bundhun, P.K.; Teeluck, A.R.; Bhurtu, A.; Huang, W.Q. Is the concomitant use of clopidogrel and Proton Pump Inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: A systematic review and meta-analysis of recently published studies (2012–2016). BMC Cardiovasc. Disord. 2017, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.L.; Qvigstad, G.; Fossmark, R.; Kleveland, P.M.; Sandvik, A.K. Rebound acid hypersecretion from a physiological, pathophysiological and clinical viewpoint. Scand. J. Gastroenterol. 2010, 45, 389–394. [Google Scholar] [CrossRef]
- Waldum, H.L.; Hauso, O.; Brenna, E.; Qvigstad, G.; Fossmark, R. Does long-term profound inhibition of gastric acid secretion increase the risk of ECL cell-derived tumors in man? Scand. J. Gastroenterol. 2016, 51, 767–773. [Google Scholar] [CrossRef]
- Tran-Duy, A.; Spaetgens, B.; Hoes, A.W.; de Wit, N.J.; Stehouwer, C.D. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1706–1719.e5. [Google Scholar] [CrossRef]
- Jianu, C.S.; Fossmark, R.; Viset, T.; Qvigstad, G.; Sordal, O.; Marvik, R.; Waldum, H.L. Gastric carcinoids after long-term use of a proton pump inhibitor. Aliment. Pharmacol. Ther. 2012, 36, 644–649. [Google Scholar] [CrossRef]
- Jianu, C.S.; Lange, O.J.; Viset, T.; Qvigstad, G.; Martinsen, T.C.; Fougner, R.; Kleveland, P.M.; Fossmark, R.; Hauso, O.; Waldum, H.L. Gastric neuroendocrine carcinoma after long-term use of proton pump inhibitor. Scand. J. Gastroenterol. 2012, 47, 64–67. [Google Scholar] [CrossRef]
- Cavalcoli, F.; Zilli, A.; Conte, D.; Ciafardini, C.; Massironi, S. Gastric neuroendocrine neoplasms and proton pump inhibitors: Fact or coincidence? Scand. J. Gastroenterol. 2015, 50, 1397–1403. [Google Scholar] [CrossRef]
- Brusselaers, N.; Wahlin, K.; Engstrand, L.; Lagergren, J. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: A nationwide population-based cohort study in Sweden. BMJ Open 2017, 7, e017739. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Wong, I.C.K.; Leung, W.K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut 2018, 67, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Israel, D.M.; Hassall, E. Omerprazole and other proton pump inhibitors: Pharmacology, efficacy, and safety, with special reference to use in children. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B.; Pottie, K.; Thompson, W.; Boghossian, T.; Pizzola, L.; Rashid, F.J.; Rojas-Fernandez, C.; Walsh, K.; Welch, V.; Moayyedi, P. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Can. Fam. Physician. 2017, 63, 354–364. [Google Scholar] [PubMed]
- Bytzer, P. Deprescribing proton pump inhibitors: Why, when and how. Med. J. Aust. 2018, 209, 436–438. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Kim, L.S.; Yang, Y.X. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology 2017, 152, 706–715. [Google Scholar] [CrossRef]
- Pilotto, A.; Leandro, G.; Franceschi, M.; Ageing and Acid-Related Disease Study Group. Short- and long-term therapy for reflux oesophagitis in the elderly: A multi-centre, placebo-controlled study with pantoprazole. Aliment. Pharmacol. Ther. 2003, 17, 1399–1406. [Google Scholar] [CrossRef]
- van der Velden, A.W.; de Wit, N.J.; Quartero, A.O.; Grobbee, D.E.; Numans, M.E. Pharmacological dependency in chronic treatment of gastroesophageal reflux disease: A randomized controlled clinical trial. Digestion 2010, 81, 43–52. [Google Scholar] [CrossRef]
- Reimer, C.; Sondergaard, B.; Hilsted, L.; Bytzer, P. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology 2009, 137, 80–87, 87.e1. [Google Scholar] [CrossRef]
- Niklasson, A.; Lindstrom, L.; Simren, M.; Lindberg, G.; Bjornsson, E. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: A double-blind placebo-controlled trial. Am. J. Gastroenterol. 2010, 105, 1531–1537. [Google Scholar] [CrossRef]
- Pregun, I.; Herszenyi, L.; Juhasz, M.; Miheller, P.; Hritz, I.; Patocs, A.; Racz, K.; Tulassay, Z. Effect of proton-pump inhibitor therapy on serum chromogranin a level. Digestion 2011, 84, 22–28. [Google Scholar] [CrossRef]
- Helgadottir, H.; Lund, S.H.; Gizurarson, S.; Waldum, H.; Bjornsson, E.S. Serum Concentration and Pharmacokinetics of Single and Repeated Oral Doses of Esomeprazole and Gastrin Elevation in Healthy Males and Females. Gastroenterology 2019, 156, abstract S-308. [Google Scholar] [CrossRef]
- Gillen, D.; Wirz, A.A.; Ardill, J.E.; McColl, K.E. Rebound hypersecretion after omeprazole and its relation to on-treatment acid suppression and Helicobacter pylori status. Gastroenterology 1999, 116, 239–247. [Google Scholar] [CrossRef]
- Waldum, H.L.; Arnestad, J.S.; Brenna, E.; Eide, I.; Syversen, U.; Sandvik, A.K. Marked increase in gastric acid secretory capacity after omeprazole treatment. Gut 1996, 39, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Farup, P.G.; Juul-Hansen, P.H.; Rydning, A. Does short-term treatment with proton pump inhibitors cause rebound aggravation of symptoms? J. Clin. Gastroenterol. 2001, 33, 206–209. [Google Scholar] [CrossRef]
- Juul-Hansen, P.; Rydning, A. Clinical and pathophysiological consequences of on-demand treatment with PPI in endoscopy-negative reflux disease. Is rebound hypersecretion of acid a problem? Scand. J. Gastroenterol. 2011, 46, 398–405. [Google Scholar] [CrossRef]
- Metz, D.C.; Pilmer, B.L.; Han, C.; Perez, M.C. Withdrawing PPI therapy after healing esophagitis does not worsen symptoms or cause persistent hypergastrinemia: Analysis of dexlansoprazole MR clinical trial data. Am. J. Gastroenterol. 2011, 106, 1953–1960. [Google Scholar] [CrossRef]
- Inadomi, J.M.; McIntyre, L.; Bernard, L.; Fendrick, A.M. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): A prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am. J. Gastroenterol. 2003, 98, 1940–1944. [Google Scholar] [CrossRef]
- McColl, K.E.; Gillen, D. Evidence that proton-pump inhibitor therapy induces the symptoms it is used to treat. Gastroenterology 2009, 137, 20–22. [Google Scholar] [CrossRef]
- Lundell, L.; Vieth, M.; Gibson, F.; Nagy, P.; Kahrilas, P.J. Systematic review: The effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment. Pharmacol. Ther. 2015, 42, 649–663. [Google Scholar] [CrossRef]
- Helgadottir, H.; Metz, D.C.; Lund, S.H.; Gizurarson, S.; Jacobsen, E.I.; Asgeirsdottir, G.A.; Yngadottir, Y.; Bjornsson, E.S. Study of Gender Differences in Proton Pump Inhibitor Dose Requirements for GERD: A Double-Blind Randomized Trial. J. Clin. Gastroenterol. 2017, 51, 486–493. [Google Scholar] [CrossRef]
- Haastrup, P.; Paulsen, M.S.; Begtrup, L.M.; Hansen, J.M.; Jarbol, D.E. Strategies for discontinuation of proton pump inhibitors: A systematic review. Fam. Pract. 2014, 31, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.K.; Walt, R.P.; Pounder, R.E.; Gomes, M.D.; Wood, E.C.; Logan, L.H. Optimal dose of oral omeprazole for maximal 24 h decrease of intragastric acidity. Gut 1984, 25, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Krol, N.; Wensing, M.; Haaijer-Ruskamp, F.; Muris, J.W.; Numans, M.E.; Schattenberg, G.; Balen, J.; Grol, R. Patient-directed strategy to reduce prescribing for patients with dyspepsia in general practice: A randomized trial. Aliment. Pharmacol. Ther. 2004, 19, 917–922. [Google Scholar] [CrossRef]
- Hungin, A.P.; Rubin, G.; O’Flanagan, H. Factors influencing compliance in long-term proton pump inhibitor therapy in general practice. Br. J. Gen. Pract. 1999, 49, 463–464. [Google Scholar] [PubMed]
- Boghossian, T.A.; Rashid, F.J.; Thompson, W.; Welch, V.; Moayyedi, P.; Rojas-Fernandez, C.; Pottie, K.; Farrell, B. Deprescribing versus continuation of chronic proton pump inhibitor use in adults. Cochrane Database Syst. Rev. 2017, 3, CD011969. [Google Scholar] [CrossRef] [PubMed]
- Bayerdorffer, E.; Bigard, M.A.; Weiss, W.; Mearin, F.; Rodrigo, L.; Dominguez Munoz, J.E.; Grundling, H.; Persson, T.; Svedberg, L.E.; Keeling, N.; et al. Randomized, multicenter study: On-demand versus continuous maintenance treatment with esomeprazole in patients with non-erosive gastroesophageal reflux disease. BMC Gastroenterol. 2016, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Bour, B.; Staub, J.L.; Chousterman, M.; Labayle, D.; Nalet, B.; Nouel, O.; Pariente, A.; Tocque, E.; Bonnot-Marlier, S. Long-term treatment of gastro-oesophageal reflux disease patients with frequent symptomatic relapses using rabeprazole: On-demand treatment compared with continuous treatment. Aliment. Pharmacol. Ther. 2005, 21, 805–812. [Google Scholar] [CrossRef]
- Janssen, W.; Meier, E.; Gatz, G.; Pfaffenberger, B. Effects of pantoprazole 20 mg in mildgastroesophageal reflux disease: Once-daily treatment in the acute phase, and comparison of on-demand versus continuous treatment in the long term. Curr. Ther. Res. Clin. Exp. 2005, 66, 345–363. [Google Scholar] [CrossRef][Green Version]
- Fass, R.; Inadomi, J.; Han, C.; Mody, R.; O’Neil, J.; Perez, M.C. Maintenance of heartburn relief after step-down from twice-daily proton pump inhibitor to once-daily dexlansoprazole modified release. Clin. Gastroenterol. Hepatol. 2012, 10, 247–253. [Google Scholar] [CrossRef]
- Cote, G.A.; Ferreira, M.R.; Rozenberg-Ben-Dror, K.; Howden, C.W. Programme of stepping down from twice daily proton pump inhibitor therapy for symptomatic gastro-oesophageal reflux disease associated with a formulary change at a VA medical center. Aliment. Pharmacol. Ther. 2007, 25, 709–714. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Okada, H.; Kawahara, Y.; Takenaka, R.; Nasu, J.; Ishioka, H.; Fujiwara, A.; Yoshinaga, F.; Yamamoto, K. Proton pump inhibitor step-down therapy for GERD: A multi-center study in Japan. World J. Gastroenterol. 2011, 17, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Zwisler, J.E.; Jarbol, D.E.; Lassen, A.T.; Kragstrup, J.; Thorsgaard, N.; Schaffalitzky de Muckadell, O.B. Placebo-Controlled Discontinuation of Long-Term Acid-Suppressant Therapy: A Randomised Trial in General Practice. Int. J. Fam. Med. 2015, 2015, 175436. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.H.; Lydeard, S.E.; Hobbs, F.D.; Kenkre, J.E.; Williams, E.I.; Jones, S.J.; Repper, J.A.; Caldow, J.L.; Dunwoodie, W.M.; Bottomley, J.M. Dyspepsia in England and Scotland. Gut 1990, 31, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, J.; Aro, P.; Storskrubb, T.; Johansson, S.E.; Lind, T.; Bolling-Sternevald, E.; Graffner, H.; Vieth, M.; Stolte, M.; Engstrand, L.; et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: A Kalixanda study report. Scand. J. Gastroenterol. 2005, 40, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Shaker, R.; Castell, D.O.; Schoenfeld, P.S.; Spechler, S.J. Nighttime heartburn is an under-appreciated clinical problem that impacts sleep and daytime function: The results of a Gallup survey conducted on behalf of the American Gastroenterological Association. Am. J. Gastroenterol. 2003, 98, 1487–1493. [Google Scholar] [CrossRef]
- Vigneri, S.; Termini, R.; Leandro, G.; Badalamenti, S.; Pantalena, M.; Savarino, V.; Di Mario, F.; Battaglia, G.; Mela, G.S.; Pilotto, A.; et al. A comparison of five maintenance therapies for reflux esophagitis. N Engl. J. Med. 1995, 333, 1106–1110. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Aguirre, T.V.; Davis, S.; Kuebeler, M.; Bhattacharyya, A.; Sampliner, R.E. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am. J. Gastroenterol. 2004, 99, 1877–1883. [Google Scholar] [CrossRef]
- Strand, D.S.; Kim, D.; Peura, D.A. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef]
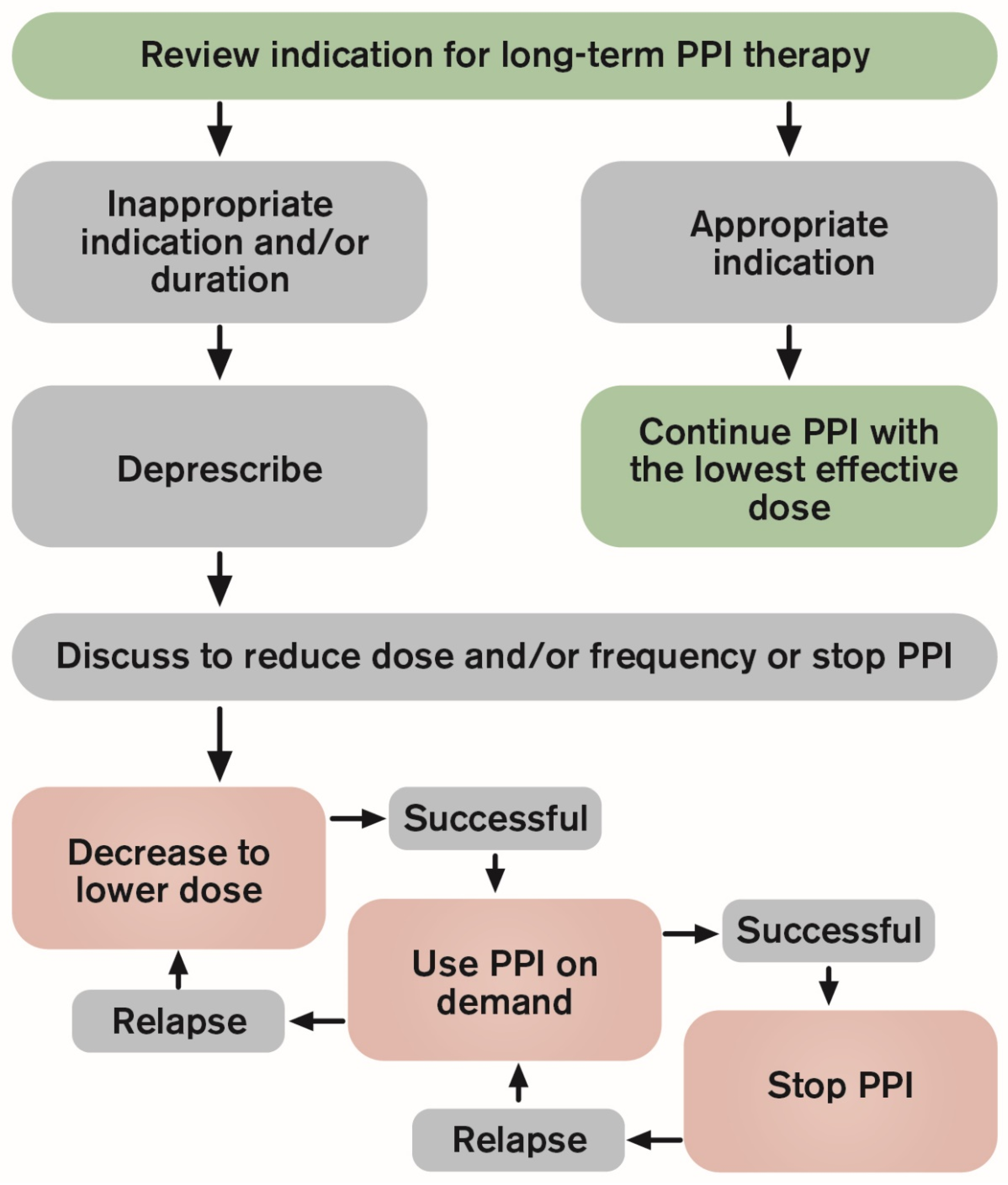
| The Steps of PPI Deprescribing | |
|---|---|
| Step 1 | Review indication and effectiveness |
| Step 2 | Assess the balance of benefits and harms |
| Step 3 | Assess patients values and preferences |
| Step 4 | Decide wether to continue, reduce dose or discontinue PPI therapy |
| Step 5 | Deprescribe and monitor |
| Step-Down Studies | |||
| Authors: Helgadottir et al. (2017) [69] | Participants: GERD patients with EE (n = 50) | Deprescribing method: Step-down dose by half vs. continuous same-dose treatment | Outcome: Step-down was successful in 76% |
| Methods: A double-blind randomized trial Country: Iceland | PPI duration: > 2 years PPI dose: 40 or 20 mg Age: median 59 years Gender (F/M): 25/25 | Setting: Hospital Follow-up time: 8 weeks | Comment: Female gender was an independent predictor for a successful step-down |
| Authors: Inadomi et al. (2003) [57] | Participants: Heartburn patients (n = 117) | Deprescribing method: Step-down from multiple- to single-dose | Outcome: Step-down was successful in 79.5% |
| Methods: A non-controlled prospective study Country: USA | PPI duration: > 8 weeks PPI dose: > 20 mg Age: median 66 years Gender (F/M): 5/112 | Setting: VA hospital and outpatient clinic Follow-up time: 6 months | Comment: Longer PPI duration before step-down was an independent predictor of PPI requirement |
| Discontinuing Studies | |||
| Authors: van der Velden et al. (2010) [47] | Participants: GERD patients on long-term PPI therapy (n = 141) | Deprescribing method: Abrupt discontinuation with 20 mg PPI as escape medication vs. daily 20 mg PPI with placebo escape medication | Outcome: 32% persisted daily PPI dosage, 43% reduced their dosage, 25% used less than 2 tablets/week. |
| Methods: A double-blind, parallel-group trial Country: The Netherlands | PPI duration: > 6 months PPI dose: 20 mg Age: mean 57 years Gender ratio: 56% | Setting: Primary care Follow-up time: 13 weeks | Comment: About 20% of long-term PPI users became satisfied on placebo with hardly any PPIs (0.7 tab-let/week) |
| Authors: Zwisler et al. (2015) [73] | Participants: Long-term PPI users without history of esophagitis, ulceration or current NSAIDs use. (n = 85) | Deprescribing method: Abrupt discontinuation vs. continuous treatment | Outcome: Discontinuation was successful in 27% of patients |
| Methods: A double-blinded randomised placebo-controlled trial Country: Denmark | PPI duration: > 8 weeks PPI dose: 40 mg Age: median 59 years Gender (F/M): 48/37 | Setting: Primary care Follow-up time: 1 year | Comment: Significantly more men had an unsuccessful discontinuation |
| Authors: Björnsson et al. (2006) [11] | Participants: Long-term PPI users without PUD or EE (n = 96) | Deprescribing method: Discontinuation: abrupt vs. 3 weeks tapering | Outcome: Discontinuation was successful in 27% (31% of tapering and 22% of abrupt discontinuation, NS) |
| Methods: A double-blind, placebo-controlled trial Country: Sweden | PPI duration: > 8 weeks PPI dose: 20 mg Age: median 63 years Gender (F/M): 52/44 | Setting: Hospital Follow-up time: 1 year | Comment: GERD and serum gastrin were independent predictors of PPI requirement |
| Authors: Pilotto et al. (2003) [46] | Participants: Erosive esophagitis patients (n = 56) | Deprescribing method: Abrupt discontinuation vs. continuous treatment | Outcome: 62.5% had a relapse of erosive esophagitis 6-months after discontinuation |
| Methods: A prospective, randomized, double-blind study Country: Italy | PPI duration: 6 months PPI dose: 20 mg Age: > 65 years of ageGender (F/M): Not given for the double-blind phase | Setting: Hospital Follow-up time: 1 year | Comment: 81% healing rate was in the maintenance phase after 4-months of 20 mg following a step-down from 40 mg for 8 weeks. |
| On-Demand Studies | |||
| Authors: Bayerdorffer et al. (2016) [66] | Participants: Symptomatic NERD patients (n = 301) | Deprescribing method: On-demand vs. continuous treatment | Outcome: On-demand was successful for 92% |
| Methods: A multicenter, open-label, randomized, parallel-group study Country: Austria, France, Germany, South Africa and Spain | PPI duration: 4 weeks PPI dose: 20 mg Age: mean 48 years Gender (F/M): 179/122 | Setting: Hospital Follow-up time: 6 months | Comment: On-demand treatment was non-inferior to continuous treatment |
| Authors: Bour et al. (2005) [67] | Participants: Non-severe GERD patients with frequent symptom relapses (n = 71) | Deprescribing method: On-demand vs. continuous treatment | Outcome: On-demand was successful, with a high symptom relief in 74.6% |
| Methods: A randomized, open-label study Country: France | PPI duration: > 1 year PPI dose: 10 mg Age: average 50 years Gender ratio: 58% men | Setting: Hospital Follow-up time: 6 months | Comment: There was a significant decrease in medication consumption in the on-demand group |
| Authors: Janssen et al. (2005) [68] | Participants: GERD patients (n = 215) | Deprescribing method: On-demand vs. continuous treatment | Outcome: On-demand was successful in 69.3% |
| Methods: A multicentre, open-label Country: Germany, France, Switzerland and Hungary. | PPI duration: 4 weeks PPI dose: 20 mg Age: mean 50 years Gender (F/M): 115/100 | Setting: Did not describe the clinical settings or type of centers Follow-up time: 6 months | Comment: Patients were satisfied with the on-demand therapy which was non-inferior to continuous therapy with regard to symptom control |
| Indications for Continuous PPI Therapy |
|---|
| Severe esophagitis (LA grade C or D) Barrets esophagus |
| Documented history of bleeding GI ulcer |
| Chronic NSAIDs use with bleeding risk factors |
| Zollinger-Ellison syndrome |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helgadottir, H.; Bjornsson, E.S. Problems Associated with Deprescribing of Proton Pump Inhibitors. Int. J. Mol. Sci. 2019, 20, 5469. https://doi.org/10.3390/ijms20215469
Helgadottir H, Bjornsson ES. Problems Associated with Deprescribing of Proton Pump Inhibitors. International Journal of Molecular Sciences. 2019; 20(21):5469. https://doi.org/10.3390/ijms20215469
Chicago/Turabian StyleHelgadottir, Holmfridur, and Einar S. Bjornsson. 2019. "Problems Associated with Deprescribing of Proton Pump Inhibitors" International Journal of Molecular Sciences 20, no. 21: 5469. https://doi.org/10.3390/ijms20215469
APA StyleHelgadottir, H., & Bjornsson, E. S. (2019). Problems Associated with Deprescribing of Proton Pump Inhibitors. International Journal of Molecular Sciences, 20(21), 5469. https://doi.org/10.3390/ijms20215469





