Monocytic Myeloid Derived Suppressor Cells in Hematological Malignancies
Abstract
1. Introduction
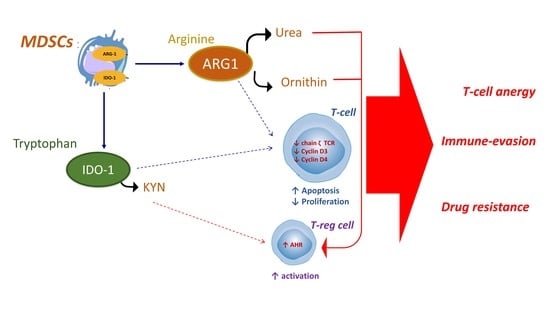
2. Monocytic Myeloid Derived Suppressor Cells in Hematological Malignancies
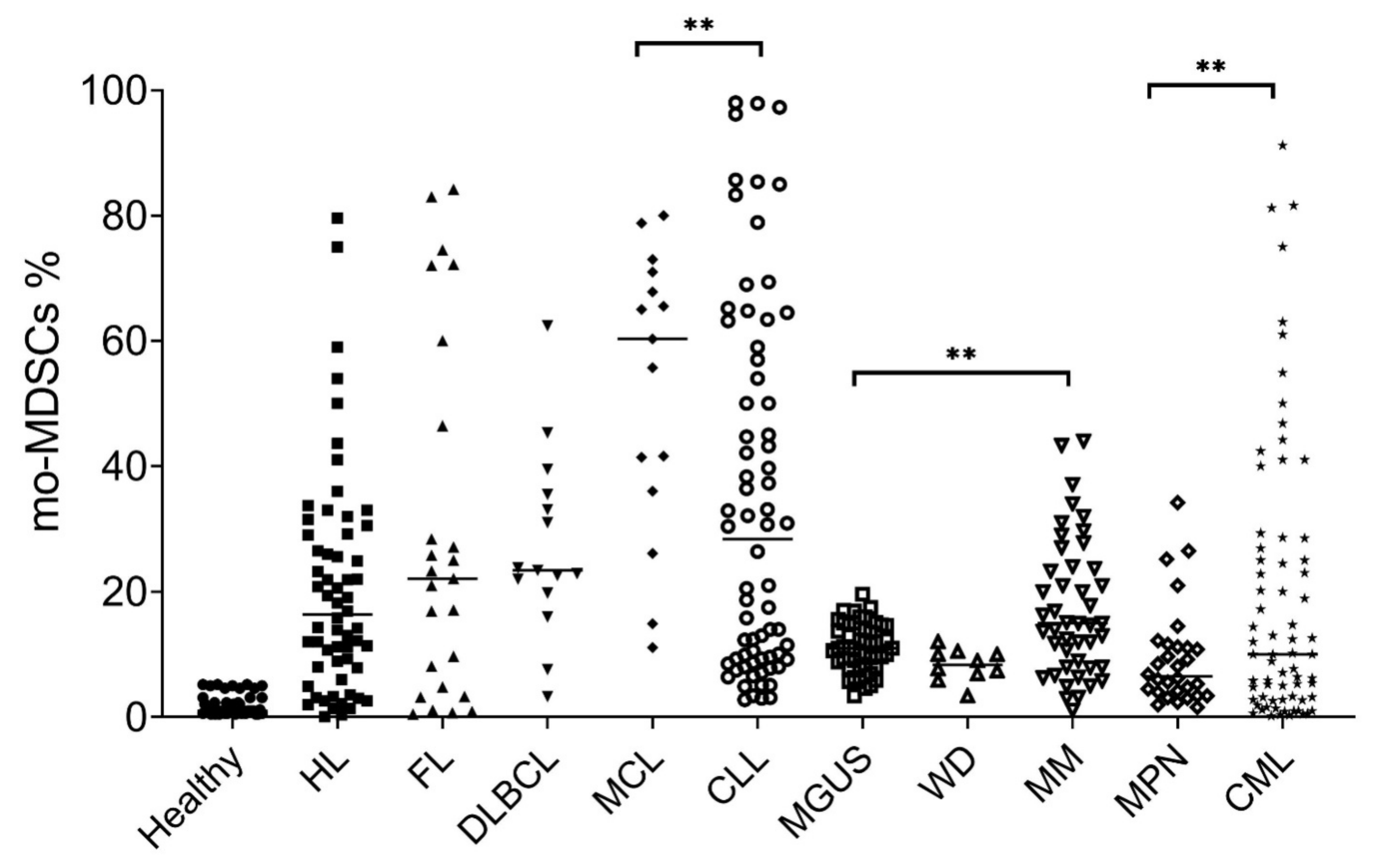
3. Monocytic Myeloid Derived Suppressor Cells in Lymphoma
4. Monocytic Myeloid Derived Suppressor Cells in Hodgkin Lymphoma
5. Monocytic Myeloid Derived Suppressor Cells in Non-Hodgkin Lymphoma
6. Monocytic Myeloid Derived Suppressor Cells in Chronic Lymphocytic Leukemia
7. Monocytic Myeloid Derived Suppressor Cells in Plasma Cell Dyscrasias
8. Monocytic Myeloid Derived Suppressor Cells in Myeloproliferative Neoplasms
9. Conclusions
- 1)
- MDSC function: phosphodiesterase inhibitors, nitroaspirins, synthetic triterpenoids, COX2 inhibitors, ARG1 inhibitors, anti-glycan antibodies, and IL-17 inhibitors can affect the immune-suppressive and restore T-cell activity;
- 2)
- MDSC maturation: ATRA, vitamins A or D3 or IL-12 can promote differentiation of g-MDSCs in neutrophils; while N-Bisphosphonates, modulators of tyrosine kinases, and STAT3 inhibitors can affect the transcriptional program of all sub-types of MDSCs;
- 3)
- MDSC depletion: several conventional chemotherapeutic agents (e.g., gemcitabine, fludarabine and cyclophosphamide) or novel immune agents (e.g., daratumumab) can affect viability of MDSCs and exert off-target effects, relevant in a different way for each specific tumor sub-type.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MDSC | Myeloid derived suppressor cells |
| MM | Multiple myeloma |
| CLL | Chronic lymphatic leukemia |
| CML | Chronic myeloid leukemia |
References
- Nagaraj, S.; Schrum, A.G.; Cho, H.I.; Celis, E.; Gabrilovich, D.I. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J. Immunol. 2010, 184, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Condamine, T.; Gabrilovich, D.I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011, 32, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Kumar, V.; Cheng, P.; Condamine, T.; Mony, S.; Languino, L.R.; McCaffrey, J.C.; Hockstein, N.; Guarino, M.; Masters, G.; Penman, E.; et al. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity 2016, 44, 303–315. [Google Scholar] [CrossRef]
- Strauss, L.; Sangaletti, S.; Consonni, F.M.; Szebeni, G.; Morlacchi, S.; Totaro, M.G.; Porta, C.; Anselmo, A.; Tartari, S.; Doni, A.; et al. RORC1 Regulates Tumor-Promoting “Emergency” Granulo-Monocytopoiesis. Cancer Cell 2015, 28, 253–269. [Google Scholar] [CrossRef]
- Schlecker, E.; Stojanovic, A.; Eisen, C.; Quack, C.; Falk, C.S.; Umansky, V.; Cerwenka, A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J. Immunol. 2012, 189, 5602–5611. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Sinha, P.; Beury, D.W.; Clements, V.K. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin. Cancer Biol. 2012, 22, 275–281. [Google Scholar] [CrossRef]
- Xu, Z.; Ji, J.; Xu, J.; Li, D.; Shi, G.; Liu, F.; Ding, L.; Ren, J.; Dou, H.; Wang, T.; et al. MiR-30a increases MDSC differentiation and immunosuppressive function by targeting SOCS3 in mice with B-cell lymphoma. FEBS J. 2017, 284, 2410–2424. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Youn, J.I.; Gabrilovich, D.I. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur. J. Immunol. 2010, 40, 2969–2975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Maric, I.; DiPrima, M.J.; Khan, J.; Orentas, R.J.; Kaplan, R.N.; Mackall, C.L. Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood 2013, 122, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Zoso, A.; Mazza, E.M.; Bicciato, S.; Mandruzzato, S.; Bronte, V.; Serafini, P.; Inverardi, L. Human fibrocytic myeloid-derived suppressor cells express IDO and promote tolerance via Treg-cell expansion. Eur. J. Immunol. 2014, 44, 3307–3319. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Kesikli, S.A.; Guc, D. Cancer associated fibroblasts have phenotypic and functional characteristics similar to the fibrocytes that represent a novel MDSC subset. Oncoimmunology 2015, 4, e1034918. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Ernstoff, M.S.; Hernandez, C.; Atkins, M.; Zabaleta, J.; Sierra, R.; Ochoa, A.C. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009, 69, 1553–1560. [Google Scholar] [CrossRef]
- Brandau, S.; Trellakis, S.; Bruderek, K.; Schmaltz, D.; Steller, G.; Elian, M.; Suttmann, H.; Schenck, M.; Welling, J.; Zabel, P.; et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J. Leukoc. Biol. 2011, 89, 311–317. [Google Scholar] [CrossRef]
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.I.; Cheng, P.; Cho, H.I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010, 207, 2439–2453. [Google Scholar] [CrossRef]
- Ku, A.W.; Muhitch, J.B.; Powers, C.A.; Diehl, M.; Kim, M.; Fisher, D.T.; Sharda, A.P.; Clements, V.K.; O’Loughlin, K.; Minderman, H.; et al. Tumor-induced MDSC act via remote control to inhibit L-selectin-dependent adaptive immunity in lymph nodes. Elife 2016, 5. [Google Scholar] [CrossRef]
- Romano, A.; Parrinello, N.L.; Chiarenza, A.; Motta, G.; Tibullo, D.; Giallongo, C.; la Cava, P.; Camiolo, G.; Puglisi, F.; Palumbo, G.A.; et al. Immune off-target effects of Brentuximab Vedotin in relapsed/refractory Hodgkin Lymphoma. Br. J. Haematol. 2019, 185, 468–479. [Google Scholar] [CrossRef]
- Romano, A.; Parrinello, N.L.; Vetro, C.; Forte, S.; Chiarenza, A.; Figuera, A.; Motta, G.; Palumbo, G.A.; Ippolito, M.; Consoli, U.; et al. Circulating myeloid-derived suppressor cells correlate with clinical outcome in Hodgkin Lymphoma patients treated up-front with a risk-adapted strategy. Br. J. Haematol. 2015, 168, 689–700. [Google Scholar] [CrossRef]
- Agostinelli, C.; Gallamini, A.; Stracqualursi, L.; Agati, P.; Tripodo, C.; Fuligni, F.; Sista, M.T.; Fanti, S.; Biggi, A.; Vitolo, U.; et al. The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin’s lymphoma: A retrospective, European, multicentre cohort study. Lancet Haematol. 2016, 3, e467–e479. [Google Scholar] [CrossRef]
- Romano, A.; Parrinello, N.L.; Vetro, C.; Tibullo, D.; Giallongo, C.; la Cava, P.; Chiarenza, A.; Motta, G.; Caruso, A.L.; Villari, L.; et al. The prognostic value of the myeloid-mediated immunosuppression marker Arginase-1 in classic Hodgkin lymphoma. Oncotarget 2016, 7, 67333–67346. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Vetro, C.; Caocci, G.; Greco, M.; Parrinello, N.L.; di Raimondo, F.; la Nasa, G. Immunological deregulation in classic hodgkin lymphoma. Mediterr. J. Hematol. Infect. Dis. 2014, 6, e2014039. [Google Scholar] [PubMed]
- Vetro, C.; Romano, A.; Ancora, F.; Coppolino, F.; Brundo, M.V.; Raccuia, S.A.; Puglisi, F.; Tibullo, D.; la Cava, P.; Giallongo, C.; et al. Clinical Impact of the Immunome in Lymphoid Malignancies: The Role of Myeloid-Derived Suppressor Cells. Front. Oncol. 2015, 5, 104. [Google Scholar] [CrossRef]
- Manson, G.; Houot, R. Next-generation immunotherapies for lymphoma: One foot in the future. Ann. Oncol. 2018, 29, 588–601. [Google Scholar] [CrossRef]
- De Charette, M.; Marabelle, A.; Houot, R. Turning tumour cells into antigen presenting cells: The next step to improve cancer immunotherapy? Eur. J. Cancer 2016, 68, 134–147. [Google Scholar] [CrossRef]
- Wiktorin, H.G.; Nilsson, M.S.; Kiffin, R.; Sander, F.E.; Lenox, B.; Rydstrom, A.; Hellstrand, K.; Martner, A. Histamine targets myeloid-derived suppressor cells and improves the anti-tumor efficacy of PD-1/PD-L1 checkpoint blockade. Cancer Immunol. Immunother. 2019, 68, 163–174. [Google Scholar] [CrossRef]
- Serafini, P.; Mgebroff, S.; Noonan, K.; Borrello, I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008, 68, 5439–5449. [Google Scholar] [CrossRef]
- Serafini, P.; Meckel, K.; Kelso, M.; Noonan, K.; Califano, J.; Koch, W.; Dolcetti, L.; Bronte, V.; Borrello, I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 2006, 203, 2691–2702. [Google Scholar] [CrossRef]
- McKee, S.J.; Tuong, Z.K.; Kobayashi, T.; Doff, B.L.; Soon, M.S.; Nissen, M.; Lam, P.Y.; Keane, C.; Vari, F.; Moi, D.; et al. B cell lymphoma progression promotes the accumulation of circulating Ly6Clo monocytes with immunosuppressive activity. Oncoimmunology 2018, 7, e1393599. [Google Scholar] [CrossRef]
- Hanson, E.M.; Clements, V.K.; Sinha, P.; Ilkovitch, D.; Ostrand-Rosenberg, S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J. Immunol. 2009, 183, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.H.; Horn, L.A.; Ostrand-Rosenberg, S. High-mobility group box protein 1 promotes the survival of myeloid-derived suppressor cells by inducing autophagy. J. Leukoc. Biol. 2016, 100, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.H.; Beury, D.W.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv. Cancer Res. 2015, 128, 95–139. [Google Scholar] [PubMed]
- Parker, K.H.; Sinha, P.; Horn, L.A.; Clements, V.K.; Yang, H.; Li, J.; Tracey, K.J.; Ostrand-Rosenberg, S. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014, 74, 5723–5733. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Liu, H.; Zhao, P.; Chen, Y.; Luo, Y.; Zhang, Z.; Wang, X. Mesenchymal stromal cells enhance the suppressive effects ofmyeloid-derived suppressor cells of multiple myeloma. Leuk. Lymphoma 2017, 58, 2668–2676. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Bulur, P.A.; Dogan, A.; Gastineau, D.A.; Dietz, A.B.; Lin, Y. Immune independent crosstalk between lymphoma and myeloid suppressor CD14(+)HLA-DR(low/neg) monocytes mediates chemotherapy resistance. Oncoimmunology 2015, 4, e996470. [Google Scholar] [CrossRef]
- Gallamini, A.; di Raimondo, F.; la Nasa, G.; Romano, A.; Borra, A.; Greco, M. Standard therapies versus novel therapies in Hodgkin lymphoma. Immunol. Lett. 2013, 55, 56–59. [Google Scholar] [CrossRef]
- Marini, O.; Spina, C.; Mimiola, E.; Cassaro, A.; Malerba, G.; Todeschini, G.; Perbellini, O.; Scupoli, M.; Carli, G.; Facchinelli, D.; et al. Identification of granulocytic myeloid-derived suppressor cells (G-MDSCs) in the peripheral blood of Hodgkin and non-Hodgkin lymphoma patients. Oncotarget 2016, 7, 27676–27688. [Google Scholar] [CrossRef]
- Amini, R.M.; Enblad, G.; Hollander, P.; Laszlo, S.; Eriksson, E.; Gustafsson, K.A.; Loskog, A.; Thörn, I. Altered profile of immune regulatory cells in the peripheral blood of lymphoma patients. BMC Cancer 2019, 19, 316. [Google Scholar] [CrossRef]
- Von Hohenstaufen, K.A.; Conconi, A.; de Campos, C.P.; Franceschetti, S.; Bertoni, F.; Casaluci, G.M.; Stathis, A.; Ghielmini, M.; Stussi, G.; Cavalli, F.; et al. Prognostic impact of monocyte count at presentation in mantle cell lymphoma. Br. J. Haematol. 2013, 162, 465–473. [Google Scholar] [CrossRef]
- Porrata, L.F.; Ristow, K.; Markovic, S.N. Absolute monocyte count at diagnosis and survival in mantle cell lymphoma. Br. J. Haematol. 2013, 163, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, T.; Fell, R.; Polliack, A.; Attias, D. Absolute monocytosis at diagnosis correlates with survival in diffuse large B-cell lymphoma-possible link with monocytic myeloid-derived suppressor cells. Hematol. Oncol. 2013, 31, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gustafson, M.P.; Bulur, P.A.; Gastineau, D.A.; Witzig, T.E.; Dietz, A.B. Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood 2011, 117, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.S.; Wright, G.; Tan, B.; Rosenwald, A.; Gascoyne, R.D.; Chan, W.C.; Fisher, R.I.; Braziel, R.M.; Rimsza, L.M.; Grogan, T.M.; et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N. Engl. J. Med. 2004, 351, 2159–2169. [Google Scholar] [CrossRef]
- Stevens, W.B.C.; Mendeville, M.; Redd, R.; Clear, A.J.; Bladergroen, R.; Calaminici, M.; Rosenwald, A.; Hoster, E.; Hiddemann, W.; Gaulard, P.; et al. Prognostic relevance of CD163 and CD8 combined with EZH2 and gain of chromosome 18 in follicular lymphoma: A study by the Lunenburg Lymphoma Biomarker Consortium. Haematologica 2017, 102, 1413–1423. [Google Scholar] [CrossRef]
- Kridel, R.; Xerri, L.; Gelas-Dore, B.; Tan, K.; Feugier, P.; Vawda, A.; Canioni, D.; Farinha, P.; Boussetta, S.; Moccia, A.A.; et al. The Prognostic Impact of CD163-Positive Macrophages in Follicular Lymphoma: A Study from the BC Cancer Agency and the Lymphoma Study Association. Clin. Cancer Res. 2015, 21, 3428–3435. [Google Scholar] [CrossRef]
- Roussel, M.; Irish, J.M.; Menard, C.; Lhomme, F.; Tarte, K.; Fest, T. Regulatory myeloid cells: An underexplored continent in B-cell lymphomas. Cancer Immunol. Immunother. 2017, 66, 1103–1111. [Google Scholar] [CrossRef]
- Azzaoui, I.; Uhel, F.; Rossille, D.; Pangault, C.; Dulong, J.; le Priol, J.; Lamy, T.; Houot, R.; le Gouill, S.; Cartron, G.; et al. T-cell defect in diffuse large B-cell lymphomas involves expansion of myeloid-derived suppressor cells. Blood 2016, 128, 1081–1092. [Google Scholar] [CrossRef]
- Sato, Y.; Shimizu, K.; Shinga, J.; Hidaka, M.; Kawano, F.; Kakimi, K.; Yamasaki, S.; Asakura, M.; Fujii, S.I. Characterization of the myeloid-derived suppressor cell subset regulated by NK cells in malignant lymphoma. Oncoimmunology 2015, 4, e995541. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Liu, X.; Yang, P.; Xu, J.; Chai, Y.; Guo, Q.; Wang, Z.; Zhang, L. Prognostic Significance of Monocytes and Monocytic Myeloid-Derived Suppressor Cells in Diffuse Large B-Cell Lymphoma Treated with R-CHOP. Cell Physiol. Biochem. 2016, 39, 521–530. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Zhang, X.; Chai, Y.; Guo, Q.; Li, L.; Yue, L.; Bai, J.; Wang, Z.; Zhang, L. Prognostic significance of peripheral monocytic myeloid-derived suppressor cells and monocytes in patients newly diagnosed with diffuse large b-cell lymphoma. Int. J. Clin. Exp. Med. 2015, 8, 15173–15181. [Google Scholar] [PubMed]
- Jitschin, R.; Braun, M.; Buttner, M.; Dettmer-Wilde, K.; Bricks, J.; Berger, J.; Eckart, M.J.; Krause, S.W.; Oefner, P.J.; le Blanc, K.; et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood 2014, 124, 750–760. [Google Scholar] [CrossRef] [PubMed]
- De Charette, M.; Houot, R. Hide or defend, the two strategies of lymphoma immune evasion: Potential implications for immunotherapy. Haematologica 2018, 103, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, S.; Hara, T.; Tsurumi, H.; Hoshi, M.; Kanemura, N.; Goto, N.; Kasahara, S.; Shimizu, M.; Ito, H.; Saito, K.; et al. Indoleamine 2,3-dioxygenase in tumor tissue indicates prognosis in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann. Hematol. 2011, 90, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Hara, T.; Tsurumi, H.; Goto, N.; Hoshi, M.; Kitagawa, J.; Kanemura, N.; Kasahara, S.; Ito, H.; Takemura, M.; et al. Serum concentration of L-kynurenine predicts the clinical outcome of patients with diffuse large B-cell lymphoma treated with R-CHOP. Eur. J. Haematol. 2010, 84, 304–309. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Lu, K.; Feng, L.-L.; Ding, M.; Gao, J.M.; Ge, X.L.; Wang, X. Up-regulated expression of indoleamine 2,3-dioxygenase 1 in non-Hodgkin lymphoma correlates with increased regulatory T-cell infiltration. Leuk. Lymphoma 2014, 55, 405–414. [Google Scholar] [CrossRef]
- Giannoni, P.; Pietra, G.; Travaini, G.; Quarto, R.; Shyti, G.; Benelli, R.; Ottaggio, L.; Mingari, M.C.; Zupo, S.; Cutrona, G.; et al. Chronic lymphocytic leukemia nurse-like cells express hepatocyte growth factor receptor (c-MET) and indoleamine 2,3-dioxygenase and display features of immunosuppressive type 2 skewed macrophages. Haematologica 2014, 99, 1078–1087. [Google Scholar] [CrossRef]
- Ninomiya, S.; Narala, N.; Huye, L.; Yagyu, S.; Savoldo, B.; Dotti, G.; Heslop, H.E.; Brenner, M.K.; Rooney, C.M.; Ramos, C.A. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood 2015, 125, 3905–3916. [Google Scholar] [CrossRef]
- Stiff, A.; Trikha, P.; Wesolowski, R.; Kendra, K.; Hsu, V.; Uppati, S.; McMichael, E.; Duggan, M.; Campbell, A.; Keller, K.; et al. Carson WE 3rd. Myeloid-Derived Suppressor Cells Express Bruton’s Tyrosine Kinase and Can Be Depleted in Tumor-Bearing Hosts by Ibrutinib Treatment. Cancer Res. 2016, 76, 2125–2136. [Google Scholar] [CrossRef]
- Gustafson, M.P.; Abraham, R.S.; Lin, Y.; Wu, W.; Gastineau, D.A.; Zent, C.S.; Dietz, A.B. Association of an increased frequency of CD14+ HLA-DR lo/neg monocytes with decreased time to progression in chronic lymphocytic leukaemia (CLL). Br. J. Haematol. 2012, 156, 674–676. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Huang, Q.; Qiu, L. CD14(+)HLA-DR(low/-) expression: A novel prognostic factor in chronic lymphocytic leukemia. Oncol. Lett. 2015, 9, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Korde, N. Multiple myeloma precursor disease: Current clinical and epidemiological insights and future opportunities. Oncology 2011, 25, 589–590. [Google Scholar] [PubMed]
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef]
- Agarwal, A.; Ghobrial, I.M. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: A review of the current understanding of epidemiology, biology, risk stratification, and management of myeloma precursor disease. Clin. Cancer Res. 2013, 19, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; DeBusk, L.M.; Fukuda, K.; Fingleton, B.; Green-Jarvis, B.; Shyr, Y.; Matrisian, L.M.; Carbone, D.P.; Lin, P.C. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004, 6, 409–421. [Google Scholar] [CrossRef]
- Yang, L.; Edwards, C.M.; Mundy, G.R. Gr-1+CD11b+ myeloid-derived suppressor cells: Formidable partners in tumor metastasis. J. Bone Miner. Res. 2010, 25, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.; Deshane, J.; Jules, J.; Lee, C.M.; Harris, B.A.; Feng, X.; Ponnazhagan, S. Myeloid-Derived Suppressor Cells Function as Novel Osteoclast Progenitors Enhancing Bone Loss in Breast Cancer. Cancer Res. 2013, 73, 672–682. [Google Scholar] [CrossRef]
- Binsfeld, M.; Muller, J.; Lamour, V.; de Veirman, K.; de Raeve, H.; Bellahcene, A.; van Valckenborgh, E.; Baron, F.; Beguin, Y.; Caers, J.; et al. Granulocytic myeloid-derived suppressor cells promote angiogenesis in the context of multiple myeloma. Oncotarget 2016, 7, 37931–37943. [Google Scholar] [CrossRef]
- Melani, C.; Sangaletti, S.; Barazzetta, F.; Werb, Z.; Colombo, M. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007, 67, 11438–11446. [Google Scholar] [CrossRef]
- Tadmor, T.; Levy, I.; Vadasz, Z. Hierarchical Involvement of Myeloid-Derived Suppressor Cells and Monocytes Expressing Latency-Associated Peptide in Plasma Cell Dyscrasias. Turk. J. Haematol. 2018, 35, 116–121. [Google Scholar] [CrossRef]
- Ai, L.; Mu, S.; Sun, C.; Fan, F.; Yan, H.; Qin, Y.; Cui, G.; Wang, Y.; Guo, T.; Mei, H.; et al. Myeloid-derived suppressor cells endow stem-like qualities to multiple myeloma cells by inducing piRNA-823 expression and DNMT3B activation. Mol. Cancer 2019, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Giallongo, C.; Tibullo, D.; Parrinello, N.L.; La Cava, P.; Di Rosa, M.; Bramanti, V.; Di Raimondo, C.; Conticello, C.; Chiarenza, A.; Palumbo, G.A.; et al. Granulocyte-like myeloid derived suppressor cells (G-MDSC) are increased in multiple myeloma and are driven by dysfunctional mesenchymal stem cells (MSC). Oncotarget 2016, 7, 85764–85775. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; de Veirman, K.; Faict, S.; Frassanito, M.A.; Ribatti, D.; Vacca, A.; Menu, E. Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J. Pathol. 2016, 239, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; De Veirman, K.; De Beule, N.; Maes, K.; De Bruyne, E.; van Valckenborgh, E.; Vanderkerken, K.; Menu, E. The bone marrow microenvironment enhances multiple myeloma progression by exosome-mediated activation of myeloid-derived suppressor cells. Oncotarget 2015, 6, 43992–44004. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, T. The growing link between multiple myeloma and myeloid derived suppressor cells. Leuk. Lymphoma 2014, 55, 2681–2682. [Google Scholar] [CrossRef][Green Version]
- De Veirman, K.; Van Valckenborgh, E.; Lahmar, Q.; Geeraerts, X.; De Bruyne, E.; Menu, E.; Van Riet, I.; Vanderkerken, K.; Van Ginderachter, J.A. Myeloid-derived suppressor cells as therapeutic target in hematological malignancies. Front. Oncol. 2014, 4, 349. [Google Scholar] [CrossRef]
- Ramachandran, I.R.; Martner, A.; Pisklakova, A.; Condamine, T.; Chase, T.; Vogl, T.; Roth, J.; Gabrilovich, D.; Nefedova, Y. Myeloid-Derived Suppressor Cells Regulate Growth of Multiple Myeloma by Inhibiting T Cells in Bone Marrow. J. Immunol. 2013, 190, 3815–3823. [Google Scholar] [CrossRef]
- Gorgun, G.T.; Whitehill, G.; Anderson, J.L.; Hideshima, T.; Maguire, C.; Laubach, J.; Raje, N.; Munshi, N.C.; Richardson, P.G.; Anderson, K.C. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood 2013, 121, 2975–2987. [Google Scholar] [CrossRef]
- Van Valckenborgh, E.; Schouppe, E.; Movahedi, K.; De Bruyne, E.; Menu, E.; De Baetselier, P.; Vanderkerken, K.; Van Ginderachter, J.A. Multiple myeloma induces the immunosuppressive capacity of distinct myeloid-derived suppressor cell subpopulations in the bone marrow. Leukemia 2012, 26, 2424–2428. [Google Scholar] [CrossRef]
- De Veirman, K.; Van Ginderachter, J.A.; Lub, S.; De Beule, N.; Thielemans, K.; Bautmans, I.; Oyajobi, B.O.; De Bruyne, E.; Menu, E.; Lemaire, M.; et al. Multiple myeloma induces Mcl-1 expression and survival of myeloid-derived suppressor cells. Oncotarget 2015, 6, 10532–10547. [Google Scholar] [CrossRef]
- De Veirman, K.; Menu, E.; Maes, K.; De Beule, N.; De Smedt, E.; Maes, A.; Vlummens, P.; Fostier, K.; Kassambara, A.; Moreaux, J.; et al. Myeloid-derived suppressor cells induce multiple myeloma cell survival by activating the AMPK pathway. Cancer Lett. 2019, 442, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Parrinello, N.L.; La Cava, P.; Tibullo, D.; Giallongo, C.; Camiolo, G.; Puglisi, F.; Parisi, M.; Pirosa, M.C.; Martino, E.; et al. PMN-MDSC and arginase are increased in myeloma and may contribute to resistance to therapy. Expert Rev. Mol. Diagn. 2018, 18, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Mitsiades, N.; Mitsiades, C.S.; Poulaki, V.; Chauhan, D.; Richardson, P.G.; Hideshima, T.; Munshi, N.; Treon, S.P.; Anderson, K.C. Biologic sequelae of nuclear factor-kappaB blockade in multiple myeloma: Therapeutic applications. Blood 2002, 99, 4079–4086. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.R.M.; Kovarova, L.; Hajek, R. Induction by Lenalidomide and Dexamethasone Combination Increases Regulatory Cells of Previously Untreated Multiple Myeloma Patients. Leuk. Lymphoma 2011, 53, 1406–1408. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki, I.; Kwak, L.W.; Cha, S.C.; Yi, Q.; Lerman, B.; Chen, J.; Surapaneni, S.; Bateman, S.; Qin, H. Lenalidomide enhances the protective effect of a therapeutic vaccine and reverses immune suppression in mice bearing established lymphomas. Leukemia 2013, 28, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lim, J.Y.; Ryu, D.B.; Kim, T.W.; Yoon, J.H.; Cho, B.S.; Eom, K.S.; Kim, Y.J.; Kim, H.J.; Lee, S.; et al. Circulating immune cell phenotype can predict the outcome of lenalidomide plus low-dose dexamethasone treatment in patients with refractory/relapsed multiple myeloma. Cancer Immunol. Immunother. 2016, 65, 983–994. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Wang, H.; Xiong, S.; Li, Y.; Tao, Q.; Xiao, W.; Qin, H.; Wang, Y.; Zhai, Z. Tumor-induced CD14+HLA-DR (-/low) myeloid-derived suppressor cells correlate with tumor progression and outcome of therapy in multiple myeloma patients. Cancer Immunol. Immunother. 2015, 64, 389–399. [Google Scholar] [CrossRef]
- Malek, E.; De Lima, M.; Letterio, J.J.; Kim, B.G.; Finke, J.H.; Driscoll, J.J.; Giralt, S.A. Myeloid-derived suppressor cells: The green light for myeloma immune escape. Blood Rev. 2016, 30, 341–348. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Diao, W.; Jin, F.; Shi, L.; Meng, J.; Liu, H.; Zhang, J.; Zeng, C.-H.; Zhang, M.-C.; et al. Role of Myeloid-Derived Suppressor Cells in Glucocorticoid-Mediated Amelioration of FSGS. J. Am. Soc. Nephrol. 2015, 26, 2183–2197. [Google Scholar] [CrossRef]
- Busch, A.; Zeh, D.; Janzen, V.; Mugge, L.O.; Wolf, D.; Fingerhut, L.; Hahn-Ast, C.; Maurer, O.; Brossart, P.; Von Lilienfeld-Toal, M. Treatment with lenalidomide induces immunoactivating and counter-regulatory immunosuppressive changes in myeloma patients. Clin. Exp. Immunol. 2014, 177, 439–453. [Google Scholar] [CrossRef]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; Van De Donk, N.W.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shen, Q.; Lin, H.; Hu, L.; Li, G.; Zhang, X. Decitabine shows potent anti-myeloma activity by depleting monocytic myeloid-derived suppressor cells in the myeloma microenvironment. J. Cancer Res. Clin. Oncol. 2019, 145, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Smyth, M.J.; Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 2013, 39, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jaffar, J.; Hellstrom, I.; Hellstrom, K.E. Administration of cyclophosphamide changes the immune profile of tumor-bearing mice. J. Immunother. 2010, 33, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Sistigu, A.; Viaud, S.; Chaput, N.; Bracci, L.; Proietti, E.; Zitvogel, L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin. Immunopathol. 2011, 33, 369–383. [Google Scholar] [CrossRef]
- Alizadeh, D.; Trad, M.; Hanke, N.T.; Larmonier, C.B.; Janikashvili, N.; Bonnotte, B.; Katsanis, E.; Larmonier, N. Doxorubicin Eliminates Myeloid-Derived Suppressor Cells and Enhances the Efficacy of Adoptive T Cell Transfer in Breast Cancer. Cancer Res. 2013, 74, 104–118. [Google Scholar] [CrossRef]
- Lee, S.E.; Lim, J.Y.; Kim, T.W.; Ryu, D.B.; Park, S.S.; Jeon, Y.W.; Yoon, J.H.; Cho, B.S.; Eom, K.S.; Kim, Y.J.; et al. Different role of circulating myeloid-derived suppressor cells in patients with multiple myeloma undergoing autologous stem cell transplantation. J. Immunother. Cancer 2019, 7, 35. [Google Scholar] [CrossRef]
- Franssen, L.E.; Van De Donk, N.W.; Emmelot, M.E.; Roeven, M.W.; Schaap, N.; Dolstra, H.; Hobo, W.; Lokhorst, H.M.; Mutis, T. The impact of circulating suppressor cells in multiple myeloma patients on clinical outcome of DLIs. Bone Marrow Transplant. 2015, 50, 822–828. [Google Scholar] [CrossRef]
- Terpos, E.; Berenson, J.; Raje, N.; Roodman, G.D. Management of bone disease in multiple myeloma. Expert Rev. Hematol. 2014, 7, 113–125. [Google Scholar] [CrossRef]
- Morgan, G.J.; Davies, F.E.; Gregory, W.M.; Bell, S.E.; Szubert, A.J.; Cook, G.; Drayson, M.T.; Owen, R.G.; Ross, F.M.; Jackson, G.H.; et al. Long-term follow-up of MRC Myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clin. Cancer Res. 2013, 19, 6030–6038. [Google Scholar] [CrossRef]
- Aviles, A.; Neri, N.; Huerta-Guzman, J.; Nambo, M.J. Randomized clinical trial of zoledronic acid in multiple myeloma patients undergoing high-dose chemotherapy and stem-cell transplantation. Curr. Oncol. 2013, 20, e13–e20. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, R.; Markowitz, J.; Carson, W. Myeloid derived suppressor cells—A new therapeutic target in the treatment of cancer. J. Immunother. Cancer 2013, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.M.; Branford, S.; Seymour, J.F.; Schwarer, A.P.; Arthur, C.; Yeung, D.T.; Dang, P.; Goyne, J.M.; Slader, C.; Filshie, R.J.; et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: Results from the TWISTER study. Blood 2013, 122, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Clarson, J.; Tang, C.; Vidovic, L.; White, D.L.; Hughes, T.P.; Yong, A.S. CML patients with deep molecular responses to TKI have restored immune effectors and decreased PD-1 and immune suppressors. Blood 2017, 129, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Giallongo, C.; Parrinello, N.; Tibullo, D.; La Cava, P.; Romano, A.; Chiarenza, A.; Barbagallo, I.; Palumbo, G.A.; Stagno, F.; Vigneri, P.; et al. Myeloid derived suppressor cells (MDSCs) are increased and exert immunosuppressive activity together with polymorphonuclear leukocytes (PMNs) in chronic myeloid leukemia patients. PLoS ONE 2014, 9, e101848. [Google Scholar] [CrossRef]
- Giallongo, C.; Parrinello, N.L.; La Cava, P.; Camiolo, G.; Romano, A.; Scalia, M.; Stagno, F.; Palumbo, G.A.; Avola, R.; Li Volti, G.; et al. Monocytic myeloid-derived suppressor cells as prognostic factor in chronic myeloid leukaemia patients treated with dasatinib. J. Cell Mol. Med. 2018, 22, 1070–1080. [Google Scholar] [CrossRef]
- Giallongo, C.; Parrinello, N.; Brundo, M.V.; Raccuia, S.A.; Di Rosa, M.; La Cava, P.; Tibullo, D. Myeloid derived suppressor cells in chronic myeloid leukemia. Front. Oncol. 2015, 5, 107. [Google Scholar] [CrossRef]
- Christiansson, L.; Söderlund, S.; Svensson, E.; Mustjoki, S.; Bengtsson, M.; Simonsson, B.; Olsson-Strömberg, U.; Loskog, A.S. Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in Sokal high risk chronic myeloid leukemia. PLoS ONE 2013, 8, e55818. [Google Scholar] [CrossRef]
- Christiansson, L.; Söderlund, S.; Mangsbo, S.; Hjorth-Hansen, H.; Höglund, M.; Markevärn, B.; Richter, J.; Stenke, L.; Mustjoki, S.; Loskog, A.; et al. The tyrosine kinase inhibitors imatinib and dasatinib reduce myeloid suppressor cells and release effector lymphocyte responses. Mol. Cancer Ther. 2015, 14, 1181–1191. [Google Scholar] [CrossRef]
- Schmitt, A.; Drouin, A.; Masse, J.M.; Guichard, J.; Shagraoui, H.; Cramer, E.M. Polymorphonuclear neutrophil and megakaryocyte mutual involvement in myelofibrosis pathogenesis. Leuk. Lymphoma 2002, 43, 719–724. [Google Scholar] [CrossRef]
- Ciaffoni, F.; Cassella, E.; Varricchio, L.; Massa, M.; Barosi, G.; Migliaccio, A.R. Activation of non-canonical TGF-β1 signaling indicates an autoimmune mechanism for bone marrow fibrosis in primary myelofibrosis. Blood Cells Mol. Dis. 2015, 54, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, A.R.; Centurione, L.; Di Baldassarre, A.; Zingariello, M.; Bosco, D.; Gatta, V.; Rana, R.A.; Langella, V.; Di Virgilio, A.; Vannucchi, A.M. Increased and Pathological Emperipolesis of Neutrophils within Megakaryocytes Associated with Myelofibrosis in GATA-1LowMice. Blood 2004, 104, 2430. [Google Scholar] [CrossRef]
- Abdelouahab, H.; Zhang, Y.; Wittner, M.; Oishi, S.; Fujii, N.; Besancenot, R.; Plo, I.; Ribrag, V.; Solary, E.; Vainchenker, W.; et al. CXCL12/CXCR4 pathway is activated by oncogenic JAK2 in a PI3K-dependent manner. Oncotarget 2017, 8, 54082–54095. [Google Scholar] [CrossRef] [PubMed]
- Barosi, G. An immune dysregulation in MPN. Curr. Hematol. Malig. Rep. 2014, 9, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Kundra, A.; Andrei, M.; Baptiste, S.; Chen, C.; Wong, C.; Sindhu, H. Myeloid-derived suppressor cells in patients with myeloproliferative neoplasm. Leuk. Res. 2016, 43, 39–43. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, G.A.; Parrinello, N.L.; Giallongo, C.; D’Amico, E.; Zanghì, A.; Puglisi, F.; Conticello, C.; Chiarenza, A.; Tibullo, D.; Di Raimondo, F.; et al. Monocytic Myeloid Derived Suppressor Cells in Hematological Malignancies. Int. J. Mol. Sci. 2019, 20, 5459. https://doi.org/10.3390/ijms20215459
Palumbo GA, Parrinello NL, Giallongo C, D’Amico E, Zanghì A, Puglisi F, Conticello C, Chiarenza A, Tibullo D, Di Raimondo F, et al. Monocytic Myeloid Derived Suppressor Cells in Hematological Malignancies. International Journal of Molecular Sciences. 2019; 20(21):5459. https://doi.org/10.3390/ijms20215459
Chicago/Turabian StylePalumbo, Giuseppe Alberto, Nunziatina Laura Parrinello, Cesarina Giallongo, Emanuele D’Amico, Aurora Zanghì, Fabrizio Puglisi, Concetta Conticello, Annalisa Chiarenza, Daniele Tibullo, Francesco Di Raimondo, and et al. 2019. "Monocytic Myeloid Derived Suppressor Cells in Hematological Malignancies" International Journal of Molecular Sciences 20, no. 21: 5459. https://doi.org/10.3390/ijms20215459
APA StylePalumbo, G. A., Parrinello, N. L., Giallongo, C., D’Amico, E., Zanghì, A., Puglisi, F., Conticello, C., Chiarenza, A., Tibullo, D., Di Raimondo, F., & Romano, A. (2019). Monocytic Myeloid Derived Suppressor Cells in Hematological Malignancies. International Journal of Molecular Sciences, 20(21), 5459. https://doi.org/10.3390/ijms20215459