Autophagy Modulation in Human Thyroid Cancer Cells following Aloperine Treatment
Abstract
:1. Introduction
2. Results
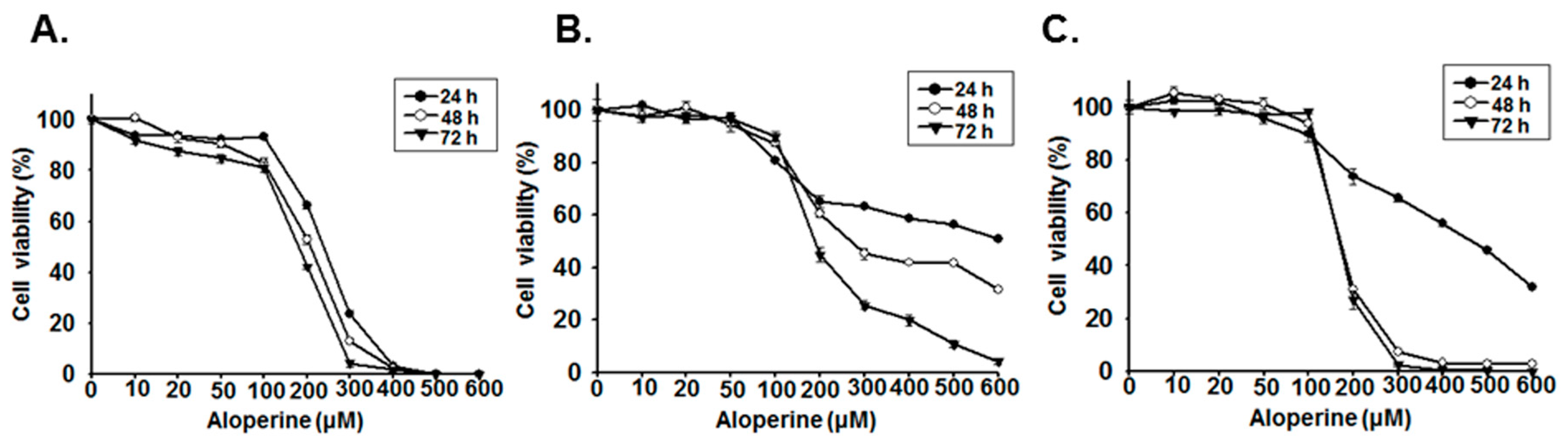
2.1. Aloperine Reduces Cellular Viability in Human Thyroid Cancer Cells
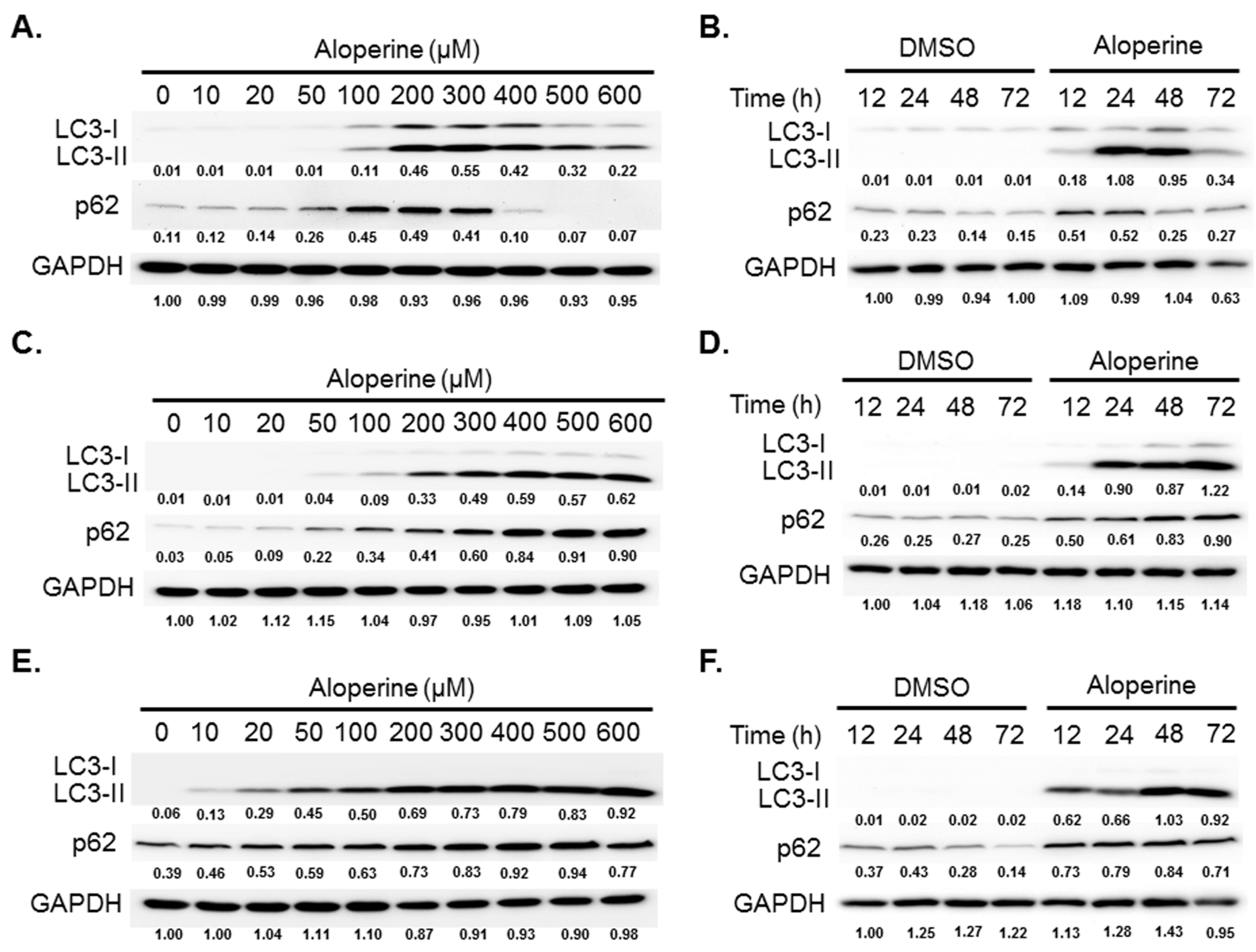
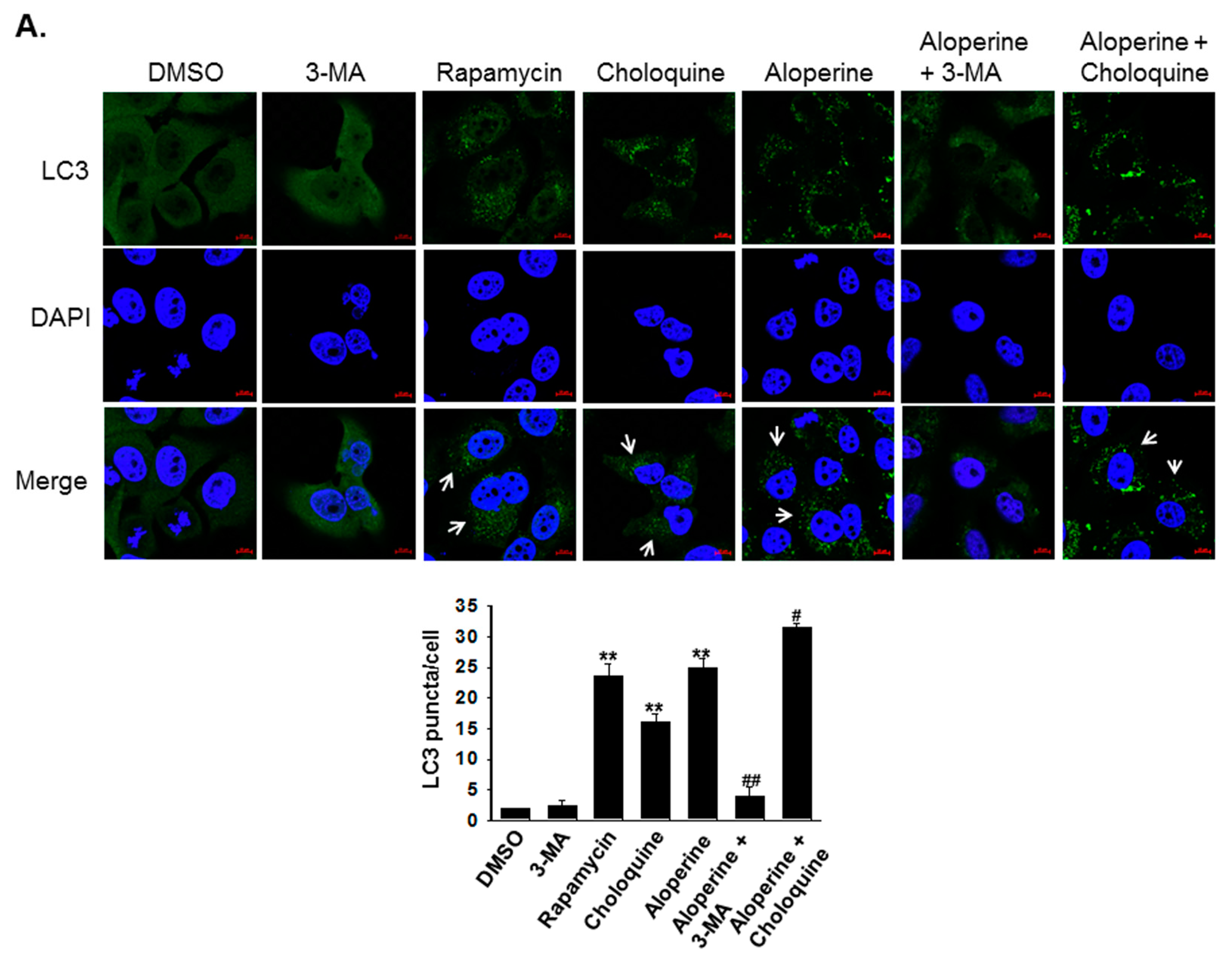
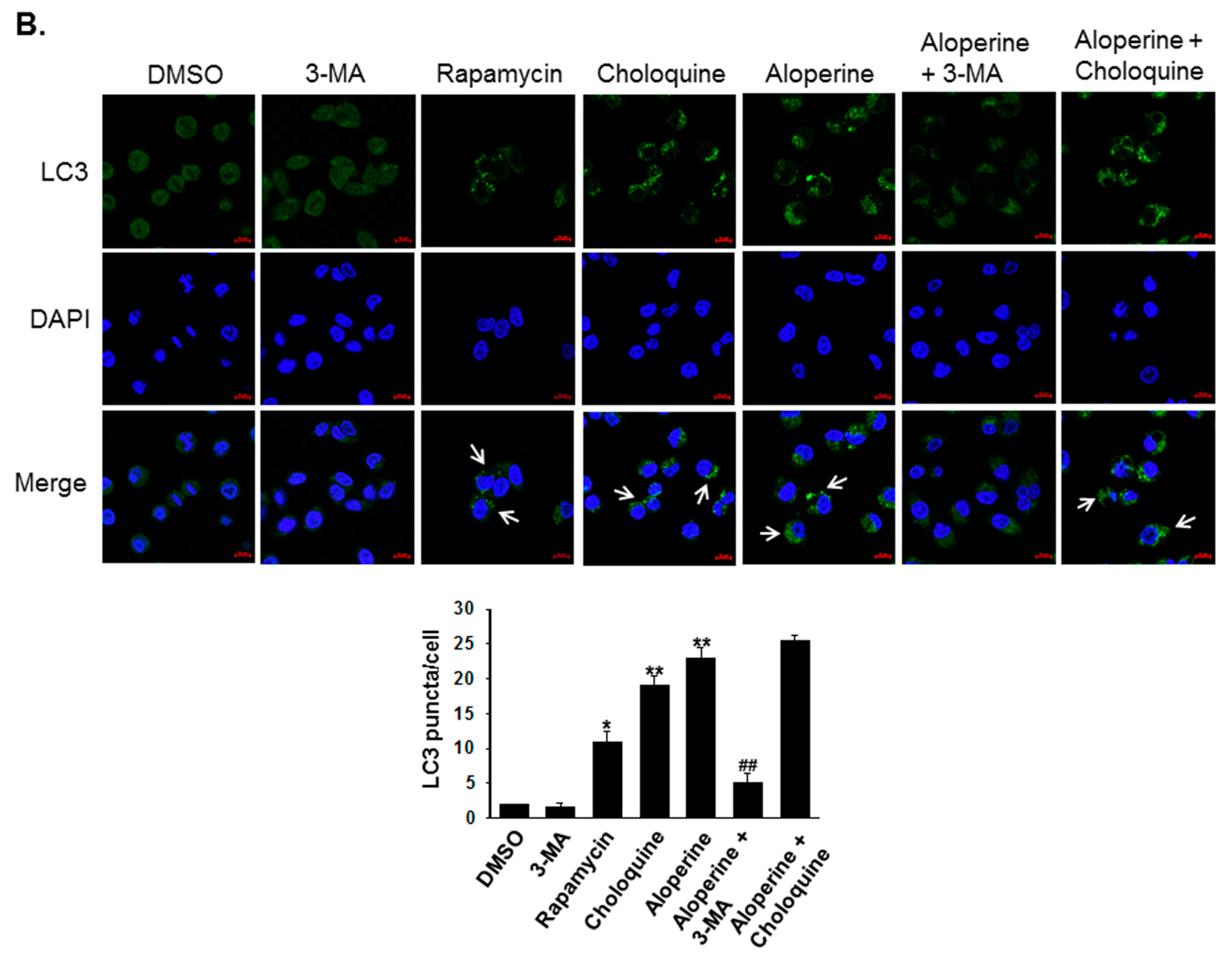
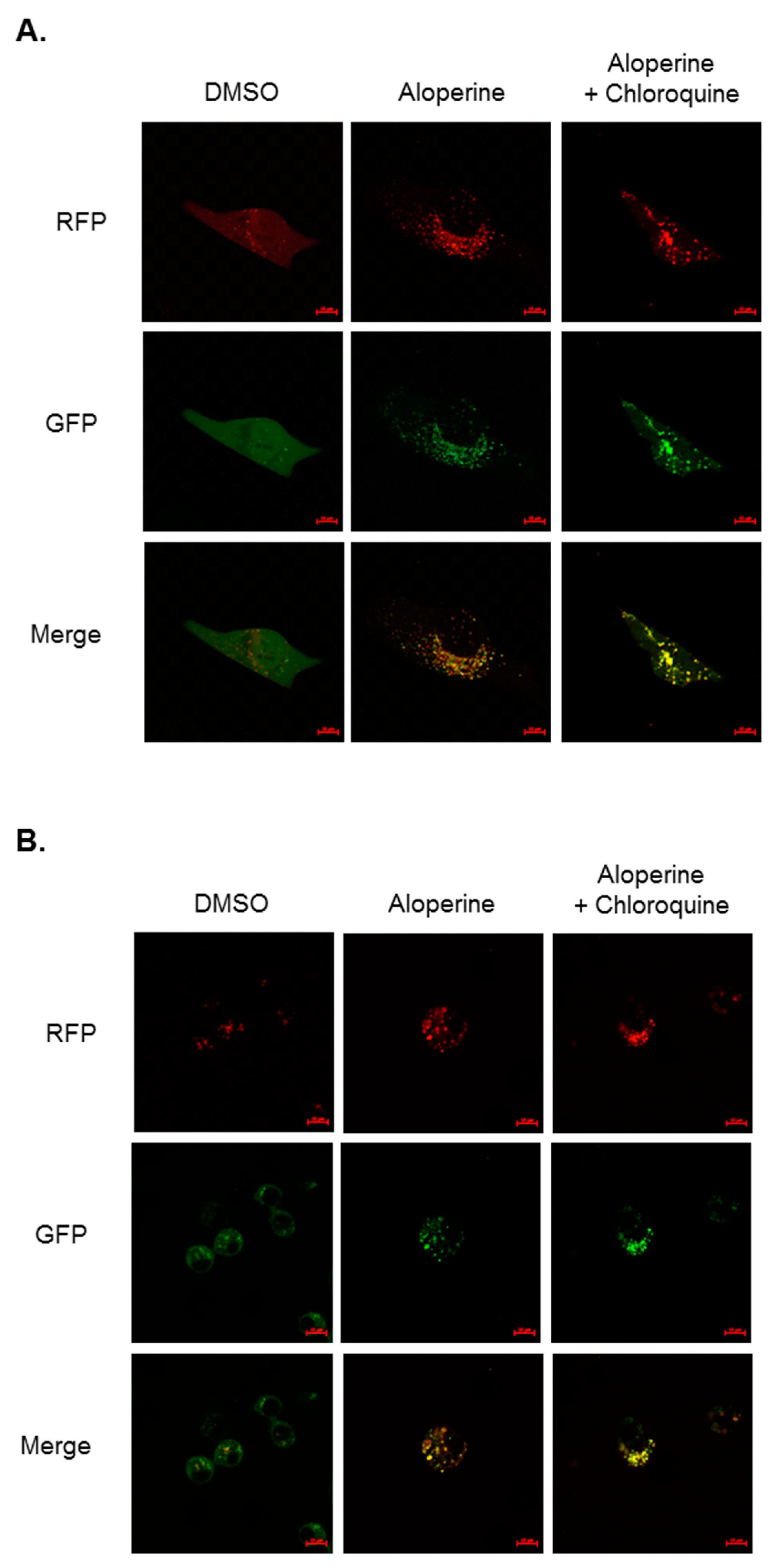
2.2. Aloperine Promotes Autophagy Activation in Human Thyroid Cancer Cells
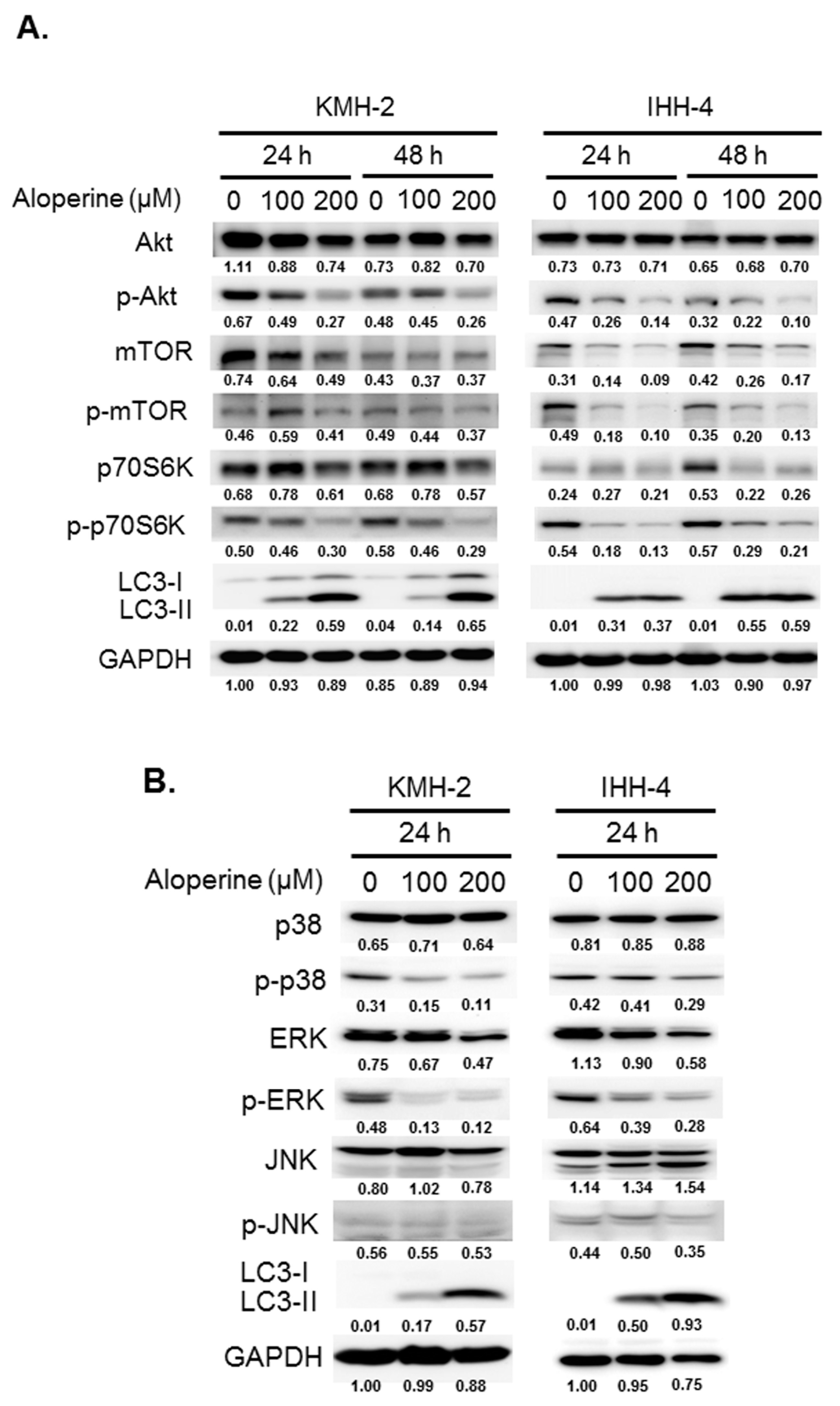
2.3. Modulations of Signaling Pathways with Aloperine in Human Thyroid Cancer Cells
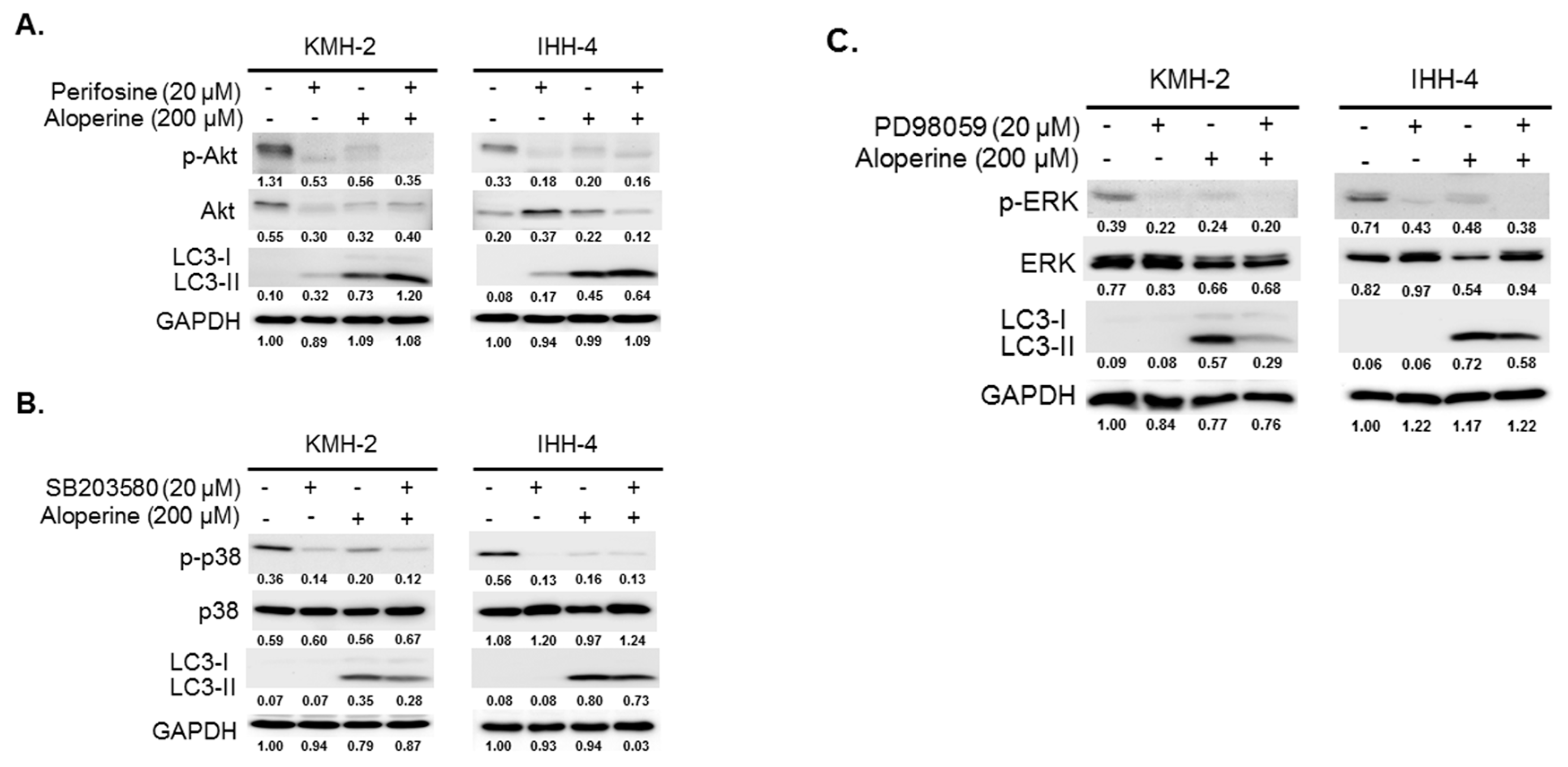
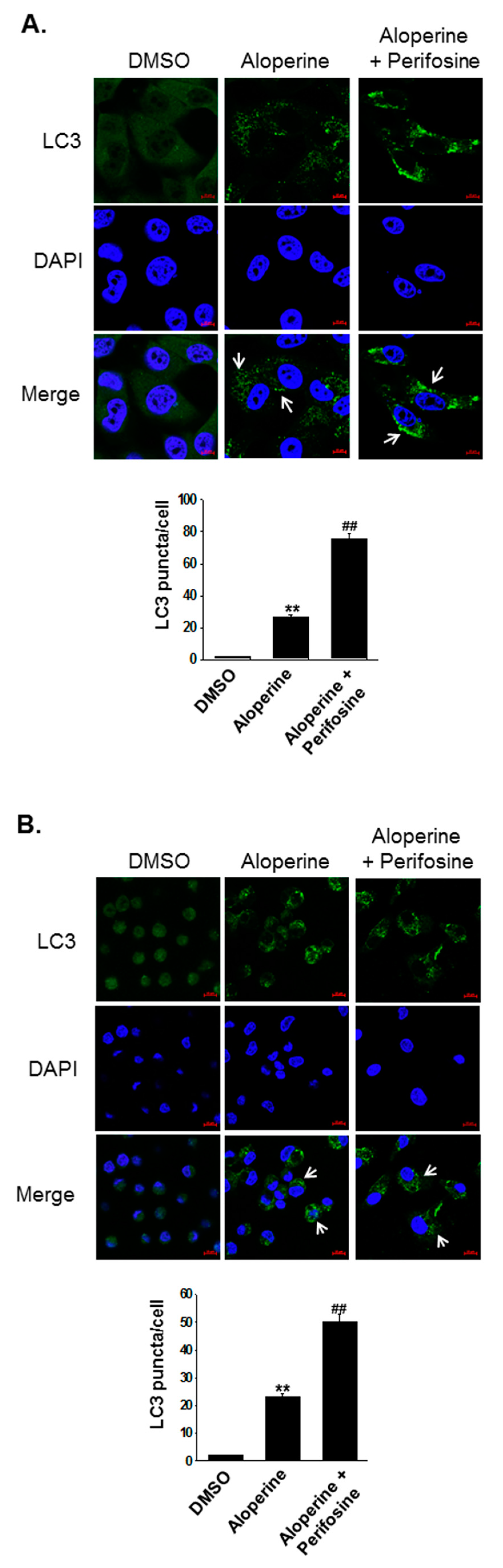
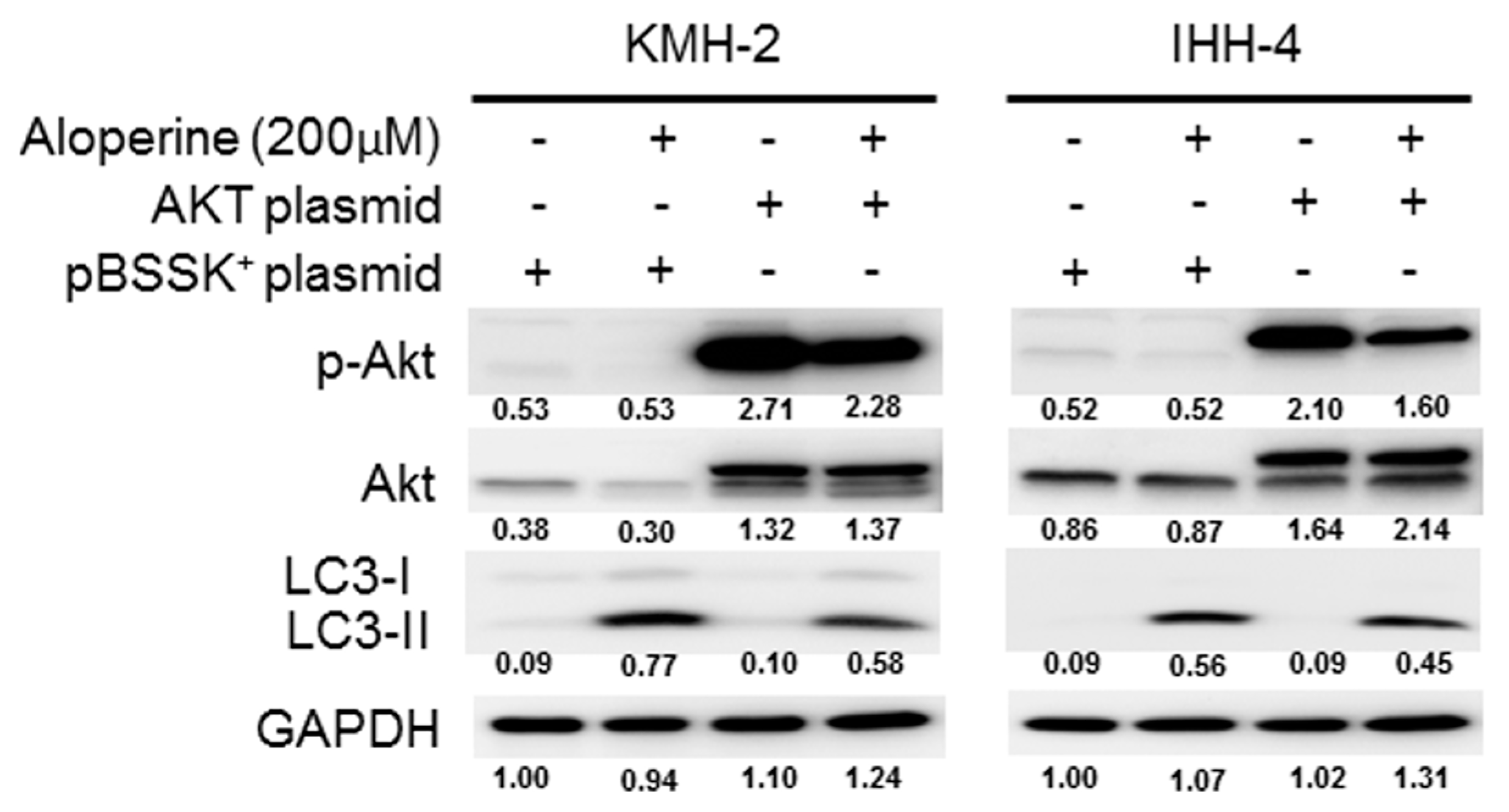
2.4. Akt Signaling Pathway Contributes to Aloperine-mediated Autophagy Induction in Human Thyroid Cancer Cells
2.5. Aloperine-Mediated Autophagy Exerts a Cytotoxic Effect in Human Thyroid Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Line and Cell Culture
4.2. Cell Viability Assay
4.3. Autophagosome Detection
4.4. Western Blotting
4.5. Plasmid Transfection
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Zheng, Y.; Deng, H.; Liang, L.; Peng, J. Aloperine Induces G2/M Phase Cell Cycle Arrest and Apoptosis in Hct116 Human Colon Cancer Cells. Int. J. Mol. Med. 2014, 33, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Gao, H.B.; Sun, X.B.; Shi, H.B.; Liu, W.; Yuan, H.N.; Wang, Z.X. Anti-Inflammatory and Anti-Allergic Action of Aloperine. Zhongguo Yao Li Xue Bao 1989, 10, 360–365. [Google Scholar] [PubMed]
- Yuan, X.Y.; Liu, W.; Zhang, P.; Wang, R.Y.; Guo, J.Y. Effects and Mechanisms of Aloperine on 2, 4-Dinitrofluorobenzene-Induced Allergic Contact Dermatitis in Balb/C Mice. Eur. J. Pharmacol. 2010, 629, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Huang, C.F.; Liu, X.S.; Jiang, J. In Vitro Anti-Tumour Activities of Quinolizidine Alkaloids Derived from Sophora Flavescens Ait. Basic Clin. Pharmacol. Toxicol. 2011, 108, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Lin, J.Y. Five Bitter Compounds Display Different Anti-Inflammatory Effects through Modulating Cytokine Secretion Using Mouse Primary Splenocytes in Vitro. J. Agric. Food Chem. 2011, 59, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.T.; Zhou, R.; Chang, R.Y.; Hao, Y.J.; Ma, L.; Jin, S.J.; Du, J.; Zheng, J.; Zhao, C.J.; Niu, Y.; et al. Protective Effects of Aloperine on Neonatal Rat Primary Cultured Hippocampal Neurons Injured by Oxygen-Glucose Deprivation and Reperfusion. J. Nat. Med. 2015, 69, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, S.; Zhou, H.; Sun, M.; Du, L.; Wei, M.; Luo, M.; Huang, J.; Deng, H.; Feng, Y.; et al. Aloperine Executes Antitumor Effects against Multiple Myeloma through Dual Apoptotic Mechanisms. J. Hematol. Oncol. 2015, 8, 26. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Y.; Zhang, M.; Guo, Y.; Yang, P.; Zhang, S.; Simsekyilmaz, S.; Xu, J.F.; Li, J.; Xiang, X.; et al. Aloperine Protects Mice against Ischemia-Reperfusion (Ir)-INDUCED Renal Injury by Regulating Pi3k/Akt/Mtor Signaling and Ap-1 Activity. Mol. Med. 2016, 21, 912–923. [Google Scholar] [CrossRef]
- Dang, Z.; Zhu, L.; Lai, W.; Bogerd, H.; Lee, K.H.; Huang, L.; Chen, C.H. Aloperine and Its Derivatives as a New Class of Hiv-1 Entry Inhibitors. ACS Med. Chem. Lett. 2016, 7, 240–244. [Google Scholar] [CrossRef]
- Ren, D.; Ma, W.; Guo, B.; Wang, S. Aloperine Attenuates Hydrogen Peroxide-Induced Injury Via Anti-Apoptotic Activity and Suppression of the Nuclear Factor-Kappab Signaling Pathway. Exp. Ther. Med. 2017, 13, 315–320. [Google Scholar] [CrossRef]
- Wu, F.; Hao, Y.; Yang, J.; Yao, W.; Xu, Y.; Yan, L.; Niu, Y.; Sun, T.; Yu, J.; Zhou, R. Protective Effects of Aloperine on Monocrotaline-Induced Pulmonary Hypertension in Rats. Biomed. Pharmacother. 2017, 89, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jin, Z.; Dai, L.; Wu, H.; Wang, J.; Wang, L.; Zhou, Z.; Yang, L.; Gao, W. Aloperine Induces Apoptosis and Inhibits Invasion in Mg-63 and U2os Human Osteosarcoma Cells. Biomed. Pharmacother. 2018, 97, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Xie, H.; Zhu, L.; Zhang, Q.; Li, Z.; Huang, L.; Chen, C.H. Structure Optimization of Aloperine Derivatives as Hiv-1 Entry Inhibitors. ACS Med. Chem. Lett. 2017, 8, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, X.Q.; Tang, S.; Mei, L.; Li, Y.H.; Zhang, J.P.; Jiang, J.D.; Peng, Z.G.; Song, D.Q. Discovery and Evolution of Aloperine Derivatives as a New Family of Hcv Inhibitors with Novel Mechanism. Eur. J. Med. Chem. 2018, 143, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Chen, S.H.; Lin, C.Y.; Chao, W.Y.; Lim, Y.P.; Yu, H.I.; Lu, C.H. In Vitro Antitumor Activity of Aloperine on Human Thyroid Cancer Cells through Caspase-Dependent Apoptosis. Int. J. Mol. Sci. 2018, 19, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Q.; Zhang, N.; Li, Q.Q.; Liu, Z.D.; Li, Y.H.; Gao, L.M.; Wang, Y.C.; Deng, H.B.; Song, D.Q. Discovery and Evolution of Aloperine Derivatives as Novel Anti-Filovirus Agents through Targeting Entry Stage. Eur. J. Med. Chem. 2018, 149, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Han, J.; Zhang, Z.; Han, Z.; Wang, S. Aloperine Protects Mice against Bleomycin-Induced Pulmonary Fibrosis by Attenuating Fibroblast Proliferation and Differentiation. Sci. Rep. 2018, 8, 6265. [Google Scholar] [CrossRef]
- Ling, Z.; Guan, H.; You, Z.; Wang, C.; Hu, L.; Zhang, L.; Wang, Y.; Chen, S.; Xu, B.; Chen, M. Aloperine Executes Antitumor Effects through the Induction of Apoptosis and Cell Cycle Arrest in Prostate Cancer in Vitro and in Vivo. Onco Targets Ther. 2018, 11, 2735–2743. [Google Scholar] [CrossRef]
- Tian, D.; Li, Y.; Li, X.; Tian, Z. Aloperine Inhibits Proliferation, Migration and Invasion and Induces Apoptosis by Blocking the Ras Signaling Pathway in Human Breast Cancer Cells. Mol. Med. Rep. 2018, 18, 3699–3710. [Google Scholar] [CrossRef]
- Zhang, N.; Dou, Y.; Liu, L.; Zhang, X.; Liu, X.; Zeng, Q.; Liu, Y.; Yin, M.; Liu, X.; Deng, H.; et al. Sa-49, a Novel Aloperine Derivative, Induces Mitf-Dependent Lysosomal Degradation of Pd-L1. EBioMedicine 2019, 40, 151–162. [Google Scholar] [CrossRef]
- Suzuki, K.; Ohsumi, Y. Molecular Machinery of Autophagosome Formation in Yeast, Saccharomyces Cerevisiae. FEBS Lett. 2007, 581, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and Function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Arozena, A.A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (3rd Edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed]
- Vakifahmetoglu-Norberg, H.; Xia, H.G.; Yuan, J. Pharmacologic Agents Targeting Autophagy. J. Clin. Investig. 2015, 125, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Hosseini, M.; Shahidsales, S.; Maftouh, M.; Ferns, G.A.; Ghayour-Mobarhan, M.; Hassanian, S.M.; Avan, A. Targeting the Akt/Pi3k Signaling Pathway as a Potential Therapeutic Strategy for the Treatment of Pancreatic Cancer. Curr. Med. Chem. 2017, 24, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.S.; Huo, C.Y.; Cao, H.H.; Fan, C.L.; Hu, J.Y.; Deng, L.J.; Lu, Z.B.; Yang, H.Y.; Yu, L.Z.; Mo, Z.X.; et al. Aloperine Induces Apoptosis and G2/M Cell Cycle Arrest in Hepatocellular Carcinoma Cells through the Pi3k/Akt Signaling Pathway. Phytomedicine 2019, 61, 152843. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and Molecular Mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Gewirtz, D.A. The Four Faces of Autophagy: Implications for Cancer Therapy. Cancer Res. 2014, 74, 647–651. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y. Elaborating the Role of Natural Products-Induced Autophagy in Cancer Treatment: Achievements and Artifacts in the State of the Art. BioMed Res. Int. 2015, 2015, 934207. [Google Scholar] [CrossRef]
- Marino, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-Consumption: The Interplay of Autophagy and Apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting Pi3k/Akt/Mtor Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.P.; Shah, S.V. Autophagy in Acute Kidney Injury. Kidney Int. 2016, 89, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, S.; Li, Z.; Lin, C.; Zhu, Z.; Sun, D.; Bai, R.; Qian, J.; Gao, X.; Chen, G.; et al. Autophagic Flux Blockage in Alveolar Epithelial Cells Is Essential in Silica Nanoparticle-Induced Pulmonary Fibrosis. Cell Death Dis. 2019, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Noda, T.; Yoshimori, T. Dissection of the Autophagosome Maturation Process by a Novel Reporter Protein, Tandem Fluorescent-Tagged Lc3. Autophagy 2007, 3, 452–460. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-I.; Shen, H.-C.; Chen, S.-H.; Lim, Y.-P.; Chuang, H.-H.; Tai, T.-S.; Kung, F.-P.; Lu, C.-H.; Hou, C.-Y.; Lee, Y.-R. Autophagy Modulation in Human Thyroid Cancer Cells following Aloperine Treatment. Int. J. Mol. Sci. 2019, 20, 5315. https://doi.org/10.3390/ijms20215315
Yu H-I, Shen H-C, Chen S-H, Lim Y-P, Chuang H-H, Tai T-S, Kung F-P, Lu C-H, Hou C-Y, Lee Y-R. Autophagy Modulation in Human Thyroid Cancer Cells following Aloperine Treatment. International Journal of Molecular Sciences. 2019; 20(21):5315. https://doi.org/10.3390/ijms20215315
Chicago/Turabian StyleYu, Hui-I, Hui-Ching Shen, Shu-Hsin Chen, Yun-Ping Lim, Hsiang-Hsun Chuang, Tsai-Sung Tai, Fang-Ping Kung, Chieh-Hsiang Lu, Chia-Yi Hou, and Ying-Ray Lee. 2019. "Autophagy Modulation in Human Thyroid Cancer Cells following Aloperine Treatment" International Journal of Molecular Sciences 20, no. 21: 5315. https://doi.org/10.3390/ijms20215315
APA StyleYu, H.-I., Shen, H.-C., Chen, S.-H., Lim, Y.-P., Chuang, H.-H., Tai, T.-S., Kung, F.-P., Lu, C.-H., Hou, C.-Y., & Lee, Y.-R. (2019). Autophagy Modulation in Human Thyroid Cancer Cells following Aloperine Treatment. International Journal of Molecular Sciences, 20(21), 5315. https://doi.org/10.3390/ijms20215315





