The Significance of the Intrinsically Disordered Regions for the Functions of the bHLH Transcription Factors
Abstract
1. Introduction
2. The Role of the bHLH Proteins in Transcription
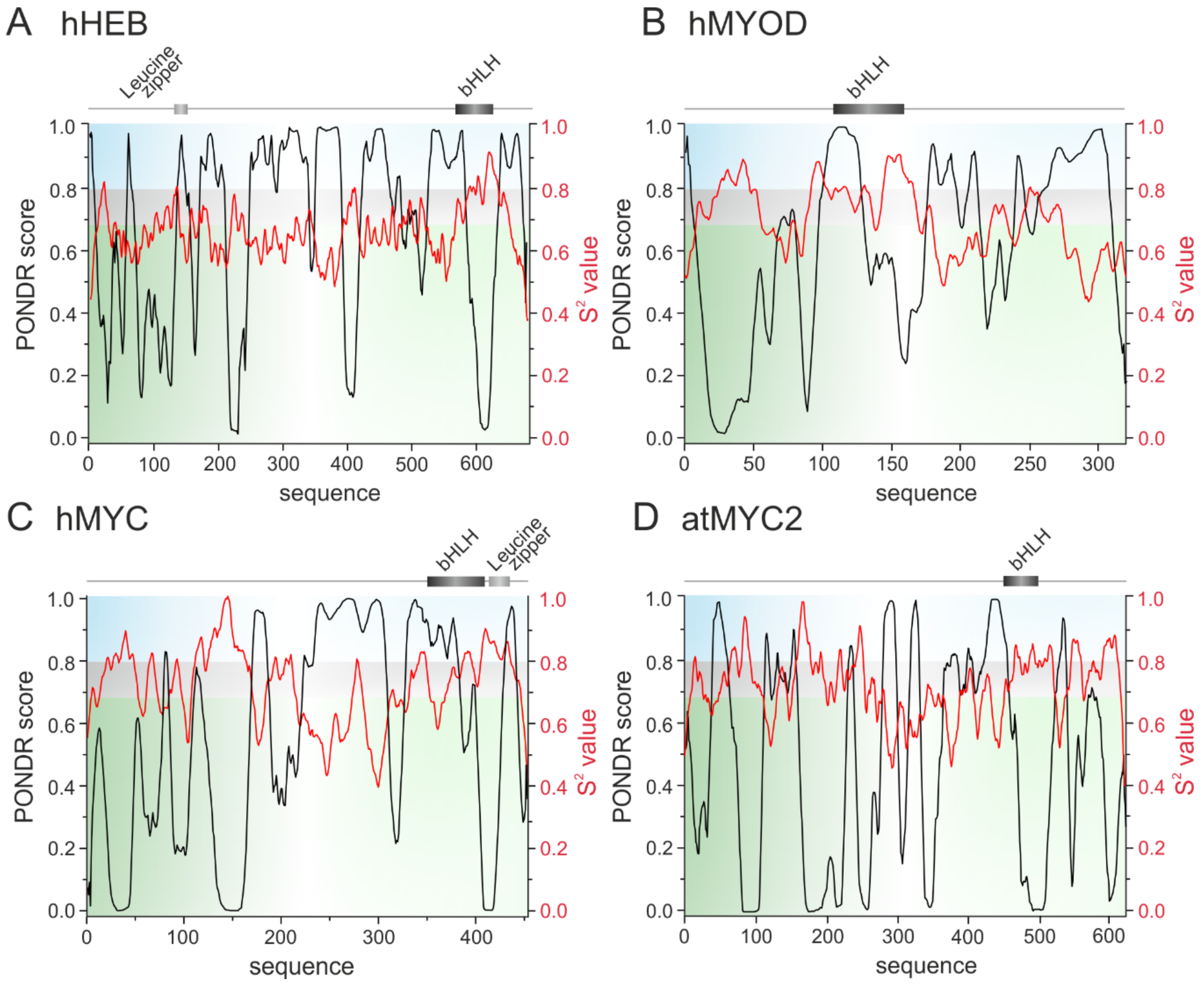
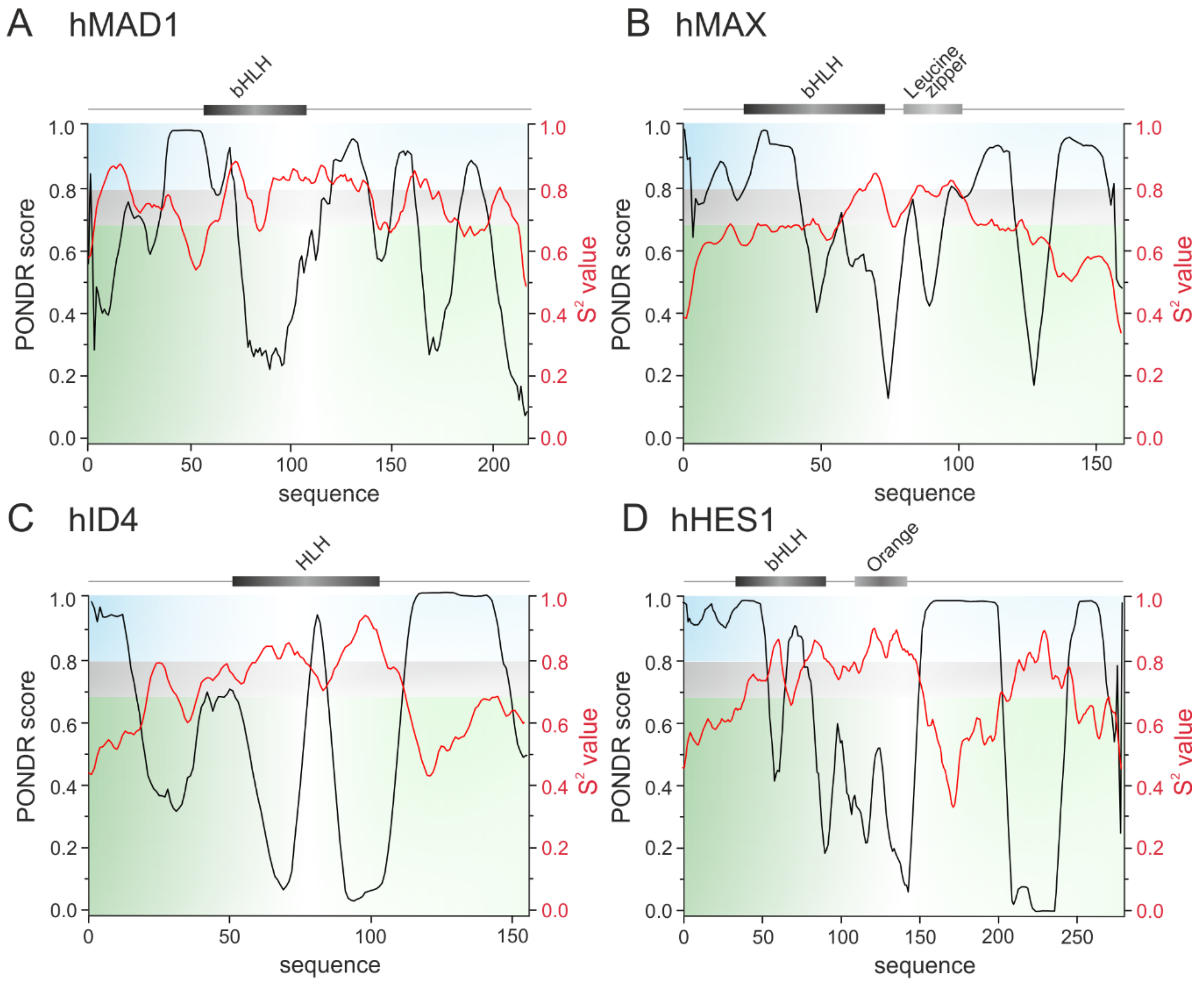
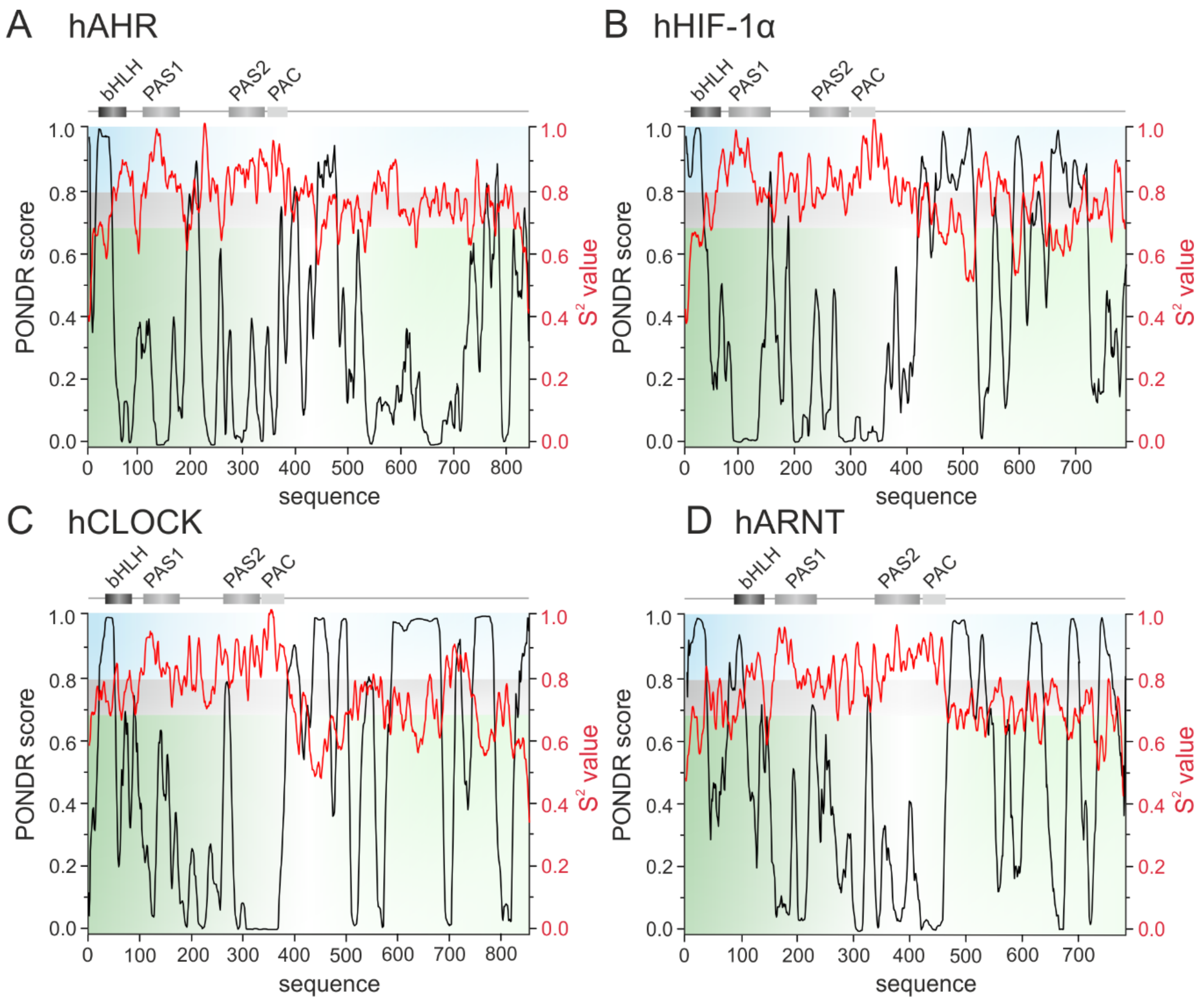
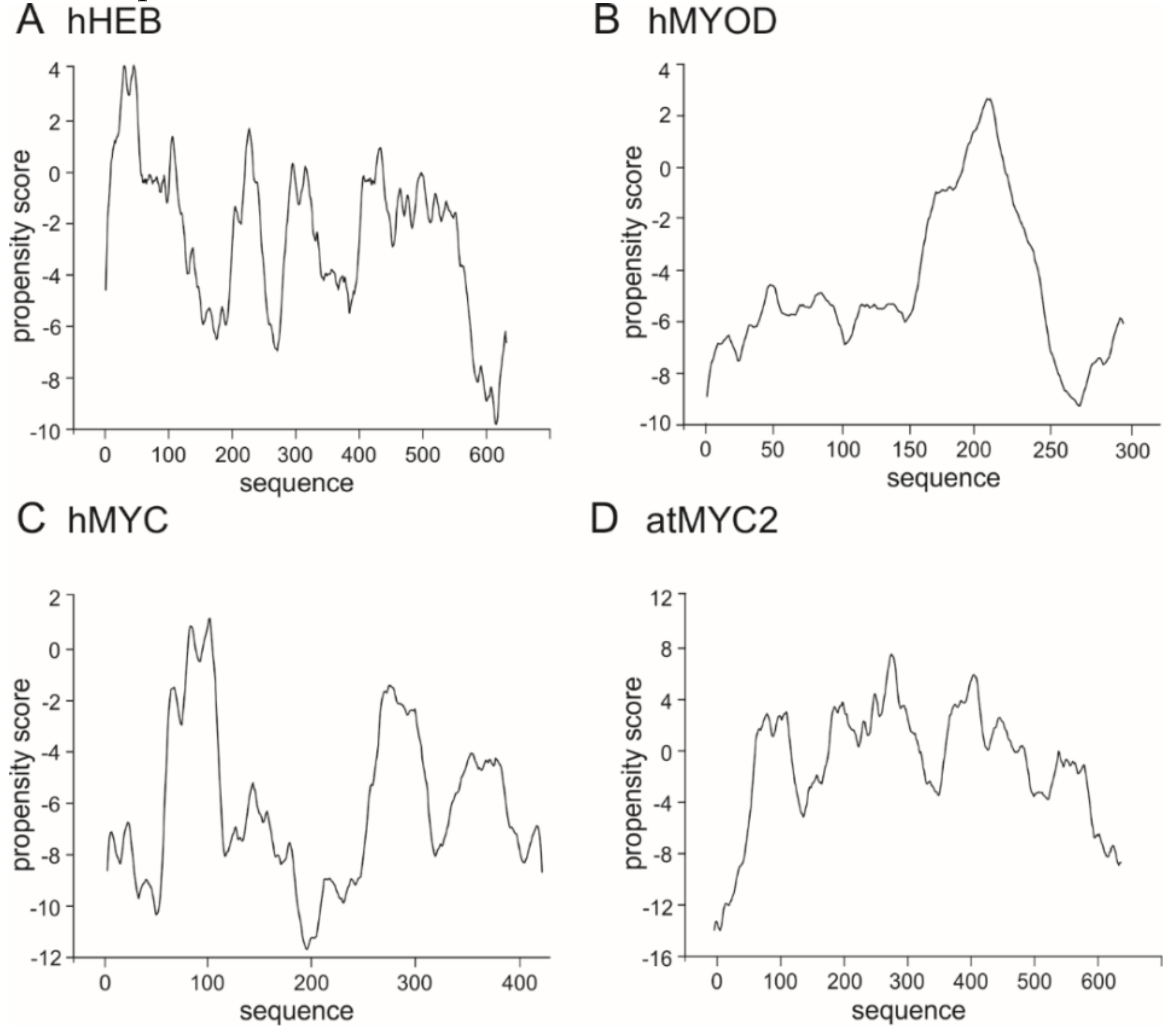
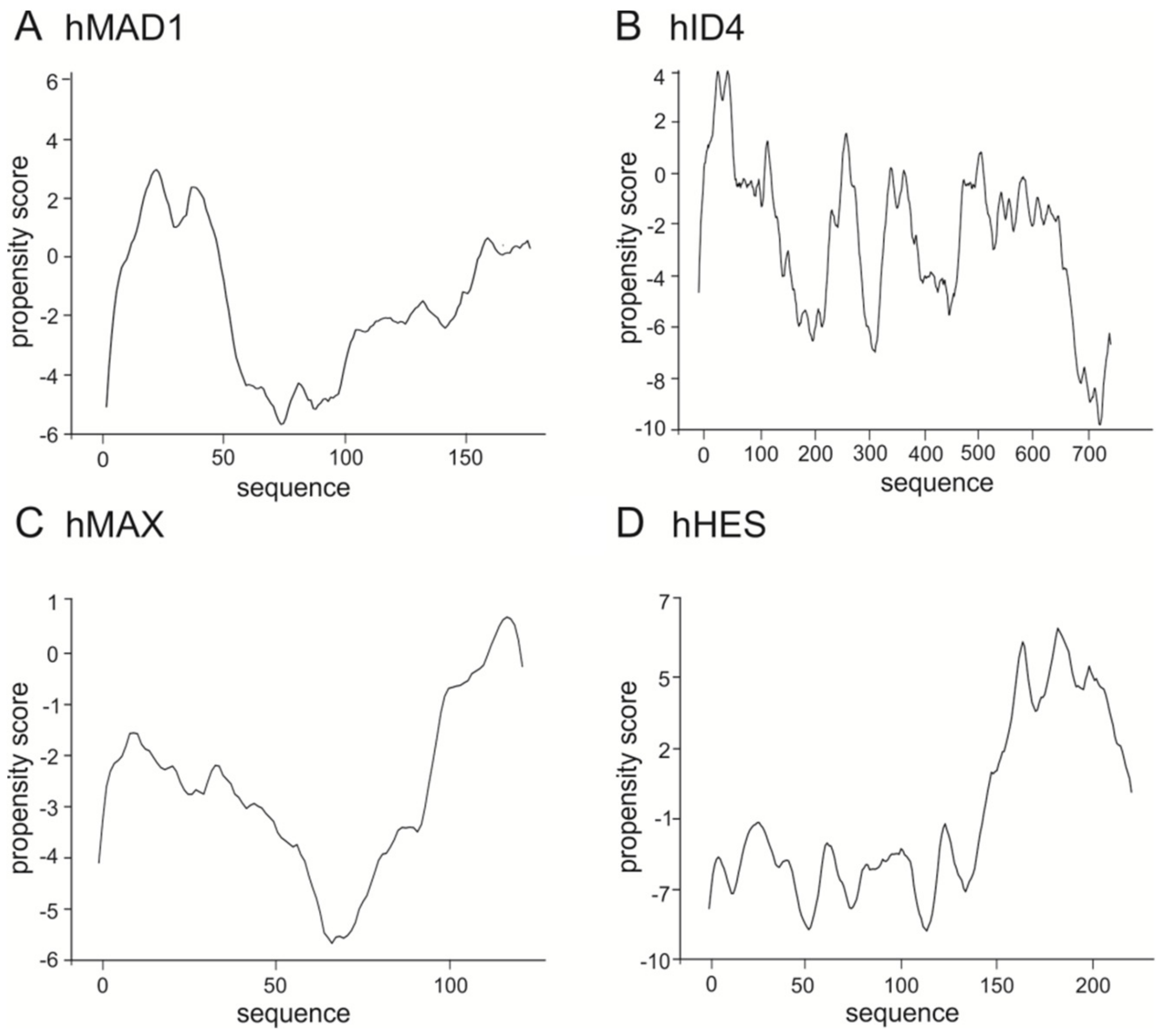
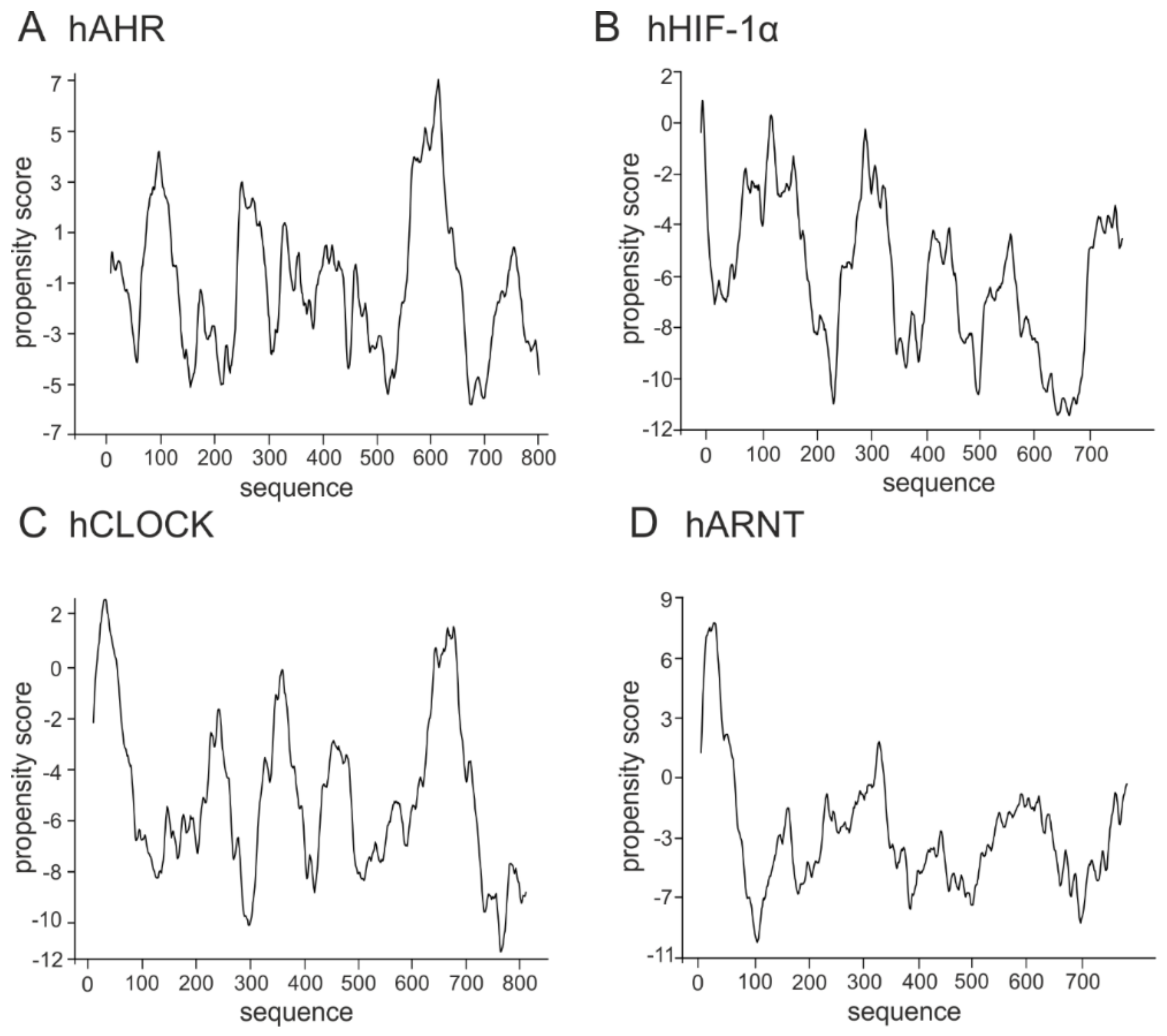
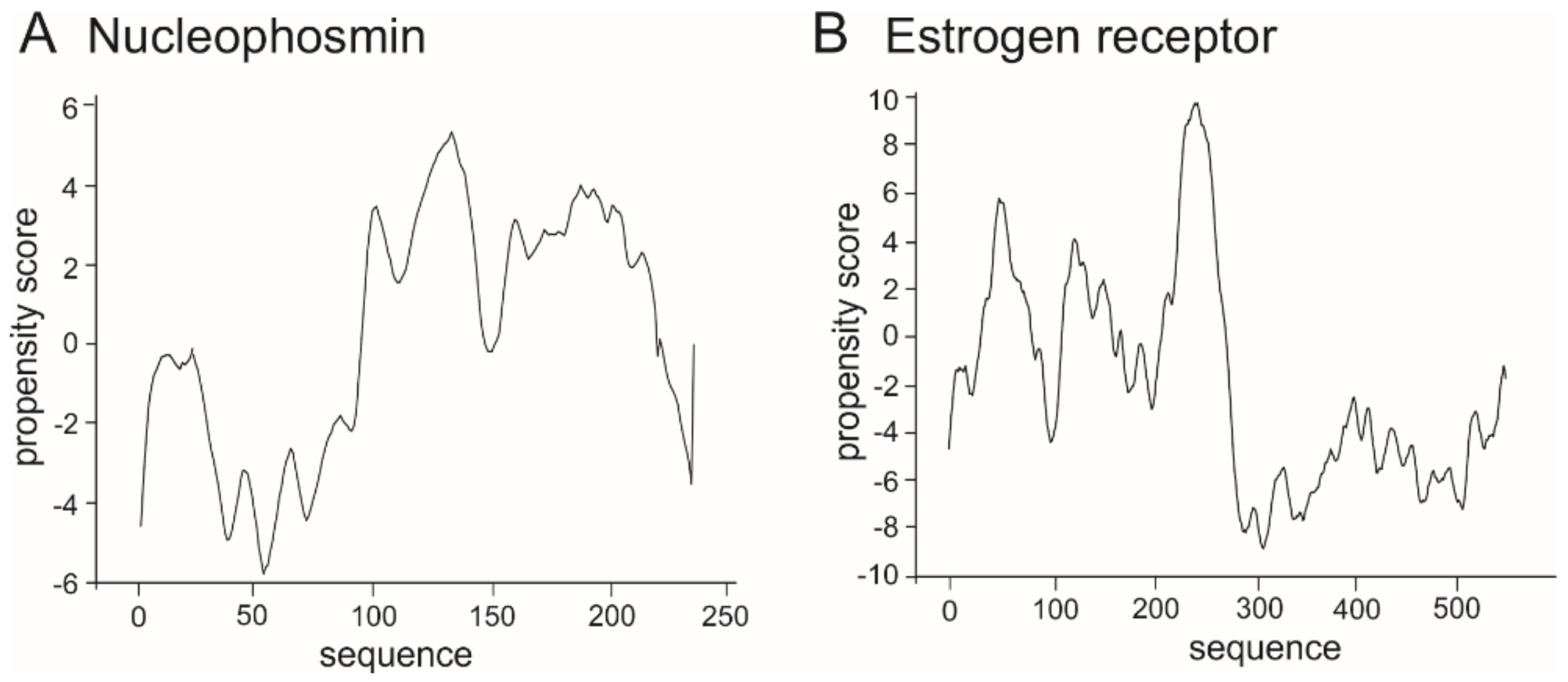
3. The bHLH Transcription Factors as IDPs
4. The Role of IDPs in Maintaining/Creation of LLPS
5. The Transcription Regulation and LLPS
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AHR | Aryl hydrocarbon receptor |
| AS-C | Achaete scute complex |
| ARNT | Aryl hydrocarbon receptor nuclear translocator |
| bHLH | Helix–loop–helix |
| ccRCC | Clear cell renal cell carcinoma |
| CLOCK | Circadian locomotor output cycles protein kaput |
| CTD | C-terminal domain |
| CycT1 | Cyclin T1 |
| EMC | Extramacrochaetae |
| E(spI) | Enhancer of split |
| ER | Estrogen receptor |
| ESC | Embryonic stem cells |
| GCE | Germ cell-expressed protein |
| GRO | Groucho |
| HAT | Histone transacetylase |
| HIF | Hypoxia-inducible factor |
| HSCa | Hematopoietic stem cells |
| HRD | histidine reach domain |
| ID | Inhibitor of DNA binding |
| IDPs | Intrinsically disordered proteins |
| IDRs | Intrinsically disordered regions |
| LLPS | liquid-liquid phase separation |
| LZ | Leucine zipper motif |
| MET | Methoprene-tolerant protein |
| MXI1 | Max interacting protein |
| Ngn2 | Neurogenin |
| NPAS | Neuronal PAS domain-containing protein |
| PAS | Period-arylhydrocarbon nuclear translocator-single minded domain |
| Pol II | RNA polymerase II |
| SE | Super-enhancer |
| SIM | Single-minded protein |
| SIMA | Similar protein |
| S/MARs | Scaffold/matrix associate regions |
| SREBP | Sterol-responsive element-binding protein |
| TAD | Transactivation domain |
| TAZ | Tafazzin |
| TFs | Transcription factors |
| TRH | Trachealess protein |
| USF | Upstream stimulatory factor |
| VHL | Von Hippel-Lindau tumor suppressor |
References
- Robinson, K.A. A network of yeast basic helix-loop-helix interactions. Nucleic Acids Res. 2000, 28, 4460–4466. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.A. SURVEY AND SUMMARY: Saccharomyces cerevisiae basic helix-loop-helix proteins regulate diverse biological processes. Nucleic Acids Res. 2000, 28, 1499–1505. [Google Scholar] [PubMed]
- Sailsbery, J.K.; Atchley, W.R.; Dean, R.A. Phylogenetic analysis and classification of the fungal bHLH domain. Mol. Biol. Evol. 2012, 29, 1301–1318. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.; Dolan, L. Origin and diversification of basic-helix-loop-helix proteins in plants. Mol. Biol. Evol. 2010, 27, 862–874. [Google Scholar]
- Massari, M.E.; Murre, C. Helix-loop-helix proteins: Regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 2000, 20, 429–440. [Google Scholar] [CrossRef]
- Simionato, E.; Ledent, V.; Richards, G.; Thomas-Chollier, M.; Kerner, P.; Coornaert, D.; Degnan, B.M.; Vervoort, M. Origin and diversification of the basic helix-loop-helix gene family in metazoans: Insights from comparative genomics. BMC Evol. Biol. 2007, 7, 33. [Google Scholar] [CrossRef]
- Ledent, V.; Paquet, O.; Vervoort, M. Phylogenetic analysis of the human basic helix-loop-helix proteins. Genome Biol. 2002, 3, 1–18. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.; Yao, Q.; Zheng, X.; Yang, Z. Phylogenetic Analysis of Zebrafish Basic Helix-Loop-Helix Transcription Factors. J. Mol. Evol. 2009, 68, 629–640. [Google Scholar]
- Sailsbery, J.K.; Dean, R.A. Accurate discrimination of bHLH domains in plants, animals, and fungi using biologically meaningful sites. BMC Evol. Biol. 2012, 12, 154. [Google Scholar]
- Murre, C. Helix–loop–helix proteins and the advent of cellular diversity: 30 years of discovery. Genes Dev. 2019, 33, 6–25. [Google Scholar]
- Murre, C.; McCaw, P.S.; Baltimore, D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell 1989, 56, 777–783. [Google Scholar] [CrossRef]
- Murre, C.; Bain, G.; van Dijk, M.A.; Engel, I.; Furnari, B.A.; Massari, M.E.; Matthews, J.R.; Quong, M.W.; Rivera, R.R.; Stuiver, M.H. Structure and function of helix-loop-helix proteins. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1994, 1281, 129–135. [Google Scholar] [CrossRef]
- Ephrussi, A.; Church, G.; Tonegawa, S.; Gilbert, W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science 1985, 227, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Atchley, W.R.; Fitch, W.M. A natural classification of the basic helix-loop-helix class of transcription factors. Proc. Natl. Acad. Sci. USA 1997, 94, 5172–5176. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Rawls, A.; Wilson-Rawls, J.; Roalson, E.H. Basic helix-loop-helix transcription factor gene family phylogenetics and nomenclature. Differ. Res. Biol. Divers. 2010, 80, 1–8. [Google Scholar] [CrossRef]
- Jones, S. An overview of the basic helix-loop-helix proteins. Genome Biol. 2004, 5, 226. [Google Scholar] [CrossRef]
- Wöhner, M.; Tagoh, H.; Bilic, I.; Jaritz, M.; Poliakova, D.K.; Fischer, M.; Busslinger, M. Molecular functions of the transcription factors E2A and E2-2 in controlling germinal center B cell and plasma cell development. J. Exp. Med. 2016, 213, 1201–1221. [Google Scholar] [CrossRef]
- Yi, S.; Yu, M.; Yang, S.; Miron, R.J.; Zhang, Y. Tcf12, A Member of Basic Helix-Loop-Helix Transcription Factors, Mediates Bone Marrow Mesenchymal Stem Cell Osteogenic Differentiation In Vitro and In Vivo. Stem Cells 2017, 35, 386–397. [Google Scholar] [CrossRef]
- Li, Y.; Brauer, P.M.; Singh, J.; Xhiku, S.; Yoganathan, K.; Zúñiga-Pflücker, J.C.; Anderson, M.K. Targeted Disruption of TCF12 Reveals HEB as Essential in Human Mesodermal Specification and Hematopoiesis. Stem Cell Rep. 2017, 9, 779–795. [Google Scholar] [CrossRef]
- Quednow, B.B.; Brzózka, M.M.; Rossner, M.J. Transcription factor 4 (TCF4) and schizophrenia: Integrating the animal and the human perspective. Cell. Mol. Life Sci. 2014, 71, 2815–2835. [Google Scholar] [CrossRef]
- Huang, C.; Chan, J.A.; Schuurmans, C. Proneural bHLH Genes in Development and Disease. In Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 2014; Volume 110, pp. 75–127. [Google Scholar]
- Tan, T.K.; Zhang, C.; Sanda, T. Oncogenic transcriptional program driven by TAL1 in T-cell acute lymphoblastic leukemia. Int. J. Hematol. 2019, 109, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S. Genomics of the OLIG family of a bHLH transcription factor associated with oligo dendrogenesis. Bioinformation 2019, 15, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Bouard, C.; Terreux, R.; Honorat, M.; Manship, B.; Ansieau, S.; Vigneron, A.M.; Puisieux, A.; Payen, L. Deciphering the molecular mechanisms underlying the binding of the TWIST1/E12 complex to regulatory E-box sequences. Nucleic Acids Res. 2016, 44, 5470–5489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whitfield, J.R.; Beaulieu, M.-E.; Soucek, L. Strategies to Inhibit Myc and Their Clinical Applicability. Front. Cell Dev. Biol. 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Amati, B.; Land, H. Myc-Max-Mad: A transcription factor network controlling cell cycle progression, differentiation and death. Curr. Opin. Genet. Dev. 1994, 4, 102–108. [Google Scholar] [CrossRef]
- Lasorella, A.; Benezra, R.; Iavarone, A. The ID proteins: Master regulators of cancer stem cells and tumour aggressiveness. Nat. Rev. Cancer 2014, 14, 77–91. [Google Scholar] [CrossRef]
- Weber, D.; Wiese, C.; Gessler, M. Hey bHLH transcription factors. In Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 2014; Volume 110, pp. 285–315. [Google Scholar]
- Iso, T.; Kedes, L.; Hamamori, Y. HES and HERP families: Multiple effectors of the notch signaling pathway. J. Cell. Physiol. 2003, 194, 237–255. [Google Scholar] [CrossRef]
- Saha, T.T.; Shin, S.W.; Dou, W.; Roy, S.; Zhao, B.; Hou, Y.; Wang, X.-L.; Zou, Z.; Girke, T.; Raikhel, A.S. Hairy and Groucho mediate the action of juvenile hormone receptor Methoprene-tolerant in gene repression. Proc. Natl. Acad. Sci. USA 2016, 113, E735–E743. [Google Scholar] [CrossRef]
- Wright, E.J.; Pereira De Castro, K.; Joshi, A.D.; Elferink, C.J. Canonical and non-canonical aryl hydrocarbon receptor signaling pathways. Curr. Opin. Toxicol. 2017, 2, 87–92. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Moffett, P.; Pelletier, J. Different transcriptional properties of mSim-1 and mSim-2. FEBS Lett. 2000, 466, 80–86. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Kwon, I.; Nakajima, Y.; Ohmiya, Y.; Son, G.H.; Lee, K.H.; Kim, K. Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. J. Cell Sci. 2010, 123, 3547–3557. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.H.L.; Lam, K.K.Y.; Loh, C.C.; Loo, J.M.; Yan, T.; Lim, T.M. Neuronal PAS domain protein 1 is a transcriptional repressor and requires arylhydrocarbon nuclear translocator for its nuclear localization. J. Biol. Chem. 2006, 281, 34617–34629. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, S.; Hamada, S.; Kakinuma, Y.; Yagi, T.; Miura, M. Novel function of neuronal PAS domain protein 1 in erythropoietin expression in neuronal cells. J. Neurosci. Res. 2005, 79, 451–458. [Google Scholar] [CrossRef]
- Gilles-Gonzalez, M.-A.; Gonzales, G. Signal transduction by heme-containing PAS-domain proteins. J. Appl. Physiol. 2004, 96, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Kamnasaran, D. Disruption of the neuronal PAS3 gene in a family affected with schizophrenia. J. Med Genet. 2003, 40, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ooe, N.; Saito, K.; Mikami, N.; Nakatuka, I.; Kaneko, H. Identification of a Novel Basic Helix-Loop-Helix-PAS Factor, NXF, Reveals a Sim2 Competitive, Positive Regulatory Role in Dendritic-Cytoskeleton Modulator Drebrin Gene Expression. Mol. Cell. Biol. 2003, 24, 608–616. [Google Scholar] [CrossRef]
- Li, M.; Mead, E.A.; Zhu, J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl. Acad. Sci. USA 2011, 2011, 638–643. [Google Scholar] [CrossRef]
- Brunnberg, S.; Pettersson, K.; Rydin, E.; Matthews, J.; Hanberg, A.; Pongratz, I. The basic helix-loop-helix-PAS protein ARNT functions as a potent coactivator of estrogen receptor-dependent transcription. Proc. Natl. Acad. Sci. USA 2003, 100, 6517–6522. [Google Scholar] [CrossRef]
- Sharma, P.; Chinaranagari, S.; Chaudhary, J. Inhibitor of differentiation 4 (ID4) acts as an inhibitor of ID-1, -2 and -3 and promotes basic helix loop helix (bHLH) E47 DNA binding and transcriptional activity. Biochimie 2015, 112, 139–150. [Google Scholar] [CrossRef]
- Zelzer, E.; Wappner, P.; Shilo, B.-Z. The PAS domain confers target gene specificity of Drosophila bHLH/PAS proteins. Genes Dev. 1997, 11, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Cusanovich, M.A.; Meyer, T.E. Photoactive yellow protein: A prototypic PAS domain sensory protein and development of a common signaling mechanism. Biochemistry 2003, 42, 4759–4770. [Google Scholar] [CrossRef] [PubMed]
- Mö Glich, A.; Ayers, R.A.; Moffat, K. Structure and Signaling Mechanism of Per-ARNT-Sim Domains. Struct. Fold. Des. 2009, 17, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Roeder, R.G. Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett. 2005, 579, 909–915. [Google Scholar] [CrossRef]
- Ansari, S.A.; Morse, R.H. Mechanisms of Mediator complex action in transcriptional activation. Cell. Mol. Life Sci. 2013, 70, 2743–2756. [Google Scholar] [CrossRef]
- Conaway, R.C.; Conaway, J.W. Function and regulation of the Mediator complex. Curr. Opin. Genet. Dev. 2011, 21, 225–230. [Google Scholar] [CrossRef]
- Poss, Z.C.; Ebmeier, C.C.; Taatjes, D.J. The Mediator complex and transcription regulation. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 575–608. [Google Scholar] [CrossRef]
- Quevedo, M.; Meert, L.; Dekker, M.R.; Dekkers, D.H.W.; Brandsma, J.H.; van den Berg, D.L.C.; Ozgür, Z.; van IJcken, W.F.J.; Demmers, J.; Fornerod, M.; et al. Mediator complex interaction partners organize the transcriptional network that defines neural stem cells. Nat. Commun. 2019, 10, 2669. [Google Scholar] [CrossRef]
- Malik, S.; Roeder, R.G. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 2010, 11, 761–772. [Google Scholar] [CrossRef]
- Malik, N.; Agarwal, P.; Tyagi, A. Emerging functions of multi-protein complex Mediator with special emphasis on plants. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 475–502. [Google Scholar] [CrossRef]
- An, C.; Mou, Z. The function of the Mediator complex in plant immunity. Plant Signal. Behav. 2013, 8, e23182. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jiang, H.; Li, L.; Zhai, Q.; Qi, L.; Zhou, W.; Liu, X.; Li, H.; Zheng, W.; Sun, J.; et al. The Arabidopsis Mediator Subunit MED25 Differentially Regulates Jasmonate and Abscisic Acid Signaling through Interacting with the MYC2 and ABI5 Transcription Factors. Plant Cell 2012, 24, 2898–2916. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Li, L.; Zhai, Q.; You, Y.; Deng, L.; Wu, F.; Chen, R.; Jiang, H.; Wang, H.; Chen, Q.; et al. Mediator subunit MED25 links the jasmonate receptor to transcriptionally active chromatin. Proc. Natl. Acad. Sci. USA 2017, 114, E8930–E8939. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, M.; Deng, L.; Shen, J.; Fang, M.; Chen, Q.; Lu, Y.; Wang, Q.; Li, C.; Zhai, Q. Myc2 regulates the termination of jasmonate signaling via an autoregulatory negative feedback loop[open]. Plant Cell 2019, 31, 106–127. [Google Scholar] [CrossRef]
- Li, X.; Yang, R.; Chen, H. The arabidopsis thaliana mediator subunit MED8 regulates plant immunity to botrytis cinerea through interacting with the basic helix-loop-helix (bHLH) transcription factor FAMA. PLoS ONE 2018, 13, e0193458. [Google Scholar] [CrossRef]
- Oliner, J.D.; Andresen, J.M.; Hansen, S.K.; Zhou, S.; Tjian, R. SREBP transcriptional activity is mediated through an interaction with the CREB-binding protein. Genes Dev. 1996, 10, 2903–2911. [Google Scholar] [CrossRef]
- Yang, F.; Vought, B.W.; Satterlee, J.S.; Walker, A.K.; Jim Sun, Z.Y.; Watts, J.L.; DeBeaumont, R.; Mako Saito, R.; Hyberts, S.G.; Yang, S.; et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature 2006, 442, 700–704. [Google Scholar] [CrossRef]
- Pacheco, D.; Warfield, L.; Brajcich, M.; Robbins, H.; Luo, J.; Ranish, J.; Hahn, S. Transcription Activation Domains of the Yeast Factors Met4 and Ino2: Tandem Activation Domains with Properties Similar to the Yeast Gcn4 Activator. Mol. Cell. Biol. 2018, 38, e00038-18. [Google Scholar] [CrossRef]
- Lazrak, M.; Deleuze, V.; Noel, D.; Haouzi, D.; Chalhoub, E.; Dohet, C.; Robbins, I.; Mathieu, D. The bHLH TAL-1/SCL regulates endothelial cell migration and morphogenesis. J. Cell Sci. 2004, 117, 1161–1171. [Google Scholar] [CrossRef]
- Huang, S.; Qiu, Y.; Stein, R.W.; Brandt, S.J. p300 functions as a transcriptional coactivator for the TAL1/SCL oncoprotein. Oncogene 1999, 18, 4958–4967. [Google Scholar] [CrossRef]
- Puri, P.L.; Avantaggiati, M.L. p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription arrest in the G 0 phase, irreversible exit from the cell cycle. EMBO J. 1997, 16, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yao, Z.; Sarkar, D.; Lawrence, M.; Sanchez, G.J.; Parker, M.H.; MacQuarrie, K.L.; Davison, J.; Morgan, M.T.; Ruzzo, W.L.; et al. Genome-wide MyoD Binding in Skeletal Muscle Cells: A Potential for Broad Cellular Reprogramming. Dev. Cell 2010, 18, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Raposo, A.A.S.F.; Vasconcelos, F.F.; Drechsel, D.; Marie, C.; Johnston, C.; Dolle, D.; Bithell, A.; Gillotin, S.; van den Berg, D.L.C.; Ettwiller, L.; et al. Ascl1 coordinately regulates gene expression and the chromatin landscape during neurogenesis. Cell Rep. 2015, 10, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-I.; Puga, A. Does the aryl hydrocarbon receptor regulate pluripotency? Curr. Opin. Toxicol. 2017, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tan, J.; Lim, K.J.; Koh, J.; Ooi, W.F.; Li, Z.; Huang, D.; Xing, M.; Chan, Y.S.; Qu, J.Z.; et al. VHL deficiency drives enhancer activation of oncogenes in clear cell renal cell carcinoma. Cancer Discov. 2017, 7, 1284–1305. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Hirayama, J.; Sassone-Corsi, P. Circadian regulator CLOCK is a histone acetyltransferase. Cell 2006, 125, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Ohkawa, Y.; Imbalzano, A.N. Temporal regulation of chromatin during myoblast differentiation. Semin. Cell Dev. Biol. 2017, 72, 77–86. [Google Scholar] [CrossRef]
- Heisig, J.; Weber, D.; Englberger, E.; Winkler, A.; Kneitz, S.; Sung, W.-K.; Wolf, E.; Eilers, M.; Wei, C.-L.; Gessler, M. Target gene analysis by microarrays and chromatin immunoprecipitation identifies HEY proteins as highly redundant bHLH repressors. PLoS Genet. 2012, 8, e1002728. [Google Scholar] [CrossRef]
- Rahl, P.B.; Lin, C.Y.; Seila, A.C.; Flynn, R.A.; McCuine, S.; Burge, C.B.; Sharp, P.A.; Young, R.A. c-Myc Regulates Transcriptional Pause Release. Cell 2010, 141, 432–445. [Google Scholar] [CrossRef]
- Anfinsen, C.B. Principles that govern the folding of protein chains. Science 1973, 181, 223–230. [Google Scholar] [CrossRef]
- Uversky, V.N. A decade and a half of protein intrinsic disorder: Biology still waits for physics. Protein Sci. A Publ. Protein Soc. 2013, 22, 693–724. [Google Scholar] [CrossRef] [PubMed]
- Dyson, H.J.; Wright, P.E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 2005, 6, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Lawson, J.D.; Brown, C.J.; Williams, R.M.; Romero, P.; Oh, J.S.; Oldfield, C.J.; Campen, A.M.; Ratliff, C.M.; Hipps, K.W.; et al. Intrinsically disordered protein. J. Mol. Graph. Model. 2001, 19, 26–59. [Google Scholar] [CrossRef]
- Lu, H.; Yu, D.; Hansen, A.S.; Ganguly, S.; Liu, R.; Heckert, A.; Darzacq, X.; Zhou, Q. Phase-separation mechanism for C-terminal hyperphosphorylation of RNA polymerase II. Nature 2018, 558, 318. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Dunker, A.K. Understanding protein non-folding. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2010, 1804, 1231–1264. [Google Scholar] [CrossRef]
- Dosztanyi, Z.; Csizmok, V.; Tompa, P.; Simon, I. The pairwise energy content estimated from amino acid composition discriminates between folded and intrinsically unstructured proteins. J. Mol. Biol. 2005, 347, 827–839. [Google Scholar] [CrossRef]
- Campen, A.; Williams, R.; Brown, C.; Meng, J.; Uversky, V.; Dunker, A. TOP-IDP-Scale: A New Amino Acid Scale Measuring Propensity for Intrinsic Disorder. Protein Pept. Lett. 2008, 15, 956–963. [Google Scholar] [CrossRef]
- Williams, R.M.; Obradovi, Z.; Mathura, V.; Braun, W.; Garner, E.C.; Young, J.; Takayama, S.; Brown, C.J.; Dunker, A.K. The protein non-folding problem: Amino acid determinants of intrinsic order and disorder. Pac. Symp. Biocomput. 2001, 2001, 89–100. [Google Scholar]
- Vacic, V.; Uversky, V.N.; Dunker, A.K.; Lonardi, S. Composition Profiler: A tool for discovery and visualization of amino acid composition differences. BMC Bioinform. 2007, 8, 211. [Google Scholar] [CrossRef]
- Uversky, V.N.; Gillespie, J.R.; Fink, A.L. Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins Struct. Funct. Genet. 2000, 41, 415–427. [Google Scholar] [CrossRef]
- Dunker, A.K.; Obradovic, Z. The protein trinity—Linking function and disorder. Nat. Biotechnol. 2001, 19, 805. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P. The interplay between structure and function in intrinsically unstructured proteins. FEBS Lett. 2005, 579, 3346–3354. [Google Scholar] [CrossRef] [PubMed]
- Van Der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Yan, J.; Fan, X.; Mizianty, M.J.; Xue, B.; Wang, K.; Hu, G.; Uversky, V.N.; Kurgan, L. Exceptionally abundant exceptions: Comprehensive characterization of intrinsic disorder in all domains of life. Cell. Mol. Life Sci. 2014, 72, 137–151. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef]
- Tompa, P.; Fuxreiter, M. Fuzzy complexes: Polymorphism and structural disorder in protein-protein interactions. Trends Biochem. Sci. 2008, 33, 2–8. [Google Scholar] [CrossRef]
- Iakoucheva, L.M.; Radivojac, P.; Brown, C.J.; O’Connor, T.R.; Sikes, J.G.; Obradovic, Z.; Dunker, A.K. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004, 32, 1037–1049. [Google Scholar] [CrossRef]
- Hatakeyama, J.; Kageyama, R. Retinal cell fate determination and bHLH factors. Semin. Cell Dev. Biol. 2004, 15, 83–89. [Google Scholar] [CrossRef]
- Sölter, M.; Locker, M.; Boy, S.; Taelman, V.; Bellefroid, E.J.; Perron, M.; Pieler, T. Characterization and function of the bHLH-O protein XHes2: Insight into the mechanism controlling retinal cell fate decision. Development 2006, 133, 4097–4108. [Google Scholar] [CrossRef][Green Version]
- Parker, M.H.; Perry, R.L.S.; Fauteux, M.C.; Berkes, C.A.; Rudnicki, M.A. MyoD Synergizes with the E-Protein HEB To Induce Myogenic Differentiation. Mol. Cell. Biol. 2006, 26, 5771–5783. [Google Scholar] [CrossRef]
- McDowell, G.S.; Hindley, C.J.; Lippens, G.; Landrieu, I.; Philpott, A. Phosphorylation in intrinsically disordered regions regulates the activity of Neurogenin2. BMC Biochem. 2014, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Aguado-Llera, D.; Goormaghtigh, E.; de Geest, N.; Quan, X.-J.; Prieto, A.; Hassan, B.A.; Gómez, J.; Neira, J.L. The basic helix-loop-helix region of human neurogenin 1 is a monomeric natively unfolded protein which forms a “fuzzy” complex upon DNA binding. Biochemistry 2010, 49, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Panova, S.; Cliff, M.J.; Macek, P.; Blackledge, M.; Jensen, M.R.; Nissink, J.W.M.; Embrey, K.J.; Davies, R.; Waltho, J.P. Mapping Hidden Residual Structure within the Myc bHLH-LZ Domain Using Chemical Denaturant Titration. Structure 2019, 27, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Obradovic, Z.; Dunker, A.K. Sequence Data Analysis for Long Disordered Regions Prediction in the Calcineurin Family. Genome Inform. Workshop Genome Inform. 1997, 8, 110–124. [Google Scholar]
- Li, X.; Romero, P.; Rani, M.; Dunker, A.; Obradovic, Z. Predicting Protein Disorder for N-, C-, and Internal Regions. Genome Inform. Workshop Genome Inform. 1999, 10, 30–40. [Google Scholar]
- Cilia, E.; Pancsa, R.; Tompa, P.; Lenaerts, T.; Vranken, W.F. From protein sequence to dynamics and disorder with DynaMine. Nat. Commun. 2013, 4, 2741. [Google Scholar] [CrossRef]
- Cilia, E.; Pancsa, R.; Tompa, P.; Lenaerts, T.; Vranken, W.F. The DynaMine webserver: Predicting protein dynamics from sequence. Nucleic Acids Res. 2014, 42, W264–W270. [Google Scholar] [CrossRef]
- Furness, S.G.B.; Lees, M.J.; Whitelaw, M.L. The dioxin (aryl hydrocarbon) receptor as a model for adaptive responses of bHLH/PAS transcription factors. FEBS Lett. 2007, 581, 3616–3625. [Google Scholar] [CrossRef]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Jülicher, F.; Hyman, A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef]
- Molliex, A.; Temirov, J.; Lee, J.; Coughlin, M.; Kanagaraj, A.P.; Kim, H.J.; Mittag, T.; Taylor, J.P. Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization. Cell 2015, 163, 123–133. [Google Scholar] [CrossRef]
- Nott, T.J.; Petsalaki, E.; Farber, P.; Jervis, D.; Fussner, E.; Plochowietz, A.; Craggs, T.D.; Bazett-Jones, D.P.; Pawson, T.; Forman-Kay, J.D.; et al. Phase Transition of a Disordered Nuage Protein Generates Environmentally Responsive Membraneless Organelles. Mol. Cell 2015, 57, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Elbaum-Garfinkle, S.; Kim, Y.; Szczepaniak, K.; Chen, C.C.H.; Eckmann, C.R.; Myong, S.; Brangwynne, C.P. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. USA 2015, 112, 7189–7194. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Koh, C.H.; Tjota, M.; Pieuchot, L.; Raman, V.; Chandrababu, K.B.; Yang, D.; Wong, L.; Jedd, G. Intrinsically disordered proteins aggregate at fungal cell-to-cell channels and regulate intercellular connectivity. Proc. Natl. Acad. Sci. USA 2012, 109, 15781–15786. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, D.M.; Cika, J.A.; Guy, C.S.; Ban, D.; Banerjee, P.R.; Stanley, C.B.; Nourse, A.; Deniz, A.A.; Kriwacki, R.W. Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying R-rich linear motifs and rRNA. eLife 2016, 5, e13571. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P.; Tompa, P.; Pappu, R.V. Polymer physics of intracellular phase transitions. Nat. Phys. 2015, 11, 899–904. [Google Scholar] [CrossRef]
- Banani, S.F.; Rice, A.M.; Peeples, W.B.; Lin, Y.; Jain, S.; Parker, R.; Rosen, M.K. Compositional Control of Phase-Separated Cellular Bodies. Cell 2016, 166, 651–663. [Google Scholar] [CrossRef]
- Ditlev, J.A.; Case, L.B.; Rosen, M.K. Who’s In and Who’s Out—Compositional Control of Biomolecular Condensates. J. Mol. Biol. 2018, 430, 4666–4684. [Google Scholar] [CrossRef]
- Harmon, T.S.; Holehouse, A.S.; Rosen, M.K.; Pappu, R.V. Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. eLife 2017, 6, e30294. [Google Scholar] [CrossRef]
- Posey, A.E.; Holehouse, A.S.; Pappu, R.V. Phase Separation of Intrinsically Disordered Proteins. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128156490. [Google Scholar]
- Berezney, R.; Coffey, D.S. Identification of a nuclear protein matrix. Biochem. Biophys. Res. Commun. 1974, 60, 1410–1417. [Google Scholar] [CrossRef]
- Linnemann, A.K.; Platts, A.E.; Krawetz, S.A. Differential nuclear scaffold/matrix attachment marks expressed genes. Hum. Mol. Genet. 2009, 18, 645–654. [Google Scholar] [CrossRef]
- Engelke, R.; Riede, J.; Hegermann, J.; Wuerch, A.; Eimer, S.; Dengjel, J.; Mittler, G. The quantitative nuclear matrix proteome as a biochemical snapshot of nuclear organization. J. Proteome Res. 2014, 13, 3940–3956. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.C.; Brangwynne, C.P. Inverse size scaling of the nucleolus by a concentration-dependent phase transition. Curr. Biol. 2015, 25, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Weber, S.C.; Vaidya, N.; Haataja, M.; Brangwynne, C.P. RNA transcription modulates phase transition-driven nuclear body assembly. Proc. Natl. Acad. Sci. USA 2015, 112, E5237–E5245. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.A.; Doolittle, L.K.; Jensen, L.E.; Gamarra, N.; Redding, S.; Rosen, M.K. Organization and Regulation of Chromatin by Liquid-Liquid Phase Separation. bioRxiv 2019. [Google Scholar] [CrossRef]
- Duronio, R.J.; Marzluff, W.F. Coordinating cell cycle-regulated histone gene expression through assembly and function of the Histone Locus Body. RNA Biol. 2017, 14, 726–738. [Google Scholar] [CrossRef]
- Mitrea, D.M.; Kriwacki, R.W. Phase separation in biology; Functional organization of a higher order Short linear motifs—The unexplored frontier of the eukaryotic proteome. Cell Commun. Signal. 2016, 14, 1–20. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Gutman, O.; Lu, H.; Zhou, Q.; Henis, Y.I.; Luo, K. Phase separation of TAZ compartmentalizes the transcription machinery to promote gene expression. bioRxiv 2019. [Google Scholar] [CrossRef]
- Sabari, B.R.; Dall’Agnese, A.; Boija, A.; Klein, I.A.; Coffey, E.L.; Shrinivas, K.; Abraham, B.J.; Hannett, N.M.; Zamudio, A.V.; Manteiga, J.C.; et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 2018, 361, eaar3958. [Google Scholar] [CrossRef]
- Boija, A.; Klein, I.A.; Sabari, B.R.; Dall’Agnese, A.; Coffey, E.L.; Zamudio, A.V.; Li, C.H.; Shrinivas, K.; Manteiga, J.C.; Hannett, N.M.; et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 2018, 175, 1842–1855.e16. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Yuan, P.; Fang, F.; Huss, M.; Vega, V.B.; Wong, E.; Orlov, Y.L.; Zhang, W.; Jiang, J.; et al. Integration of External Signaling Pathways with the Core Transcriptional Network in Embryonic Stem Cells. Cell 2008, 133, 1106–1117. [Google Scholar] [CrossRef]
- Whyte, W.A.; Orlando, D.A.; Hnisz, D.; Abraham, B.J.; Lin, C.Y.; Kagey, M.H.; Rahl, P.B.; Lee, T.I.; Young, R.A. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 2013, 153, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Hnisz, D.; Shrinivas, K.; Young, R.A.; Chakraborty, A.K.; Sharp, P.A. A Phase Separation Model for Transcriptional Control. Cell 2017, 169, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.R.; Abraham, B.J.; Anders, L.; Berezovskaya, A.; Gutierrez, A.; Durbin, A.D.; Etchin, J.; Lee, L.; Sallan, S.E.; Silverman, L.B.; et al. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. Science 2014, 346, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D.; Lin, C.Y.; Duan, Q.; Griffin, G.; Federation, A.J.; Paranal, R.M.; Bair, S.; Newton, G.; Lichtman, A.H.; Kung, A.L.; et al. Nf-kb directs dynamic super enhancer formation in inflammation and atherogenesis. Mol. Cell 2014, 56, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Lovén, J.; Hoke, H.A.; Lin, C.Y.; Lau, A.; Orlando, D.A.; Vakoc, C.R.; Bradner, J.E.; Lee, T.I.; Young, R.A. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 2013, 153, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, E.; Ferrari, F.; Walsh, R.M.; Bar-Nur, O.; Stadtfeld, M.; Cheloufi, S.; Stuart, H.T.; Polo, J.M.; Ohsumi, T.K.; Borowsky, M.L.; et al. Genome-wide Chromatin Interactions of the Nanog Locus in Pluripotency, Differentiation, and Reprogramming. Cell Stem Cell 2013, 12, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Manavathi, B.; Samanthapudi, V.S.K.; Gajulapalli, V.N.R. Estrogen receptor coregulators and pioneer factors: The orchestrators of mammary gland cell fate and development. Front. Cell Dev. Biol. 2014, 12, 34. [Google Scholar] [CrossRef]
- Kagey, M.H.; Newman, J.J.; Bilodeau, S.; Zhan, Y.; Orlando, D.A.; Van Berkum, N.L.; Ebmeier, C.C.; Goossens, J.; Rahl, P.B.; Levine, S.S.; et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature 2010, 467, 430. [Google Scholar] [CrossRef]
- Heery, D.M.; Kalkhoven, E.; Hoare, S.; Parker, M.G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 1997, 387, 733. [Google Scholar] [CrossRef]
- Cagas, P.M.; Corden, J.L. Structural studies of a synthetic peptide derived from the carboxyl-terminal domain of RNA polymerase II. Proteins Struct. Funct. Bioinform. 1995, 21, 149–160. [Google Scholar] [CrossRef]
- Portz, B.; Lu, F.; Gibbs, E.B.; Mayfield, J.E.; Rachel Mehaffey, M.; Zhang, Y.J.; Brodbelt, J.S.; Showalter, S.A.; Gilmour, D.S. Structural heterogeneity in the intrinsically disordered RNA polymerase II C-terminal domain. Nat. Commun. 2017, 8, 15231. [Google Scholar] [CrossRef] [PubMed]
- Corden, J.L.; Cadena, D.L.; Ahearn, J.M.; Dahmus, M.E. A unique structure at the carboxyl terminus of the largest subunit of eukaryotic RNA polymerase II. Proc. Natl. Acad. Sci. USA 1985, 82, 7934–7938. [Google Scholar] [CrossRef] [PubMed]
- Dahmus, M.E. Reversible Phosphorylation of the C-terminal Domain of RNA Polymerase II. J. Biol. Chem. 1996, 271, 19009–19012. [Google Scholar] [CrossRef]
- Wippich, F.; Bodenmiller, B.; Trajkovska, M.G.; Wanka, S.; Aebersold, R.; Pelkmans, L. Dual specificity kinase DYRK3 couples stress granule condensation/ dissolution to mTORC1 signaling. Cell 2013, 152, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Chen, J.X.; Selbach, M.; Pelkmans, L. Kinase-controlled phase transition of membraneless organelles in mitosis. Nature 2018, 559, 211. [Google Scholar] [CrossRef] [PubMed]
- Boehning, M.; Dugast-Darzacq, C.; Rankovic, M.; Hansen, A.S.; Yu, T.; Marie-Nelly, H.; McSwiggen, D.T.; Kokic, G.; Dailey, G.M.; Cramer, P.; et al. RNA polymerase II clustering through carboxy-terminal domain phase separation. Nat. Struct. Mol. Biol. 2018, 25, 833. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, B.; Lorenzo Gotor, N.; Dhar, R.; Cirillo, D.; Baldrighi, M.; Tartaglia, G.G.; Lehner, B. A Concentration-Dependent Liquid Phase Separation Can Cause Toxicity upon Increased Protein Expression. Cell Rep. 2016, 16, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, B.; Erdős, G.; Szabó, B.; Schád, É.; Tantos, Á.; Abukhairan, R.; Horváth, T.; Murvai, N.; Kovács, O.P.; Kovács, M.; et al. PhaSePro: The database of proteins driving liquid–liquid phase separation. Nucleic Acids Res. 2019. [Google Scholar] [CrossRef]
- Darling, A.L.; Liu, Y.; Oldfield, C.J.; Uversky, V.N. Intrinsically Disordered Proteome of Human Membrane-Less Organelles. Proteomics 2018, 18, 1700193. [Google Scholar] [CrossRef]
- Hyman, A.A.; Weber, C.A.; Jülicher, F. Liquid-Liquid Phase Separation in Biology. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58. [Google Scholar] [CrossRef]
- Uversky, V.N. Protein intrinsic disorder-based liquid–liquid phase transitions in biological systems: Complex coacervates and membrane-less organelles. Adv. Colloid Interface Sci. 2017, 239, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef] [PubMed]
| Structural Motif Dimerization | Representative Members | Short Description |
|---|---|---|
| class I (E proteins)/ group A | ||
| bHLH, homo- and heterodimerization | Vertebrate: E12, E47 [17], HEB [18,19], TCF4 [20] Invertebrate: Daughterless | transcription activators, ubiquitous expression, neurogenesis, immune cell development, sex development, gonadogenesis |
| class II/ group A | ||
| bHLH, preferred heterodimerization with class I partners | Vertebrate: MYOD, Myogenin, MYF5-6, Ngn1-3, ATOH, NeuroD, NDRF, MATH, MASH, ASCL1 [21], TAL1/SCL [22], OLIG1-3 [23] Invertebrate: TWIST [24], AS-C | transcription activators, tissue specific expression, muscle development, neuro-genesis, generation of autonomic and olfactory neurons, development of granule neurons and external germinal layer of cerebellum, oligodendrocyte development, specification of blood lineage and maturation of several hematopoietic cells, pancreatic development |
| class III/ group B | ||
| bHLH-LZ | Vertebrate: MYC [25], USF, TFE3, SREBP1-2 Drosophila: MYC Plants: MYC2 | transcription activators/represors, oncogenic transformation, apoptosis, cellular differentiation, proliferation, cholesterol-mediated induction of the low-density lipoprotein receptor, jasmonate signaling (plants) |
| class IV/ group B | ||
| bHLH, heterodimerisation with each other and MYC proteins | Vertabrate: MAD, MAX [26], MXI1 Drosophila: MNT, MAX | transcription regulators lacking transactivation domain (TAD) |
| class V/ group D | ||
| HLH (no basic region) | Vertebrate: ID1-4 [27] Invertebrate:EMC | negative transcription regulators of class I and II (group A) proteins, no DNA binding, regulation by sequestration. |
| class VI/ group B | ||
| bHLH-O, (presence of proline in basic region) | Vertebrate: HES, HEY1-3 [28], STRA13, HERP1-2 [29] Drosophila: HAIRY [30], E(spI) | negative transcription regulators interacting with corepressors (Groucho); neurogenesis, vasculogenesis, mesoderm segmentation, myogenesis, T lymphocyte development, cardiovascular development and homeostasis; effectors of Notch signalling [28]; in Drosophila: regulation of differentiation, anteroposterior segmentation and sex determination |
| class VII/ group C - subclass I | ||
| bHLH-PAS, heterodimerization with subclass II | Vertebrate: AHR [31], HIF1-3α [32], SIM1-2 [33], CLOCK [34], NPAS1-4 [35,36,37,38,39] Drosophila: MET [40], GCE, SIMA, TRH | transcription regulation in response to physiological and environmental signals: xenobiotics, hypoxia, development, circadian rhytms |
| class VII/ group C - subclass II | ||
| bHLH-PAS, homo- and heterodimerization with subclass I | Vertebrate: ARNT [41], ARNT2, BMAL1, BMAL2 Drosophila: TANGO, CYCLE | general partners for subclass I bHLH-PAS proteins |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarczewska, A.; Greb-Markiewicz, B. The Significance of the Intrinsically Disordered Regions for the Functions of the bHLH Transcription Factors. Int. J. Mol. Sci. 2019, 20, 5306. https://doi.org/10.3390/ijms20215306
Tarczewska A, Greb-Markiewicz B. The Significance of the Intrinsically Disordered Regions for the Functions of the bHLH Transcription Factors. International Journal of Molecular Sciences. 2019; 20(21):5306. https://doi.org/10.3390/ijms20215306
Chicago/Turabian StyleTarczewska, Aneta, and Beata Greb-Markiewicz. 2019. "The Significance of the Intrinsically Disordered Regions for the Functions of the bHLH Transcription Factors" International Journal of Molecular Sciences 20, no. 21: 5306. https://doi.org/10.3390/ijms20215306
APA StyleTarczewska, A., & Greb-Markiewicz, B. (2019). The Significance of the Intrinsically Disordered Regions for the Functions of the bHLH Transcription Factors. International Journal of Molecular Sciences, 20(21), 5306. https://doi.org/10.3390/ijms20215306





