Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin
Abstract
1. Introduction
2. Results
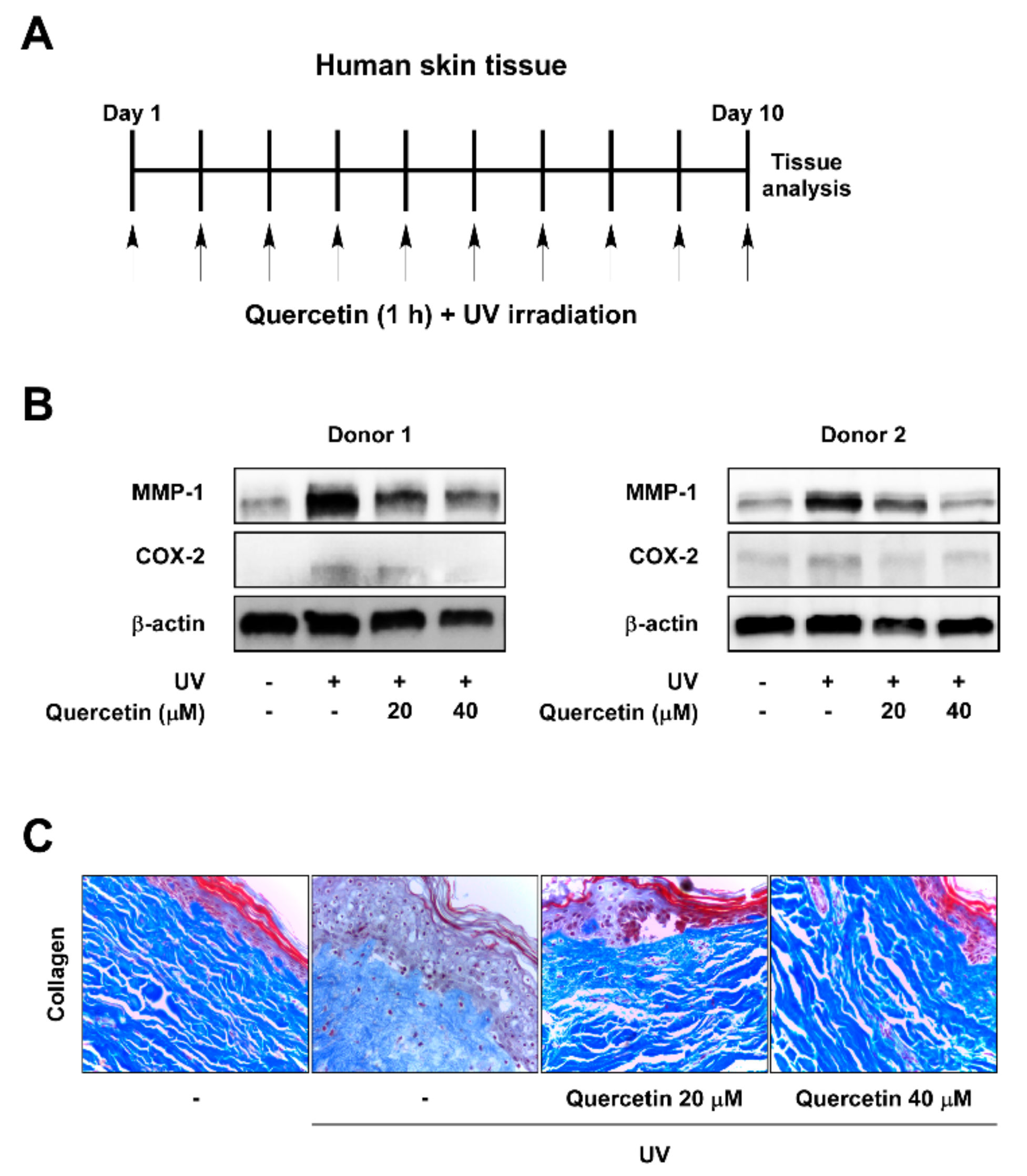
2.1. Quercetin Prevents UV-Induced Skin Aging in Human Skin Tissue
2.2. Quercetin Inhibits UV-Induced AP-1 and NF-κB Activation
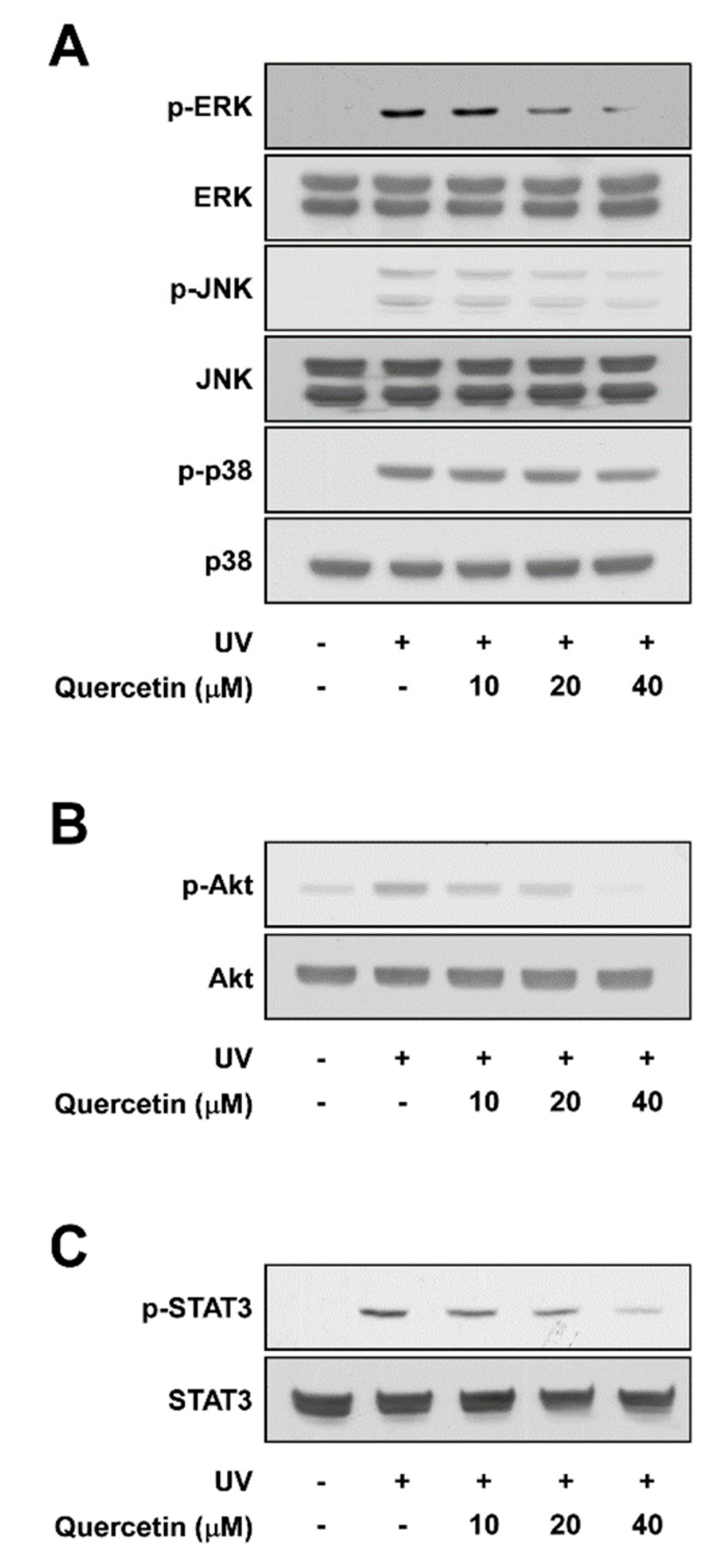
2.3. Quercetin Suppresses UV-Induced Phosphorylations of ERK, JNK, Akt, and STAT3
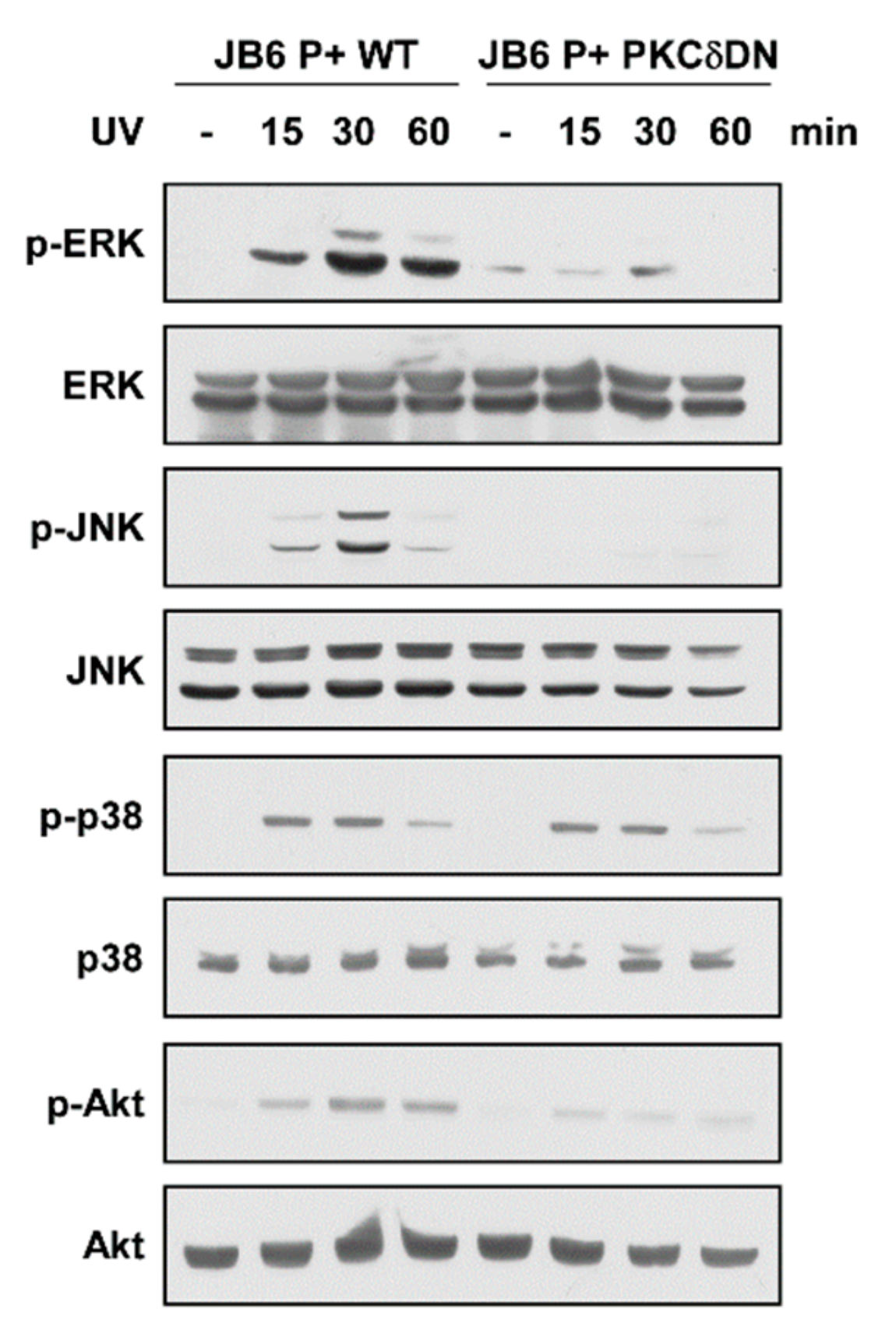
2.4. Expression of Dominant Negative PKCδ Suppresses UV-Induced MAPK and Akt Activation
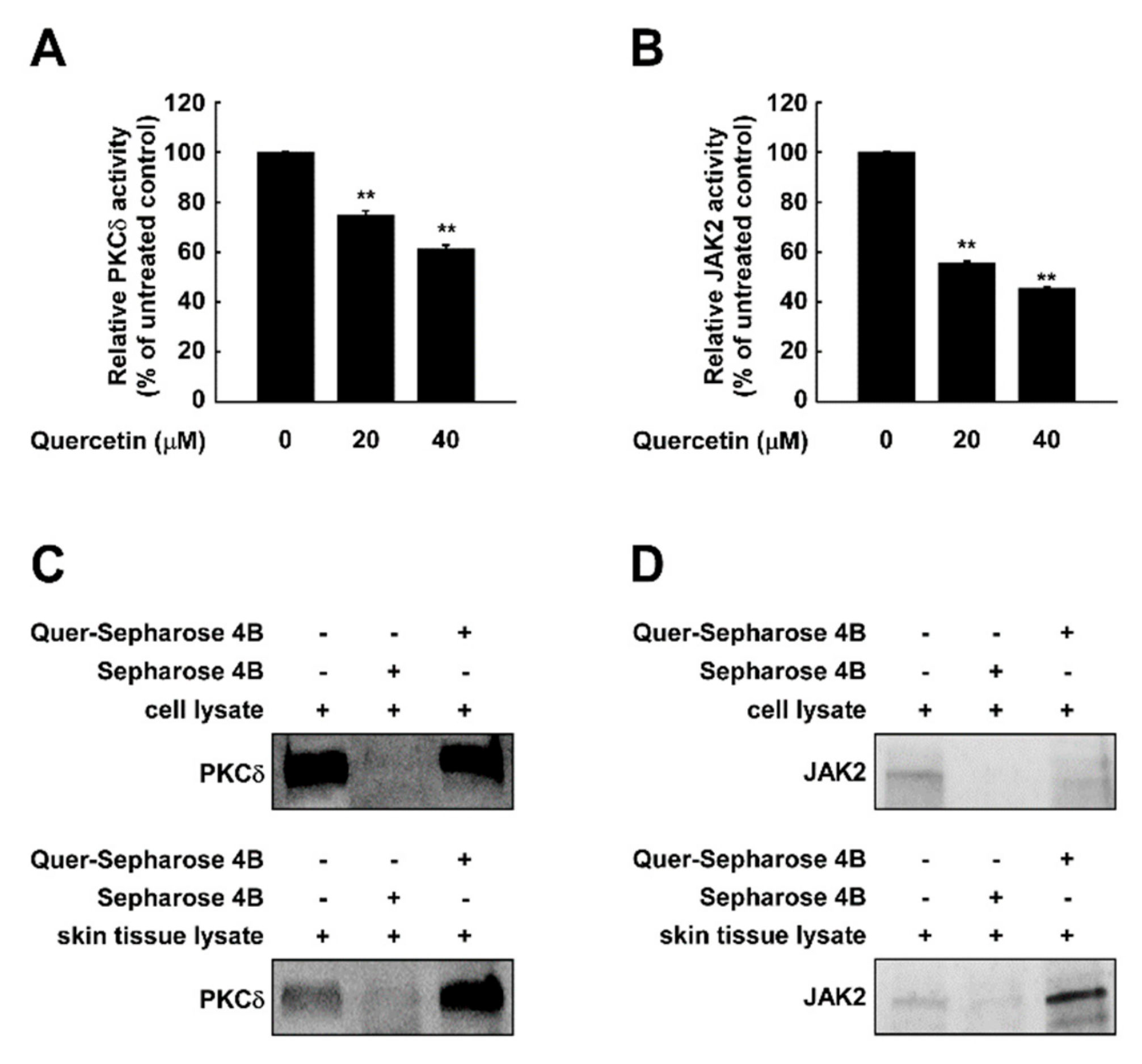
2.5. Quercetin Directly Binds to and Attenuates the Activity of PKCδ and JAK2 Kinase
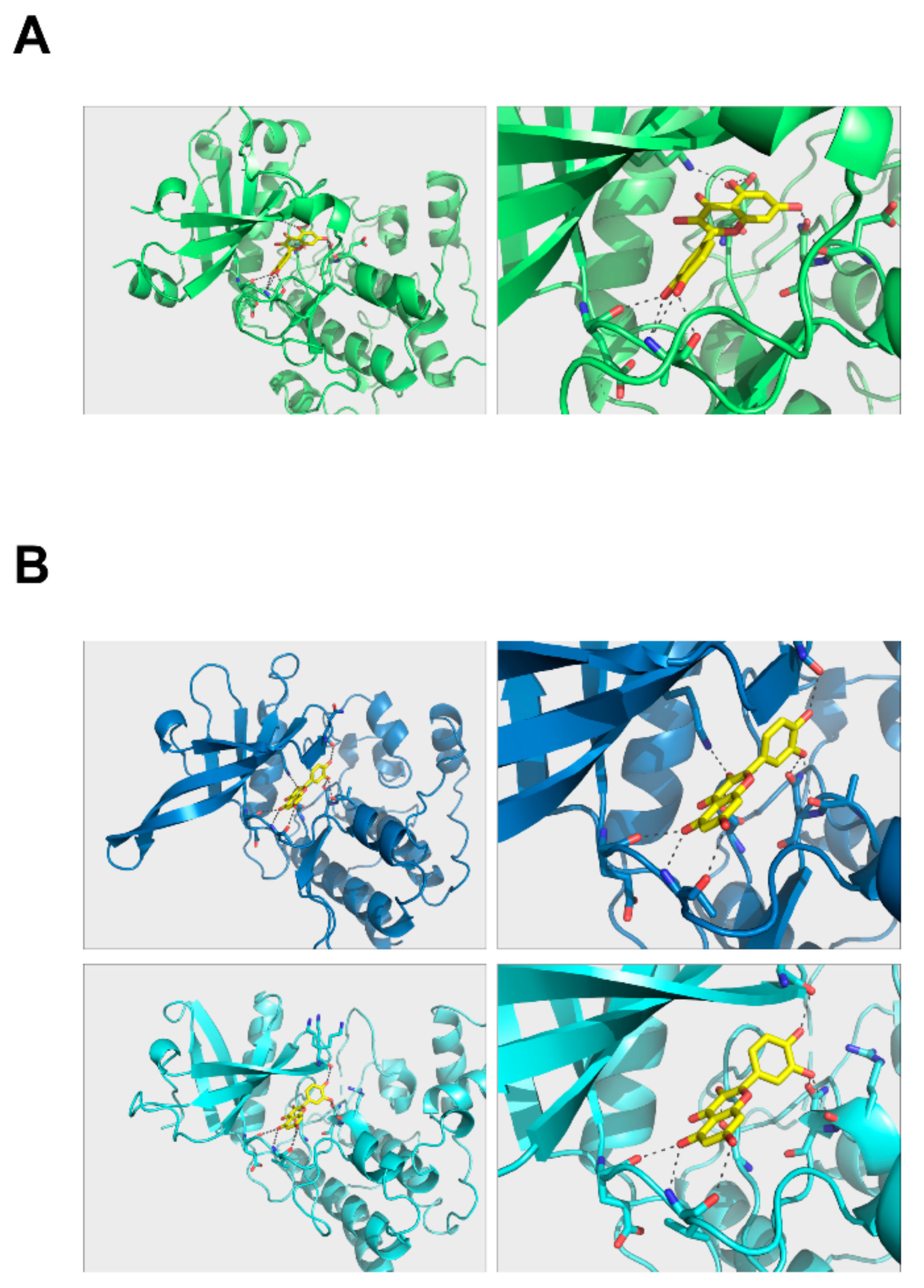
2.6. Docking Model of Quercetin with PKCδ and JAK2
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and UV Irradiation
4.3. Ethic Statement
4.4. Excised Human Skin and UV Irradiation
4.5. COX-2 Promoter Reporter and AP-1 and NF-κB Activation Luciferase Assays
4.6. Kinase Assays
4.7. Immunoblotting
4.8. Histological Analaysis
4.9. Preparation of Quercetin-Sepharose 4B Beads
4.10. In Vitro and Ex Vivo Pull-Down Assays
4.11. Complex Structure Computational Modeling
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| UV | Ultraviolet |
| MMP-1 | Matrix metalloprotease-1 |
| COX-2 | Cyclooxygenase-2 |
| NF-κB | Nuclear factor-κB |
| AP-1 | Activator protein-1 |
| MAPK | Mitogen-activated protein kinase |
| ERKs | Extracellular signal-regulated kinases |
| JNKs | c-Jun N terminal kinases |
| PKC-delta | Protein kinase C delta |
| JAK2 | Janus kinase 2 |
| STAT-3 | Signal transducer the and activator of transcription 3 |
References
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet Radiation, Aging and the Skin: Prevention of Damage by Topical cAMP Manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Rundhaug, J.E.; Mikulec, C.; Pavone, A.; Fischer, S.M. A role for cyclooxygenase-2 in ultraviolet light-induced skin carcinogenesis. Mol. Carcinog. 2007, 46, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Camarillo, C.; Ocampo, E.A.; Casamichana, M.L.; Perez-Plasencia, C.; Alvarez-Sanchez, E.; Marchat, L.A. Protein kinases and transcription factors activation in response to UV-radiation of skin: Implications for carcinogenesis. Int. J. Mol. Sci. 2012, 13, 142–172. [Google Scholar] [CrossRef]
- Bossi, O.; Gartsbein, M.; Leitges, M.; Kuroki, T.; Grossman, S.; Tennenbaum, T. UV irradiation increases ROS production via PKCdelta signaling in primary murine fibroblasts. J. Cell Biochem. 2008, 105, 194–207. [Google Scholar] [CrossRef]
- Dempsey, E.C.; Newton, A.C.; Mochly-Rosen, D.; Fields, A.P.; Reyland, M.E.; Insel, P.A.; Messing, R.O. Protein kinase C isozymes and the regulation of diverse cell responses. Am. J. Physiol Lung Cell Mol. Physiol. 2000, 279, L429–L438. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE 2003 2003. [Google Scholar] [CrossRef]
- Cooper, S.J.; Bowden, G.T. Ultraviolet B regulation of transcription factor families: Roles of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef]
- Bell, S.; Degitz, K.; Quirling, M.; Jilg, N.; Page, S.; Brand, K. Involvement of NF-kappaB signalling in skin physiology and disease. Cell Signal. 2003, 15, 1–7. [Google Scholar] [CrossRef]
- Chen, W.D.; Zhang, J.L.; Wang, X.Y.; Hu, Z.W.; Qian, Y.B. The JAK2/STAT3 signaling pathway is required for inflammation and cell death induced by cerulein in AR42J cells. Eur. Rev. Med. Pharmaco. 2019, 23, 1770–1777. [Google Scholar]
- Kasembeli, M.M.; Bharadwaj, U.; Robinson, P.; Tweardy, D.J. Contribution of STAT3 to Inflammatory and Fibrotic Diseases and Prospects for its Targeting for Treatment. Int. J. Mol. Sci. 2018, 19, 2299. [Google Scholar] [CrossRef]
- Seo, M.J.; Lee, Y.J.; Hwang, J.H.; Kim, K.J.; Lee, B.Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J. Nutr. Biochem. 2015, 26, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [PubMed]
- Kim, A.L.; Labasi, J.M.; Zhu, Y.; Tang, X.; McClure, K.; Gabel, C.A.; Athar, M.; Bickers, D.R. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J. Invest. Dermatol. 2005, 124, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Mao, R.; Yang, J. NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef]
- Chen, N.; Ma, W.; Huang, C.; Dong, Z. Translocation of protein kinase Cepsilon and protein kinase Cdelta to membrane is required for ultraviolet B-induced activation of mitogen-activated protein kinases and apoptosis. J. Biol. Chem. 1999, 274, 15389–15394. [Google Scholar] [CrossRef]
- Seo, J.Y.; Kim, E.K.; Lee, S.H.; Park, K.C.; Kim, K.H.; Eun, H.C.; Chung, J.H. Enhanced expression of cylooxygenase-2 by UV in aged human skin in vivo. Mech. Ageing Dev. 2003, 124, 903–910. [Google Scholar] [CrossRef]
- C Tanaka, K.; Asamitsu, K.; Uranishi, H.; Iddamalgoda, A.; Ito, K.; Kojima, H.; Okamoto, T. Protecting skin photoaging by NF-kappaB inhibitor. Curr. Drug Metab. 2010, 11, 431–435. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chan, S.Y.; Chu, Y.; Wen, K.C. Fisetin Ameliorated Photodamage by Suppressing the Mitogen-Activated Protein Kinase/Matrix Metalloproteinase Pathway and Nuclear Factor-kappaB Pathways. J. Agric. Food Chem. 2015, 63, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sampat, K.; Hu, N.; Zakari, J.; Yuspa, S.H. Protein kinase C negatively regulates Akt activity and modifies UVC-induced apoptosis in mouse keratinocytes. J. Biol. Chem. 2006, 281, 3237–3243. [Google Scholar] [CrossRef] [PubMed]
- Page, K.; Li, J.; Zhou, L.M.; Iasvovskaia, S.; Corbit, K.C.; Soh, J.W.; Weinstein, I.B.; Brasier, A.R.; Lin, A.N.; Hershenson, M.B. Regulation of airway epithelial cell NF-kappa B-dependent gene expression by protein kinase C delta. J. Immunol. 2003, 170, 5681–5689. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, P.J.; Addya, S.; Jimenez, S.A. Effect of Protein Kinase C delta (PKC-delta) Inhibition on the Transcriptome of Normal and Systemic Sclerosis Human Dermal Fibroblasts In Vitro. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, Y.K.; Kim, K.H.; Park, S.J.; Kim, S.J.; Chung, J.H. A novel role for the TRPV1 channel in UV-induced matrix metalloproteinase (MMP)-1 expression in HaCaT cells. J. Cell Physiol. 2009, 219, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Erkasap, N.; Ozyurt, R.; Ozkurt, M.; Yasar, F.; Erkasap, S.; Ihtiyar, E. The role of JAK/STAT signaling pathway and TNF-alpha crosstalk in human colorectal cancer. Gene Rep. 2016, 3, 1–4. [Google Scholar] [CrossRef]
- Byun, S.; Lee, K.W.; Jung, S.K.; Lee, E.J.; Hwang, M.K.; Lim, S.H.; Bode, A.M.; Lee, H.J.; Dong, Z.G. Luteolin Inhibits Protein Kinase C epsilon and c-Src Activities and UVB-Induced Skin Cancer. Cancer Res. 2010, 70, 2415–2423. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.-G.; Byun, S. Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. https://doi.org/10.3390/ijms20215262
Shin EJ, Lee JS, Hong S, Lim T-G, Byun S. Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin. International Journal of Molecular Sciences. 2019; 20(21):5262. https://doi.org/10.3390/ijms20215262
Chicago/Turabian StyleShin, Eun Ju, Ji Su Lee, Seungpyo Hong, Tae-Gyu Lim, and Sanguine Byun. 2019. "Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin" International Journal of Molecular Sciences 20, no. 21: 5262. https://doi.org/10.3390/ijms20215262
APA StyleShin, E. J., Lee, J. S., Hong, S., Lim, T.-G., & Byun, S. (2019). Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin. International Journal of Molecular Sciences, 20(21), 5262. https://doi.org/10.3390/ijms20215262





