Chemical Modification of Microcin J25 Reveals New Insights on the Stereospecific Requirements for Antimicrobial Activity
Abstract
:1. Introduction
2. Results
2.1. Chemical Treatment
2.2. Minimum Inhibitory Concentration (MIC)
2.3. Synergy Study
2.4. Minimal Biofilm Eradication Concentration (MBEC)
2.5. Stability Assays
2.6. MS/MS Analysis
2.7. Ion-Mobility Mass Spectrometry (IM-MS) Analysis
2.8. Circular Dichroism (CD) Spectroscopy
2.9. Nuclear Magnetic Ressonance (NMR) Spectroscopy
2.10. Structure Calculation
3. Discussion
4. Materials and Methods
4.1. Heterologous Expression of MccJ25
4.2. Minimum Inhibitory Concentration (MIC)
4.3. Synergy Study
4.4. Minimal Biofilm Eradication Concentration (MBEC)
4.5. NMR Spectroscopy
4.6. Ion-Mobility Mass Spectrometry (IM-MS)
4.7. MS/MS Analysis
4.8. Structure Calculation
4.9. Circular Dichroism (CD)
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rosengren, K.J.; Clark, R.J.; Daly, N.L.; Göransson, U.; Jones, A.; Craik, D.J. Microcin J25 has a threaded sidechain-to-backbone ring structure and not a head-to-tail cyclized backbone. J. Am. Chem. Soc. 2003, 125, 12464–12474. [Google Scholar] [CrossRef]
- Knappe, T.A.; Manzenrieder, F.; Mas-Moruno, C.; Linne, U.; Sasse, F.; Kessler, H.; Xie, X.; Marahiel, M.A. Introducing Lasso Peptides as Molecular Scaffolds for Drug Design: Engineering of an Integrin Antagonist. Angew. Chem. Int. Ed. 2011, 50, 8714–8717. [Google Scholar] [CrossRef] [Green Version]
- Hegemann, J.D.; De Simone, M.; Zimmermann, M.; Knappe, T.A.; Xie, X.; Di Leva, F.S.; Marinelli, L.; Novellino, E.; Zahler, S.; Kessler, H.; et al. Rational improvement of the affinity and selectivity of integrin binding of grafted lasso peptides. J. Med. Chem. 2014, 57, 5829–5834. [Google Scholar] [CrossRef]
- Salomón, R.A.; Farías, R.N. Microcin 25, a novel antimicrobial peptide produced by Escherichia coli. J. Bacteriol. 1992, 174, 7428–7435. [Google Scholar] [CrossRef] [Green Version]
- Blond, A.; Péduzzi, J.; Goulard, C.; Chiuchiolo, M.J.; Barthélémy, M.; Prigent, Y.; Salomón, R.A.; Farías, R.N.; Moreno, F.; Rebuffat, S. The cyclic structure of microcin J25, a 21-residue peptide antibiotic from Escherichia coli. Eur. J. Biochem. 1999, 259, 747–755. [Google Scholar] [CrossRef]
- Delgado, M.A.; Rintoul, M.R.; Farias, R.N.; Salomon, R.A. Escherichia coli RNA Polymerase Is the Target of the Cyclopeptide Antibiotic Microcin J25. J. Bacteriol. 2001, 183, 4543–4550. [Google Scholar] [CrossRef]
- Selsted, M.E.; Novotny, M.J.; Morris, W.L.; Tang, Y.Q.; Smith, W.; Cullor, J.S. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J. Biol. Chem. 1992, 267, 4292–4295. [Google Scholar] [PubMed]
- Turner, J.; Cho, Y.; Dinh, N.-N.; Waring, A.J.; Lehrer, R.I. Activities of LL-37, a Cathelin-Associated Antimicrobial Peptide of Human Neutrophils. Antimicrob. Agents Chemother. 1998, 42, 2206–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37—A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta—Biomembr. 2016, 1858, 546–566. [Google Scholar] [CrossRef] [PubMed]
- Falla, T.J.; Karunaratne, D.N.; Hancock, R.E.W. Mode of Action of the Antimicrobial Peptide Indolicidin. J. Biol. Chem. 1996, 271, 19298–19303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rintoul, M.R.; De Arcuri, B.F.; Salomón, R.A.; Farías, R.N.; Morero, R.D. The antibacterial action of microcin J25: Evidence for disruption of cytoplasmic membrane energization in Salmonella newport. FEMS Microbiol. Lett. 2001, 204, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Vincent, P.A.; Bellomio, A.; De Arcuri, B.F.; Farías, R.N.; Morero, R.D. MccJ25 C-terminal is involved in RNA-polymerase inhibition but not in respiration inhibition. Biochem. Biophys. Res. Commun. 2005, 331, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, J.; Sineva, E.; Knight, J.; Levy, R.M.; Ebright, R.H. Antibacterial peptide Microcin J25 inhibits transcription by binding within and obstructing the RNA polymerase secondary channel. Mol. Cell 2004, 14, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Bellomio, A.; Vincent, P.A.; De Arcuri, B.F.; Farias, R.N.; Morero, R.D. Microcin J25 has dual and independent mechanisms of action in Escherichia coli: RNA polymerase inhibition and increased superoxide production. J. Bacteriol. 2007, 189, 4180–4186. [Google Scholar] [CrossRef]
- Bonhivers, M.; Plançon, L.; Ghazi, A.; Boulanger, P.; Le Maire, M.; Lambert, O.; Rigaud, J.L.; Letellier, L. FhuA, an Escherichia coli outer membrane protein with a dual function of transporter and channel which mediates the transport of phage DNA. Biochimie 1998, 80, 363–369. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Duquesne, S.; Peduzzi, J.; Goulard, C.; Desmadril, M.; Letellier, L.; Rebuffat, S.; Boulanger, P. The iron–siderophore transporter FhuA is the receptor for the antimicrobial peptide microcin J25: Role of the microcin Val11 –Pro16 β-hairpin region in the recognition mechanism. Biochem. J. 2005, 389, 869–876. [Google Scholar] [CrossRef]
- Salomon, R.A.; Farias, R.N. The fhuA protein is involved in microcin 25 uptake. J. Bacteriol. 1993, 175, 7741–7742. [Google Scholar] [CrossRef]
- Bellomio, A.; Vincent, P.A.; de Arcuri, B.F.; Salomón, R.A.; Morero, R.D.; Farías, R.N. The microcin J25 β-hairpin region is important for antibiotic uptake but not for RNA polymerase and respiration inhibition. Biochem. Biophys. Res. Commun. 2004, 325, 1454–1458. [Google Scholar] [CrossRef]
- Pavlova, O.; Mukhopadhyay, J.; Sineva, E.; Ebright, R.H.; Severinov, K. Systematic structure-activity analysis of microcin J25. J. Biol. Chem. 2008, 283, 25589–25595. [Google Scholar] [CrossRef]
- Knappe, T.A.; Linne, U.; Zirah, S.; Rebuffat, S.; Xie, X.; Marahiel, M.A. Isolation and structural characterization of capistruin, a lasso peptide predicted from the genome sequence of Burkholderia thailandensis E264. J. Am. Chem. Soc. 2008, 130, 11446–11454. [Google Scholar] [CrossRef]
- Rudilla, H.; Fusté, E.; Cajal, Y.; Rabanal, F.; Vinuesa, T.; Viñas, M. Synergistic antipseudomonal effects of synthetic peptide AMP38 and carbapenems. Molecules 2016, 21, 1223. [Google Scholar] [CrossRef] [PubMed]
- Ducasse, R.; Yan, K.P.; Goulard, C.; Blond, A.; Li, Y.; Lescop, E.; Guittet, E.; Rebuffat, S.; Zirah, S. Sequence determinants governing the topology and biological activity of a lasso peptide, microcin J25. ChemBioChem 2012, 13, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, K.J.; Blond, A.; Afonso, C.; Tabet, J.C.; Rebuffat, S.; Craik, D.J. Structure of Thermolysin Cleaved Microcin J25: Extreme Stability of a Two-Chain Antimicrobial Peptide Devoid of Covalent Links. Biochemistry 2004, 43, 4696–4702. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.-A.A.; Kalkum, M.; Ottesen, J.; Yuzenkova, J.; Chait, B.T.; Landick, R.; Muir, T.; Severinov, K.; Darst, S.A. Structure of microcin J25, a peptide inhibitor of bacterial RNA polymerase, is a lassoed tail. J. Am. Chem. Soc. 2003, 125, 12475–12483. [Google Scholar] [CrossRef]
- Blond, A.; Cheminant, M.; Destoumieux-Garzón, D.; Ségalas-Milazzo, I.; Peduzzi, J.; Goulard, C.; Rebuffat, S. Thermolysin-linearized microcin J25 retains the structured core of the native macrocyclic peptide and displays antimicrobial activity. Eur. J. Biochem. 2002, 269, 6212–6222. [Google Scholar] [CrossRef]
- Fouque, K.J.D.; Lavanant, H.; Zirah, S.; Hegemann, J.D.; Zimmermann, M.; Marahiel, M.A.; Rebuffat, S.; Afonso, C. Signatures of Mechanically Interlocked Topology of Lasso Peptides by Ion Mobility–Mass Spectrometry: Lessons from a Collection of Representatives. J. Am. Soc. Mass Spectrom. 2017, 28, 315–322. [Google Scholar] [CrossRef]
- Jeanne Dit Fouque, K.; Afonso, C.; Zirah, S.; Hegemann, J.D.; Zimmermann, M.; Marahiel, M.A.; Rebuffat, S.; Lavanant, H. Ion mobility-mass spectrometry of lasso peptides: Signature of a rotaxane topology. Anal. Chem. 2015, 87, 1166–1172. [Google Scholar] [CrossRef]
- Blond, A.; Cheminant, M.; Ségalas-Milazzo, I.; Péduzzi, J.; Barthélémy, M.; Goulard, C.; Salomón, R.; Moreno, F.; Farías, R.; Rebuffat, S. Solution structure of microcin J25, the single macrocyclic antimicrobial peptide from Escherichia coli. Eur. J. Biochem. 2001, 268, 2124–2133. [Google Scholar] [CrossRef]
- Soudy, R.; Wang, L.; Kaur, K. Synthetic peptides derived from the sequence of a lasso peptide microcin J25 show antibacterial activity. Bioorganic Med. Chem. 2012, 20, 1794–1800. [Google Scholar] [CrossRef] [Green Version]
- Woody, R.W. Aromatic side-chain contributions to the far ultraviolet circular dichroism of peptides and proteins. Biopolymers 1978, 17, 1451–1467. [Google Scholar] [CrossRef]
- Bayro, M.J.; Mukhopadhyay, J.; Swapna, G.V.T.; Huang, J.Y.; Ma, L.C.; Sineva, E.; Dawson, P.E.; Montelione, G.T.; Ebright, R.H. Structure of antibacterial peptide microcin J25: A 21-residue lariat protoknot. J. Am. Chem. Soc. 2003, 125, 12382–12383. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Bigam, C.G.; Holm, A.; Hodges, R.S.; Sykes, B.D. 1H, 13C and 15N random coil NMR chemical shifts of the common amino acids. I. Investigations of nearest-neighbor effects. J. Biomol. NMR 1995, 5, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Watts, K.S.; Dalal, P.; Tebben, A.J.; Cheney, D.L.; Shelley, J.C. Macrocycle Conformational Sampling with MacroModel. J. Chem. Inf. Model. 2014, 54, 2680–2696. [Google Scholar] [CrossRef]
- Semenova, E.; Yuzenkova, Y.; Peduzzi, J.; Rebuffat, S.; Severinov, K. Structure-activity analysis of microcin J25: Distinct parts of the threaded lasso molecule are responsible for interaction with bacterial RNA polymerase. J. Bacteriol. 2005, 187, 3859–3863. [Google Scholar] [CrossRef] [PubMed]
- Socias, S.B.; Severinov, K.; Salomon, R.A. The Ile13 residue of microcin J25 is essential for recognition by the receptor FhuA, but not by the inner membrane transporter SbmA. FEMS Microbiol. Lett. 2009, 301, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.M.; Fusté, E.; Rabanal, F.; Vinuesa, T.; Viñas, M. An overview of antimicrobial peptides and the latest advances in their development. Expert Opin. Biol. Ther. 2017, 17, 663–676. [Google Scholar] [CrossRef]
- Committee, T.E.; Testing, A.S.; Changes, N.; Pseudomonas, E. European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters 0–77. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf (accessed on 12 January 2018).
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Moskowitz, S.M.; Foster, J.M.; Emerson, J.; Burns, J.L. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J. Clin. Microbiol. 2004, 42, 1915–1922. [Google Scholar] [CrossRef]
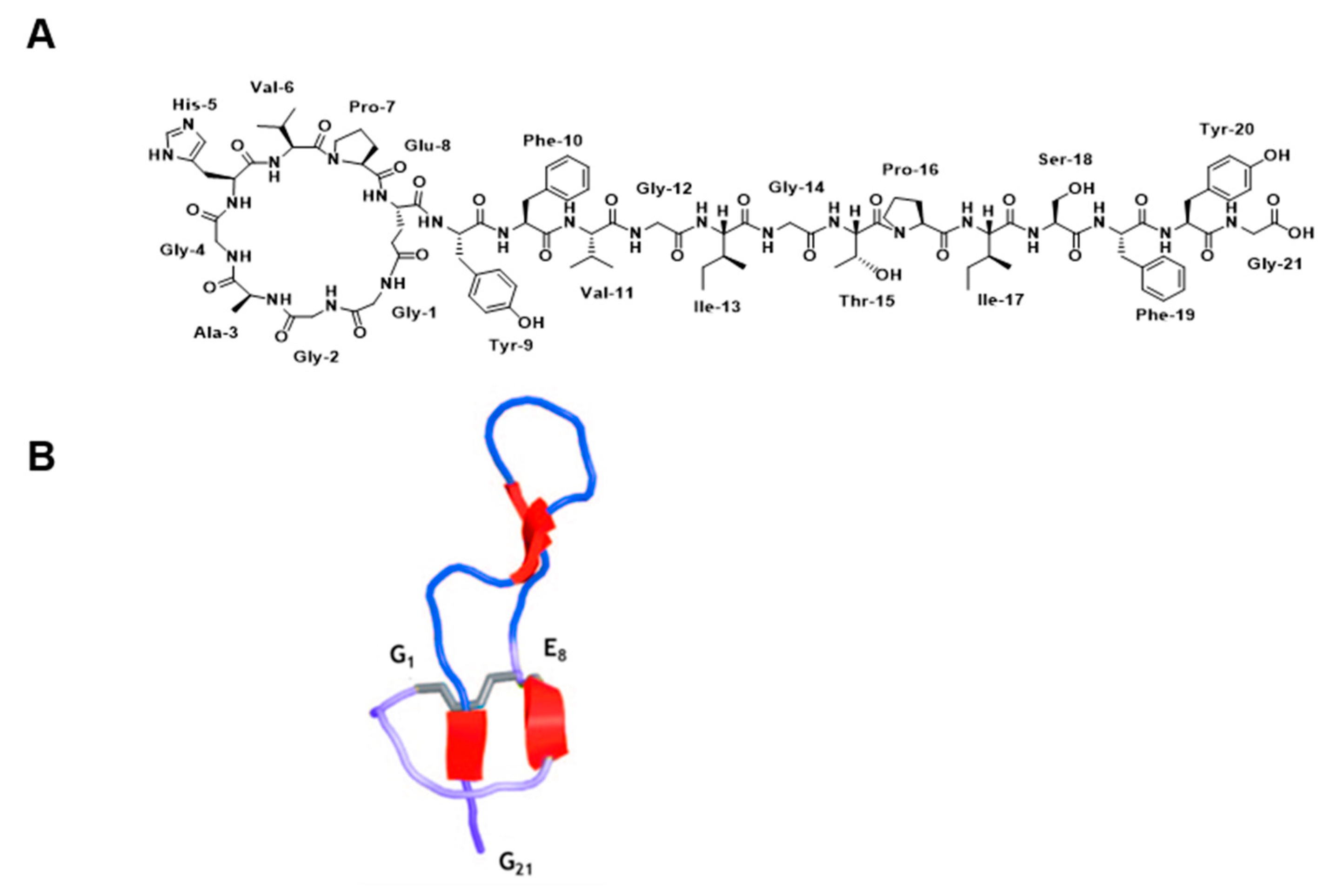


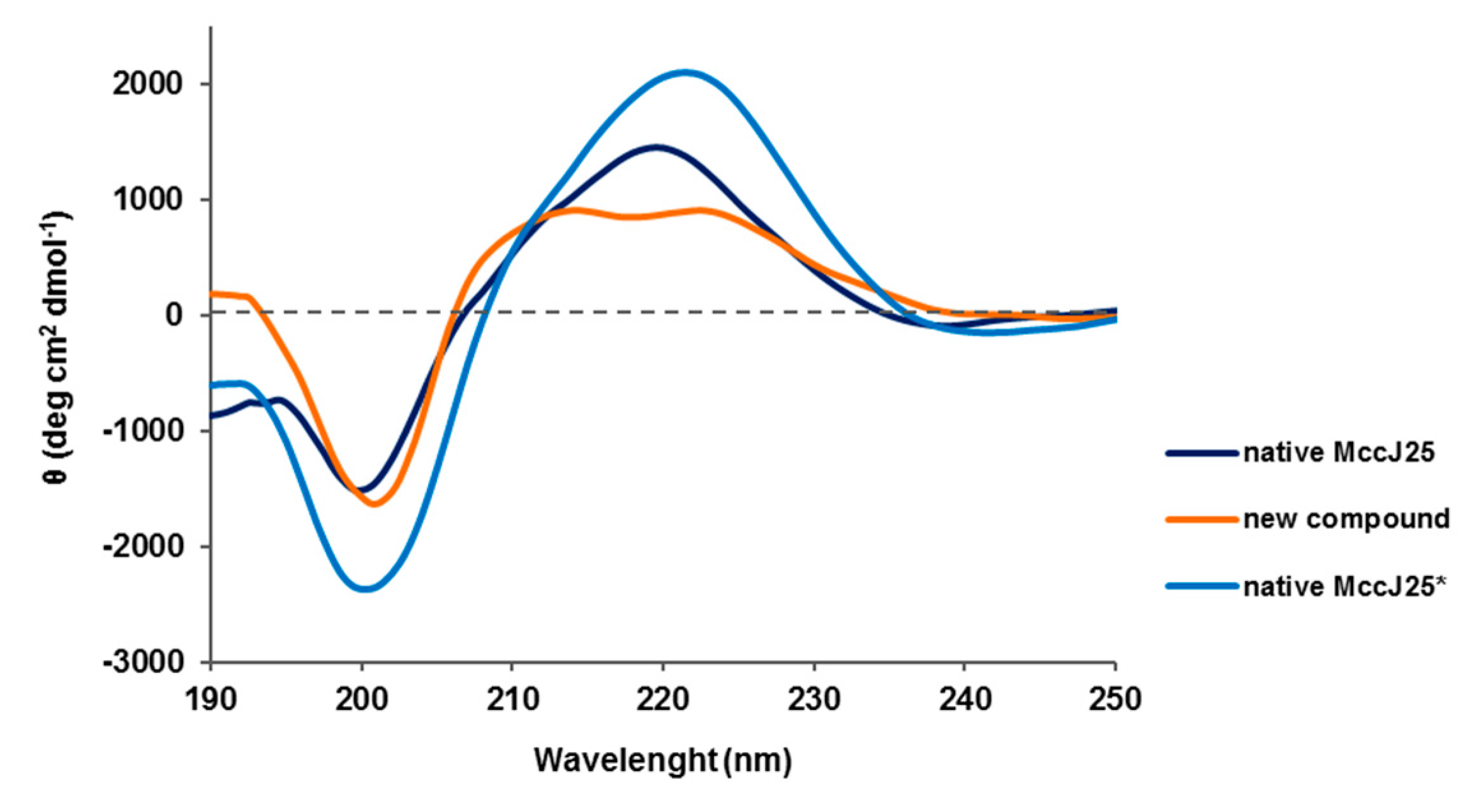

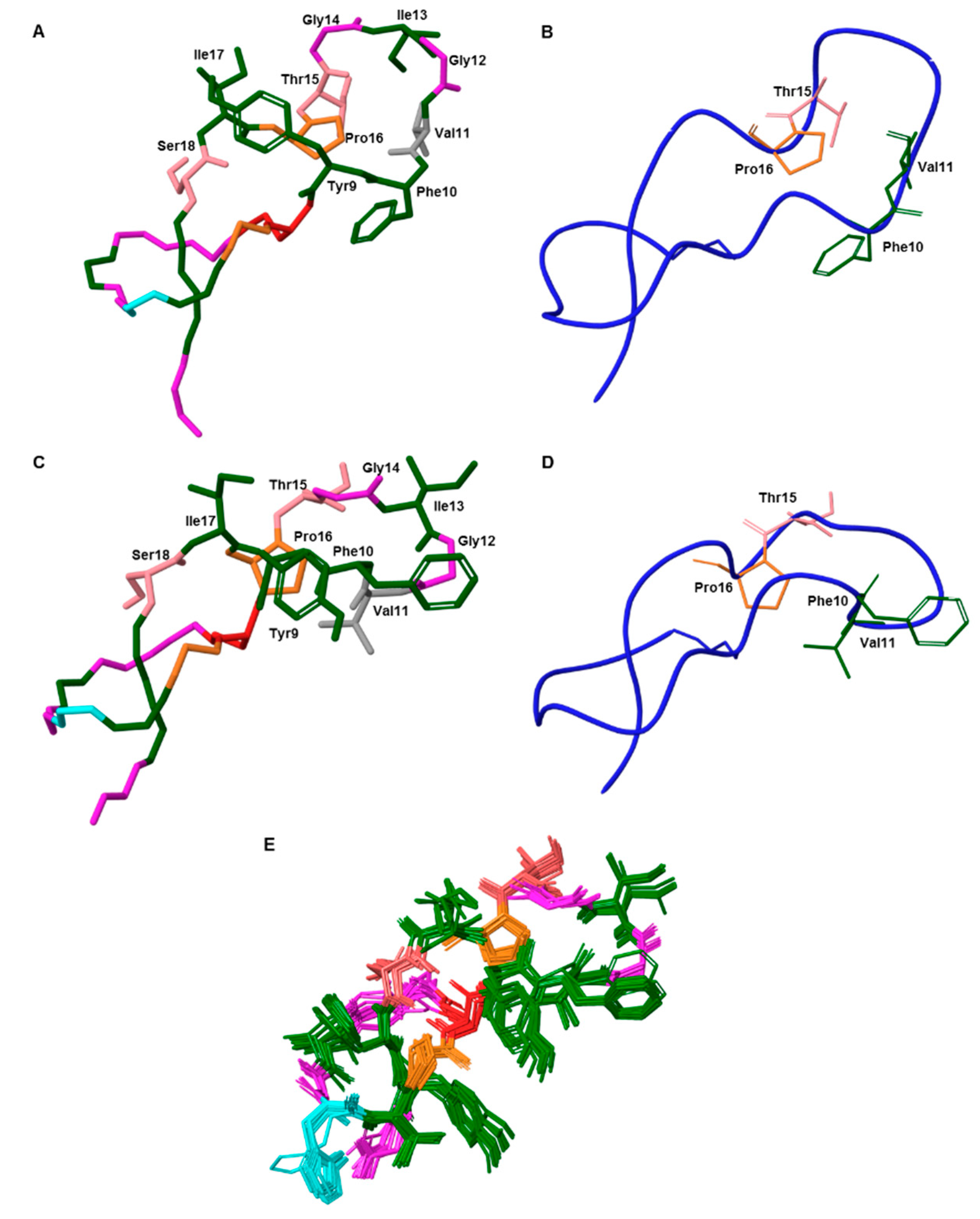
| Strains[a] | Native MccJ25 | New Compound |
|---|---|---|
| Escherichia coli MDR 39255 | 0.5 | >256 |
| Escherichia coli MDR 208691 | >128 | >128 |
| Escherichia coli MDR 246415 | 00625 | >128 |
| Escherichia coli MDR 239910 | >128 | >128 |
| Salmonella enterica ATCC 14028 | >128 | >128 |
| Salmonella enterica ATCC 13076 | <0.0625 | >128 |
| Salmonella enterica ATCC 49214 | 0.8 | >128 |
| Salmonella typhimurium SY5015 | >128 | >128 |
| Residue | δ (ppm)[a] | Residue | δ (ppm)[a] |
|---|---|---|---|
| Ser18 | 7.80 | Glu8 | 7.90 |
| Gly4 | 7.80 | Ile13 | 7.89 |
| Tyr9 | 7.84 | Ala3 | 8.44 |
| His5 | 7.79 | Val11 | 8.42 |
| Gly12 | 8.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Gómez, H.; Jorba, M.; Albericio, F.; Viñas, M.; Tulla-Puche, J. Chemical Modification of Microcin J25 Reveals New Insights on the Stereospecific Requirements for Antimicrobial Activity. Int. J. Mol. Sci. 2019, 20, 5152. https://doi.org/10.3390/ijms20205152
Martin-Gómez H, Jorba M, Albericio F, Viñas M, Tulla-Puche J. Chemical Modification of Microcin J25 Reveals New Insights on the Stereospecific Requirements for Antimicrobial Activity. International Journal of Molecular Sciences. 2019; 20(20):5152. https://doi.org/10.3390/ijms20205152
Chicago/Turabian StyleMartin-Gómez, Helena, Marta Jorba, Fernando Albericio, Miguel Viñas, and Judit Tulla-Puche. 2019. "Chemical Modification of Microcin J25 Reveals New Insights on the Stereospecific Requirements for Antimicrobial Activity" International Journal of Molecular Sciences 20, no. 20: 5152. https://doi.org/10.3390/ijms20205152
APA StyleMartin-Gómez, H., Jorba, M., Albericio, F., Viñas, M., & Tulla-Puche, J. (2019). Chemical Modification of Microcin J25 Reveals New Insights on the Stereospecific Requirements for Antimicrobial Activity. International Journal of Molecular Sciences, 20(20), 5152. https://doi.org/10.3390/ijms20205152








