Naturally Prefabricated Marine Biomaterials: Isolation and Applications of Flat Chitinous 3D Scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida)
Abstract
:1. Introduction
2. Results and Discussion
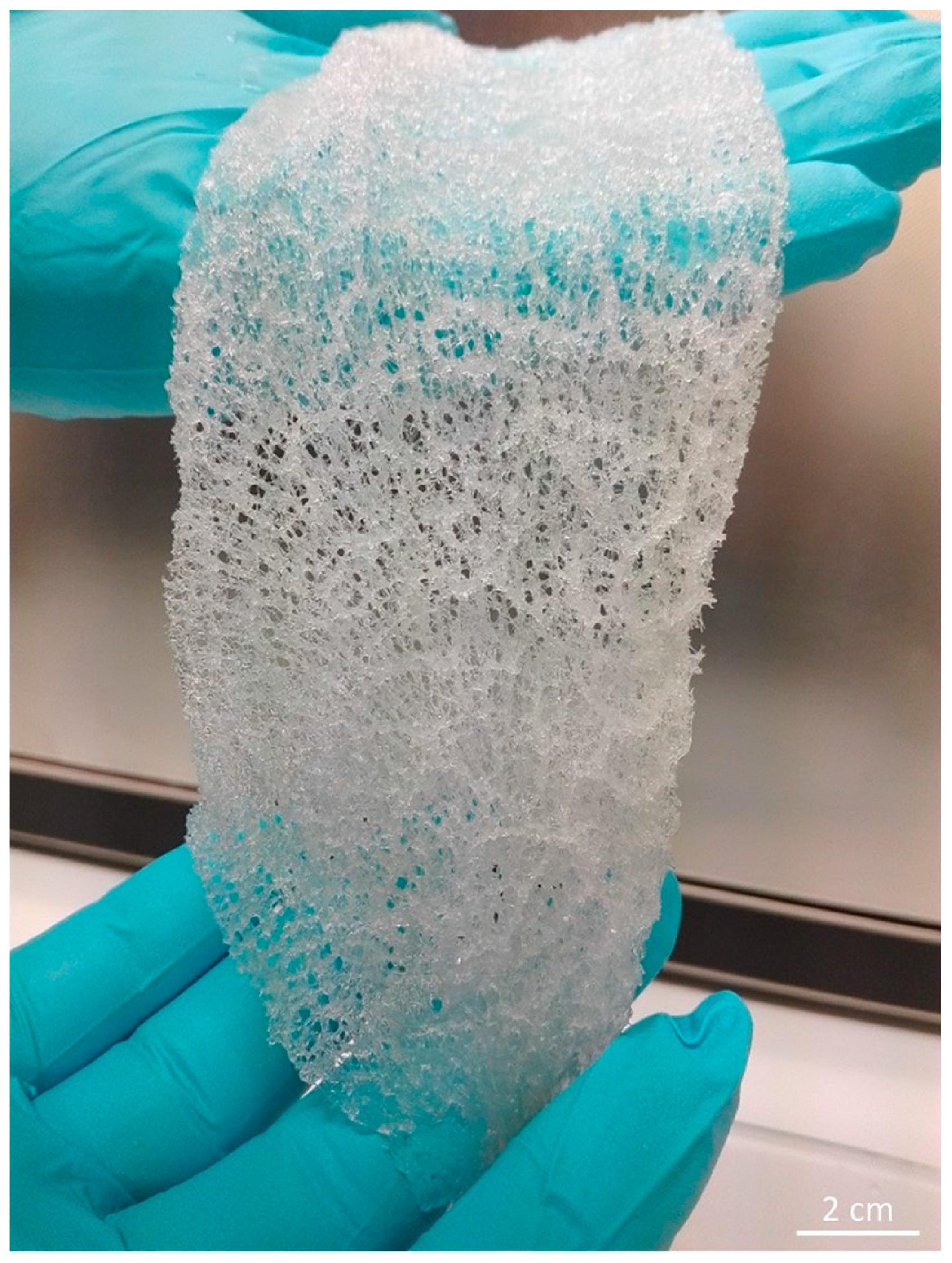
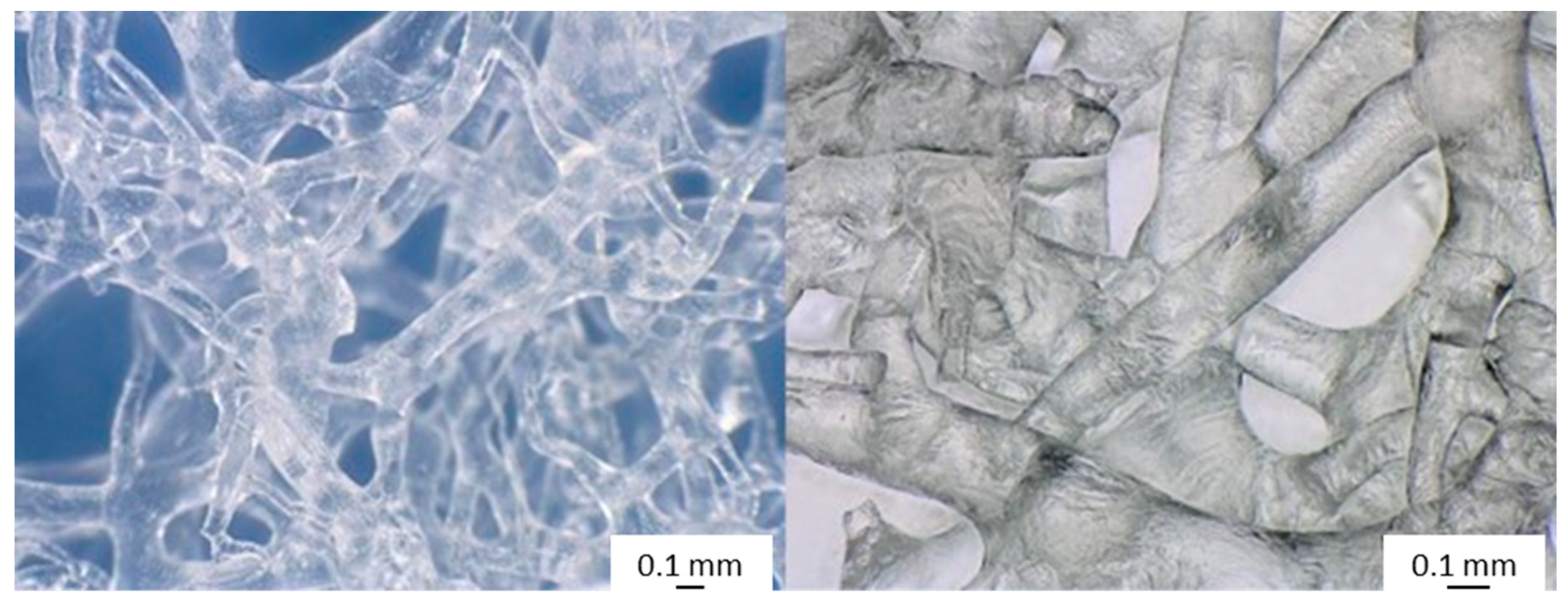
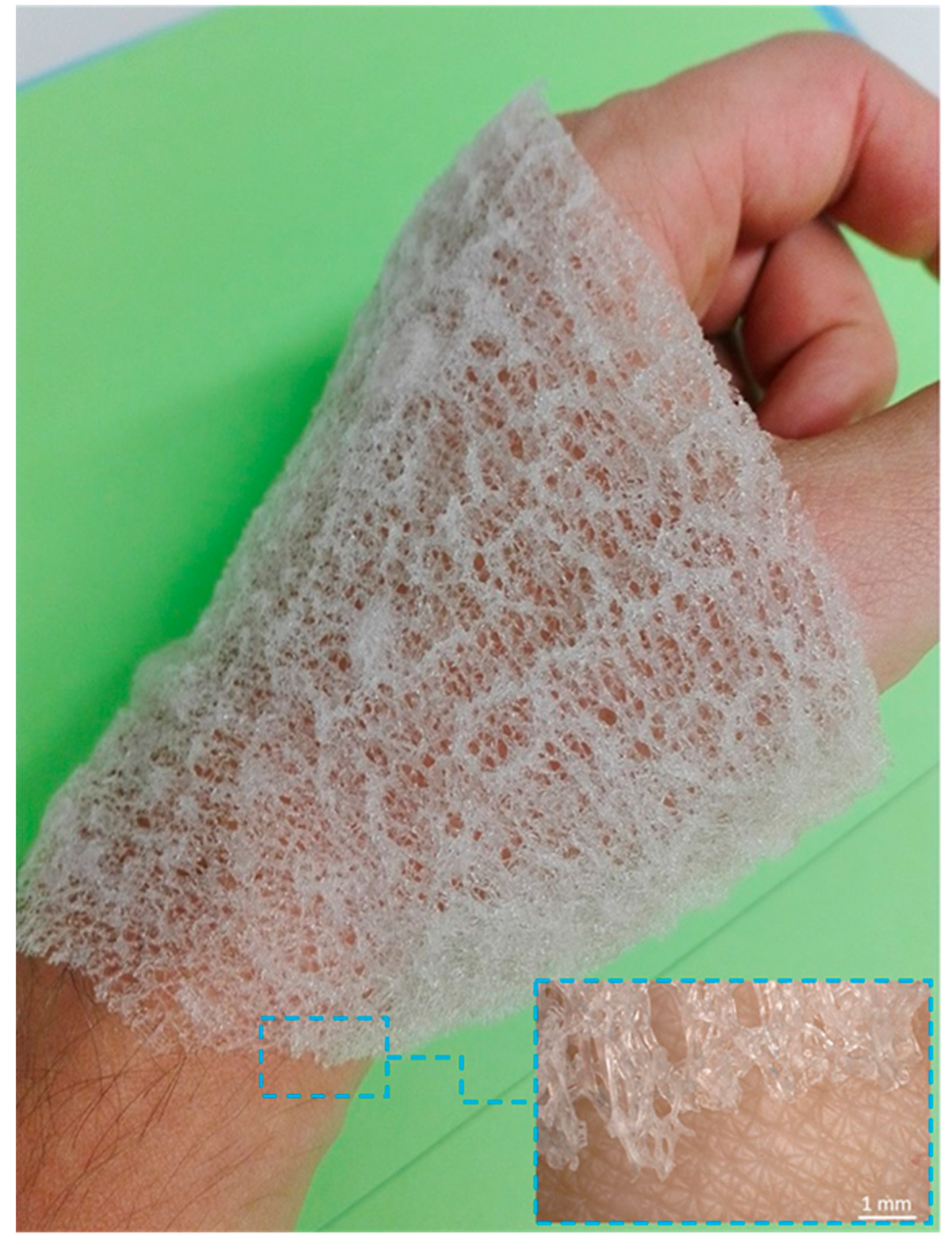
2.1. Isolation of 3D Chitin Scaffolds
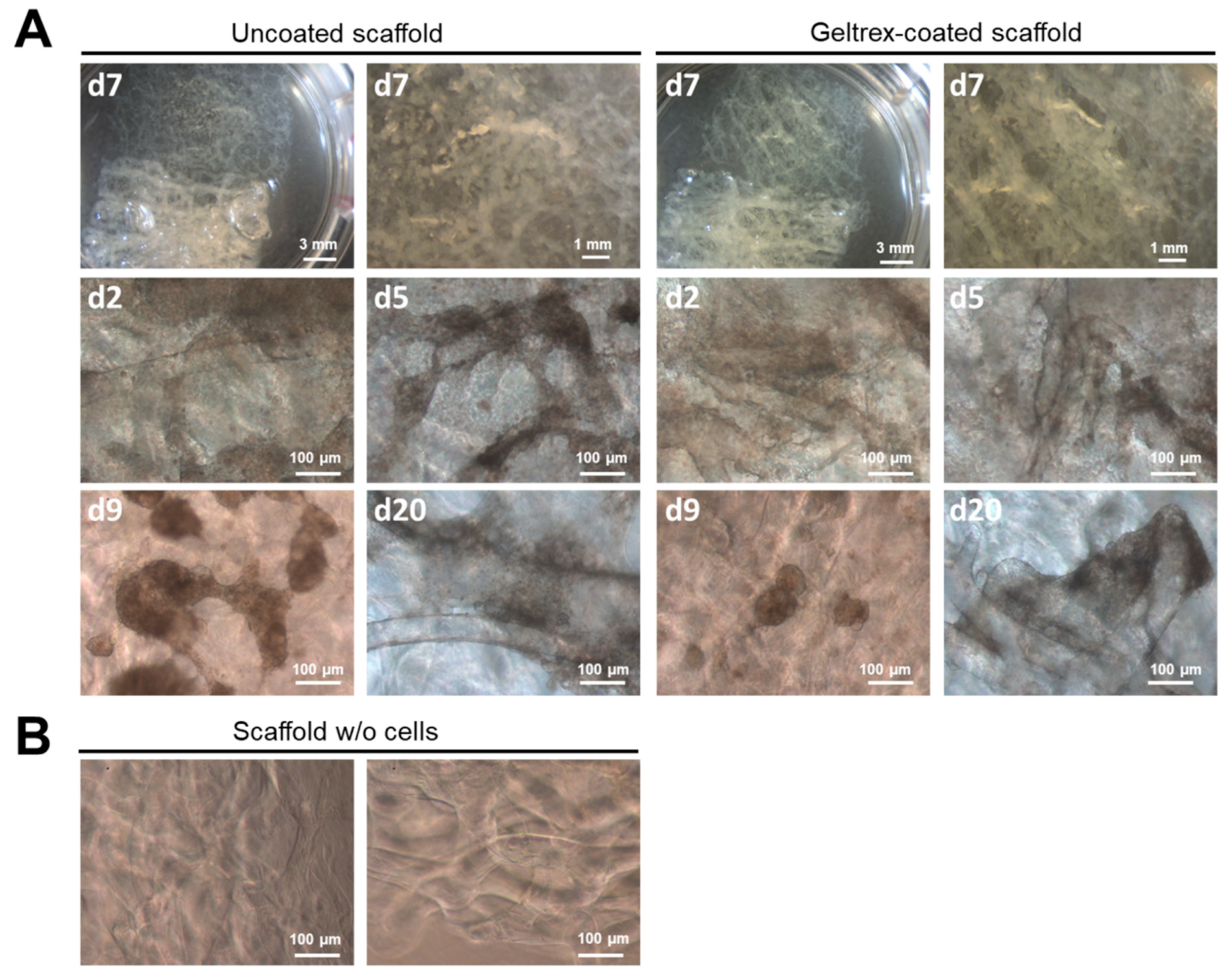
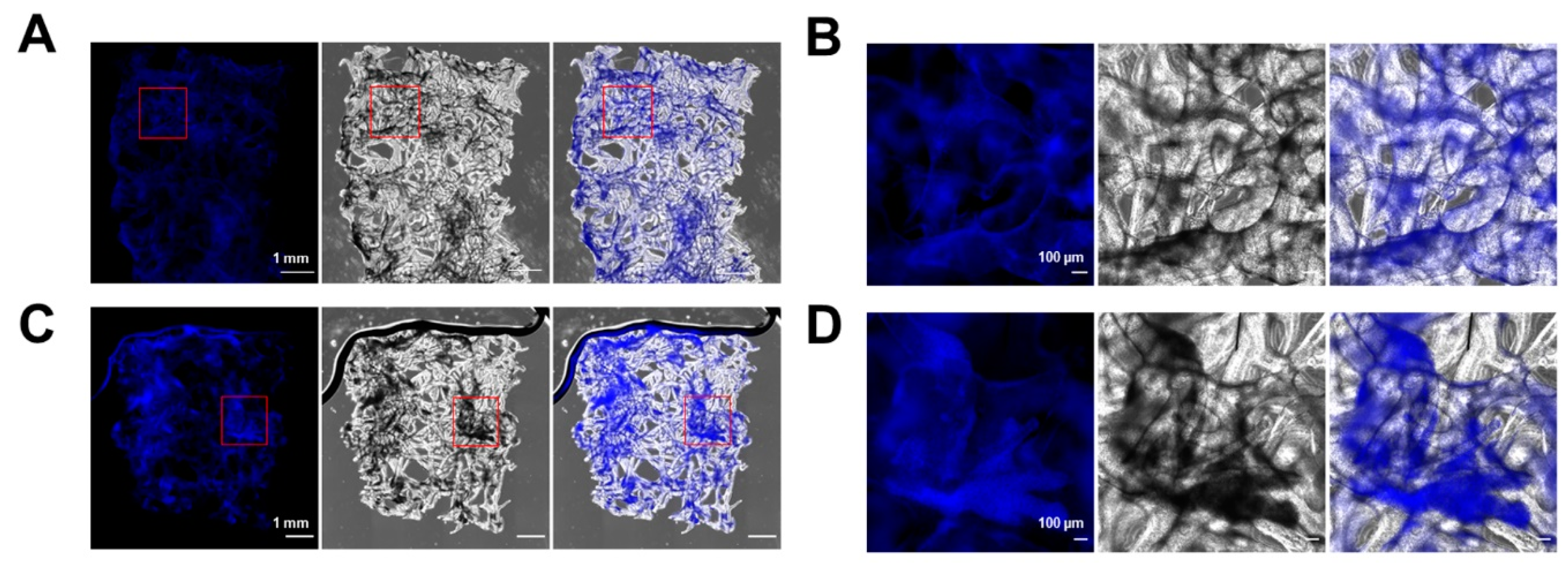
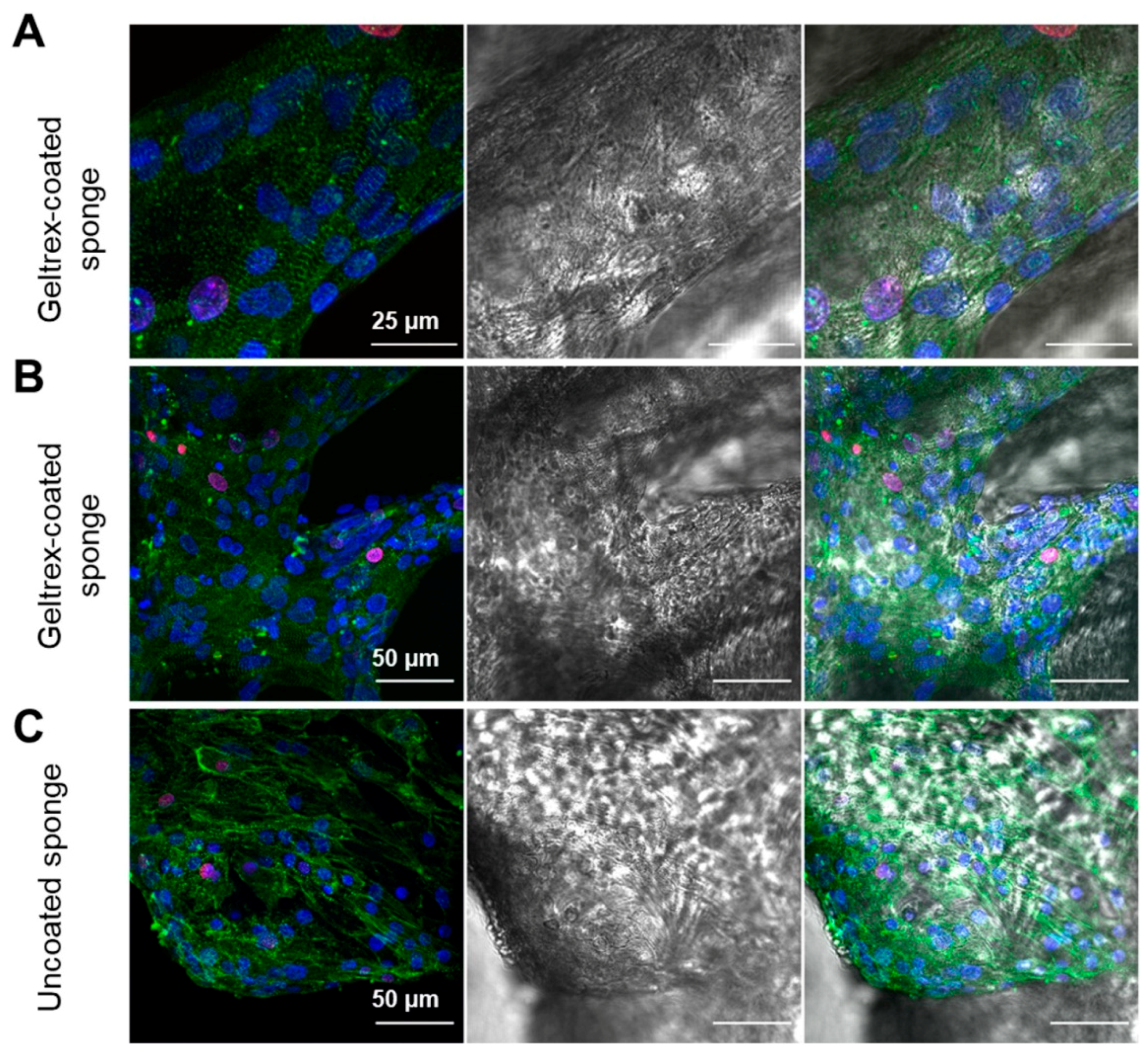
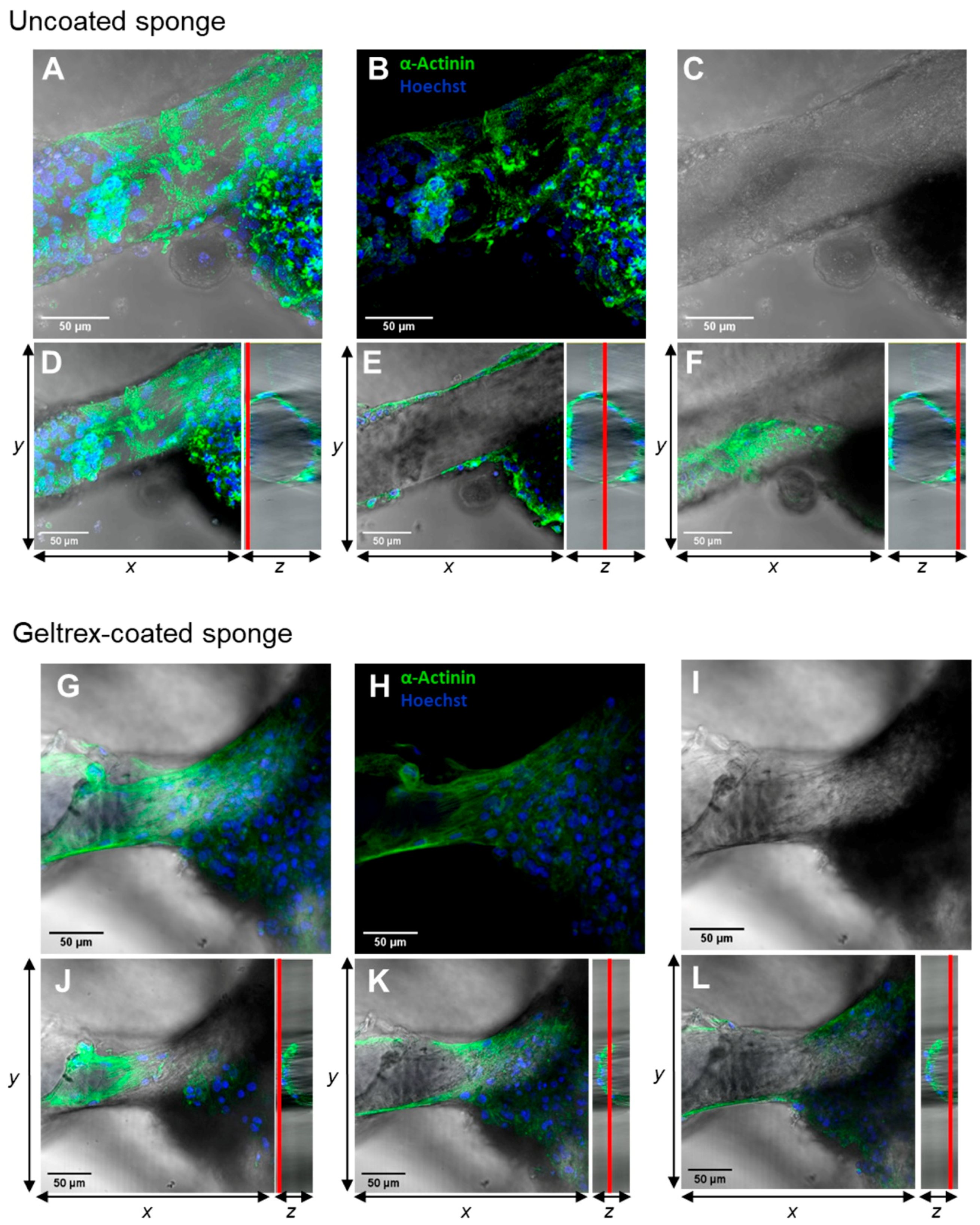
2.2. 3D Chitin Scaffold from I. labyrinthus as Model System for Tissue Engineering of Cardiomyocytes
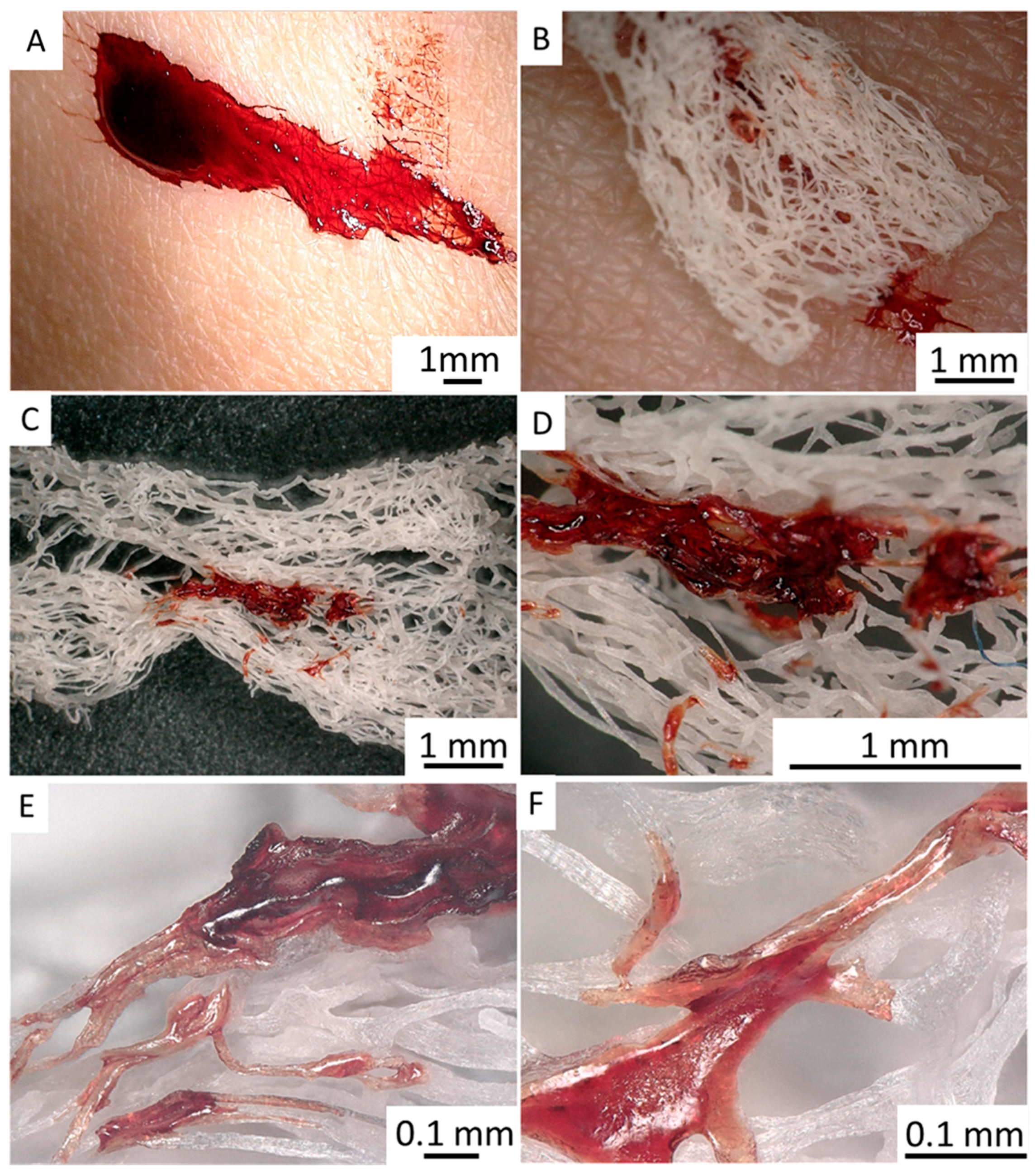
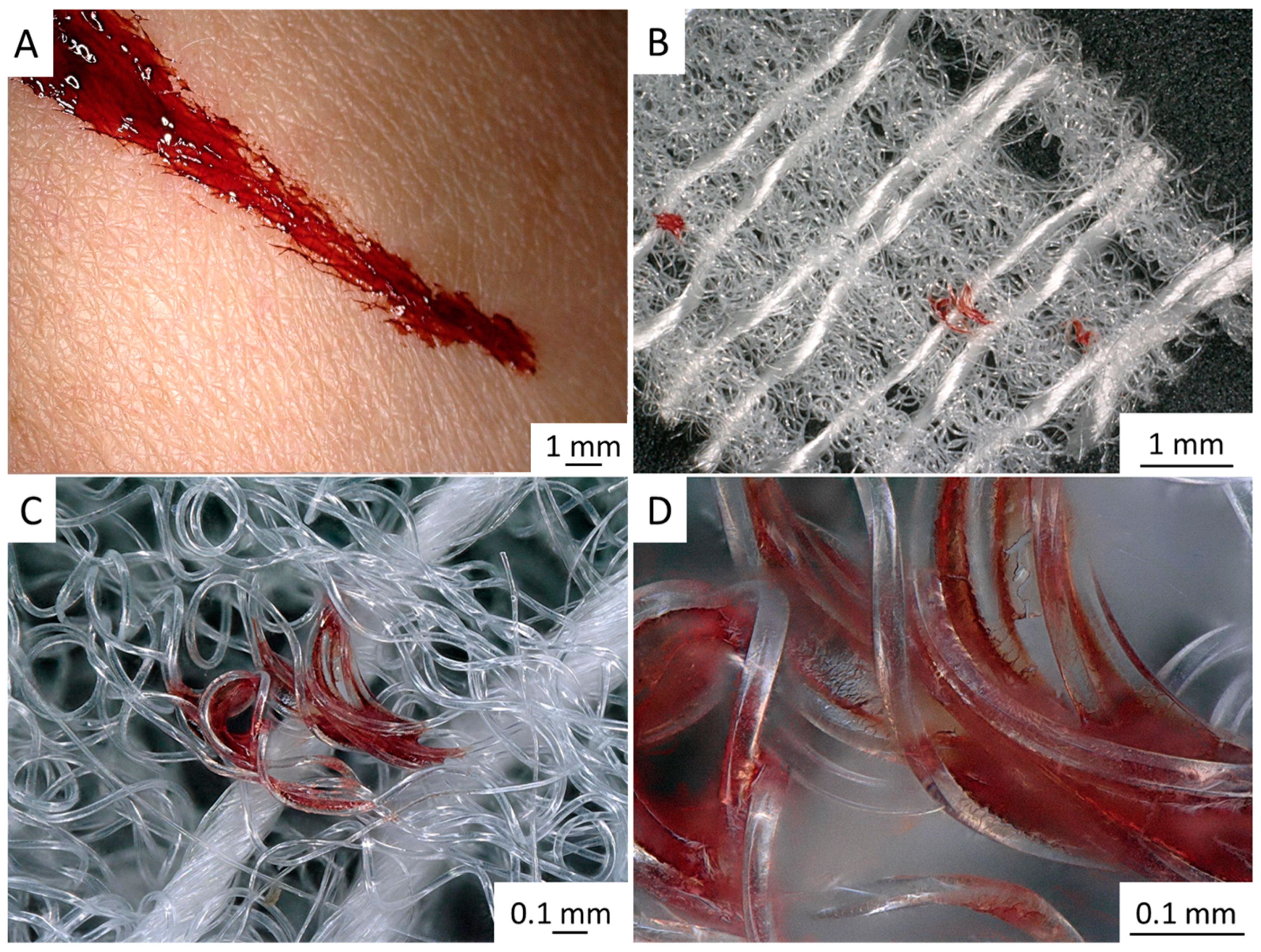
2.3. 3D Chitin Scaffolds of Poriferan Origin as Alternative Gauze Fabrics
3. Materials and Methods
3.1. Location and Collection
3.2. Isolation of Chitinous Skeleton from the Sponge and Identification of Selected Bromotyrosines
3.3. Stereomicroscopy Imaging
3.4. Differentiation and Culture of Human iPSC-CMs
3.5. Immunostaining and Fluorescence Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2001, 18, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, N.; Ehrlich, H.; Eisenhofer, G.; Ehrlich, A.; Meschke, S.; Ziegler, C.G.; Bornstein, S.R. Anti-Tumorigenic and Anti-Metastatic Activity of the Sponge-Derived Marine Drugs Aeroplysinin-1 and Isofistularin-3 against Pheochromocytoma. In Vitro Mar. Drugs 2018, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Normann, M.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Joseph, Y.; Ehrlich, H. Marine Spongin: Naturally Prefabricated 3D Scaffold-Based Biomaterial. Mar. Drugs 2018, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Maldonado, M.; Spindler, K.D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (demospongia: Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2007, 308B, 347–356. [Google Scholar] [CrossRef]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef]
- Żółtowska-Aksamitowska, S.; Shaala, L.A.; Youssef, D.T.A.; Elhady, S.S.; Tsurkan, M.V.; Petrenko, I.; Wysokowski, M.; Tabachnick, K.; Meissner, H.; Ivanenko, V.N.; et al. First report on chitin in a non-Verongiid marine Demosponge: the Mycale euplectellioides case. Mar. Drugs 2018, 16, 68. [Google Scholar] [CrossRef]
- Fromont, J.; Żółtowska-Aksamitowska, S.; Galli, R.; Meissner, H.; Erpenbeck, D.; Vacelet, J.; Diaz, C.; Tsurkan, M.V.; Petrenko, I.; Youssef, D.T.A.; et al. New family and genus of a Dendrilla-like sponge with characters of Verongiida. Part II. Discovery of chitin in the skeleton of Ernstilla lacunosa. Zool. Anz. 2019, 280, 21–29. [Google Scholar]
- Klinger, C.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Petrenko, I.; Machałowski, T.; Ereskovsky, A.; Martinović, R.; Muzychka, L.; et al. Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge. Mar. Drugs 2019, 17, 131. [Google Scholar] [CrossRef]
- Laport, M.S.; Santos, O.C.; Muricy, G. Marine sponges: potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar. Drugs 2017, 15, 310. [Google Scholar] [CrossRef]
- Norman, M.; Bartczak, P.; Zdarta, J.; Tylus, W.; Szatkowski, T.; Stelling, A.L.; Ehrlich, H.; Jesionowski, T. Adsorption of C.I. Natural Red 4 onto Spongin Skeleton of Marine Demosponge. Materials 2015, 8, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Norman, M.; Smułek, W.; Moszyński, D.; Kaczorek, E.; Stelling, A.L.; Ehrlich, H.; Jesionowski, T. Spongin-Based Scaffolds from Hippospongia communis Demosponge as an Effective Support for Lipase Immobilization. Catalysts 2017, 7, 147. [Google Scholar] [CrossRef]
- Szatkowski, T.; Siwińska-Stefańska, K.; Wysokowski, M.; Stelling, A.L.; Joseph, Y.; Ehrlich, H.; Jesionowski, T. Immobilization of Titanium(IV) Oxide onto 3D Spongin Scaffolds of Marine Sponge Origin According to Extreme Biomimetics Principles for Removal of C.I. Basic Blue 9. Biomimetics 2017, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Szatkowski, T.; Wysokowski, M.; Lota, G.; Pęziak, D.; Bazhenov, V.V.; Nowaczyk, G.; Walter, J.; Molodtsov, S.L.; Stocker, H.; Himcinschi, C.; et al. Novel nanostructured hematite–spongin composite developed using an extreme biomimetic approach. RSC Adv. 2015, 5, 79031–79040. [Google Scholar] [CrossRef]
- Szatkowski, T.; Kopczyński, K.; Motylenko, M.; Borrman, H.; Mania, B.; Graś, M.; Lota, G.; Bazhenov, V.V.; Rafaja, D.; Roth, F.; et al. Extreme biomimetics: A carbonized 3D spongin scaffold as a novel support for nanostructured manganese oxide(IV) and its electrochemical applications. Nano Res. 2015, 8, 4199–4214. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Beyer, J.; Makarova, A.; Stocker, H.; Walter, J.; Galli, R.; Kaiser, S.; Vyalikh, D.; Bazhenov, V.V.; et al. Extreme biomimetic approach for developing novel chitin-GeO2 nanocomposites with photoluminescent properties. Nano Res. 2015, 8, 2288–2301. [Google Scholar] [CrossRef]
- Petrenko, I.; Bazhenov, V.V.; Galli, R.; Wysokowski, M.; Fromont, J.; Schupp, P.; Stelling, A.L.; Niederschlag, E.; Stocker, H.; Kutsova, V.Z.; et al. Chitin of poriferan origin and the bioelectrometallurgy of copper/copper oxide. Int. J. Biol. Macromol. 2017, 104, 1626–1632. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Stepniak, I.; Galiński, M.; Nowacki, K.; Wysokowski, M.; Jakubowska, P.; Bazhenov, V.V.; Leisegang, T.; Ehrlich, H.; Jesionowski, T. A novel chitosan/sponge chitin origin material as a membrane for supercapacitors – preparation and characterization. RSC Adv. 2016, 6, 4007–4013. [Google Scholar] [CrossRef]
- Machałowski, T.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Bechmann, N.; Binnewerg, B.; Schubert, M.; Guan, K.; Bornstein, S.R.; Czaczyk, K.; Pokrovsky, O.; et al. Spider Chitin. The biomimetic potential and applications of Caribena versicolor tubular chitin. Carbohydr. Polym. 2019, 226, 115301. [Google Scholar] [CrossRef]
- Green, D.W. Tissue bionics: Examples in biomimetic tissue engineering. Biomed. Mater. 2008, 3, 34010. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meissner, H.; Lode, A.; et al. 3D chitinous scaffolds derived from cultivated marine demosponge Aplysina aerophoba for tissue engineering approaches based on human mesenchymal stromal cells. Int. J. Biol. Macromol. 2017, 104, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.; Gryshkov, O.; Rogulska, O.; Lode, A.; Petrenko, A.Y.; Gelinsky, M.; Glasmacher, B.; Ehrlich, H. Chitinous scaffolds from marine sponges for tissue engineering. In Marine-Derived Biomaterials for Tissue Engineering Applications; Springer Series in Biomaterials Science and Engineering; Choi, A., Ben-Nissan, B., Eds.; Springer: Singapore, 2019; Volume 14, pp. 285–307. [Google Scholar]
- Mohamed, N.M.; Enticknap, J.J.; Lohr, J.E.; McIntosh, S.M.; Hill, R.T. Changes in bacterial communities of the marine sponge Mycale laxissima on transfer into aquaculture. Appl. Environ. Microbiol. 2008, 74, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Bazhenov, V.V.; Meschke, S.; Bürger, M.; Ehrlich, A.; Petovic, S.; Durovic, M. Marine invertebrates of Boka Kotorska Bay unique sources for bioinspired materials science. In The Boka Kotorska Bay Environment, Series: The Handbook of Environmental Chemistry; Djurović, M., Semenov, A.V., Zonn, I.S., Kostianoy, A.G., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 313–334. [Google Scholar]
- Pozzolini, M.; Valisano, L.; Cerrano, C.; Menta, M.; Schiaparelli, S.; Bavestrello, G.; Benatti, U.; Giovine, M. Influence of rocky substrata on three-dimensional sponge cells model development. In Vitro Cell Dev. Biol. Animal 2010, 46, 140. [Google Scholar] [CrossRef]
- Shaala, L.A.; Asfour, H.Z.; Youssef, D.T.A.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.; Galli, R.; Meissner, H.; Petrenko, I.; Tabachnick, K.; et al. New source of 3D chitin scaffolds: the red sea demosponge Pseudoceratina arabica (Pseudoceratinidae, Verongiida). Mar. Drugs 2019, 17, 92. [Google Scholar] [CrossRef]
- Ehrlich, H.; Shaala, L.A.; Youssef, D.T.A.; Żółtowska-Aksamitowska, S.; Tsurkan, M.; Galli, R.; Meissner, H.; Wysokowski, M.; Petrenko, I.; Tabachnick, K.R.; et al. Discovery of chitin in skeletons of non-verongiid Red Sea demosponges. PLoS ONE 2018, 13, e0195803. [Google Scholar] [CrossRef]
- Brunner, E.; Ehrlich, H.; Schupp, P.; Hedrich, R.; Hunoldt, S.; Kammer, M.; Machill, S.; Paasch, S.; Bazhenov, V.V.; Kurek, D.V.; et al. Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 2009, 168, 539–547. [Google Scholar] [CrossRef]
- Wysokowski, M.; Bazhenov, V.V.; Tsurkan, M.V.; Galli, R.; Stelling, A.L.; Stöcker, H.; Kaiser, S.; Niederschlag, E.; Gärtner, G.; Behm, T.; et al. Isolation and identification of chitin in three-dimensional skeleton of Aplysina fistularis marine sponge. Int. J. Biol. Macromol. 2013, 62, 94–100. [Google Scholar] [CrossRef]
- Kunze, K.; Niemann, H.; Ueberlein, S.; Schulze, R.; Ehrlich, H.; Brunner, E.; Proksch, P.; Pée, K.-H.V. Brominated skeletal components of the marine demosponges, Aplysina cavernicola and Ianthella basta: Analytical and Biochemical Investigations. Mar. Drugs 2013, 11, 1271–1287. [Google Scholar] [CrossRef]
- Niemann, H.; Marmann, A.; Lin, W.; Proksch, P. Sponge derived bromotyrosines: structural diversity through natural combinatorial chemistry. Nat. Prod. Commun. 2015, 10, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Lin, W.; Müller, W.E.G.; Kobbutat, M.; Lai, D.; Proksch, P. Trimeric hemibastadin congener from the marine sponge Ianthella basta. J. Nat. Prod. 2013, 76, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Vacelet, J. Les cellules a inclusions de l’éponge cornée Verongia cavernicola Vacelet. J. Micros. 1967, 6, 237–240. [Google Scholar]
- Turon, X.; Becerro, M.A.; Uriz, M.J. Distribution of brominated compounds within the sponge Aplysina aerophoba: coupling of X-ray microanalysis with cryofixation techniques. Cell Tissue Res. 2000, 301, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Tabudravu, J.N.; Ejsink, V.G.H.; Gooday, G.W.; Jaspars, M.; Komander, D.; Legg, M.; Synstad, B.; van Aalten, D.M.F. Psammaplin A, a chitinase inhibitor isolated from the fijian marine sponge Aplysinella rhax. Bioorg. Med. Chem. 2002, 10, 1123–1128. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Cross, S.S. Fistularin 3 and 11-ketofistularin 3. Feline leukemia virus active bromotyrosine metabolites from the marine sponge Aplysina archeri. J. Nat. Prod. 1992, 55, 509–512. [Google Scholar] [PubMed]
- Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Sulaiman, M.; Khedr, A. Bioactive secondary metabolites from the red sea marine Verongid sponge Suberea species. Mar. Drugs 2015, 13, 1621–1631. [Google Scholar] [CrossRef]
- Galeano, E.; Thomas, O.P.; Robledo, S.; Munoz, D.; Martinez, A. Antiparasitic bromotyrosine derivatives from the marine sponge Verongula rigida. Mar. Drugs 2011, 9, 1902–1913. [Google Scholar] [CrossRef]
- de Medeiros, A.I.; Gandolfi, R.C.; Secatto, A.; Falcucci, R.M.; Faccioli, L.H.; Hajdu, E.; Peixinho, S.; Roberto Berlinck, R.G.S. 11-Oxoaerothionin isolated from the marine sponge Aplysina fistularis shows anti-inflammatory activity in LPS-stimulated macrophages. Immunopharm. Immunot. 2012, 34, 919–924. [Google Scholar]
- Florean, C.; Schnekenburger, M.; Lee, Y.J.; Kim, K.R.; Mazumder, A.; Song, S.; Kim, J.M.; Grandjenette, C.; Kim, J.G.; Yoon, A.Y.; et al. Discovery and characterization of Isofistularin-3, a marine brominated alkaloid, as a new DNA demethylating agent inducing cell cycle arrest and sensitization to TRAIL in cancer cells. Oncotarget 2016, 7, 24027–24049. [Google Scholar] [CrossRef]
- Rohde, S.; Schupp, P.J. Growth and regeneration of the elephant ear sponge Ianthella basta (Porifera). Hydrobiologia 2012, 687, 219–226. [Google Scholar] [CrossRef]
- Fromont, J.; Abdul Wahab, M.A.; Gomez, O.; Ekins, M.; Grol, M.; Hooper, J.N.A. Patterns of sponge biodiversity in the Pilbara, Northwestern Australia. Diversity 2016, 8, 21. [Google Scholar] [CrossRef]
- Bergquist, P.R.; Kelly-Borges, M. Systematics and biogeography of the genus Ianthella (Demospongiae: Verongida: Ianthellida) in the South-West Pacific. The Beagle, Rec. Northern Terr. Mus. Arts Sci. 1995, 12, 151–176. [Google Scholar]
- Kazlauskas, R.; Lidgard, R.O.; Murphy, P.T.; Wells, R.J.; Blount, J.F. Brominated tyrosine-derived metabolites from the sponge Ianthella basta. Austr. J. Chem. 1981, 34, 765–786. [Google Scholar] [CrossRef]
- Franklin, M.A.; Penn, S.G.; Lebrilla, C.B.; Lam, T.H.; Pessah, I.N.; Molinski, T.F. Bastadin 20 and bastadin O-sulfate esters from Ianthella basta: novel modulators of the Ry1R FKBP12 receptor complex. J. Nat. Prod. 1996, 59, 1121–1127. [Google Scholar] [CrossRef]
- Greve, H.; Kehraus, S.; Krick, A.; Kelter, G.; Maier, A.; Fiebig, H.-H.; Wright, A.D.; König, G.M. Cytotoxic bastadin 24 from the Australian sponge Ianthella quadrangulata. J. Nat. Prod. 2008, 71, 309–312. [Google Scholar] [CrossRef]
- Ehrlich, H.; Rigby, J.K.; Botting, J.; Tsurkan, M.; Werner, C.; Schwille, P.; Petrasek, Z.; Pisera, A.; Simon, P.; Sivkov, V.; et al. Discovery of 505–million–year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013, 3, 3497. [Google Scholar] [CrossRef]
- Wollert, K.C.; Bethmann, K.; Drexler, H. Cell-Based Therapies and Tissue Engineering in Heart Failure. In Heart Failure: A Companion to Braunwald’s Heart Disease, 2nd ed.; Mann, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Pilarczyk, G.; Raulf, A.; Gunkel, M.; Fleischmann, B.K.; Lemor, R.; Hausmann, M. Tissue-mimicking, geometrical constraints stimulate tissue-like constitution and activity of mouse neonatal and human-induced pluripotent stem cell-derived cardiac myocytes. J. Funct. Biomater. 2016, 7, 1. [Google Scholar] [CrossRef]
- Seeger, T.; Wu, J.C. Cardiac Remodeling and Regeneration. In Cardiac Electrophysiology: From Cell to Bedside, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Devalla, H.D.; Passier, R. Cardiac differentiation of pluripotent stem cells and implications for modeling the heart in health and disease. Sci. Transl. Med. 2018, 4, eaah5457. [Google Scholar] [CrossRef]
- Ruan, J.L.; Tulloch, N.L.; Razumova, M.V.; Saiget, M.; Muskheli, V.; Pabon, L.; Reinecke, H.; Regnier, M.; Murry, C.E. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016, 134, 1557–1567. [Google Scholar] [CrossRef]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Chang Liao, M.L.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined Engineered Human Myocardium with Advanced Maturation for Applications in Heart Failure Modelling and Repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, W.J.; Yuan, F.; Nakane, T.; Masumoto, H. Quantification of Cardiomyocyte Alignment from Three-Dimensional (3D) Confocal Microscopy of Engineered Tissue. Mirosc. Microanal. 2017, 23, 826–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Beyazoglu, T.; Hefner, E.; Gurkan, U.A.; Demirci, U. Automated and adaptable quantification of cellular alignment from microscopic images for tissue engineering applications. Tissue Eng. Part C Methods 2011, 17, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, S.; Miki, K.; Takaki, T.; Okubo, C.; Hatani, T.; Chonobayashi, K.; Nishikawa, M.; Takei, I.; Oishi, A.; Narita, M.; et al. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 2016, 6, 19111. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.V.; Kensah, G.; Roraermel, A.; Baraki, H.; Kutschka, I.; Zweigerdt, R.; Martin, U.; Haverich, A.; Gruh, I.; Martens, A. Transplantation of purified iPSC-derived cardiomyocytes in myocardial infarction. PLoS ONE 2017, 12, e0173222. [Google Scholar] [CrossRef]
- Pawan, K.C.; Yi, H.; Ge, Z. Cardiac tissue-derived extracellular matrix scaffolds for myocardial repair: advantages and challenges. Regen. Biomater. 2019, 6, 185–199. [Google Scholar]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing Kingdoms: Using Decellularized Plants as Perfusable Tissue Engineering Scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Kaiser, N.J.; Kant, R.J.; Minor, A.J.; Coulombe, K.L.K. Optimizing Blended Collagen-Fibrin Hydrogels for Cardiac Tissue Engineering with Human iPSC-derived Cardiomyocytes. ACS Biomater. Sci. Eng. 2019, 5, 887–899. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, T.; Zhang, L.; Gong, W.; Sun, W. Biomimetic design and fabrication of scaffolds integrating oriented micro-pores with branched channel networks for myocardial tissue engineering. Biofabrication 2019, 11, 3. [Google Scholar] [CrossRef]
- Li, T.-T.; Lou, C.-W.; Chen, A.-P.; Lee, M.-C.; Ho, T.-F.; Chen, Y.-S.; Lin, J.-H. Highly Absorbent Antibacterial Hemostatic Dressing for Healing Severe Hemorrhagic Wounds. Materials 2016, 9, 793. [Google Scholar] [CrossRef]
- Lv, L.; Tang, F.; Lan, G. Preparation and characterization of a chitin/platelet-poor plasma composite as a hemostatic material. RSC Adv. 2016, 6, 95358–95368. [Google Scholar] [CrossRef]
- Ohshima, Y.; Nashino, K.; Okuda, R.; Minami, A.; Kihune, K. Clinical application of new chitin non-woven fabric and new chitin sponge sheet as wound dressing. Eur. J. Plast. Surg. 1991, 14, 202–211. [Google Scholar] [CrossRef]
- Drozd, N.N.; Torlopov, M.A.; Udoratina, E.V.; Logvinova, Y.S. Effect of nanocrystalline particles of chitin on blood components in humans and experimental animals. Bull. Exp. Biol. Med. 2018, 164, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Khoshmohabat, H.; Paydar, S.; Kazemi, H.M.; Dalfardi, B. Overview of agents used for emergency hemostasis. Trauma Mon. 2016, 21, e26023. [Google Scholar] [CrossRef]
- Pogorielov, M.; Kalinkevich, O.; Deineka, V.; Garbuzova, V.; Solodovnik, A.; Kalinkevich, A.; Kalinichenko, T.; Gapchenko, A.; Sklyar, A. Danilchenko, Haemostatic chitosan coated gauze: in vitro interaction with human blood and in-vivo effectiveness. Biomat. Res. 2015, 19, 22. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Huang, Y. Comparative evaluation of biological performance, biosecurity, and availability of cellulose-based absorbable hemostats. Clin. Appl. Thromb. Hemos. 2018, 24, 566–574. [Google Scholar] [CrossRef]
- Erpenbeck, D.; Voigt, O.; Al-Aidaroos, A.M.; Berumen, M.L.; Büttner, G.; Catania, D.; Guirguis, A.N.; Paulay, G.; Schätzle, S.; Wörheide, G. Molecular biodiversity of Red Sea demosponges. Mar. Poll. Bull. 2016, 105, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Wysokowski, M.; Behm, T.; Born, R.; Bazhenov, V.V.; Meissner, H.; Richter, G.; Szwarc-Rzepka, K.; Makarova, A.; Vyalikh, D.; Schupp, P.; et al. Preparation of chitin–silica composites by in vitro silicification of two-dimensional Ianthella basta demosponge chitinous scaffolds under modified Stöber conditions. Mat. Sci. Eng. C 2013, 33, 3935–3941. [Google Scholar] [CrossRef]
- Jurek, J.; Yoshida, W.Y.; Scheuer, P.J.; Kelly-Borges, M. Three new bromotyrosine-derived metabolites of the sponge Psammaplysilla purpurea. J. Nat. Prod. 1993, 56, 1609–1612. [Google Scholar] [CrossRef]
- Gotsbacher, M.P.; Karuso, P. New Antimicrobial Bromotyrosine Analogues from the Sponge Pseudoceratina purpurea and Its Predator Tylodina corticalis. Mar. Drugs 2015, 13, 1389–1409. [Google Scholar] [CrossRef]
- Cyganek, L.; Tiburcy, M.; Sekeres, K.; Gerstenberg, K.; Bohnenberger, H.; Lenz, C.; Guan, K. Deep phenotyping of human induced pluripotent stem cell–derived atrial and ventricular cardiomyocytes. JCI Insight 2018, 3, e99941. [Google Scholar] [CrossRef] [PubMed]
- Streckfuss-Bömeke, K.; Tiburcy, M.; Fomin, A.; Luo, X.; Li, W.; Fischer, C.; Özcelik, C.; Perrot, A.; Sossalla, S.; Haas, J.; et al. Severe DCM phenotype of patient harboring RBM20 mutation S635A can be modeled by patient-specific induced pluripotent stem cell-derived cardiomyocytes. J. Mol. Cell Cardiol. 2017, 113, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahama, H.; Nwe, N.; Jayakumar, R.; Koiwa, S.; Furuike, T.; Tamura, H. Novel biodegradable chitin membranes for tissue engineering applications. Carbohydr. Polym. 2008, 73, 295–302. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, M.; Binnewerg, B.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; Bechmann, N.; et al. Naturally Prefabricated Marine Biomaterials: Isolation and Applications of Flat Chitinous 3D Scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida). Int. J. Mol. Sci. 2019, 20, 5105. https://doi.org/10.3390/ijms20205105
Schubert M, Binnewerg B, Voronkina A, Muzychka L, Wysokowski M, Petrenko I, Kovalchuk V, Tsurkan M, Martinovic R, Bechmann N, et al. Naturally Prefabricated Marine Biomaterials: Isolation and Applications of Flat Chitinous 3D Scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida). International Journal of Molecular Sciences. 2019; 20(20):5105. https://doi.org/10.3390/ijms20205105
Chicago/Turabian StyleSchubert, Mario, Björn Binnewerg, Alona Voronkina, Lyubov Muzychka, Marcin Wysokowski, Iaroslav Petrenko, Valentine Kovalchuk, Mikhail Tsurkan, Rajko Martinovic, Nicole Bechmann, and et al. 2019. "Naturally Prefabricated Marine Biomaterials: Isolation and Applications of Flat Chitinous 3D Scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida)" International Journal of Molecular Sciences 20, no. 20: 5105. https://doi.org/10.3390/ijms20205105
APA StyleSchubert, M., Binnewerg, B., Voronkina, A., Muzychka, L., Wysokowski, M., Petrenko, I., Kovalchuk, V., Tsurkan, M., Martinovic, R., Bechmann, N., Ivanenko, V. N., Fursov, A., Smolii, O. B., Fromont, J., Joseph, Y., Bornstein, S. R., Giovine, M., Erpenbeck, D., Guan, K., & Ehrlich, H. (2019). Naturally Prefabricated Marine Biomaterials: Isolation and Applications of Flat Chitinous 3D Scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida). International Journal of Molecular Sciences, 20(20), 5105. https://doi.org/10.3390/ijms20205105









