Green Tea Polyphenol (−)-Epigallocatechin Gallate (EGCG) Attenuates Neuroinflammation in Palmitic Acid-Stimulated BV-2 Microglia and High-Fat Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Results
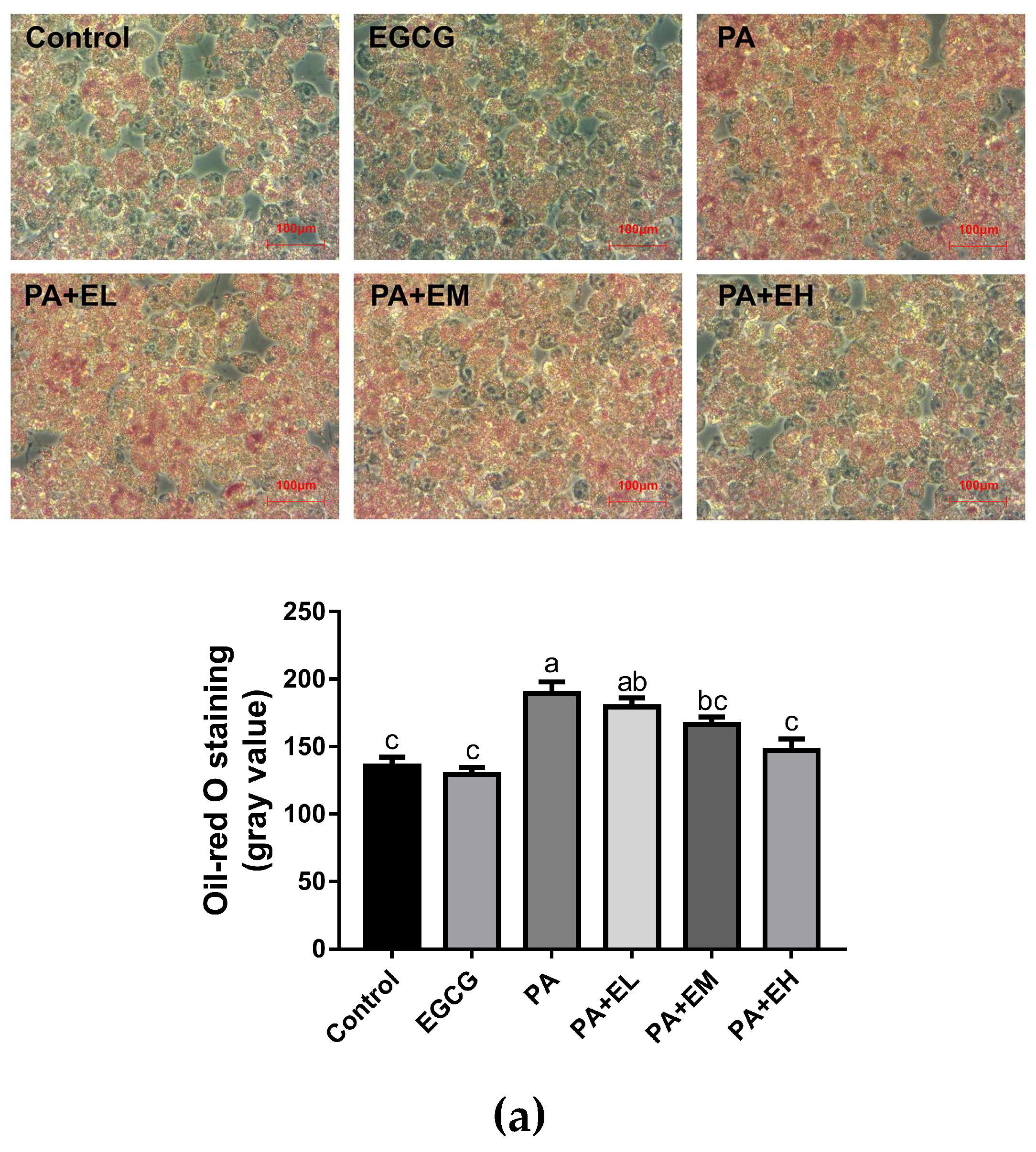
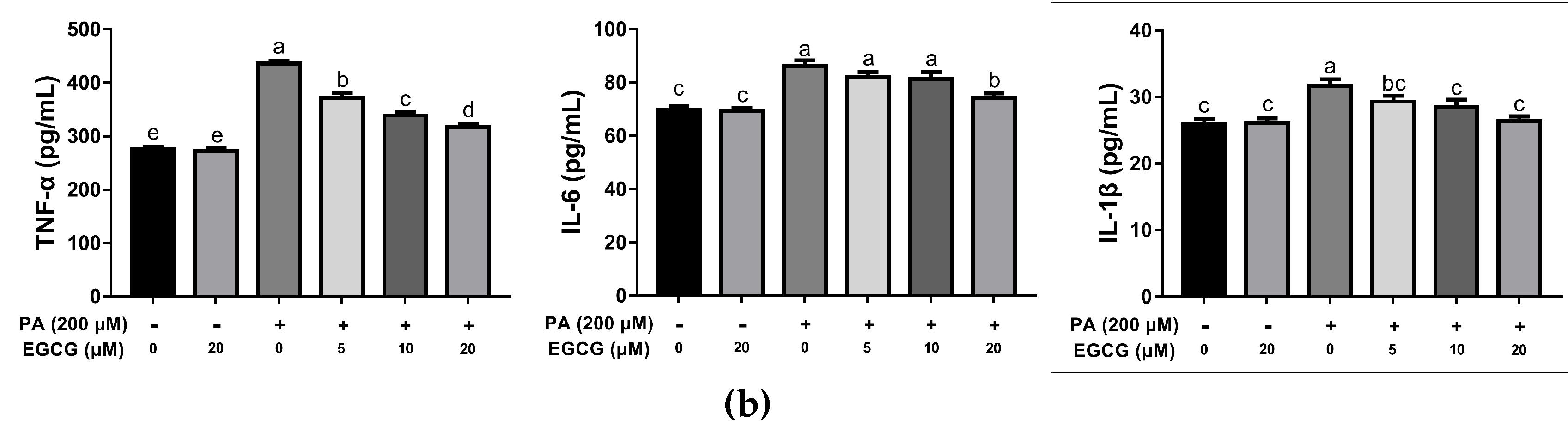
2.1. EGCG Attenuates Lipid Accumulation and Inflammatory Responses in PA-Stimulated BV-2 Cells
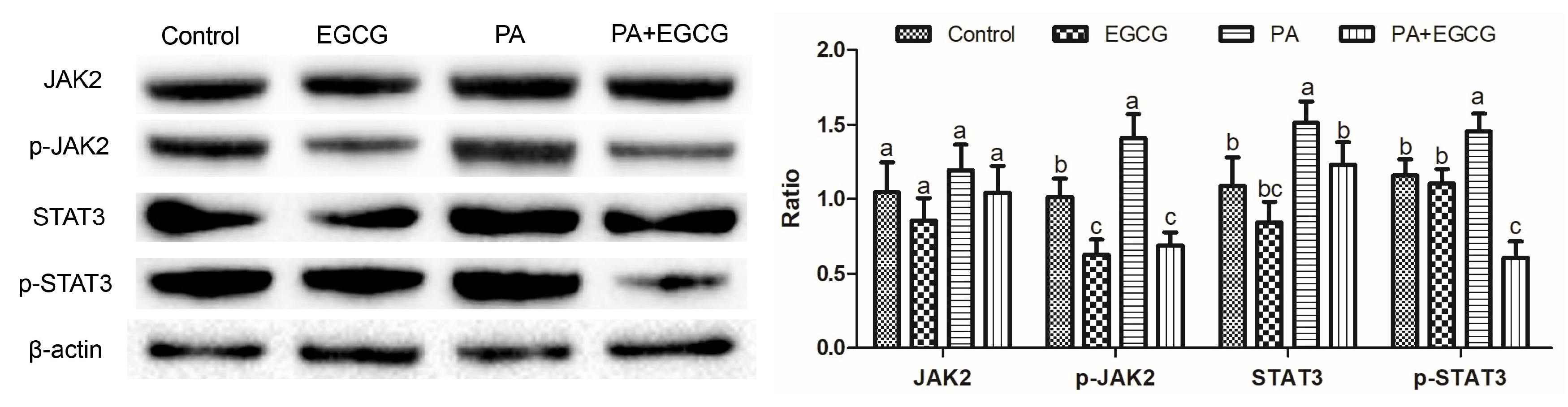
2.2. EGCG Suppresses Phosphorylation of JAK2 and STAT3 in PA-Stimulated BV-2 Cells
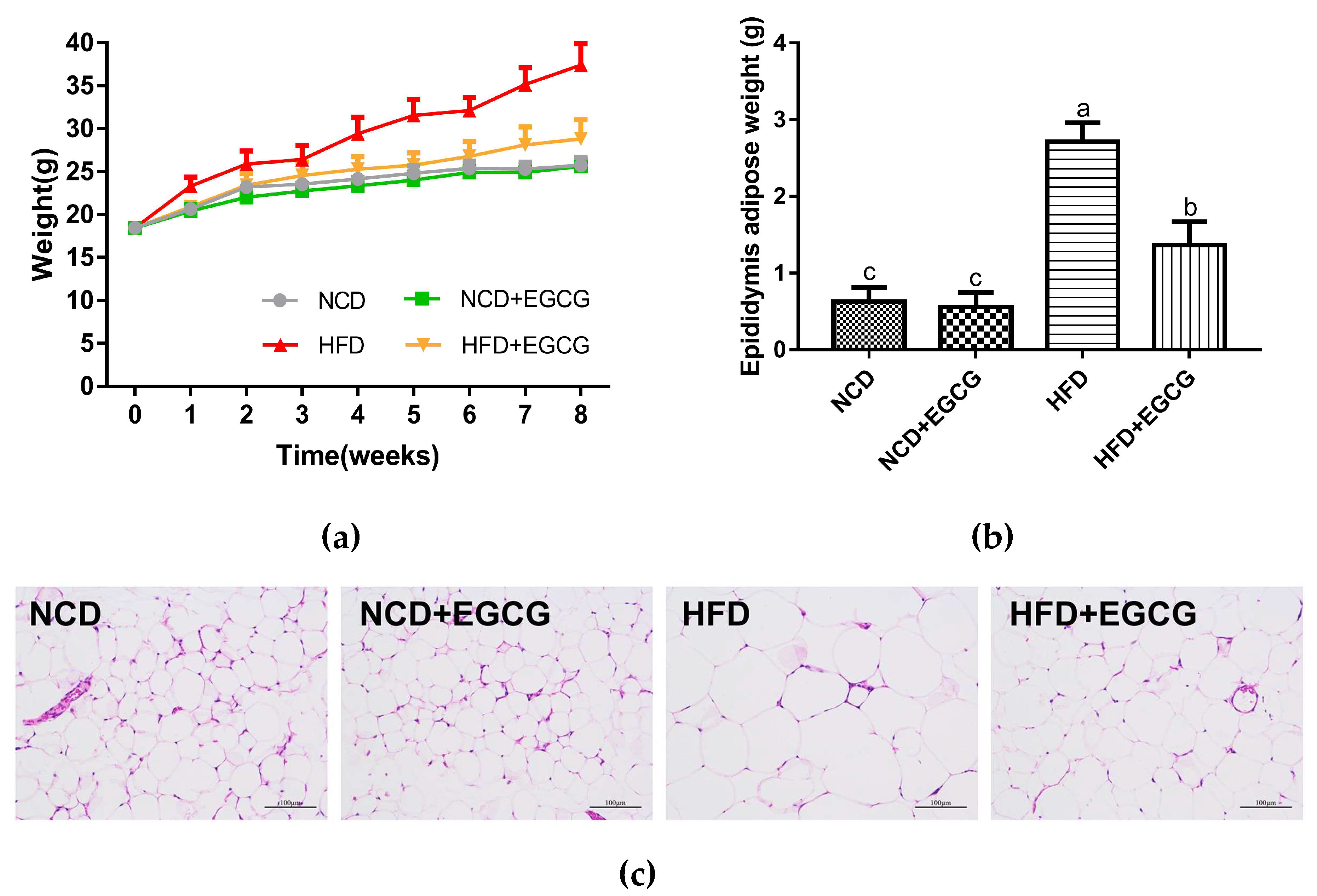
2.3. EGCG Ameliorates HFD Induced Obesity
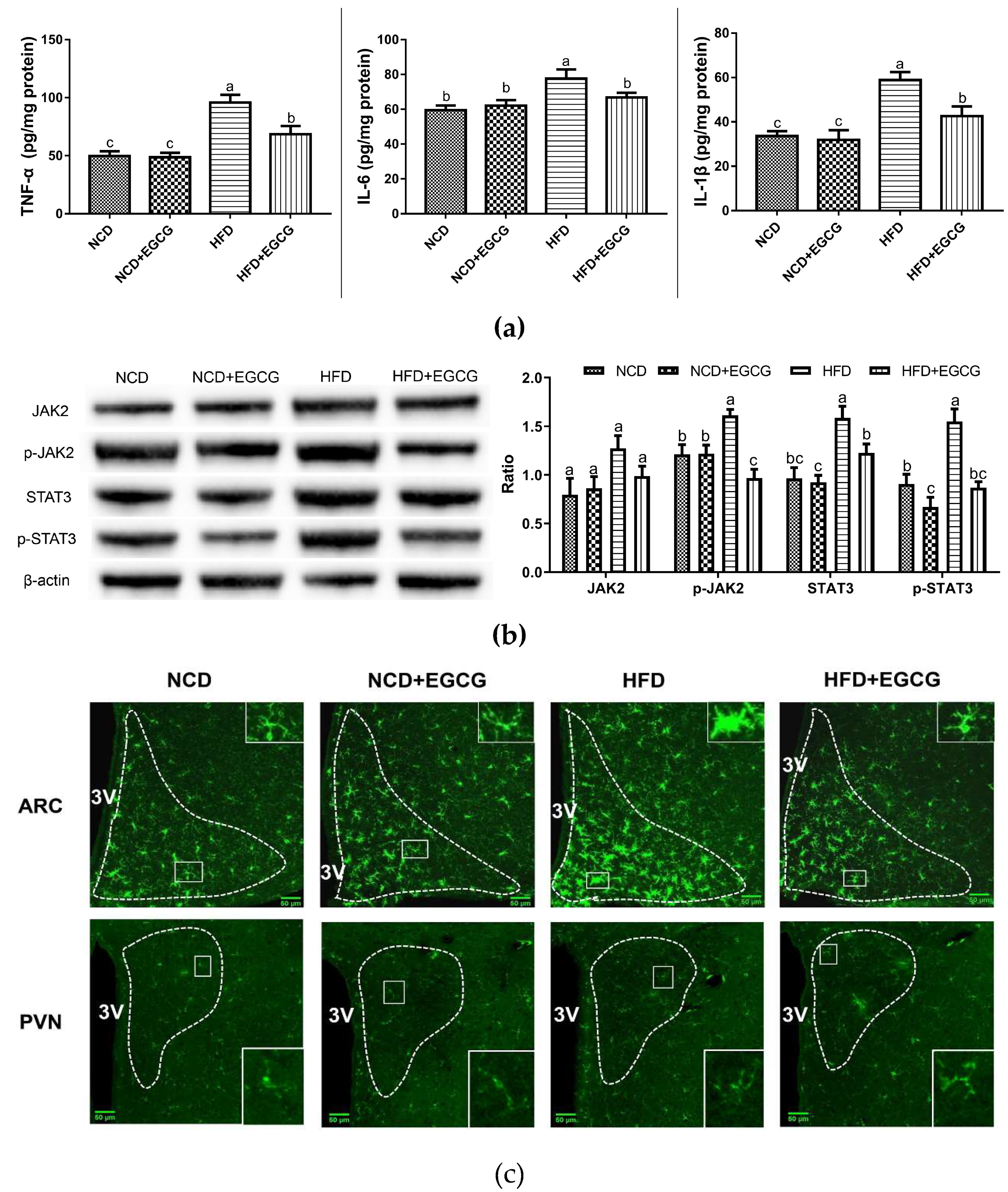
2.4. EGCG Alleviates the Obesity-Associated Neuroinflammation of the Hypothalamus
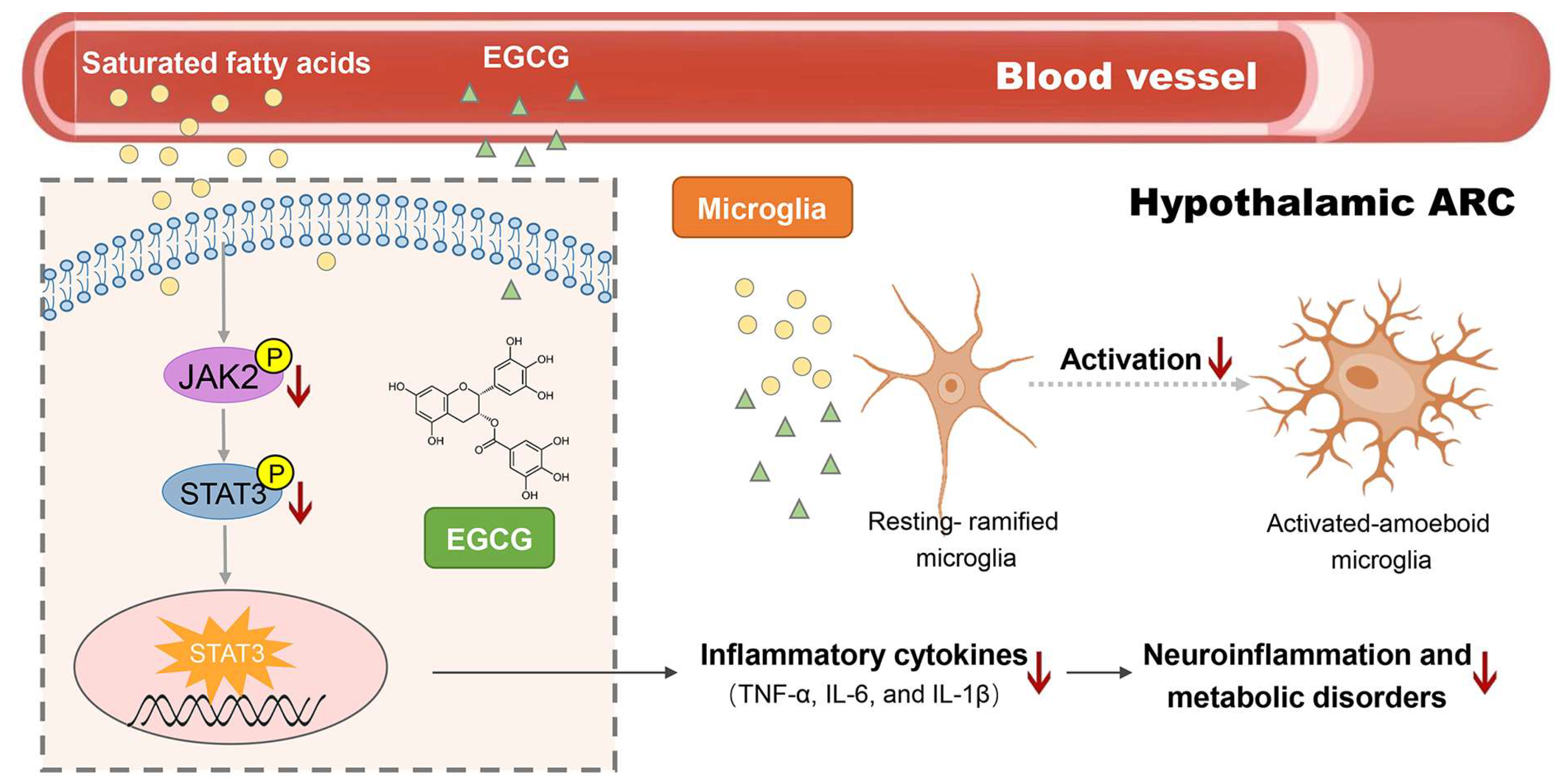
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. BV-2 Cell Culture and Treatment
4.3. Oil Red O Staining
4.4. Animals
4.5. Collection of Serum and Tissue Samples
4.6. H&E Staining
4.7. Western Blot Analysis
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Immunofluorescence
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AgRP | Agouti-related neuropeptide |
| ARC | Arcuate nucleus |
| CNS | Central nervous system |
| DIO | Diet-induced obesity |
| EGCG | (−)-Epigallocatechin gallate |
| HFD | High-fat diet |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin 1-beta |
| JAK2 | Janus kinase 2 |
| PA | Palmitic acid |
| POMC | Proopiomelanocortin |
| PVN | Paraventricular nucleus |
| SFA | Saturated fatty acids |
| STAT3 | Signal transducers and activators of transcription 3 |
| TNF-α | Tumor necrosis factor-alpha |
References
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; Federation, W.O. Obesity: A chronic relapsing progressive disease process. A position statement of the world obesity federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Cypess, A.M.; Kahn, C.R. Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 2010, 9, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.A.; Connaughton, R.M.; Lyons, C.L.; McMorrow, A.M.; Roche, H.M. Fatty acids and chronic low grade inflammation associated with obesity and the metabolic syndrome. Eur. J. Pharmacol. 2016, 785, 207–214. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.T.; Araujo, E.P.; Bordin, S.; Ashimine, R.; Zollner, R.L.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 2005, 146, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ge, K.L.; Song, C.; Ge, Y.L.; Zhang, J.Y. Effects of n-3pufas on autophagy and inflammation of hypothalamus and body weight in mice. Biochem. Biophys. Res. Commun. 2018, 501, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Lizarbe, B.; Soares, A.F.; Larsson, S.; Duarte, J.M.N. Neurochemical modifications in the hippocampus, cortex and hypothalamus of mice exposed to long-term high-fat diet. Front. Neurosci. 2019, 12, 985. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Mendes, N.F.; Kim, Y.B.; Velloso, L.A.; Araujo, E.P. Hypothalamic microglial activation in obesity: A mini-review. Front. Neurosci. 2018, 12, 846. [Google Scholar] [CrossRef]
- Meydani, M.; Hasan, S.T. Dietary polyphenols and obesity. Nutrients 2010, 2, 737–751. [Google Scholar] [CrossRef]
- Zhan, W.; Liu, Y.; Li, D.P.; Liu, Y. Advancing insights on the anti-obesity biochemical mechanism of (-)-epigallocatechin gallate (egcg) by inhibiting alpha-amylase activity. RSC Adv. 2016, 6, 96918–96927. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Chang, C.J.; Lin, C.S.; Lu, C.C.; Ko, Y.F.; Tseng, S.F.; Lai, H.C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2017, 13, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Duffy, C.M.; Yuan, C.; Wisdorf, L.E.; Billington, C.J.; Kotz, C.M.; Nixon, J.P.; Butterick, T.A. Role of orexin a signaling in dietary palmitic acid-activated microglial cells. Neurosci. Lett. 2015, 606, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Ma, R.; Sun, S.G.; Wei, G.R.; Fang, Y.; Liu, R.G.; Li, G. Jak2-stat3 signaling pathway mediates thrombin-induced proinflammatory actions of microglia in vitro. J. Neuroimmunol. 2008, 204, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Tsunekawa, T.; Banno, R.; Mizoguchi, A.; Sugiyama, M.; Tominaga, T.; Onoue, T.; Hagiwara, D.; Ito, Y.; Iwama, S.; Goto, M.; et al. Deficiency of ptp1b attenuates hypothalamic inflammation via activation of the jak2-stat3 pathway in microglia. Ebiomedicine 2017, 16, 172–183. [Google Scholar] [CrossRef] [PubMed]
- de Git, K.C.G.; Adan, R.A.H. Leptin resistance in diet-induced obesity: The role of hypothalamic inflammation. Obes. Rev. 2015, 16, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.P.; Guyenet, S.J.; Dorfman, M.D.; Wisse, B.E.; Schwartz, M.W. Hypothalamic inflammation: Marker or mechanism of obesity pathogenesis? Diabetes 2013, 62, 2629–2634. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.P.; Schwartz, M.W. Minireview: Inflammation and obesity pathogenesis: The hypothalamus heats up. Endocrinology 2010, 151, 4109–4115. [Google Scholar] [CrossRef]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2018, 27, 1356. [Google Scholar] [CrossRef]
- Santos, C.; de Lima, C.M.; Foro, C.A.R.; de Oliveira, M.A.; Magalhaes, N.G.M.; Guerreiro-Diniz, C.; Diniz, D.G.; Vasconcelos, P.F.D.; Diniz, C.W.P. Visuospatial learning and memory in the cebus apella and microglial morphology in the molecular layer of the dentate gyrus and ca1 lacunosum molecular layer. J. Chem. Neuroanat. 2014, 61–62, 176–188. [Google Scholar] [CrossRef]
- Bollinger, J.L.; Collins, K.E.; Patel, R.; Wellman, C.L. Behavioral stress alters corticolimbic microglia in a sex- and brain region-specific manner. PLoS ONE 2017, 12, e0187631. [Google Scholar] [CrossRef]
- Konstantinidi, M.; Koutelidakis, A.E. Functional foods and bioactive compounds: A review of its possible role on weight management and obesity’s metabolic consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Petzke, K.J.; Raederstorff, D.; Wolfram, S.; Klaus, S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int. J. Obes. 2012, 36, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Grove, K.A.; Sae-tan, S.; Kennett, M.J.; Lambert, J.D. (-)-epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice. Obesity 2012, 20, 2311–2313. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Ridgeway, S.D.; Kim, J.A. Effects of the green tea polyphenol epigallocatechin-3-gallate on high-fat diet-induced insulin resistance and endothelial dysfunction. Am. J. Physiol. Metab. 2013, 305, E1444–E1451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; Mao, L.M.; Xu, P.; Wang, Y.F. Effects of (-)-epigallocatechin gallate (egcg) on energy expenditure and microglia-mediated hypothalamic inflammation in mice fed a high-fat diet. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Atrens, D.M.; Menendez, J.A. Glucagon and the paraventricular hypothalamus—modulation of energy-balance. Brain Res. 1993, 630, 245–251. [Google Scholar] [CrossRef]
- Carvalheira, J.B.C.; Ribeiro, E.B.; Araujo, E.P.; Guimaraes, R.B.; Telles, M.M.; Torsoni, M.; Gontijo, J.A.R.; Velloso, L.A.; Saad, M.J.A. Selective impairment of insulin signalling in the hypothalamus of obese zucker rats. Diabetologia 2003, 46, 1629–1640. [Google Scholar] [CrossRef]
- Yi, C.X.; Tschop, M.H.; Woods, S.C.; Hofmann, S.M. High-fat-diet exposure induces igg accumulation in hypothalamic microglia. Dis. Model. Mech. 2012, 5, 686–690. [Google Scholar] [CrossRef]
- Cunningham, C. Microglia and neurodegeneration: The role of systemic inflammation. Glia 2013, 61, 71–90. [Google Scholar] [CrossRef]
- He, L.X.; Tong, X.Y.; Zeng, J.; Tu, Y.Q.; Wu, S.C.; Li, M.P.; Deng, H.M.; Zhu, M.M.; Li, X.C.; Nie, H.; et al. Paeonol suppresses neuroinflammatory responses in lps-activated microglia cells. Inflammation 2016, 39, 1904–1917. [Google Scholar] [CrossRef]
- Stentz, F.B.; Kitabchi, A.E. Palmitic acid-induced activation of human t-lymphocytes and aortic endothelial cells with production of insulin receptors, reactive oxygen species, cytokines, and lipid peroxidation. Biochem. Biophys. Res. Commun. 2006, 346, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Lanussa, O.; Avila-Rodriguez, M.; Baez-Jurado, E.; Zamudio, J.; Echeverria, V.; Garcia-Segura, L.M.; Barreto, G.E. Tibolone reduces oxidative damage and inflammation in microglia stimulated with palmitic acid through mechanisms involving estrogen receptor beta. Mol. Neurobiol. 2018, 55, 5462–5477. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.; Pfuhlmann, K.; Schriever, S.C.; Pfluger, P.T. Profound weight loss induces reactive astrogliosis in the arcuate nucleus of obese mice. Mol. Metab. 2019, 24, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, H.J.; Lee, Y.S.; Kang, G.M.; Lim, H.S.; Lee, S.H.; Song, D.K.; Kwon, O.; Hwang, I.; Son, M.; et al. Hypothalamic macrophage inducible nitric oxide synthase mediates obesity-associated hypothalamic inflammation. Cell Rep. 2018, 25, 934–946. [Google Scholar] [CrossRef]
- Arruda, A.P.; Milanski, M.; Coope, A.; Torsoni, A.S.; Ropelle, E.; Carvalho, D.P.; Carvalheira, J.B.; Velloso, L.A. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology 2011, 152, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.T.; Chen, T.C.; Lu, X.X.; Xie, H.Y.; Zhou, L.; Zheng, S.S. Overexpression of variant pnpla3 gene at i148m position causes malignant transformation of hepatocytes via il-6-jak2/stat3 pathway in low dose free fatty acid exposure: A laboratory investigation in vitro and in vivo. Am. J. Trans. Res. 2016, 8, 1319–U1341. [Google Scholar]
- Ahmad, S.F.; Attia, S.M.; Bakheet, S.A.; Zoheir, K.M.A.; Ansari, M.A.; Korashy, H.M.; Abdel-Hamied, H.E.; Ashour, A.E.; Abd-Allah, A.R.A. Naringin attenuates the development of carrageenan-induced acute lung inflammation through inhibition of nf-kappa b, stat3 and pro-inflammatory mediators and enhancement of i kappa b alpha and anti-inflammatory cytokines. Inflammation 2015, 38, 846–857. [Google Scholar] [CrossRef]
- Al-Massri, K.F.; Ahmed, L.A.; El-Abhar, H.S. Pregabalin and lacosamide ameliorate paclitaxel-induced peripheral neuropathy via inhibition of jak/stat signaling pathway and notch-1 receptor. Neurochem. Int. 2018, 120, 164–171. [Google Scholar] [CrossRef]
- Bates, S.H.; Stearns, W.H.; Dundon, T.A.; Schubert, M.; Tso, A.W.K.; Wang, Y.P.; Banks, A.S.; Lavery, H.J.; Haq, A.K.; Maratos-Flier, E.; et al. Stat3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 2003, 421, 856–859. [Google Scholar] [CrossRef]
- Wang, Y.; Zang, W.; Ji, S.; Cao, J.; Sun, C. Three polymethoxyflavones purified from ougan (citrus reticulata cv. Suavissima) inhibited lps-induced no elevation in the neuroglia bv-2 cell line via the jak2/stat3 pathway. Nutrients 2019, 11, 791. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, S.; Zang, W.; Wang, N.; Cao, J.; Li, X.; Sun, C. Identification of phenolic compounds from a unique citrus species, finger lime (citrus australasica) and their inhibition of lps-induced no-releasing in bv-2 cell line. Food Chem. Toxicol. 2019, 129, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Ibars, M.; Ardid-Ruiz, A.; Suarez, M.; Muguerza, B.; Blade, C.; Aragones, G. Proanthocyanidins potentiate hypothalamic leptin/stat3 signalling and pomc gene expression in rats with diet-induced obesity. Int. J. Obes. 2017, 41, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.J.; Qi, S.M.; Dai, W.X.; Luo, L.; Yin, Z.M. Arctigenin inhibits lipopolysaccharide-induced inos expression in raw264.7 cells through suppressing jak-stat signal pathway. Int. Immunopharmacol. 2011, 11, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, D.X.; Wang, F.W.; Liu, S.M.; Zhao, S.D.; Ling, E.A.; Hao, A.J. Saturated fatty acids activate microglia via toll-like receptor 4/nf-kappa b signalling. Br. J. Nutr. 2012, 107, 229–241. [Google Scholar] [CrossRef] [PubMed]
| Serum Biochemical Indices | NCD | NCD + EGCG | HFD | HFD + EGCG |
|---|---|---|---|---|
| Glucose (mmol/L) | 5.93 ± 0.57 c | 6.02 ± 0.48 c | 8.82 ± 0.66 a | 6.78 ± 0.34 b |
| TG (mmol/L) | 1.16 ± 0.15 b | 1.24 ± 0.11 b | 1.38 ± 0.14 a | 1.15 ± 0.19 b |
| TC (mmol/L) | 3.10 ± 0.25 c | 2.91 ± 0.15 c | 4.36 ± 0.43 a | 3.44 ± 0.16 b |
| HDL (mmol/L) | 2.96 ± 0.34 a | 2.75 ± 0.16 a | 2.89 ± 0.29 a | 3.14 ± 0.30 a |
| LDL (mmol/L) | 0.31 ± 0.09 a | 0.29 ± 0.03 a | 0.39 ± 0.11 a | 0.24 ± 0.08 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, L.; Hochstetter, D.; Yao, L.; Zhao, Y.; Zhou, J.; Wang, Y.; Xu, P. Green Tea Polyphenol (−)-Epigallocatechin Gallate (EGCG) Attenuates Neuroinflammation in Palmitic Acid-Stimulated BV-2 Microglia and High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2019, 20, 5081. https://doi.org/10.3390/ijms20205081
Mao L, Hochstetter D, Yao L, Zhao Y, Zhou J, Wang Y, Xu P. Green Tea Polyphenol (−)-Epigallocatechin Gallate (EGCG) Attenuates Neuroinflammation in Palmitic Acid-Stimulated BV-2 Microglia and High-Fat Diet-Induced Obese Mice. International Journal of Molecular Sciences. 2019; 20(20):5081. https://doi.org/10.3390/ijms20205081
Chicago/Turabian StyleMao, Limin, Danielle Hochstetter, Liyun Yao, Yueling Zhao, Jihong Zhou, Yuefei Wang, and Ping Xu. 2019. "Green Tea Polyphenol (−)-Epigallocatechin Gallate (EGCG) Attenuates Neuroinflammation in Palmitic Acid-Stimulated BV-2 Microglia and High-Fat Diet-Induced Obese Mice" International Journal of Molecular Sciences 20, no. 20: 5081. https://doi.org/10.3390/ijms20205081




