Mechanical Ventilation Impairs IL-17 Cytokine Family Expression in Ventilator-Associated Pneumonia
Abstract
:1. Introduction
2. Results
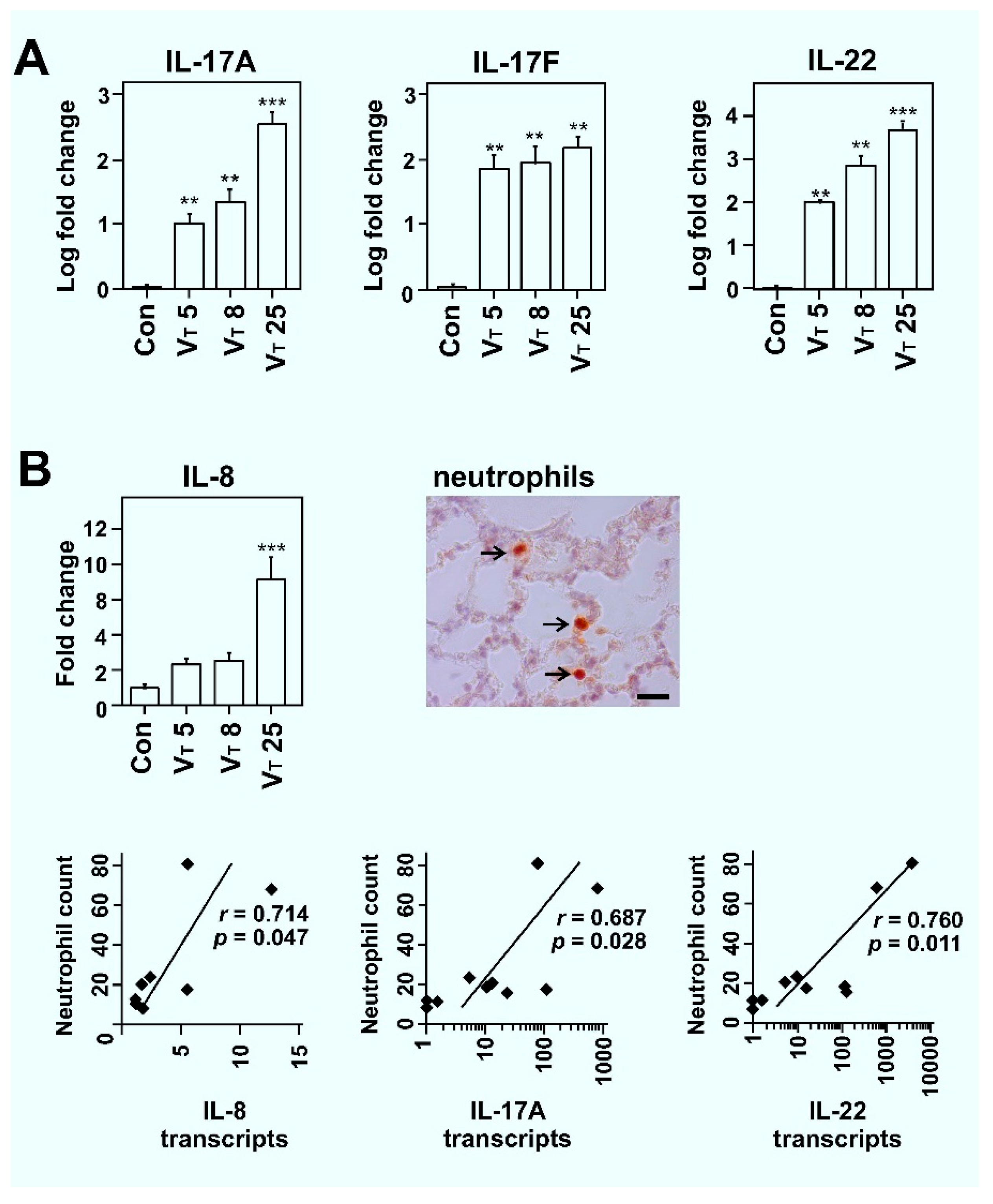
2.1. Mechanical Ventilation Induces VT Dose-Dependent TH17 Cytokine Expression in Lungs of Rats
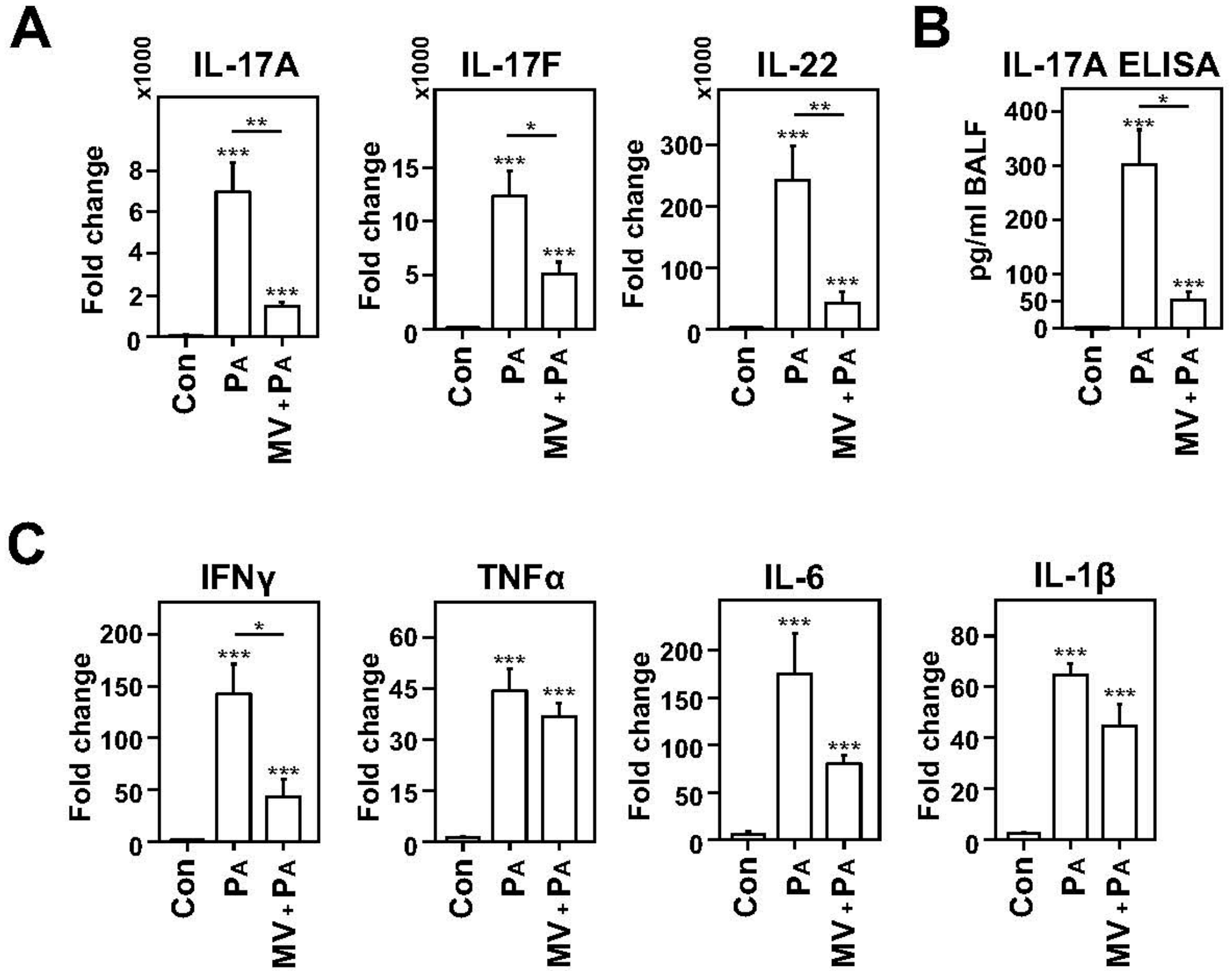
2.2. Dampened IL-17 Response after Infection in Mechanically Ventilated Rats
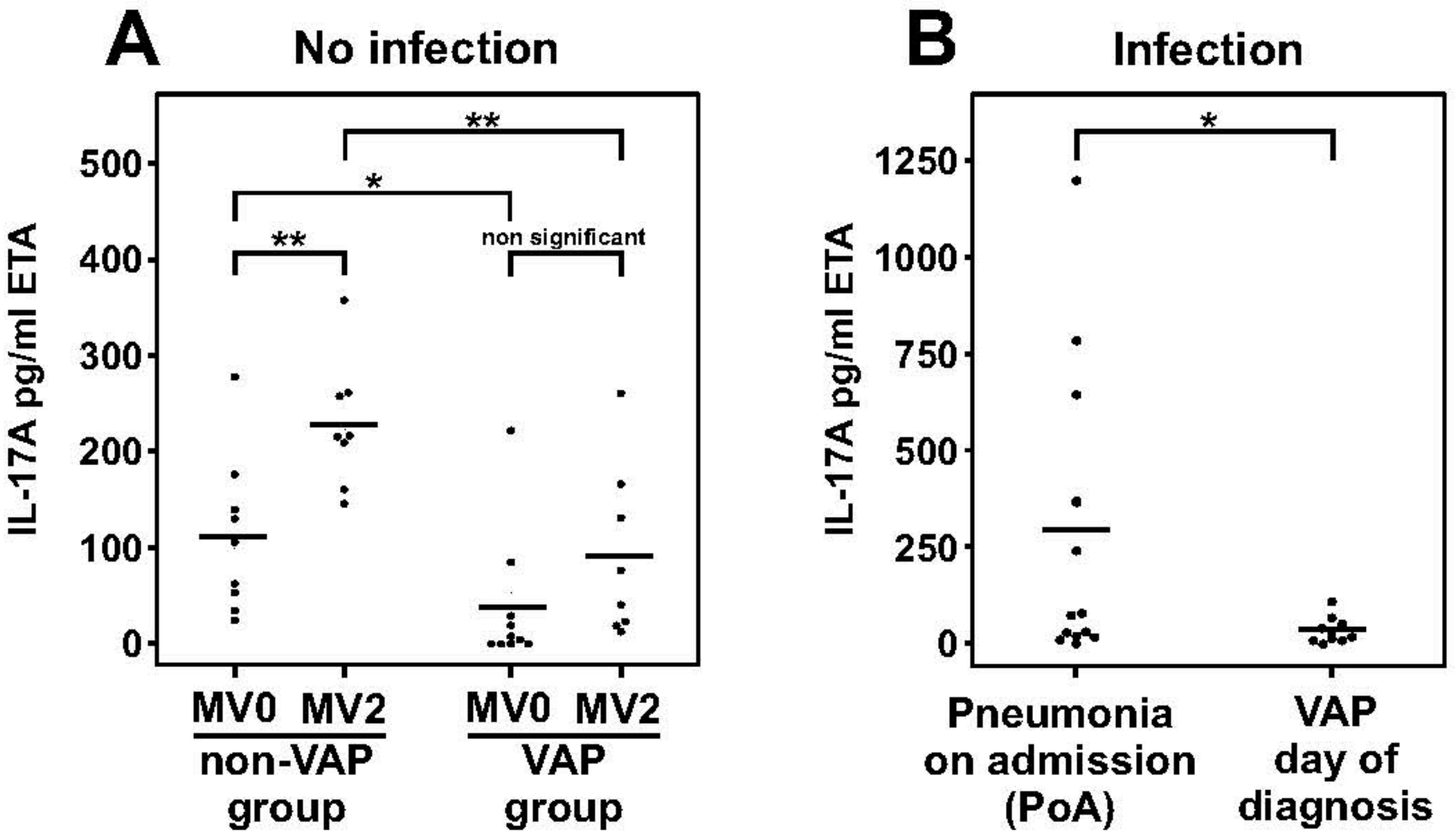
2.3. Dampened IL-17A Response in Patients with Ventilator-Associated Pneumonia
3. Discussion
4. Materials and Methods
4.1. Rat Mechanical Ventilation Model
4.2. Induction of Bacterial Pneumonia in Ventilated Rats
4.3. Patient Samples
4.4. Transcript Studies
4.5. Neutrophil Quantification
4.6. IL-17 Immunoassay
4.7. Data Analyses and Statistics
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASPIRE-ICU | The Advanced understanding of Staphylococcus aureus and Pseudomonas aeruginosa Infections in Europe—Intensive Care Units |
| BALF | Bronchoalveolar lavage fluid |
| ETA | Endotracheal aspirate |
| IBIVAP | Identification of predictive biomarkers of pneumonia in artificially ventilated patients |
| MV | Mechanical ventilation |
| PA | Pseudomonas aeruginosa |
| PEEP | Positive end expiratory pressure |
| PIP | Positive inspiratory pressure |
| PoA | Pneumonia on admission |
| VAP | Ventilator-associated pneumonia |
| VT | Tidal volume |
Appendix A
| Target | Host | Fw seq | Rv seq |
|---|---|---|---|
| Actb | Rat | GTCGTACCACTGGCATTGTG | CTCTCAGCTGTGGTGGTGAA |
| Sdha | Rat | CTCTTTTGGACCTTGTCGTCTTT | TCTCCAGCATTTGCCTTAATCGG |
| Ifn-γ | Rat | ATTCATGAGCATCGCCAAGTTC | TGACAGCTGGTGAATCACTCTGAT |
| Tnf-α | Rat | CTTCTCATTCCTGCTCGTGG | TGATCTGAGTGTGAGGGTCTG |
| IL-1β | Rat | TGCAGGCTTCGAGATGAAC | GGGATTTTGTCGTTGCTTGTC |
| IL-6 | Rat | AAGCCAGAGTCATTCAGAGC | GTCCTTAGCCACTCCTTCTG |
| IL-17A | Rat | ACTACCTCAACCGTTCCACTTCA | CTTCAGGACCAGGATCTCTTGCT |
| IL-17F | Rat | CCCGGAGACCTCTCAGAAGA | GCCCTACTTTGGGGTTCCTC |
| IL-22 | Rat | TCCAGCAGCCATACATCGTC | GGCTTTGACTCCTCGGAACA |
| IL-10 | Rat | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| IL-8 | Rat | CCCCCATGGTTCAGAAGATTG | TTGTCAGAAGCCAGCGTTCAC |
References
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Jorens, P.G. Sticking to an Old Definition of Ventilator-Associated Pneumonia Is Not Old-Fashioned. Respir. Care. 2016, 61, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.C.; Hernandez, L.A.; Peevy, K.J. Mechanisms of ventilator-induced lung injury. Crit. Care Med. 1993, 21, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Gajic, O.; Dara, S.I.; Mendez, J.L.; Adesanya, A.O.; Festic, E.; Caples, S.M.; Rana, R.; St Sauver, J.L.; Lymp, J.F.; Afessa, B.; et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit. Care Med. 2004, 32, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, L.; Valenza, F.; Ribeiro, S.P.; Li, J.; Slutsky, A.S. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J. Clin. Investig. 1997, 99, 944–952. [Google Scholar] [CrossRef]
- Serpa Neto, A.; Cardoso, S.O.; Manetta, J.A.; Pereira, V.G.; Esposito, D.C.; Pasqualucci Mde, O.; Damasceno, M.C.; Schultz, M.J. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: A meta-analysis. JAMA: J. Am. Med. Assoc. 2012, 308, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Bielen, K.; Jongers, B.; Boddaert, J.; Lammens, C.; Jorens, P.G.; Malhotra-Kumar, S.; Goossens, H.; Kumar-Singh, S. Mechanical ventilation induces IL-4 secretion in lungs and reduces the phagocytic capacity of lung macrophages. J. Infect. Dis. 2018, 217, 1645–1655. [Google Scholar] [CrossRef]
- Wienhold, S.M.; Macri, M.; Nouailles, G.; Dietert, K.; Gurtner, C.; Gruber, A.D.; Heimesaat, M.M.; Lienau, J.; Schumacher, F.; Kleuser, B.; et al. Ventilator-induced lung injury is aggravated by antibiotic mediated microbiota depletion in mice. Crit. Care 2018, 22, 282. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y.; Zhang, H.; Cheng, K.C.; Slutsky, A.S. Mechanical ventilation may increase susceptibility to the development of bacteremia. Crit. Care Med. 2003, 31, 1429–1434. [Google Scholar] [CrossRef]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef]
- Xu, X.; Shao, B.; Wang, R.; Zhou, S.; Tang, Z.; Lu, W.; Xiong, S. Role of Interleukin-17 in defense against pseudomonas aeruginosa infection in lungs. Int. J. Clin. Exp. Med. 2014, 7, 809–816. [Google Scholar] [PubMed]
- Aujla, S.J.; Chan, Y.R.; Zheng, M.; Fei, M.; Askew, D.J.; Pociask, D.A.; Reinhart, T.A.; McAllister, F.; Edeal, J.; Gaus, K.; et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008, 14, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Cavani, A.; Schmidt-Weber, C. IL-17 and IL-22: Siblings, not twins. Trends Immunol. 2010, 31, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Laan, M.; Cui, Z.H.; Hoshino, H.; Lotvall, J.; Sjostrand, M.; Gruenert, D.C.; Skoogh, B.E.; Linden, A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999, 162, 2347–2352. [Google Scholar] [PubMed]
- Liu, Y.; Sun, J.K.; Qi, X.; Chen, Y.M.; Li, J.; Chen, S.Y.; Liu, H. Expression and Significance of Th17 and Treg Cells in Pulmonary Infections with Gram-Negative Bacteria. Immunol. Investig. 2017, 46, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Orlov, M.; Dmyterko, V.; Wurfel, M.M.; Mikacenic, C. Th17 cells are associated with protection from ventilator associated pneumonia. PLoS ONE 2017, 12, e0182966. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.B.F.; Chirico, M.T.T.; Cartelle, C.T.; de Paula Costa, G.; Talvani, A.; Cangussu, S.D.; de Menezes, R.C.A.; Bezerra, F.S. High-Fat Diet Increases HMGB1 Expression and Promotes Lung Inflammation in Mice Subjected to Mechanical Ventilation. Oxidative Med. Cell. Longev. 2018, 2018, 7457054. [Google Scholar] [CrossRef] [PubMed]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef]
- Sutherasan, Y.; Vargas, M.; Pelosi, P. Protective mechanical ventilation in the non-injured lung: Review and meta-analysis. Crit. Care 2014, 18, 211. [Google Scholar] [CrossRef] [PubMed]
- Menendez, C.; Martinez-Caro, L.; Moreno, L.; Nin, N.; Moral-Sanz, J.; Morales, D.; Cogolludo, A.; Esteban, A.; Lorente, J.A.; Perez-Vizcaino, F. Pulmonary vascular dysfunction induced by high tidal volume mechanical ventilation. Crit. Care Med. 2013, 41, e149–e155. [Google Scholar] [CrossRef]
- Jorens, P.G.; Van Damme, J.; De Backer, W.; Bossaert, L.; De Jongh, R.F.; Herman, A.G.; Rampart, M. Interleukin 8 (IL-8) in the bronchoalveolar lavage fluid from patients with the adult respiratory distress syndrome (ARDS) and patients at risk for ARDS. Cytokine 1992, 4, 592–597. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, S.; Bonneau, O.; Dubois, G.R.; Jones, C.E.; Trifilieff, A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 2003, 170, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Sutherland, T.E.; Ruckerl, D. IL-17 and neutrophils: Unexpected players in the type 2 immune response. Curr. Opin. Immunol. 2015, 34, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Singh, S.; Pirici, D.; McGowan, E.; Serneels, S.; Ceuterick, C.; Hardy, J.; Duff, K.; Dickson, D.; Van Broeckhoven, C. Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am. J. Pathol. 2005, 167, 527–543. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Massion, P.P.; Inoue, H.; Richman-Eisenstat, J.; Grunberger, D.; Jorens, P.G.; Housset, B.; Pittet, J.F.; Wiener-Kronish, J.P.; Nadel, J.A. Novel Pseudomonas product stimulates interleukin-8 production in airway epithelial cells in vitro. J. Clin. Investig. 1994, 93, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Grgurich, P.E.; Hudcova, J.; Lei, Y.; Sarwar, A.; Craven, D.E. Management and prevention of ventilator-associated pneumonia caused by multidrug-resistant pathogens. Expert. Rev. Respir. Med. 2012, 6, 533–555. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Andersen, C.L.; Tesfa, D.; Siersma, V.D.; Sandholdt, H.; Hasselbalch, H.; Bjerrum, O.W.; Felding, P.; Lind, B.; Olivarius Nde, F.; Palmblad, J. Prevalence and clinical significance of neutropenia discovered in routine complete blood cell counts: A longitudinal study. J. Intern. Med. 2016, 279, 566–575. [Google Scholar] [CrossRef]
- Aydogdu, M.; Gursel, G. Predictive factors for septic shock in patients with ventilator-associated pneumonia. South. Med. J. 2008, 101, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.D. Ventilator associated pneumonia. Bmj 2012, 344, e3325. [Google Scholar] [CrossRef]
- Liu, J.; Feng, Y.; Yang, K.; Li, Q.; Ye, L.; Han, L.; Wan, H. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunol. Med. Microbiol. 2011, 61, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Garvey, P.B.; Zhang, P.; Nelson, S.; Bagby, G.; Summer, W.R.; Schwarzenberger, P.; Shellito, J.E.; Kolls, J.K. Interleukin-17 and Lung Host Defense againstKlebsiella pneumoniae Infection. Am. J. Respir. Cell Mol. Biol. 2001, 25, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Cua, D.J.; Tato, C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef]
- Isailovic, N.; Daigo, K.; Mantovani, A.; Selmi, C. Interleukin-17 and innate immunity in infections and chronic inflammation. J. Autoimmun. 2015, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.M.; Pociask, D.; Clements, J.D.; McLachlan, J.B.; Morici, L.A. Intradermal vaccination with a Pseudomonas aeruginosa vaccine adjuvanted with a mutant bacterial ADP-ribosylating enterotoxin protects against acute pneumonia. Vaccine 2019, 37, 808–816. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodriguez-Bano, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20 (Suppl. 1), 1–55. [Google Scholar] [CrossRef] [Green Version]
- Sutton, C.E.; Mielke, L.A.; Mills, K.H. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur. J. Immunol. 2012, 42, 2221–2231. [Google Scholar] [CrossRef]
- Coquet, J.M.; Chakravarti, S.; Kyparissoudis, K.; McNab, F.W.; Pitt, L.A.; McKenzie, B.S.; Berzins, S.P.; Smyth, M.J.; Godfrey, D.I. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl. Acad. Sci. USA 2008, 105, 11287–11292. [Google Scholar] [CrossRef]
- Nieuwenhuis, E.E.; Matsumoto, T.; Exley, M.; Schleipman, R.A.; Glickman, J.; Bailey, D.T.; Corazza, N.; Colgan, S.P.; Onderdonk, A.B.; Blumberg, R.S. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat. Med. 2002, 8, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Tsay, T.B.; Jiang, Y.Z.; Hsu, C.M.; Chen, L.W. Pseudomonas aeruginosa colonization enhances ventilator-associated pneumonia-induced lung injury. Respir. Res. 2016, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Fujie, H.; Niu, K.; Ohba, M.; Tomioka, Y.; Kitazawa, H.; Nagashima, K.; Ohrui, T.; Numasaki, M. A distinct regulatory role of Th17 cytokines IL-17A and IL-17F in chemokine secretion from lung microvascular endothelial cells. Inflammation 2012, 35, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Nagao, T.; Nagi-Miura, N.; Ohno, N.; Yasuhara, M.; Yamamoto, K.; Nakayama, T.; Suzuki, K. MPO-ANCA induces IL-17 production by activated neutrophils in vitro via classical complement pathway-dependent manner. J. Autoimmun. 2008, 31, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Bielen, K.; ’s Jongers, B.; Malhotra-Kumar, S.; Jorens, P.G.; Goossens, H.; Kumar-Singh, S. Animal models of hospital-acquired pneumonia: Current practices and future perspectives. Ann. Transl. Med. 2017, 5, 132. [Google Scholar] [CrossRef] [PubMed]
- Paling, F.P.; Troeman, D.P.R.; Wolkewitz, M.; Kalyani, R.; Prins, D.R.; Weber, S.; Lammens, C.; Timbermont, L.; Goossens, H.; Malhotra-Kumar, S.; et al. Rationale and design of ASPIRE-ICU: A prospective cohort study on the incidence and predictors of Staphylococcus aureus and Pseudomonas aeruginosa pneumonia in the ICU. BMC Infect. Dis. 2017, 17, 643. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Bielen, K.; ’s Jongers, B.; Boddaert, J.; Raju, T.K.; Lammens, C.; Malhotra-Kumar, S.; Jorens, P.G.; Goossens, H.; Kumar-Singh, S. Biofilm-Induced Type 2 Innate Immunity in a Cystic Fibrosis Model of Pseudomonas aeruginosa. Front Cell Infect Microbiol 2017, 7, 274. [Google Scholar] [CrossRef]
| MV Av ± SD n = 9 | VAP Av ± SD n = 10 | PoA Av ± SD n = 13 | p-Value (VAP vs. PoA) | |
|---|---|---|---|---|
| Age (years) | 61.1 ± 12.2 | 60.2 ± 11.6 | 62.6 ± 9.6 | 0.592 |
| Gender (% male) | 63% | 50% | 69% | |
| MV before sampling (days) | 0; 2 | 0; 2; 4.9 ± 2.8 | 1.9 ± 0.9 | 0.0018 |
| Total ICU stay (days) | 20.2 ± 11.2 | 24.8 ± 12.8 | 18.2 ± 13.4 | 0.243 |
| APACHE II at admission | 24.9 ± 6.6 | 23.4 ± 8.8 | 33 ± 13.5 | 0.106 |
| Prognosis (% deceased) | 33% (3/9) | 40% (4/10) | 15% (2/13) | |
| Total white blood cell count * | 13.6 ± 1.25 | 13.7 ± 1.60 | 15.4 ± 2.69 | 0.703 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Winter, F.H.R.; ’s Jongers, B.; Bielen, K.; Mancuso, D.; Timbermont, L.; Lammens, C.; Van averbeke, V.; Boddaert, J.; Ali, O.; Kluytmans, J.; et al. Mechanical Ventilation Impairs IL-17 Cytokine Family Expression in Ventilator-Associated Pneumonia. Int. J. Mol. Sci. 2019, 20, 5072. https://doi.org/10.3390/ijms20205072
De Winter FHR, ’s Jongers B, Bielen K, Mancuso D, Timbermont L, Lammens C, Van averbeke V, Boddaert J, Ali O, Kluytmans J, et al. Mechanical Ventilation Impairs IL-17 Cytokine Family Expression in Ventilator-Associated Pneumonia. International Journal of Molecular Sciences. 2019; 20(20):5072. https://doi.org/10.3390/ijms20205072
Chicago/Turabian StyleDe Winter, Fien H. R., Bart ’s Jongers, Kenny Bielen, Domenico Mancuso, Leen Timbermont, Christine Lammens, Vincent Van averbeke, Jan Boddaert, Omar Ali, Jan Kluytmans, and et al. 2019. "Mechanical Ventilation Impairs IL-17 Cytokine Family Expression in Ventilator-Associated Pneumonia" International Journal of Molecular Sciences 20, no. 20: 5072. https://doi.org/10.3390/ijms20205072
APA StyleDe Winter, F. H. R., ’s Jongers, B., Bielen, K., Mancuso, D., Timbermont, L., Lammens, C., Van averbeke, V., Boddaert, J., Ali, O., Kluytmans, J., Ruzin, A., Malhotra-Kumar, S., Jorens, P. G., Goossens, H., & Kumar-Singh, S. (2019). Mechanical Ventilation Impairs IL-17 Cytokine Family Expression in Ventilator-Associated Pneumonia. International Journal of Molecular Sciences, 20(20), 5072. https://doi.org/10.3390/ijms20205072





