Predicting Gonadal Germ Cell Cancer in People with Disorders of Sex Development; Insights from Developmental Biology
Abstract
1. Introduction
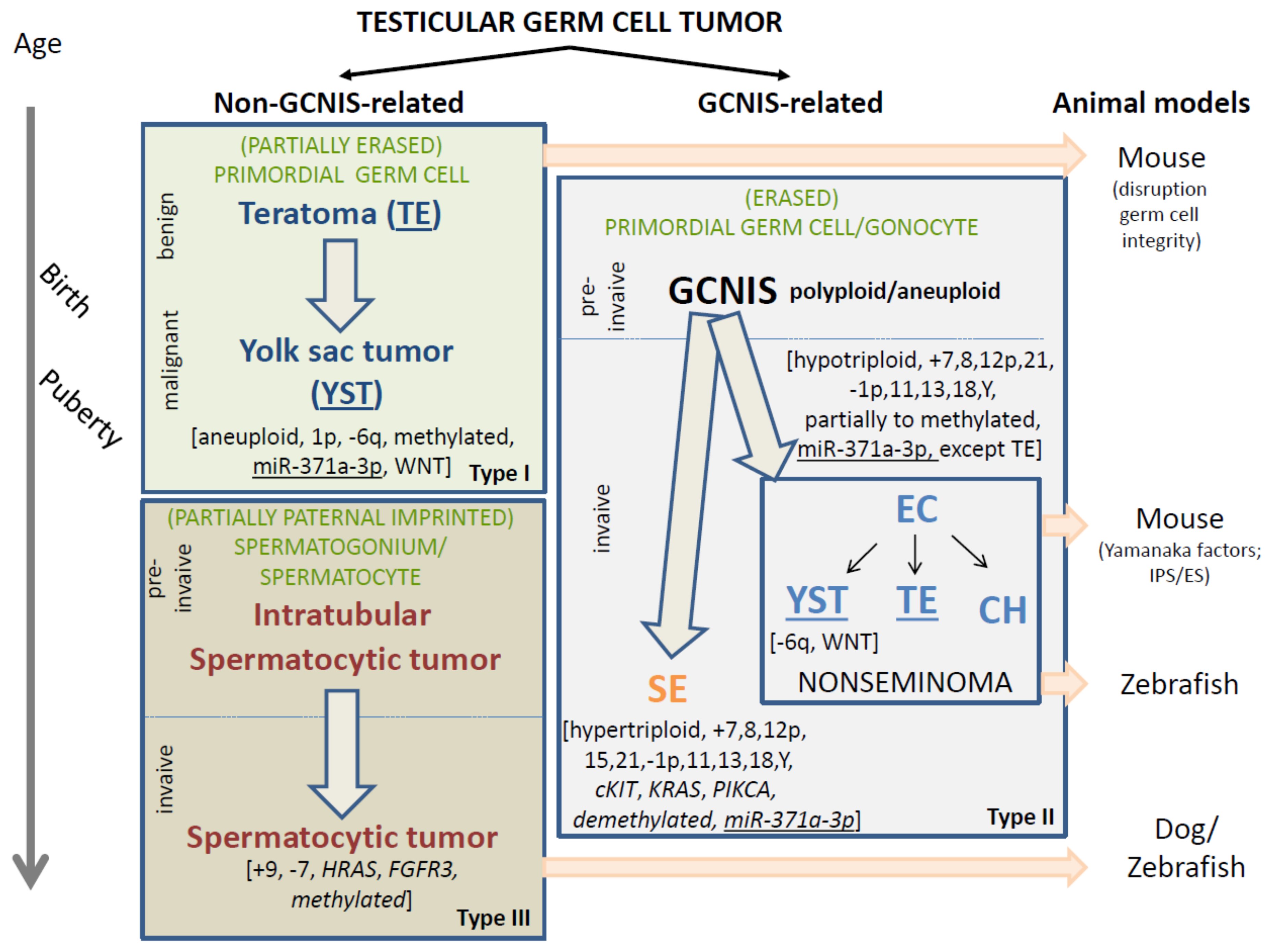
2. Classification of GCNIS- and Non-GCNIS-Related Testicular GCTs
3. Spontaneous and Laboratory-Generated GCT Animal Models
4. Non-GCNIS-Related (Types I and III) Testicular GCTs: Cells of Origin
4.1. Prepubertal-Type Teratoma and/or Yolk Sac Tumors (Type I Testicular GCT)
4.1.1. Risk Factors
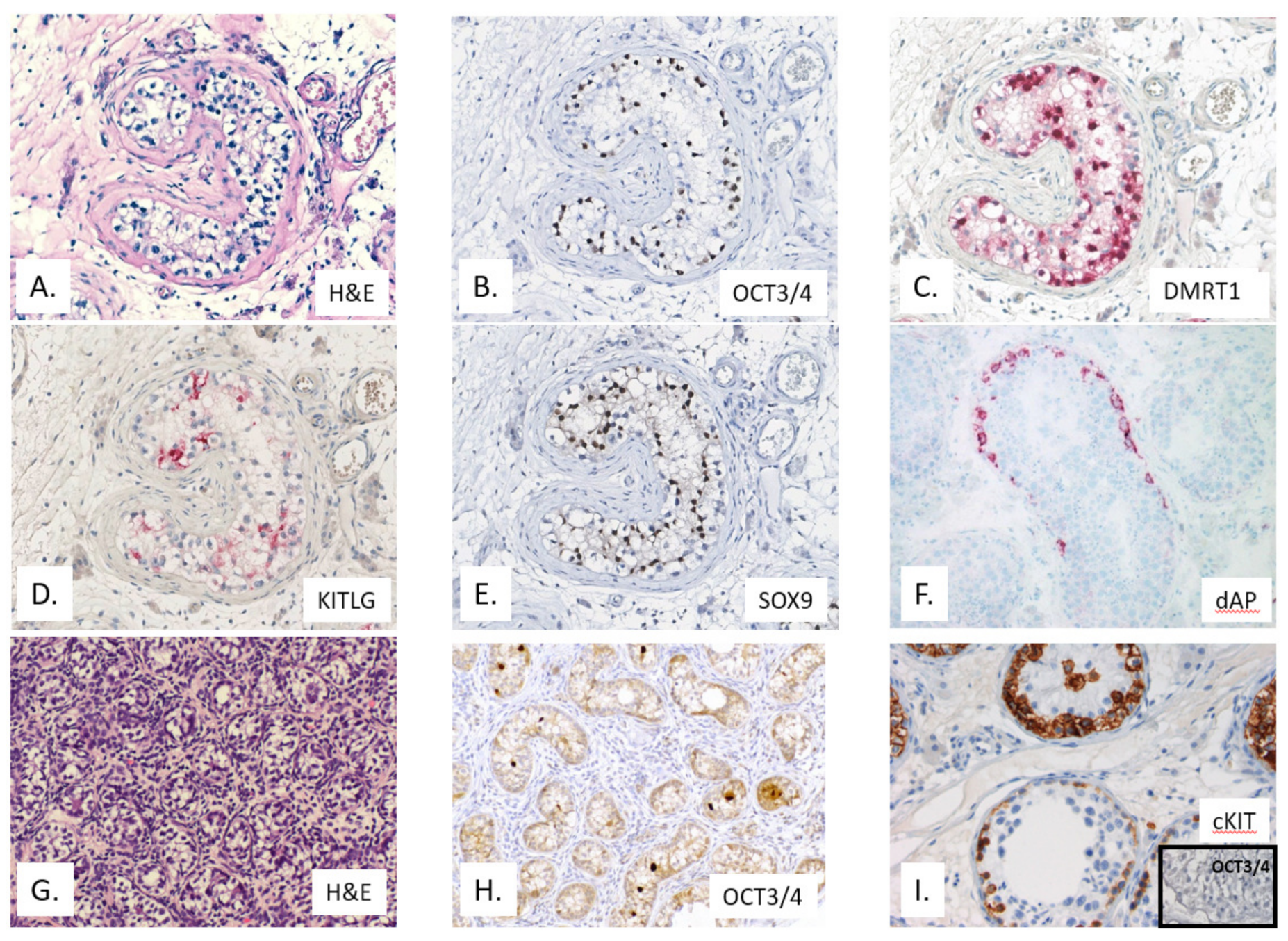
4.1.2. Immunohistochemistry
4.1.3. Molecular Genetic Constitution
4.2. Spermatocytic Tumors (Type III Testicular GCT)
4.2.1. Risk Factors
4.2.2. Immunohistochemistry
4.2.3. Molecular Genetic Constitution
4.3. GCNIS-Related Testicular GCTs: Type II—Histological Diversity and Cell of Origin
4.3.1. Risk Factors
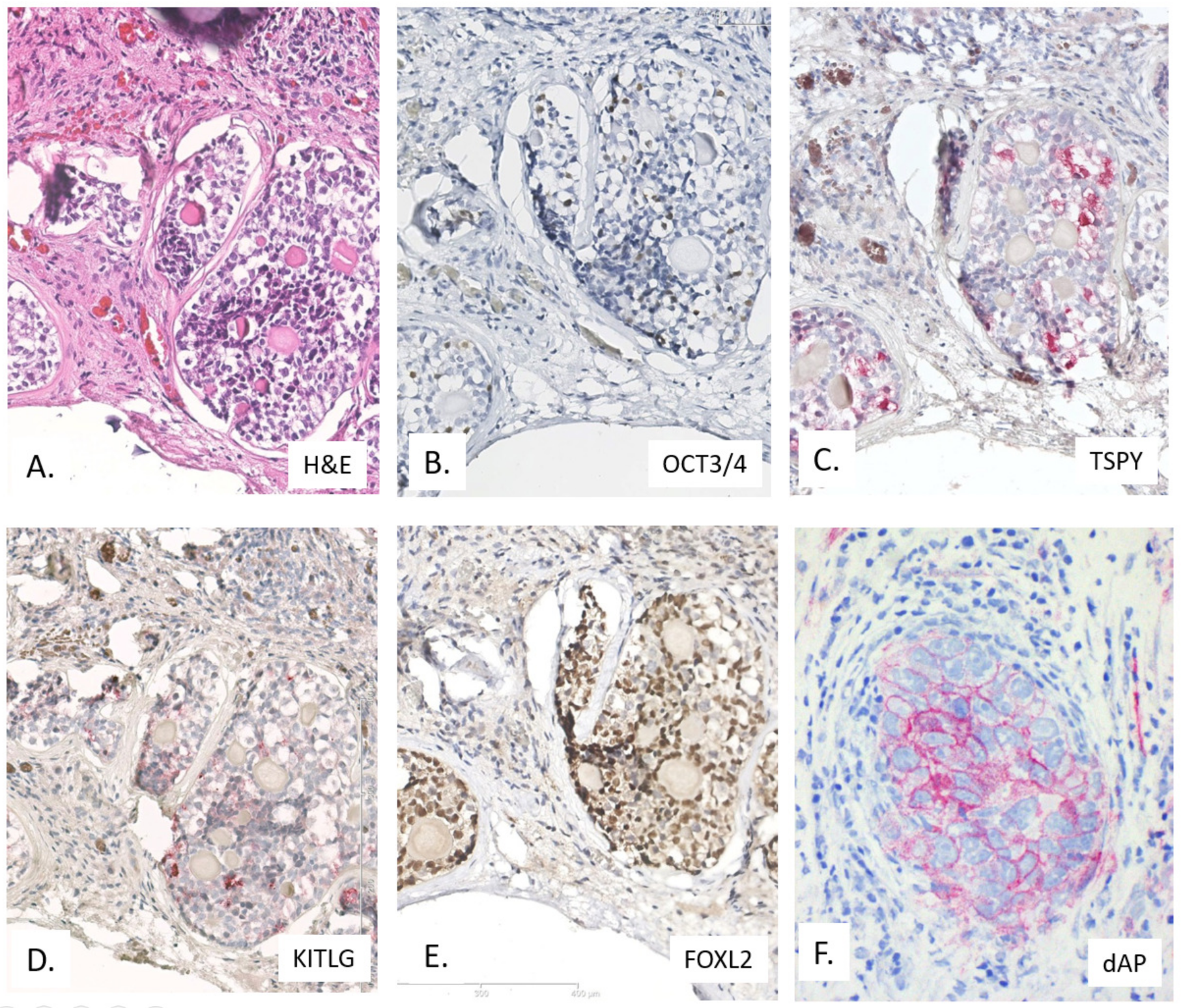
4.3.2. Immunohistochemistry
4.3.3. Molecular Genetic Constitution
5. Application of miR-371a-3p as Molecular Biomarker for Malignant GCTs in Liquid Biopsies
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Alpha Fetoprotein |
| CIS | Carcinoma In Situ |
| CNV | copy number variations |
| DSD | Disorders of Sex Development |
| EC | embryonal carcinoma |
| (F)ISH | (Fluorescent) In Situ Hybridization |
| GB | Gonadoblastoma |
| GBY | Gonadoblastoma on the Y chromosome |
| GCNIS | Germ cell neoplasia in situ |
| GCTs | Germ Cell Tumors |
| GGCC | gonadal germ cell cancer |
| GWAS | Genome wide association studies |
| hCG | human ChorioGonadotropin |
| IGCNU | Intratubular Germ Cell Neoplasia, Unclassified |
| PLAP | Placental like alkaline phosphatase |
| SE | seminoma |
| SNP | Single Nucleotide Polymorphism |
| TE | teratoma |
| TSPY | Testis specific protein on the Y chromosome |
| TIN | Testicular Intratubular Neoplasia |
| YST | yolk sac tumor |
| WHO | World Health Organization |
References
- Rosai, J.; Ackerman, L.V. Surgical Pathology, 9th ed.; Rosai & Ackerman’s Surgical Pathology: Edinburgh, UK; New York, NY, USA, 2004; p. 3080. [Google Scholar]
- Oosterhuis, J.W.; Looijenga, L.H. Testicular germ-cell tumours in a broader perspective. Nat. Rev. Cancer 2005, 5, 210–222. [Google Scholar] [CrossRef]
- Oosterhuis, J.W.; Looijenga, L.H.J. Germ cell tumors from a developmental perspective. Nat. Rev. Cancer 2019, 19, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 who classification of tumours of the urinary system and male genital organs-part a: Renal, penile, and testicular tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Humphrey, P.A.; Ulbright, T.M.; Reuter, V.E. WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed.; IARC: Lyon, France, 2016. [Google Scholar]
- Cheng, L.; Albers, P.; Berney, D.M.; Feldman, D.R.; Daugaard, G.; Gilligan, T.; Looijenga, L.H.J. Testicular cancer. Nat. Rev. Dis. Primers 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Looijenga, L.H. Testicular germ cell tumors. Pediatr. Endocrinol. Rev. 2014, 11 (Suppl. 2), 251–262. [Google Scholar]
- Youngren, K.K.; Coveney, D.; Peng, X.; Bhattacharya, C.; Schmidt, L.S.; Nickerson, M.L.; Lamb, B.T.; Deng, J.M.; Behringer, R.R.; Capel, B.; et al. The ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature 2005, 435, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Salz, H.K.; Dawson, E.P.; Heaney, J.D. Germ cell tumors: Insights from the drosophila ovary and the mouse testis. Mol. Reprod. Dev. 2017, 84, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Zechel, J.L.; MacLennan, G.T.; Heaney, J.D.; Nadeau, J.H. Spontaneous metastasis in mouse models of testicular germ-cell tumours. Int. J. Androl. 2011, 34, e278–e287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Berlo, R.J.; Oosterhuis, J.W.; Schrijnemakers, E.; Schoots, C.J.; de Jong, B.; Damjanov, I. Yolk-sac carcinoma develops spontaneously as a late occurrence in slow-growing teratoid tumors produced from transplanted 7-day mouse embryos. Int. J. Cancer 1990, 45, 153–155. [Google Scholar] [CrossRef]
- Looijenga, L.H.; Olie, R.A.; van der Gaag, I.; van Sluijs, F.J.; Matoska, J.; Ploem-Zaaijer, J.; Knepfle, C.; Oosterhuis, J.W. Seminomas of the canine testis. Counterpart of spermatocytic seminoma of men? Lab. Investig. 1994, 71, 490–496. [Google Scholar]
- Meng, X.; de Rooij, D.G.; Westerdahl, K.; Saarma, M.; Sariola, H. Promotion of seminomatous tumors by targeted overexpression of glial cell line-derived neurotrophic factor in mouse testis. Cancer Res. 2001, 61, 3267–3271. [Google Scholar] [PubMed]
- Sariola, H.; Meng, X. Gdnf-induced seminomatous tumours in mouse—An experimental model for human seminomas? APMIS 2003, 111, 192–196, discussion 196. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, H.; Lee, J.; Tanaka, T.; Ishii, K.; Toyokuni, S.; Kanatsu-Shinohara, M.; Shinohara, T. In vitro transformation of mouse testis cells by oncogene transfection. Biol. Reprod. 2012, 86, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pierpont, T.M.; Lyndaker, A.M.; Anderson, C.M.; Jin, Q.; Moore, E.S.; Roden, J.L.; Braxton, A.; Bagepalli, L.; Kataria, N.; Hu, H.Z.; et al. Chemotherapy-induced depletion of oct4-positive cancer stem cells in a mouse model of malignant testicular cancer. Cell Rep. 2017, 21, 1896–1909. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.C.; Chandler, G.L.; Damoulis, V.A.; Fustino, N.J.; Lillard, K.; Looijenga, L.; Margraf, L.; Rakheja, D.; Amatruda, J.F. Mutation in the type ib bone morphogenetic protein receptor alk6b impairs germ-cell differentiation and causes germ-cell tumors in zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 13153–13158. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.C.; Lillard, K.; Damoulis, V.; Amatruda, J.F. Zebrafish models of germ cell tumor. Methods Cell Biol. 2011, 105, 3–24. [Google Scholar] [PubMed]
- Basten, S.G.; Davis, E.E.; Gillis, A.J.; van Rooijen, E.; Stoop, H.; Babala, N.; Logister, I.; Heath, Z.G.; Jonges, T.N.; Katsanis, N.; et al. Mutations in lrrc50 predispose zebrafish and humans to seminomas. PLoS Genet. 2013, 9, e1003384. [Google Scholar] [CrossRef]
- Amatruda, J.F.; Ross, J.A.; Christensen, B.; Fustino, N.J.; Chen, K.S.; Hooten, A.J.; Nelson, H.; Kuriger, J.K.; Rakheja, D.; Frazier, A.L.; et al. DNA methylation analysis reveals distinct methylation signatures in pediatric germ cell tumors. BMC Cancer 2013, 13, 313. [Google Scholar] [CrossRef]
- Oosterhuis, J.; Looijenga, L.H.J. Germ, cell tumors: Pathology and genetics. In Encyclopedia of Cancer, 3rd ed.; Boffetta, P., Hainaut, P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 2, pp. 121–153. [Google Scholar]
- Schneider, D.T.; Calaminus, G.; Koch, S.; Teske, C.; Schmidt, P.; Haas, R.J.; Harms, D.; Gobel, U. Epidemiologic analysis of 1,442 children and adolescents registered in the german germ cell tumor protocols. Pediatr. Blood Cancer 2004, 42, 169–175. [Google Scholar] [CrossRef]
- Stang, A.; Trabert, B.; Wentzensen, N.; Cook, M.B.; Rusner, C.; Oosterhuis, J.W.; McGlynn, K.A. Gonadal and extragonadal germ cell tumours in the united states, 1973–2007. Int. J. Androl. 2012, 35, 616–625. [Google Scholar] [CrossRef]
- Poynter, J.N.; Amatruda, J.F.; Ross, J.A. Trends in incidence and survival of pediatric and adolescent patients with germ cell tumors in the united states, 1975 to 2006. Cancer 2010, 116, 4882–4891. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, A.K.; Spector, L.G.; Fortuna, G.; Marcotte, E.L.; Poynter, J.N. Trends in international incidence of pediatric cancers in children under 5 years of age: 1988–2012. JNCI Cancer Spectr. 2019, 3, pkz007. [Google Scholar] [CrossRef] [PubMed]
- Kusler, K.A.; Poynter, J.N. International testicular cancer incidence rates in children, adolescents and young adults. Cancer Epidemiol. 2018, 56, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis, J.W.; Stoop, J.A.; Rijlaarsdam, M.A.; Biermann, K.; Smit, V.T.; Hersmus, R.; Looijenga, L.H. Pediatric germ cell tumors presenting beyond childhood? Andrology 2015, 3, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Berney, D.M.; Hirsch, M.S.; Cheng, L.; Ulbright, T.M. Evidence supporting the existence of benign teratomas of the postpubertal testis: A clinical, histopathologic, and molecular genetic analysis of 25 cases. Am. J. Surg Pathol 2013, 37, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Harms, D.; Zahn, S.; Gobel, U.; Schneider, D.T. Pathology and molecular biology of teratomas in childhood and adolescence. Klin. Padiatr. 2006, 218, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Ulbright, T.M.; Tickoo, S.K.; Berney, D.M.; Srigley, J.R.; Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in testicular tumors: Report from the international society of urological pathology consensus conference. Am. J. Surg Pathol. 2014, 38, e50–e59. [Google Scholar] [PubMed]
- Oosterhuis, J.W.; Looijenga, L.H.; van Echten, J.; de Jong, B. Chromosomal constitution and developmental potential of human germ cell tumors and teratomas. Cancer Genet. Cytogenet. 1997, 95, 96–102. [Google Scholar] [CrossRef]
- Cornejo, K.M.; Cheng, L.; Church, A.; Wang, M.; Jiang, Z. Chromosome 12p abnormalities and imp3 expression in prepubertal pure testicular teratomas. Hum. Pathol 2016, 49, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Mostert, M.; Rosenberg, C.; Stoop, H.; Schuyer, M.; Timmer, A.; Oosterhuis, W.; Looijenga, L. Comparative genomic and in situ hybridization of germ cell tumors of the infantile testis. Lab. Investig. 2000, 80, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Linger, R.; Dudakia, D.; Huddart, R.; Tucker, K.; Friedlander, M.; Phillips, K.A.; Hogg, D.; Jewett, M.A.; Lohynska, R.; Daugaard, G.; et al. Analysis of the dnd1 gene in men with sporadic and familial testicular germ cell tumors. Genes Chromosomes Cancer 2008, 47, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Perlman, E.J.; Hu, J.; Ho, D.; Cushing, B.; Lauer, S.; Castleberry, R.P. Genetic analysis of childhood endodermal sinus tumors by comparative genomic hybridization. J. Pediatr. Hematol Oncol. 2000, 22, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Perlman, E.J.; Valentine, M.B.; Griffin, C.A.; Look, A.T. Deletion of 1p36 in childhood endodermal sinus tumors by two-color fluorescence in situ hybridization: A pediatric oncology group study. Genes Chromosomes Cancer 1996, 16, 15–20. [Google Scholar] [CrossRef]
- Mostert, M.M.; van de Pol, M.; van Echten, J.; Olde Weghuis, D.; Geurts van Kessel, A.; Oosterhuis, J.W.; Looijenga, L.H. Fluorescence in situ hybridization-based approaches for detection of 12p overrepresentation, in particular i(12p), in cell lines of human testicular germ cell tumors of adults. Cancer Genet. Cytogenet. 1996, 87, 95–102. [Google Scholar] [CrossRef][Green Version]
- Looijenga, L.H.; Rosenberg, C.; van Gurp, R.J.; Geelen, E.; van Echten-Arends, J.; de Jong, B.; Mostert, M.; Wolter Oosterhuis, J. Comparative genomic hybridization of microdissected samples from different stages in the development of a seminoma and a non-seminoma. J. Pathol. 2000, 191, 187–192. [Google Scholar] [CrossRef]
- Killian, J.K.; Dorssers, L.C.; Trabert, B.; Gillis, A.J.; Cook, M.B.; Wang, Y.; Waterfall, J.J.; Stevenson, H.; Smith, W.I., Jr.; Noyes, N. Imprints and DPPA3 are bypassed during pluripotency-and differentiation-coupled methylation reprogramming in testicular germ cell tumors. Genome Res. 2016, 26, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Malek, N.P.; Casper, J.; Looijenga, L.H.; Strohmeyer, T.; Schmoll, H.J.; Nordheim, A.; Janknecht, R. Quantification of additional short arms of chromosome 12 in germ cell tumours using the polymerase chain reaction. Eur J. Cancer 1997, 33, 1488–1494. [Google Scholar] [CrossRef]
- LeBron, C.; Pal, P.; Brait, M.; Dasgupta, S.; Guerrero-Preston, R.; Looijenga, L.H.; Kowalski, J.; Netto, G.; Hoque, M.O. Genome-wide analysis of genetic alterations in testicular primary seminoma using high resolution single nucleotide polymorphism arrays. Genomics 2011, 97, 341–349. [Google Scholar] [CrossRef]
- Poynter, J.N.; Hooten, A.J.; Frazier, A.L.; Ross, J.A. Associations between variants in kitlg, spry4, bak1, and dmrt1 and pediatric germ cell tumors. Genes Chromosomes Cancer 2012, 51, 266–271. [Google Scholar] [CrossRef]
- Marcotte, E.L.; Pankratz, N.; Amatruda, J.F.; Frazier, A.L.; Krailo, M.; Davies, S.; Starr, J.R.; Lau, C.C.; Roesler, M.; Langer, E.; et al. Variants in bak1, spry4, and gab2 are associated with pediatric germ cell tumors: A report from the children’s oncology group. Genes Chromosomes Cancer 2017, 56, 548–558. [Google Scholar] [CrossRef]
- Sakumi, K. Germline mutation: De novo mutation in reproductive lineage cells. Genes Genet. Syst. 2019, 94, 3–12. [Google Scholar] [CrossRef]
- Ju, Y.S.; Martincorena, I.; Gerstung, M.; Petljak, M.; Alexandrov, L.B.; Rahbari, R.; Wedge, D.C.; Davies, H.R.; Ramakrishna, M.; Fullam, A.; et al. Somatic mutations reveal asymmetric cellular dynamics in the early human embryo. Nature 2017, 543, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Moorjani, P.; Gao, Z.; Przeworski, M. Human germline mutation and the erratic evolutionary clock. PLoS Biol. 2016, 14, e2000744. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis, J.W.; Looijenga, L.H.J. Pathology and Biology of Human Germ Cell Tumors; Nogales, J.R.E., Ed.; Springer: Berlin, Germany, 2017; pp. 23–129. [Google Scholar]
- Idrees, M.T.; Ulbright, T.M.; Oliva, E.; Young, R.H.; Montironi, R.; Egevad, L.; Berney, D.; Srigley, J.R.; Epstein, J.I.; Tickoo, S.K.; et al. The world health organization 2016 classification of testicular non-germ cell tumours: A review and update from the international society of urological pathology testis consultation panel. Histopathology 2017, 70, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, M.K.; Schneider, D.T.; Schuster, A.E.; Murdoch, F.E.; Perlman, E.J. Activation of wnt/beta-catenin signaling in distinct histologic subtypes of human germ cell tumors. Pediatr. Dev. Pathol. 2006, 9, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Fustino, N.; Rakheja, D.; Ateek, C.S.; Neumann, J.C.; Amatruda, J.F. Bone morphogenetic protein signalling activity distinguishes histological subsets of paediatric germ cell tumours. Int. J. Androl. 2011, 34, e218–e233. [Google Scholar] [CrossRef]
- Pfankuchen, D.B.; Baltes, F.; Batool, T.; Li, J.P.; Schlesinger, M.; Bendas, G. Heparin antagonizes cisplatin resistance of a2780 ovarian cancer cells by affecting the wnt signaling pathway. Oncotarget 2017, 8, 67553–67566. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Pfankuchen, D.B.; Wantoch von Rekowski, K.; Schlesinger, M.; Reipsch, F.; Bendas, G. The impact of the low molecular weight heparin tinzaparin on the sensitization of cisplatin-resistant ovarian cancers-preclinical in vivo evaluation in xenograft tumor models. Molecules 2017, 22, 728. [Google Scholar] [CrossRef] [PubMed]
- Looijenga, L.H.; Stoop, H.; Hersmus, R.; Gillis, A.J.; Wolter Oosterhuis, J. Genomic and expression profiling of human spermatocytic seminomas: Pathogenetic implications. Int. J. Androl. 2007, 30, 328–335, discussion 335–326. [Google Scholar] [CrossRef]
- Scully, R.E. Spermatocytic seminoma of the testis. A report of 3 cases and review of the literature. Cancer 1961, 14, 788–794. [Google Scholar] [CrossRef]
- Barr, W.B., Jr.; Silberg, S. A case report and review of the literature on spermatocytic seminoma of the testis. J. Urol. 1963, 89, 464–466. [Google Scholar] [CrossRef]
- Rosai, J.; Silber, I.; Khodadoust, K. Spermatocytic seminoma. I. Clinicopathologic study of six cases and review of the literature. Cancer 1969, 24, 92–102. [Google Scholar] [CrossRef]
- Oosterhuis, J.W.; Castedo, S.M.; de Jong, B.; Cornelisse, C.J.; Dam, A.; Sleijfer, D.T.; Schraffordt Koops, H. Ploidy of primary germ cell tumors of the testis. Pathogenetic and clinical relevance. Lab. Investig. 1989, 60, 14–21. [Google Scholar] [PubMed]
- Pendlebury, S.; Horwich, A.; Dearnaley, D.P.; Nicholls, J.; Fisher, C. Spermatocytic seminoma: A clinicopathological review of ten patients. Clin. Oncol. 1996, 8, 316–318. [Google Scholar] [CrossRef]
- Looijenga, L.H.; Oosterhuis, J.W. Pathogenesis of testicular germ cell tumours. Rev. Reprod. 1999, 4, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.W.; Bayley, A.J.; Sweet, J.; Jewett, M.A.; Tew-George, B.; Gospodarowicz, M.K.; Warde, P.R. Spermatocytic seminoma: A review. Eur. Urol. 2004, 45, 495–498. [Google Scholar] [CrossRef]
- Looijenga, L.H.; Hersmus, R.; Gillis, A.J.; Pfundt, R.; Stoop, H.J.; van Gurp, R.J.; Veltman, J.; Beverloo, H.B.; van Drunen, E.; van Kessel, A.G.; et al. Genomic and expression profiling of human spermatocytic seminomas: Primary spermatocyte as tumorigenic precursor and dmrt1 as candidate chromosome 9 gene. Cancer Res. 2006, 66, 290–302. [Google Scholar] [CrossRef]
- Looijenga, L.H.; Gillis, A.J.; Stoop, H.J.; Hersmus, R.; Oosterhuis, J.W. Chromosomes and expression in human testicular germ-cell tumors: Insight into their cell of origin and pathogenesis. Ann. N. Y. Acad. Sci. 2007, 1120, 187–214. [Google Scholar] [CrossRef]
- Looijenga, L.H. Spermatocytic seminoma: Toward further understanding of pathogenesis. J. Pathol. 2011, 224, 431–433. [Google Scholar] [CrossRef]
- Lombardi, M.; Valli, M.; Brisigotti, M.; Rosai, J. Spermatocytic seminoma: Review of the literature and description of a new case of the anaplastic variant. Int. J. Surg. Pathol. 2011, 19, 5–10. [Google Scholar] [CrossRef]
- Giannoulatou, E.; McVean, G.; Taylor, I.B.; McGowan, S.J.; Maher, G.J.; Iqbal, Z.; Pfeifer, S.P.; Turner, I.; Burkitt Wright, E.M.; Shorto, J.; et al. Contributions of intrinsic mutation rate and selfish selection to levels of de novo hras mutations in the paternal germline. Proc. Natl. Acad. Sci. USA 2013, 110, 20152–20157. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.S.; Badve, S.S.; Ulbright, T.M. The utility of immunostaining for nut, gage7 and ny-eso-1 in the diagnosis of spermatocytic seminoma. Histopathology 2014, 65, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Gentile, G.; Giunchi, F.; Schiavina, R.; Franceschelli, A.; Borghesi, M.; Zukerman, Z.; Cevenini, M.; Vagnoni, V.; Romagnoli, D.; Colombo, F.; et al. First case of bilateral, synchronous anaplastic variant of spermatocytic seminoma treated with radical orchifunicolectomy as single approach: Case report and review of the literature. Arch. Ital. Urol. Androl. 2014, 86, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Giannoulatou, E.; Maher, G.J.; Ding, Z.; Gillis, A.J.M.; Dorssers, L.C.J.; Hoischen, A.; Rajpert-De Meyts, E.; Consortium, W.G.S.; McVean, G.; Wilkie, A.O.M.; et al. Whole-genome sequencing of spermatocytic tumors provides insights into the mutational processes operating in the male germline. PLoS ONE 2017, 12, e0178169. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Karpate, A.; Desai, S. Spermatocytic seminoma with rhabdomyosarcomatous differentiation: A case report with a review of the literature. J. Cancer Res. Ther. 2009, 5, 213–215. [Google Scholar]
- Pandey, V.; Khatib, Y.; Khade, A.L.; Pandey, R.; Khare, M.S. Spermatocytic seminoma with rhabdomyoblastic differentiation: Case report and review of literature. Indian J. Pathol. Microbiol. 2018, 61, 437–439. [Google Scholar] [PubMed]
- Stoop, H.; van Gurp, R.; de Krijger, R.; Geurts van Kessel, A.; Köberle, B.; Oosterhuis, W.; Looijenga, L. Reactivity of germ cell maturation stage-specific markers in spermatocytic seminoma: Diagnostic and etiological implications. Lab. Investig. 2001, 81, 919–928. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajpert-De Meyts, E.; Jacobsen, G.K.; Bartkova, J.; Aubry, F.; Samson, M.; Bartek, J.; Skakkebaek, N.E. The immunohistochemical expression pattern of chk2, p53, p19ink4d, mage-a4 and other selected antigens provides new evidence for the premeiotic origin of spermatocytic seminoma. Histopathology 2003, 42, 217–226. [Google Scholar] [CrossRef]
- Lim, J.; Goriely, A.; Turner, G.D.; Ewen, K.A.; Jacobsen, G.K.; Graem, N.; Wilkie, A.O.; Rajpert-De Meyts, E. Oct2, ssx and sage1 reveal the phenotypic heterogeneity of spermatocytic seminoma reflecting distinct subpopulations of spermatogonia. J. Pathol. 2011, 224, 473–483. [Google Scholar] [CrossRef]
- Krentz, A.D.; Murphy, M.W.; Kim, S.; Cook, M.S.; Capel, B.; Zhu, R.; Matin, A.; Sarver, A.L.; Parker, K.L.; Griswold, M.D.; et al. The dm domain protein dmrt1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc. Natl. Acad. Sci. USA 2009, 106, 22323–22328. [Google Scholar] [CrossRef]
- Rosenberg, C.; Mostert, M.C.; Schut, T.B.; van de Pol, M.; van Echten, J.; de Jong, B.; Raap, A.K.; Tanke, H.; Oosterhuis, J.W.; Looijenga, L.H. Chromosomal constitution of human spermatocytic seminomas: Comparative genomic hybridization supported by conventional and interphase cytogenetics. Genes Chromosomes Cancer 1998, 23, 286–291. [Google Scholar] [CrossRef]
- Lim, J.; Maher, G.J.; Turner, G.D.; Dudka-Ruszkowska, W.; Taylor, S.; Rajpert-De Meyts, E.; Goriely, A.; Wilkie, A.O. Selfish spermatogonial selection: Evidence from an immunohistochemical screen in testes of elderly men. PLoS ONE 2012, 7, e42382. [Google Scholar] [CrossRef] [PubMed]
- Maher, G.J.; Goriely, A.; Wilkie, A.O. Cellular evidence for selfish spermatogonial selection in aged human testes. Andrology 2014, 2, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Maher, G.J.; Ralph, H.K.; Ding, Z.; Koelling, N.; Mlcochova, H.; Giannoulatou, E.; Dhami, P.; Paul, D.S.; Stricker, S.H.; Beck, S.; et al. Selfish mutations dysregulating ras-mapk signaling are pervasive in aged human testes. Genome Res. 2018, 28, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.E. Possible carcinoma-in-situ of the testis. Lancet 1972, 2, 516–517. [Google Scholar] [CrossRef]
- Berney, D.M.; Looijenga, L.H.; Idrees, M.; Oosterhuis, J.W.; Rajpert-De Meyts, E.; Ulbright, T.M.; Skakkebaek, N.E. Germ cell neoplasia in situ (gcnis): Evolution of the current nomenclature for testicular pre-invasive germ cell malignancy. Histopathology 2016, 69, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Honecker, F.; Stoop, H.; Mayer, F.; Bokemeyer, C.; Castrillon, D.H.; Lau, Y.F.; Looijenga, L.H.; Oosterhuis, J.W. Germ cell lineage differentiation in non-seminomatous germ cell tumours. J. Pathol. 2006, 208, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Cools, M.; Drop, S.L.; Wolffenbuttel, K.P.; Oosterhuis, J.W.; Looijenga, L.H. Germ cell tumors in the intersex gonad: Old paths, new directions, moving frontiers. Endocr. Rev. 2006, 27, 468–484. [Google Scholar] [CrossRef]
- Ng, S.B.; Yong, M.H.; Knight, L.A.; Lee, V.K.; Nadarajah, S.; Stoop, H.; Looijenga, L.H. Gonadoblastoma-associated mixed germ cell tumour in 46,xy complete gonadal dysgenesis (swyer syndrome): Analysis of y chromosomal genotype and oct3/4 and tspy expression profile. Histopathology 2008, 52, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Hersmus, R.; Stoop, H.; White, S.J.; Drop, S.L.; Oosterhuis, J.W.; Incrocci, L.; Wolffenbuttel, K.P.; Looijenga, L.H. Delayed recognition of disorders of sex development (dsd): A missed opportunity for early diagnosis of malignant germ cell tumors. Int. J. Endocrinol. 2012, 2012, 671209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ulbright, T.M.; Young, R.H. Gonadoblastoma and selected other aspects of gonadal pathology in young patients with disorders of sex development. Semin. Diagn. Pathol. 2014, 31, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.S.; Ulbright, T.M.; Idrees, M.T. Gonadoblastoma: An immunohistochemical study and comparison to sertoli cell nodule with intratubular germ cell neoplasia, with pathogenetic implications. Histopathology 2014, 65, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwan, Y.G.; Biermann, K.; Wolffenbuttel, K.P.; Cools, M.; Looijenga, L.H. Gonadal maldevelopment as risk factor for germ cell cancer: Towards a clinical decision model. Eur. Urol. 2015, 67, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Hersmus, R.; Kalfa, N.; de Leeuw, B.; Stoop, H.; Oosterhuis, J.W.; de Krijger, R.; Wolffenbuttel, K.P.; Drop, S.L.; Veitia, R.A.; Fellous, M.; et al. Foxl2 and sox9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (dsd). J. Pathol. 2008, 215, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Audi, L.; Ahmed, S.F.; Krone, N.; Cools, M.; McElreavey, K.; Holterhus, P.M.; Greenfield, A.; Bashamboo, A.; Hiort, O.; Wudy, S.A.; et al. Genetics in endocrinology: Approaches to molecular genetic diagnosis in the management of differences/disorders of sex development (dsd): Position paper of eu cost action bm 1303 ‘dsdnet’. Eur. J. Endocrinol. 2018, 179, R197–R206. [Google Scholar] [CrossRef] [PubMed]
- Spoor, J.A.; Oosterhuis, J.W.; Hersmus, R.; Biermann, K.; Wolffenbuttel, K.P.; Cools, M.; Kazmi, Z.; Ahmed, S.F.; Looijenga, L.H.J. Histological assessment of gonads in dsd: Relevance for clinical management. Sex. Dev. 2018, 12, 106–122. [Google Scholar] [CrossRef]
- Dal Cin, P.; Drochmans, A.; Moerman, P.; Van den Berghe, H. Isochromosome 12p in mediastinal germ cell tumor. Cancer Genet. Cytogenet. 1989, 42, 243–251. [Google Scholar]
- Sung, M.T.; Maclennan, G.T.; Lopez-Beltran, A.; Zhang, S.; Montironi, R.; Cheng, L. Primary mediastinal seminoma: A comprehensive assessment integrated with histology, immunohistochemistry, and fluorescence in situ hybridization for chromosome 12p abnormalities in 23 cases. Am. J. Surg. Pathol. 2008, 32, 146–155. [Google Scholar] [CrossRef]
- Batool, A.; Karimi, N.; Wu, X.N.; Chen, S.R.; Liu, Y.X. Testicular germ cell tumor: A comprehensive review. Cell Mol. Life Sci. 2019, 76, 1713–1727. [Google Scholar] [CrossRef]
- Looijenga, L.H.; Hersmus, R.; Oosterhuis, J.W.; Cools, M.; Drop, S.L.; Wolffenbuttel, K.P. Tumor risk in disorders of sex development (dsd). Best Pract Res. Clin. Endocrinol. Metab. 2007, 21, 480–495. [Google Scholar] [CrossRef]
- Pleskacova, J.; Hersmus, R.; Oosterhuis, J.W.; Setyawati, B.A.; Faradz, S.M.; Cools, M.; Wolffenbuttel, K.P.; Lebl, J.; Drop, S.L.; Looijenga, L.H. Tumor risk in disorders of sex development. Sex. Dev. 2010, 4, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Looijenga, L.H.; Hersmus, R.; de Leeuw, B.H.; Stoop, H.; Cools, M.; Oosterhuis, J.W.; Drop, S.L.; Wolffenbuttel, K.P. Gonadal tumours and dsd. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Juniarto, A.Z.; Setyawati, B.A.; Miranti, I.P.; Santosa, A.; Hersmus, R.; Stoop, H.; Cools, M.; Oosterhuis, J.W.; Drop, S.L.; Faradz, S.M.; et al. Gonadal malignancy in 13 consecutive collected patients with disorders of sex development (dsd) from semarang (indonesia). J. Clin. Pathol. 2013, 66, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Cools, M. Germ cell cancer risk in dsd patients. Ann. Endocrinol. (Paris) 2014, 75, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Wolffenbuttel, K.P.; Hersmus, R.; Stoop, H.; Biermann, K.; Hoebeke, P.; Cools, M.; Looijenga, L.H. Gonadal dysgenesis in disorders of sex development: Diagnosis and surgical management. J. Pediatr. Urol. 2016, 12, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.A.; Nordenström, A.; Houk, C.P.; Ahmed, S.F.; Auchus, R.; Baratz, A.; Baratz Dalke, K.; Liao, L.M.; Lin-Su, K.; Looijenga, L.H.; et al. Global disorders of sex development update since 2006: Perceptions, approach and care. Horm. Res. Paediatr. 2016, 85, 158–180. [Google Scholar] [CrossRef] [PubMed]
- Hersmus, R.; van Bever, Y.; Wolffenbuttel, K.P.; Biermann, K.; Cools, M.; Looijenga, L.H. The biology of germ cell tumors in disorders of sex development. Clin. Genet. 2017, 91, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Wermann, H.; Stoop, H.; Gillis, A.J.; Honecker, F.; van Gurp, R.J.; Ammerpohl, O.; Richter, J.; Oosterhuis, J.W.; Bokemeyer, C.; Looijenga, L.H. Global DNA methylation in fetal human germ cells and germ cell tumours: Association with differentiation and cisplatin resistance. J. Pathol. 2010, 221, 433–442. [Google Scholar] [CrossRef]
- Netto, G.J.; Nakai, Y.; Nakayama, M.; Jadallah, S.; Toubaji, A.; Nonomura, N.; Albadine, R.; Hicks, J.L.; Epstein, J.I.; Yegnasubramanian, S.; et al. Global DNA hypomethylation in intratubular germ cell neoplasia and seminoma, but not in nonseminomatous male germ cell tumors. Mod. Pathol. 2008, 21, 1337–1344. [Google Scholar] [CrossRef]
- Kristensen, D.G.; Nielsen, J.E.; Jorgensen, A.; Skakkebaek, N.E.; Rajpert-De Meyts, E.; Almstrup, K. Evidence that active demethylation mechanisms maintain the genome of carcinoma in situ cells hypomethylated in the adult testis. Br. J. Cancer 2014, 110, 668–678. [Google Scholar] [CrossRef]
- Novotny, G.W.; Belling, K.C.; Bramsen, J.B.; Nielsen, J.E.; Bork-Jensen, J.; Almstrup, K.; Sonne, S.B.; Kjems, J.; Rajpert-De Meyts, E.; Leffers, H. Microrna expression profiling of carcinoma in situ cells of the testis. Endocr. Relat. Cancer 2012, 19, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Rajpert-De Meyts, E.; Skakkebaek, N.E. Pathogenesis of testicular carcinoma in situ and germ cell cancer: Still more questions than answers. Int. J. Androl. 2011, 34, e2–e6. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sung, M.T.; Cossu-Rocca, P.; Jones, T.D.; MacLennan, G.T.; De Jong, J.; Lopez-Beltran, A.; Montironi, R.; Looijenga, L.H. Oct4: Biological functions and clinical applications as a marker of germ cell neoplasia. J. Pathol. 2007, 211, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Jong, J.; Looijenga, L.H. Stem cell marker oct3/4 in tumor biology and germ cell tumor diagnostics: History and future. Crit. Rev. Oncog. 2006, 12, 171–203. [Google Scholar] [CrossRef] [PubMed]
- Looijenga, L.H.; Stoop, H.; de Leeuw, H.P.; de Gouveia Brazao, C.A.; Gillis, A.J.; van Roozendaal, K.E.; van Zoelen, E.J.; Weber, R.F.; Wolffenbuttel, K.P.; van Dekken, H.; et al. Pou5f1 (oct3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003, 63, 2244–2250. [Google Scholar] [PubMed]
- Biermann, K.; Stoop, H.; Looijenga, L. C-kit protein expression does not discriminate neoplastic from non-neoplastic intratubular germ cells. Histopathology 2012, 60, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Stoop, H.; Kirkels, W.; Dohle, G.R.; Gillis, A.J.; den Bakker, M.A.; Biermann, K.; Oosterhuis, W.; Looijenga, L.H. Diagnosis of testicular carcinoma in situ ‘(intratubular and microinvasive)’ seminoma and embryonal carcinoma using direct enzymatic alkaline phosphatase reactivity on frozen histological sections. Histopathology 2011, 58, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Stoop, H.; Honecker, F.; van de Geijn, G.J.; Gillis, A.J.; Cools, M.C.; de Boer, M.; Bokemeyer, C.; Wolffenbuttel, K.P.; Drop, S.L.; de Krijger, R.R.; et al. Stem cell factor as a novel diagnostic marker for early malignant germ cells. J. Pathol. 2008, 216, 43–54. [Google Scholar] [CrossRef] [PubMed]
- De Jong, J.; Stoop, H.; Gillis, A.J.; van Gurp, R.J.; van de Geijn, G.J.; Boer, M.; Hersmus, R.; Saunders, P.T.; Anderson, R.A.; Oosterhuis, J.W.; et al. Differential expression of sox17 and sox2 in germ cells and stem cells has biological and clinical implications. J. Pathol. 2008, 215, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Di Vizio, D.; Cito, L.; Boccia, A.; Chieffi, P.; Insabato, L.; Pettinato, G.; Motti, M.L.; Schepis, F.; D’Amico, W.; Fabiani, F.; et al. Loss of the tumor suppressor gene pten marks the transition from intratubular germ cell neoplasias (itgcn) to invasive germ cell tumors. Oncogene 2005, 24, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, C.; Rapley, E.A.; Seal, S.; Pernet, D.; Renwick, A.; Hughes, D.; Ricketts, M.; Linger, R.; Nsengimana, J.; Deloukas, P.; et al. Variants near dmrt1, tert and atf7ip are associated with testicular germ cell cancer. Nat. Genet. 2010, 42, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Rapley, E.A.; Nathanson, K.L. Predisposition alleles for testicular germ cell tumour. Curr. Opin. Genet. Dev. 2010, 20, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, K.; Loveday, C.; Levy, M.; Dudakia, D.; Rapley, E.; Nsengimana, J.; Bishop, D.T.; Reid, A.; Huddart, R.; Broderick, P.; et al. Large-scale sequencing of testicular germ cell tumour (tgct) cases excludes major tgct predisposition gene. Eur. Urol. 2018, 73, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; McGlynn, K.A.; Rajpert-De Meyts, E.; Bishop, D.T.; Chung, C.C.; Dalgaard, M.D.; Greene, M.H.; Gupta, R.; Grotmol, T.; Haugen, T.B.; et al. Meta-analysis of five genome-wide association studies identifies multiple new loci associated with testicular germ cell tumor. Nat. Genet. 2017, 49, 1141–1147. [Google Scholar] [CrossRef]
- Litchfield, K.; Levy, M.; Orlando, G.; Loveday, C.; Law, P.J.; Migliorini, G.; Holroyd, A.; Broderick, P.; Karlsson, R.; Haugen, T.B.; et al. Identification of 19 new risk loci and potential regulatory mechanisms influencing susceptibility to testicular germ cell tumor. Nat. Genet. 2017, 49, 1133–1140. [Google Scholar] [CrossRef]
- Kanetsky, P.A.; Mitra, N.; Vardhanabhuti, S.; Li, M.; Vaughn, D.J.; Letrero, R.; Ciosek, S.L.; Doody, D.R.; Smith, L.M.; Weaver, J.; et al. Common variation in kitlg and at 5q31.3 predisposes to testicular germ cell cancer. Nat. Genet. 2009, 41, 811–815. [Google Scholar] [CrossRef]
- Mayer, F.; Gillis, A.J.; Dinjens, W.; Oosterhuis, J.W.; Bokemeyer, C.; Looijenga, L.H. Microsatellite instability of germ cell tumors is associated with resistance to systemic treatment. Cancer Res. 2002, 62, 2758–2760. [Google Scholar]
- Litchfield, K.; Levy, M.; Dudakia, D.; Proszek, P.; Shipley, C.; Basten, S.; Rapley, E.; Bishop, D.T.; Reid, A.; Huddart, R.; et al. Rare disruptive mutations in ciliary function genes contribute to testicular cancer susceptibility. Nat. Commun. 2016, 7, 13840. [Google Scholar] [CrossRef]
- Cools, M.; Wolffenbuttel, K.P.; Hersmus, R.; Mendonca, B.B.; Kaprová, J.; Drop, S.L.S.; Stoop, H.; Gillis, A.J.M.; Oosterhuis, J.W.; Costa, E.M.F.; et al. Malignant testicular germ cell tumors in postpubertal individuals with androgen insensitivity: Prevalence, pathology and relevance of single nucleotide polymorphism-based susceptibility profiling. Hum. Reprod. 2017, 32, 2561–2573. [Google Scholar] [CrossRef]
- Atkin, N.B.; Baker, M.C. Specific chromosome change, i(12p), in testicular tumours? Lancet 1982, 2, 1349. [Google Scholar] [CrossRef]
- Van Echten, J.; Oosterhuis, J.W.; Looijenga, L.H.; van de Pol, M.; Wiersema, J.; te Meerman, G.J.; Schaffordt Koops, H.; Sleijfer, D.T.; de Jong, B. No recurrent structural abnormalities apart from i(12p) in primary germ cell tumors of the adult testis. Genes Chromosomes Cancer 1995, 14, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Mostert, M.M.; van de Pol, M.; Olde Weghuis, D.; Suijkerbuijk, R.F.; Geurts van Kessel, A.; van Echten, J.; Oosterhuis, J.W.; Looijenga, L.H. Comparative genomic hybridization of germ cell tumors of the adult testis: Confirmation of karyotypic findings and identification of a 12p-amplicon. Cancer Genet. Cytogenet. 1996, 89, 146–152. [Google Scholar] [CrossRef]
- Zafarana, G.; Gillis, A.J.; van Gurp, R.J.; Olsson, P.G.; Elstrodt, F.; Stoop, H.; Millán, J.L.; Oosterhuis, J.W.; Looijenga, L.H. Coamplification of dad-r, sox5, and eki1 in human testicular seminomas, with specific overexpression of dad-r, correlates with reduced levels of apoptosis and earlier clinical manifestation. Cancer Res. 2002, 62, 1822–1831. [Google Scholar] [PubMed]
- Clark, A.T.; Rodriguez, R.T.; Bodnar, M.S.; Abeyta, M.J.; Cedars, M.I.; Turek, P.J.; Firpo, M.T.; Reijo Pera, R.A. Human stellar, nanog, and gdf3 genes are expressed in pluripotent cells and map to chromosome 12p13, a hotspot for teratocarcinoma. Stem Cells 2004, 22, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, C.; Van Gurp, R.J.; Geelen, E.; Oosterhuis, J.W.; Looijenga, L.H. Overrepresentation of the short arm of chromosome 12 is related to invasive growth of human testicular seminomas and nonseminomas. Oncogene 2000, 19, 5858–5862. [Google Scholar] [CrossRef] [PubMed]
- Dorssers, L.C.J.; Gillis, A.J.M.; Stoop, H.; van Marion, R.; Nieboer, M.M.; van Riet, J.; van de Werken, H.J.G.; Oosterhuis, J.W.; de Ridder, J.; Looijenga, L.H.J. Molecular heterogeneity and early metastatic clone selection in testicular germ cell cancer development. Br. J. Cancer 2019, 120, 444–452. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Summersgill, B.; Jafer, O.; Rodriguez, S.; Zafarana, G.; Oosterhuis, J.W.; Gillis, A.J.; Looijenga, L.; Cooper, C.; Huddart, R.; et al. Defining minimum genomic regions of imbalance involved in testicular germ cell tumors of adolescents and adults through genome wide microarray analysis of cdna clones. Oncogene 2004, 23, 9142–9147. [Google Scholar] [CrossRef]
- Zafarana, G.; Grygalewicz, B.; Gillis, A.J.; Vissers, L.E.; van de Vliet, W.; van Gurp, R.J.; Stoop, H.; Debiec-Rychter, M.; Oosterhuis, J.W.; van Kessel, A.G.; et al. 12p-amplicon structure analysis in testicular germ cell tumors of adolescents and adults by array cgh. Oncogene 2003, 22, 7695–7701. [Google Scholar] [CrossRef]
- Looijenga, L.H.; Zafarana, G.; Grygalewicz, B.; Summersgill, B.; Debiec-Rychter, M.; Veltman, J.; Schoenmakers, E.F.; Rodriguez, S.; Jafer, O.; Clark, J.; et al. Role of gain of 12p in germ cell tumour development. APMIS 2003, 111, 161–171, discussion 172–163. [Google Scholar] [CrossRef]
- Roelofs, H.; Mostert, M.C.; Pompe, K.; Zafarana, G.; van Oorschot, M.; van Gurp, R.J.; Gillis, A.J.; Stoop, H.; Beverloo, B.; Oosterhuis, J.W.; et al. Restricted 12p amplification and ras mutation in human germ cell tumors of the adult testis. Am. J. Pathol. 2000, 157, 1155–1166. [Google Scholar] [CrossRef]
- Shen, H.; Shih, J.; Hollern, D.P.; Wang, L.; Bowlby, R.; Tickoo, S.K.; Thorsson, V.; Mungall, A.J.; Newton, Y.; Hegde, A.M.; et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep. 2018, 23, 3392–3406. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.; Jafer, O.; Goker, H.; Summersgill, B.M.; Zafarana, G.; Gillis, A.J.; van Gurp, R.J.; Oosterhuis, J.W.; Lu, Y.J.; Huddart, R.; et al. Expression profile of genes from 12p in testicular germ cell tumors of adolescents and adults associated with i(12p) and amplification at 12p11.2-p12.1. Oncogene 2003, 22, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, K.; Summersgill, B.; Yost, S.; Sultana, R.; Labreche, K.; Dudakia, D.; Renwick, A.; Seal, S.; Al-Saadi, R.; Broderick, P.; et al. Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumours. Nat. Commun. 2015, 6, 5973. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Weiner, A.; Zack, T.; O’Donnell, E.; Guerriero, J.L.; Bernard, B.; Reddy, A.; Han, G.C.; AlDubayan, S.; Amin-Mansour, A.; Schumacher, S.E.; et al. Genomic evolution and chemoresistance in germ-cell tumours. Nature 2016, 540, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Cutcutache, I.; Suzuki, Y.; Tan, I.B.; Ramgopal, S.; Zhang, S.; Ramnarayanan, K.; Gan, A.; Lee, H.H.; Tay, S.T.; Ooi, A.; et al. Exome-wide sequencing shows low mutation rates and identifies novel mutated genes in seminomas. Eur. Urol. 2015, 68, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yamaguchi, S.; Burstein, M.D.; Terashima, K.; Chang, K.; Ng, H.K.; Nakamura, H.; He, Z.; Doddapaneni, H.; Lewis, L.; et al. Novel somatic and germline mutations in intracranial germ cell tumours. Nature 2014, 511, 241–245. [Google Scholar] [CrossRef]
- Goddard, N.C.; McIntyre, A.; Summersgill, B.; Gilbert, D.; Kitazawa, S.; Shipley, J. Kit and ras signalling pathways in testicular germ cell tumours: New data and a review of the literature. Int. J. Androl. 2007, 30, 337–348, discussion 349. [Google Scholar] [CrossRef]
- McIntyre, A.; Summersgill, B.; Grygalewicz, B.; Gillis, A.J.; Stoop, J.; van Gurp, R.J.; Dennis, N.; Fisher, C.; Huddart, R.; Cooper, C.; et al. Amplification and overexpression of the kit gene is associated with progression in the seminoma subtype of testicular germ cell tumors of adolescents and adults. Cancer Res. 2005, 65, 8085–8089. [Google Scholar] [CrossRef]
- Samaniego, F.; Rodriguez, E.; Houldsworth, J.; Murty, V.V.; Ladanyi, M.; Lele, K.P.; Chen, Q.G.; Dmitrovsky, E.; Geller, N.L.; Reuter, V.; et al. Cytogenetic and molecular analysis of human male germ cell tumors: Chromosome 12 abnormalities and gene amplification. Genes Chromosomes Cancer 1990, 1, 289–300. [Google Scholar] [CrossRef]
- Rijlaarsdam, M.A.; Rijlaarsdam, D.J.; Gillis, A.J.; Dorssers, L.C.; Looijenga, L.H. Mimsg: A target enrichment algorithm for predicted mir-mrna interactions based on relative ranking of matched expression data. Bioinformatics 2013, 29, 1638–1646. [Google Scholar] [CrossRef][Green Version]
- Bagrodia, A.; Lee, B.H.; Lee, W.; Cha, E.K.; Sfakianos, J.P.; Iyer, G.; Pietzak, E.J.; Gao, S.P.; Zabor, E.C.; Ostrovnaya, I.; et al. Genetic determinants of cisplatin resistance in patients with advanced germ cell tumors. J. Clin. Oncol. 2016, 34, 4000–4007. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Muhlenberg, T.; Leahy, M.; Hoiczyk, M.; Gauler, T.; Schuler, M.; Looijenga, L. Therapeutic potential of mdm2 inhibition in malignant germ cell tumours. Eur. Urol. 2010, 57, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Koster, R.; Timmer-Bosscha, H.; Bischoff, R.; Gietema, J.A.; de Jong, S. Disruption of the mdm2-p53 interaction strongly potentiates p53-dependent apoptosis in cisplatin-resistant human testicular carcinoma cells via the fas/fasl pathway. Cell Death Dis. 2011, 2, e148. [Google Scholar] [CrossRef] [PubMed]
- Honecker, F.; Wermann, H.; Mayer, F.; Gillis, A.J.; Stoop, H.; van Gurp, R.J.; Oechsle, K.; Steyerberg, E.; Hartmann, J.T.; Dinjens, W.N.; et al. Microsatellite instability, mismatch repair deficiency, and braf mutation in treatment-resistant germ cell tumors. J. Clin. Oncol. 2009, 27, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Looijenga, L.H.; Oosterhuis, J.W. Pathobiology of testicular germ cell tumors: Views and news. Anal. Quant. Cytol. Histol. 2002, 24, 263–279. [Google Scholar]
- Cools, M.; Wolffenbuttel, K.P.; Drop, S.L.; Oosterhuis, J.W.; Looijenga, L.H. Gonadal development and tumor formation at the crossroads of male and female sex determination. Sex. Dev. 2011, 5, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Van Der Zwan, Y.G.; Stoop, H.; Rossello, F.; White, S.J.; Looijenga, L.H. Role of epigenetics in the etiology of germ cell cancer. Int. J. Dev. Biol. 2013, 57, 299–308. [Google Scholar] [CrossRef]
- Looijenga, L.H.; Van Agthoven, T.; Biermann, K. Development of malignant germ cells—the genvironmental hypothesis. Int. J. Dev. Biol. 2013, 57, 241–253. [Google Scholar] [CrossRef]
- Rijlaarsdam, M.A.; Looijenga, L.H. An oncofetal and developmental perspective on testicular germ cell cancer. Semin. Cancer Biol. 2014, 29, 59–74. [Google Scholar] [CrossRef]
- Lobo, J.; Gillis, A.J.M.; Jeronimo, C.; Henrique, R.; Looijenga, L.H.J. Human germ cell tumors are developmental cancers: Impact of epigenetics on pathobiology and clinic. Int. J. Mol. Sci. 2019, 20, 258. [Google Scholar] [CrossRef]
- Honorio, S.; Agathanggelou, A.; Wernert, N.; Rothe, M.; Maher, E.R.; Latif, F. Frequent epigenetic inactivation of the rassf1a tumour suppressor gene in testicular tumours and distinct methylation profiles of seminoma and nonseminoma testicular germ cell tumours. Oncogene 2003, 22, 461–466. [Google Scholar] [CrossRef]
- Looijenga, L.H.; Gillis, A.J.; van Gurp, R.J.; Verkerk, A.J.; Oosterhuis, J.W. X inactivation in human testicular tumors. Xist expression and androgen receptor methylation status. Am. J. Pathol. 1997, 151, 581–590. [Google Scholar]
- Kawakami, T.; Okamoto, K.; Ogawa, O.; Okada, Y. Xist unmethylated DNA fragments in male-derived plasma as a tumour marker for testicular cancer. Lancet 2004, 363, 40–42. [Google Scholar] [CrossRef]
- Voorhoeve, P.M.; le Sage, C.; Schrier, M.; Gillis, A.J.; Stoop, H.; Nagel, R.; Liu, Y.P.; van Duijse, J.; Drost, J.; Griekspoor, A.; et al. A genetic screen implicates mirna-372 and mirna-373 as oncogenes in testicular germ cell tumors. Cell 2006, 124, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.U.; Son, Y.; Kim, C.H.; Kim, K.S.; Hyun, S.H.; Woo, H.G.; Jee, B.A.; Choi, J.H.; Sung, H.K.; Choi, H.C.; et al. Embryonic stem cell-derived mmu-mir-291a-3p inhibits cellular senescence in human dermal fibroblasts through the tgf--receptor 2 pathway. J. Gerontol. A Biol. Sci Med. Sci. 2019, 74, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.J.; Stoop, H.J.; Hersmus, R.; Oosterhuis, J.W.; Sun, Y.; Chen, C.; Guenther, S.; Sherlock, J.; Veltman, I.; Baeten, J.; et al. High-throughput micrornaome analysis in human germ cell tumours. J. Pathol. 2007, 213, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Rijlaarsdam, M.A.; van Agthoven, T.; Gillis, A.J.; Patel, S.; Hayashibara, K.; Lee, K.Y.; Looijenga, L.H. Identification of known and novel germ cell cancer-specific (embryonic) mirs in serum by high-throughput profiling. Andrology 2015, 3, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Vilela-Salgueiro, B.; Barros-Silva, D.; Lobo, J.; Costa, A.L.; Guimaraes, R.; Cantante, M.; Lopes, P.; Braga, I.; Oliveira, J.; Henrique, R.; et al. Germ cell tumour subtypes display differential expression of microrna371a-3p. Philos. Trans. R Soc. Lond B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef]
- Gillis, A.J.; Rijlaarsdam, M.A.; Eini, R.; Dorssers, L.C.; Biermann, K.; Murray, M.J.; Nicholson, J.C.; Coleman, N.; Dieckmann, K.P.; Belge, G.; et al. Targeted serum mirna (tsmir) test for diagnosis and follow-up of (testicular) germ cell cancer patients: A proof of principle. Mol. Oncol. 2013, 7, 1083–1092. [Google Scholar] [CrossRef]
- Murray, M.J.; Bell, E.; Raby, K.L.; Rijlaarsdam, M.A.; Gillis, A.J.; Looijenga, L.H.; Brown, H.; Destenaves, B.; Nicholson, J.C.; Coleman, N. A pipeline to quantify serum and cerebrospinal fluid micrornas for diagnosis and detection of relapse in paediatric malignant germ-cell tumours. Br. J. Cancer 2016, 114, 151–162. [Google Scholar] [CrossRef]
- Murray, M.J.; Coleman, N. Testicular cancer: A new generation of biomarkers for malignant germ cell tumours. Nat. Rev. Urol. 2012, 9, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Leão, R.; van Agthoven, T.; Figueiredo, A.; Jewett, M.A.S.; Fadaak, K.; Sweet, J.; Ahmad, A.E.; Anson-Cartwright, L.; Chung, P.; Hansen, A.; et al. Serum mirna predicts viable disease after chemotherapy in patients with testicular nonseminoma germ cell tumor. J. Urol. 2018, 200, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; van Agthoven, T.; Gronesova, P.; Chovanec, M.; Miskovska, V.; Mardiak, J.; Looijenga, L.H.J. Clinical utility of plasma mir-371a-3p in germ cell tumors. J. Cell Mol. Med. 2019, 23, 1128–1136. [Google Scholar] [PubMed]
- Salvatori, D.C.F.; Dorssers, L.C.J.; Gillis, A.J.M.; Perretta, G.; van Agthoven, T.; Gomes Fernandes, M.; Stoop, H.; Prins, J.B.; Oosterhuis, J.W.; Mummery, C.; et al. The microrna-371 family as plasma biomarkers for monitoring undifferentiated and potentially malignant human pluripotent stem cells in teratoma assays. Stem Cell Rep. 2018, 11, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Van Agthoven, T.; Eijkenboom, W.M.H.; Looijenga, L.H.J. Microrna-371a-3p as informative biomarker for the follow-up of testicular germ cell cancer patients. Cell Oncol. 2017, 40, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Van Agthoven, T.; Looijenga, L.H. Accurate primary germ cell cancer diagnosis using serum based microrna detection (amptsmir test). Oncotarget 2016, 8, 58037–58049. [Google Scholar] [CrossRef]
- Spiekermann, M.; Belge, G.; Winter, N.; Ikogho, R.; Balks, T.; Bullerdiek, J.; Dieckmann, K.P. Microrna mir-371a-3p in serum of patients with germ cell tumours: Evaluations for establishing a serum biomarker. Andrology 2015, 3, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.P.; Spiekermann, M.; Balks, T.; Ikogho, R.; Anheuser, P.; Wosniok, W.; Loening, T.; Bullerdiek, J.; Belge, G. Microrna mir-371a-3p—a novel serum biomarker of testicular germ cell tumors: Evidence for specificity from measurements in testicular vein blood and in neoplastic hydrocele fluid. Urol. Int. 2016, 97, 76–83. [Google Scholar] [CrossRef]
- Flor, I.; Spiekermann, M.; Loning, T.; Dieckmann, K.P.; Belge, G.; Bullerdiek, J. Expression of micrornas of c19mc in different histological types of testicular germ cell tumour. Cancer Genomics Proteomics 2016, 13, 281–289. [Google Scholar]
- Dieckmann, K.P.; Radtke, A.; Spiekermann, M.; Balks, T.; Matthies, C.; Becker, P.; Ruf, C.; Oing, C.; Oechsle, K.; Bokemeyer, C.; et al. Serum levels of microrna mir-371a-3p: A sensitive and specific new biomarker for germ cell tumours. Eur. Urol. 2017, 71, 213–220. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Radtke, A.; Geczi, L.; Matthies, C.; Anheuser, P.; Eckardt, U.; Sommer, J.; Zengerling, F.; Trenti, E.; Pichler, R.; et al. Serum levels of microrna-371a-3p (m371 test) as a new biomarker of testicular germ cell tumors: Results of a prospective multicentric study. J. Clin. Oncol. 2019, 37, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, W.P.A.; Gillis, A.J.M.; van Leenders, G.; Stoop, H.; van Agthoven, T.; Dorssers, L.C.J.; Dinkelman-Smit, M.; Boormans, J.L.; Looijenga, L.H.J. Cellular origin of microrna-371a-3p in healthy males based on systematic urogenital tract tissue evaluation. Andrology 2019, 7, 463–468. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Looijenga, L.H.J.; Kao, C.-S.; Idrees, M.T. Predicting Gonadal Germ Cell Cancer in People with Disorders of Sex Development; Insights from Developmental Biology. Int. J. Mol. Sci. 2019, 20, 5017. https://doi.org/10.3390/ijms20205017
Looijenga LHJ, Kao C-S, Idrees MT. Predicting Gonadal Germ Cell Cancer in People with Disorders of Sex Development; Insights from Developmental Biology. International Journal of Molecular Sciences. 2019; 20(20):5017. https://doi.org/10.3390/ijms20205017
Chicago/Turabian StyleLooijenga, Leendert H. J., Chia-Sui Kao, and Muhammad T. Idrees. 2019. "Predicting Gonadal Germ Cell Cancer in People with Disorders of Sex Development; Insights from Developmental Biology" International Journal of Molecular Sciences 20, no. 20: 5017. https://doi.org/10.3390/ijms20205017
APA StyleLooijenga, L. H. J., Kao, C.-S., & Idrees, M. T. (2019). Predicting Gonadal Germ Cell Cancer in People with Disorders of Sex Development; Insights from Developmental Biology. International Journal of Molecular Sciences, 20(20), 5017. https://doi.org/10.3390/ijms20205017




