Control of Arms of Au Stars Size and its Dependent Cytotoxicity and Photosensitizer Effects in Photothermal Anticancer Therapy
Abstract
:1. Introduction
2. Results and Discussion
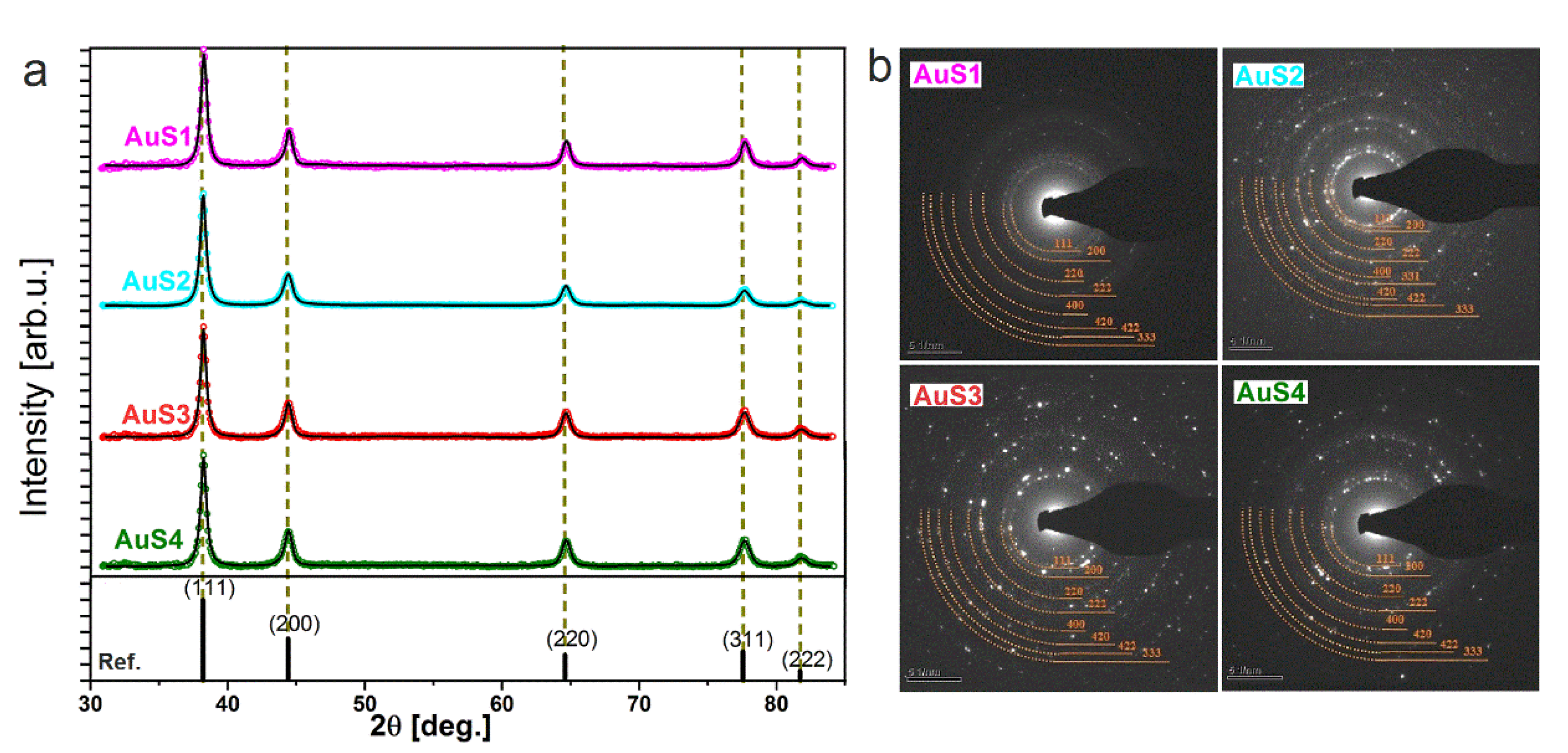
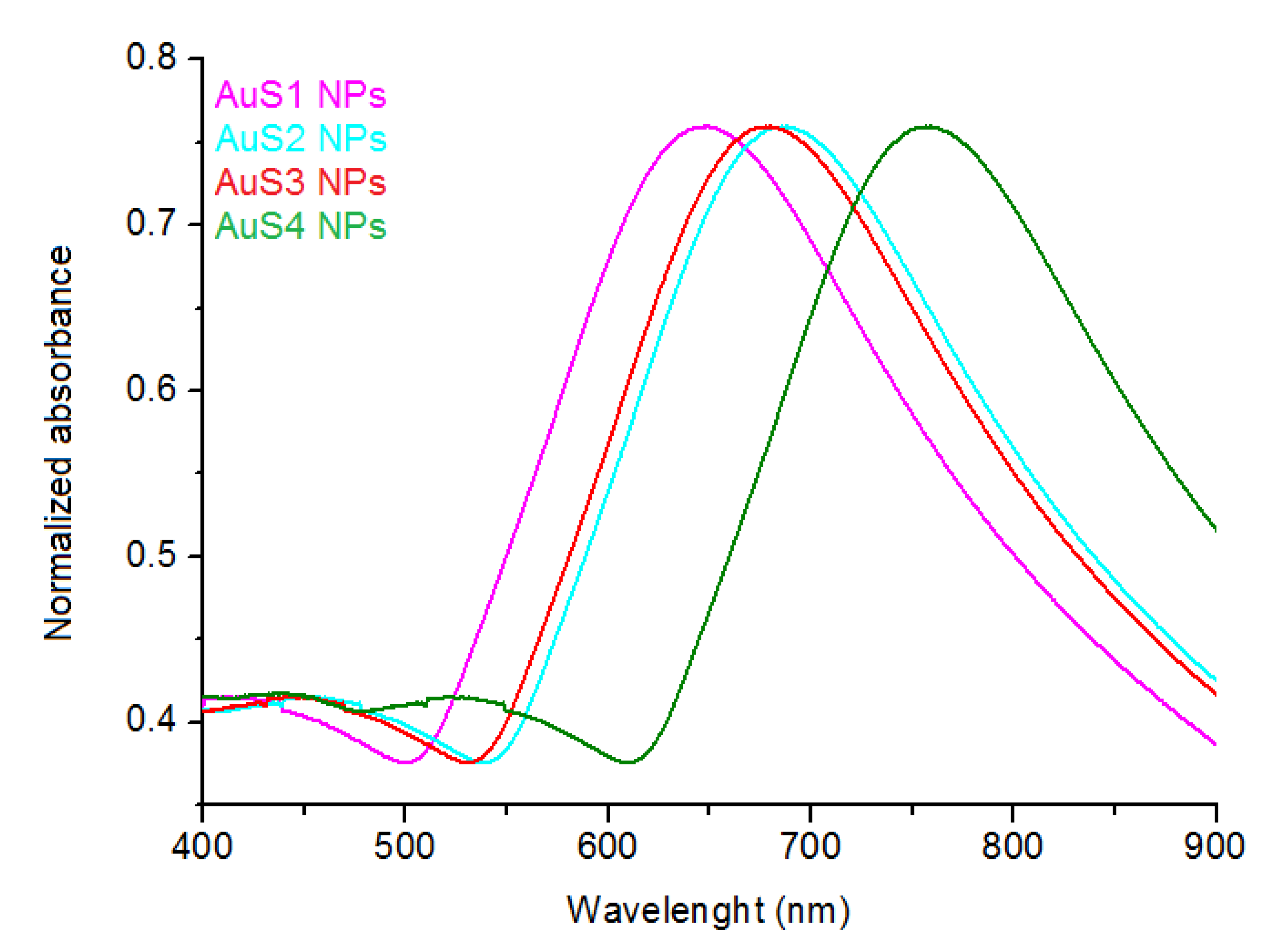
2.1. Morphology, Structure, and Optical Properties of Nanoparticles
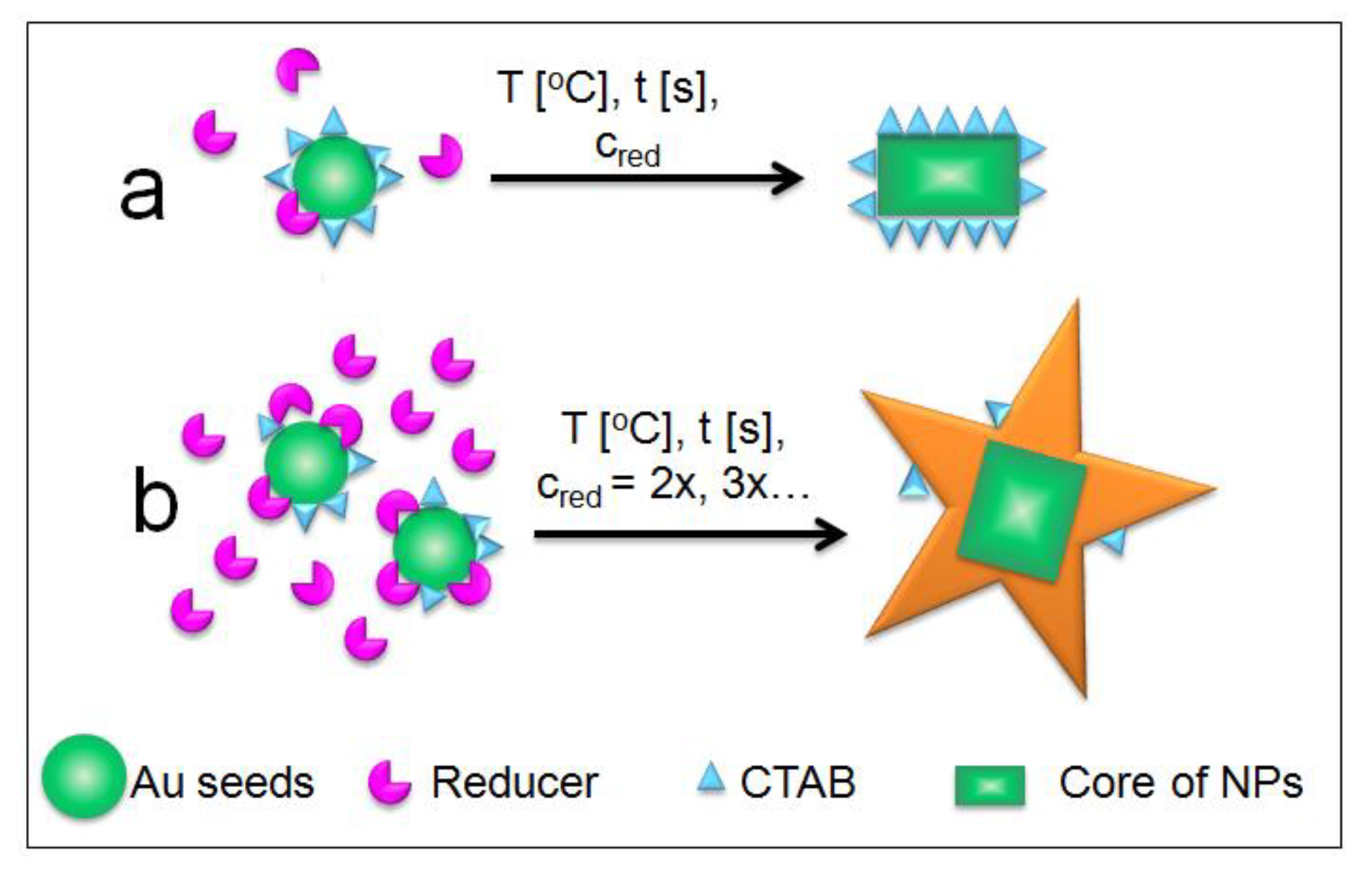
2.2. Mechanism of Formation of AuS NPs with Control of Star Arms Lengths
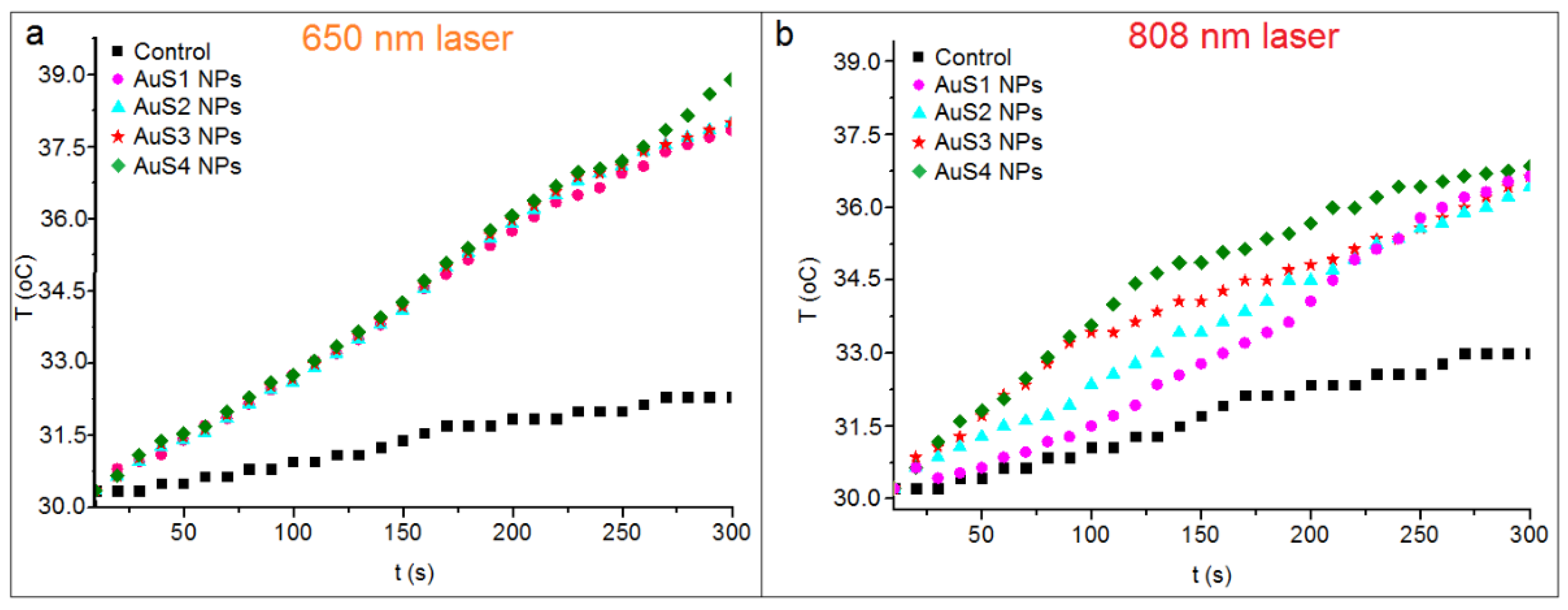
2.3. Photothermal Conversion Efficiency of Obtained AuS NPs
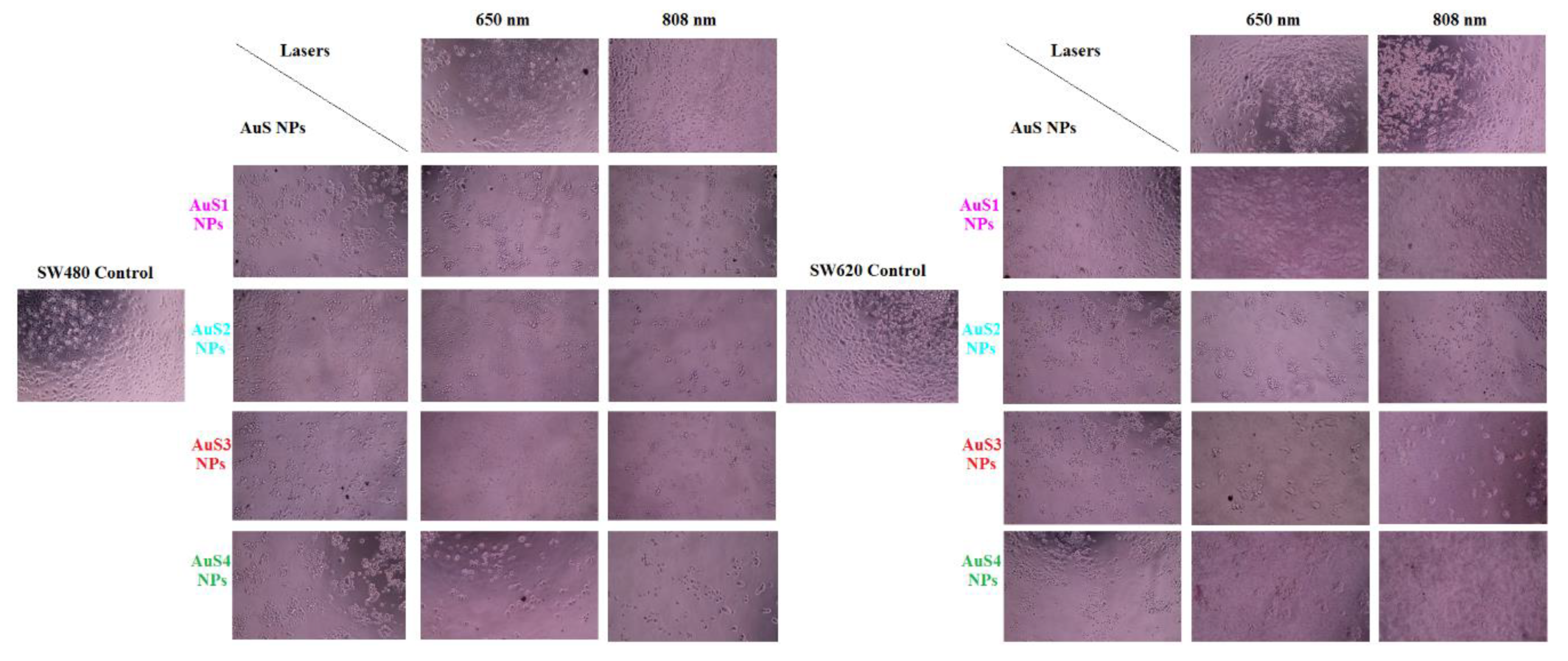
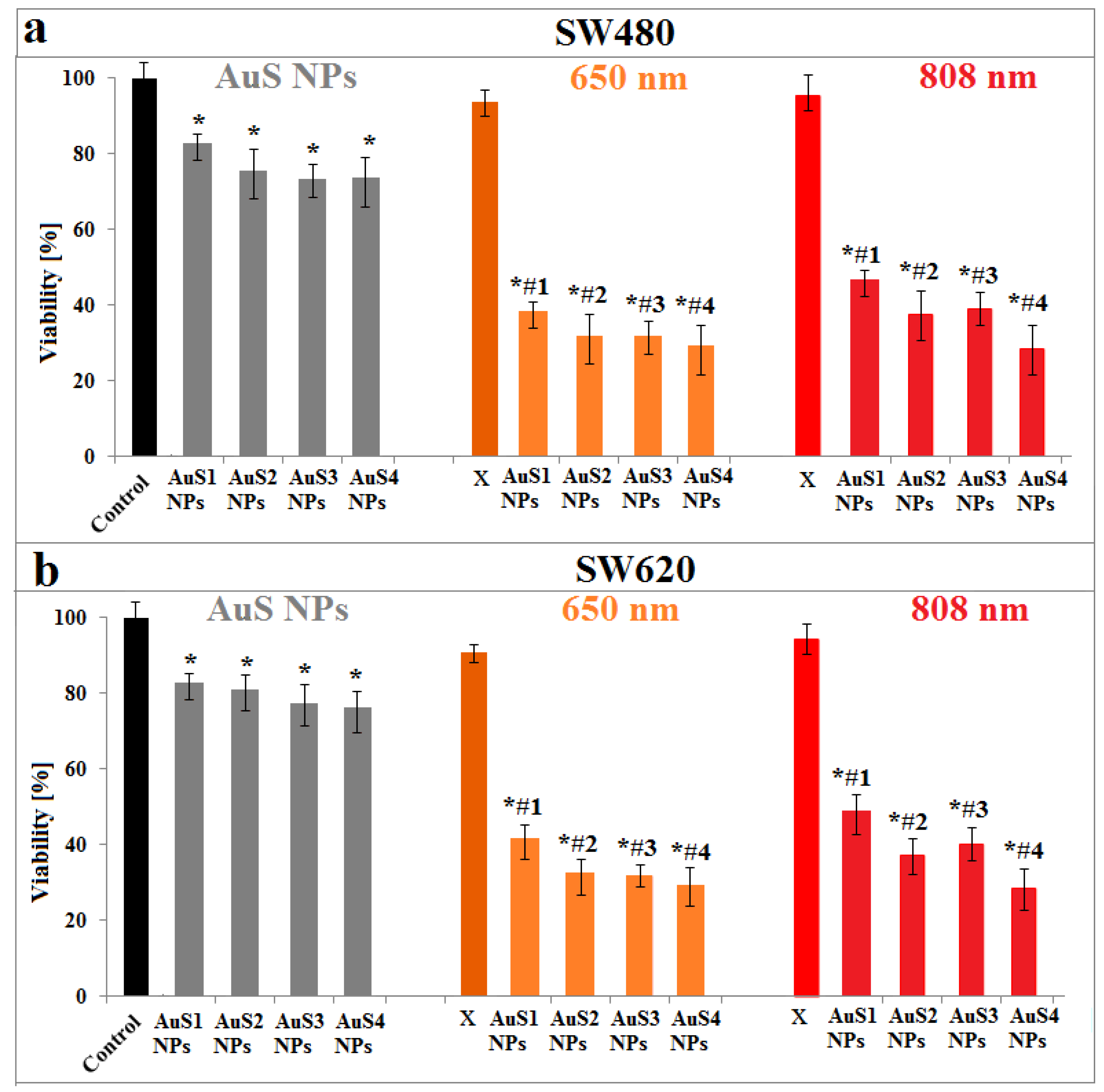
2.4. Simulation of PTT of Cancer Cells in the Presence of AuS NPs
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Au Nanostars with Different Lengths of Star Arms
3.3. TEM Characterization
3.4. X-Ray Diffraction
3.5. UV-Vis Spectroscopy
3.6. Cell Culture
3.7. Optical Microscopy Images of Cells
3.8. MTS Assay
3.9. Photothermal Conversion Efficiency Determined from the Time Constant
3.10. Light Source and Cells Irradiation Protocols
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Draz, M.S.; Shafiee, H. Applications of gold nanoparticles in virus detection. Theranostics 2018, 8, 1985–2017. [Google Scholar] [CrossRef]
- Wang, C.C.; Wu, S.M.; Li, H.W.; Chang, H.T. Biomedical applications of DNA-conjugated gold nanoparticles. Chembiochem 2016, 17, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Austin, L.A.; Kang, B.; El-Sayed, M.A. Probing molecular cell event dynamics at the single-cell level with targeted plasmonic gold nanoparticles: A review. Nano Today 2015, 10, 542–558. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iati, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Solveyra, E.; Szleifer, I. What is the role of curvature on the properties of nanomaterials for biomedical applications? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 334–354. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured plasmonic sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Senthil, P.; Pastoriza-Santos, I.; Rodriguez-Gonzalez, B.; Javier Garcia de Abajo, F.J.; Liz-Marzan, L.M. High-yield synthesis and optical response of gold nanostars. Nanotechnology 2008, 19, 015606. [Google Scholar] [CrossRef] [PubMed]
- Song, H.M.; Wei, W.; Ong, Q.K.; Wei, A. Plasmon-resonant nanoparticles and nanostars with magnetic cores: Synthesis and magnetomotive imaging. ACS Nano 2010, 4, 5163–5173. [Google Scholar] [CrossRef]
- Gao, J.; Sanchez-Purra, M.; Huang, H.; Wang, S.; Chen, Y.; Yu, X.; Luo, Q.; Hamad-Schifferli, K.; Liu, S. Synthesis of different-sized gold nanostars for Raman bioimaging and photothermal therapy in cancer nanotheranostics. Sci. China Chem. 2017, 60, 1219–1229. [Google Scholar] [CrossRef]
- Zhao, W.; Karp, J.M. Tumour targeting: Nanoantennas heat up. Nat. Mater. 2009, 8, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, H.; Rahman, D.S.; Sengupta, M.; Ghosh, S.K. Gold nanostars in plasmonic photothermal therapy: The role of tip heads in the thermoplasmonic landscape. J. Phys. Chem. C 2018, 122, 13082–13094. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Khlebstov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar]
- Sun, Y.N.; Wang, C.D.; Zhang, X.M.; Ren, L.; Tian, X.H. Shape dependence of gold nanoparticles on in vivo acute toxicological effects and biodistribution. J. Nanosci. Nanotechnol. 2011, 11, 1210–1216. [Google Scholar] [CrossRef]
- Kah, J.C.Y.; Grabinski, C.; Untener, E.; Garrett, C.; Chen, J.; Zhu, D.; Hussain, S.M.; Hamad-Schifferli, K. Protein coronas on gold nanorods passivated with amphiphilic ligands affects cytotoxicity and cellular response to penicillin/streptomycin. ACS Nano 2014, 8, 4608–4620. [Google Scholar] [CrossRef]
- Jurkiewicz, K.; Kamiński, M.; Glajcar, W.; Woźnica, N.; Julienne, F.; Bartczak, P.; Polański, J.; Lelątko, J.; Zubko, M.; Burian, A. Paracrystalline structure of gold, silver, palladium and platinum nanoparticles. J. Appl. Crystallogr. 2018, 51, 411–419. [Google Scholar] [CrossRef]
- Swanson, H.; Tatge, E. Circular of the Bureau of Standards No 539: Standard X-ray Diffraction Powder Patterns; United States Department of Commerce: Washington, DC, USA, 1953; pp. 1–108. [Google Scholar]
- Guo, G.Y.; Ebert, H. First-principles study of the magnetic hyperfine field in Fe and Co multilayers. Phys. Rev. B 1996, 53, 2492–2503. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Snyder, B.; El-Sayed, M.E. Synthesis and optical properties of small Au nanorods using a seedless growth technique. Langmuir 2012, 28, 9807–9815. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guyot-Sionnest, P. Mechanism of silver(I)-assisted growth of gold nanorods and bipyramids. J. Phys. Chem. B 2005, 109, 22192–22200. [Google Scholar] [CrossRef] [PubMed]
- Keul, H.A.; Moller, M.; Bockstaller, M.R. Structural evolution of gold nanorods during controlled secondary growth. Langmuir 2007, 23, 10307–10315. [Google Scholar] [CrossRef] [PubMed]
- Shishir, V.K.; Adarsh, P.B.; Prasad, P.; Ashiqur, R.; Si Amar, D.; Clayton, S.J. High conversion synthesis of <10 nm starch-stabilized silver nanoparticles using microwave technology. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Jordi, P.; Neus, G.B.; Victor, P. Size-Controlled Synthesis of Sub-10-nanometer Citrate-Stabilized Gold Nanoparticles and Related Optical Properties. Chem. Mater. 2016, 28, 1066–1075. [Google Scholar]
- Vallyathan, V.; Shi, X. The role of oxygen free radicals in occupational and environmental lung diseases. Environ. Health Perspect. 1997, 105, 165–177. [Google Scholar] [PubMed]
- Vo-Ding, T.; Liu, Y.; Crawford, B.M.; Wang, H.N.; Yuan, H.; Register, J.K.; Khoury, C.G. Shining gold nanostars: From cancer diagnostics to photothermal treatment and immunotherapy. J. Immunol. Sci. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Schins, R.P. Mechanism of genotoxicity of particles and fibers. Inhal. Toxicol. 2002, 14, 57–78. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Ding, Y.; Sun, S. Understanding the photothermal effect of gold nanostars and nanorods for biomedical applications. RSC Adv. 2014, 4, 30375–30383. [Google Scholar] [CrossRef]
- Lacerda, S.H.; Park, J.J.; Meuse, C.; Pristinski, D.; Becker, M.L.; Karim, A.; Douglas, J.F. Interaction of gold nanoparticles with common human blood proteins. ACS Nano 2010, 4, 365–379. [Google Scholar] [CrossRef]
- Cigler, P.; Lytton-Jean, A.K.; Anderson, D.G.; Finn, M.G.; Park, S.Y. DNA-controlled assembly of a NaTI lattice structure from gold nanoparticles and protein nanoparticles. Nat. Mater. 2010, 9, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, F.; Guo, X.; Chen, W.; Dong, C.; Zhang, J.; Zhang, J.; Wang, L. Gold nanostars for cancer cell-targeted SERS-imaging and NIR light-triggered plasmonic photothermal therapy (PPTT) in the first and second biological windows. J. Mater. Chem. B 2019, 7, 2001–2008. [Google Scholar] [CrossRef]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, X.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y.; et al. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics 2015, 5, 946–960. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Abstract of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr. Toulouse, France, 1990; p. 127. Available online: http://www.scirp.org/ (accessed on 17 September 2019).
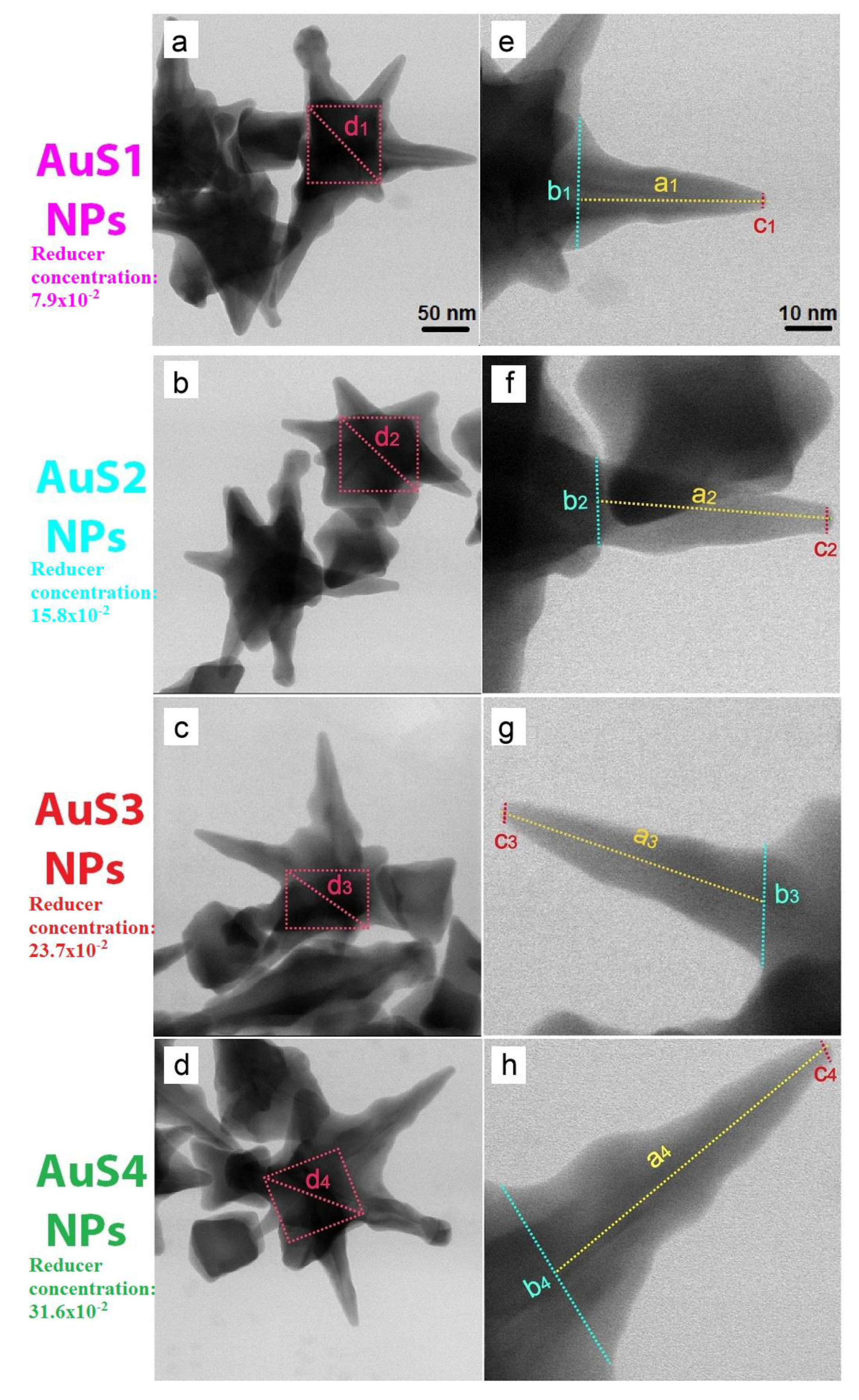
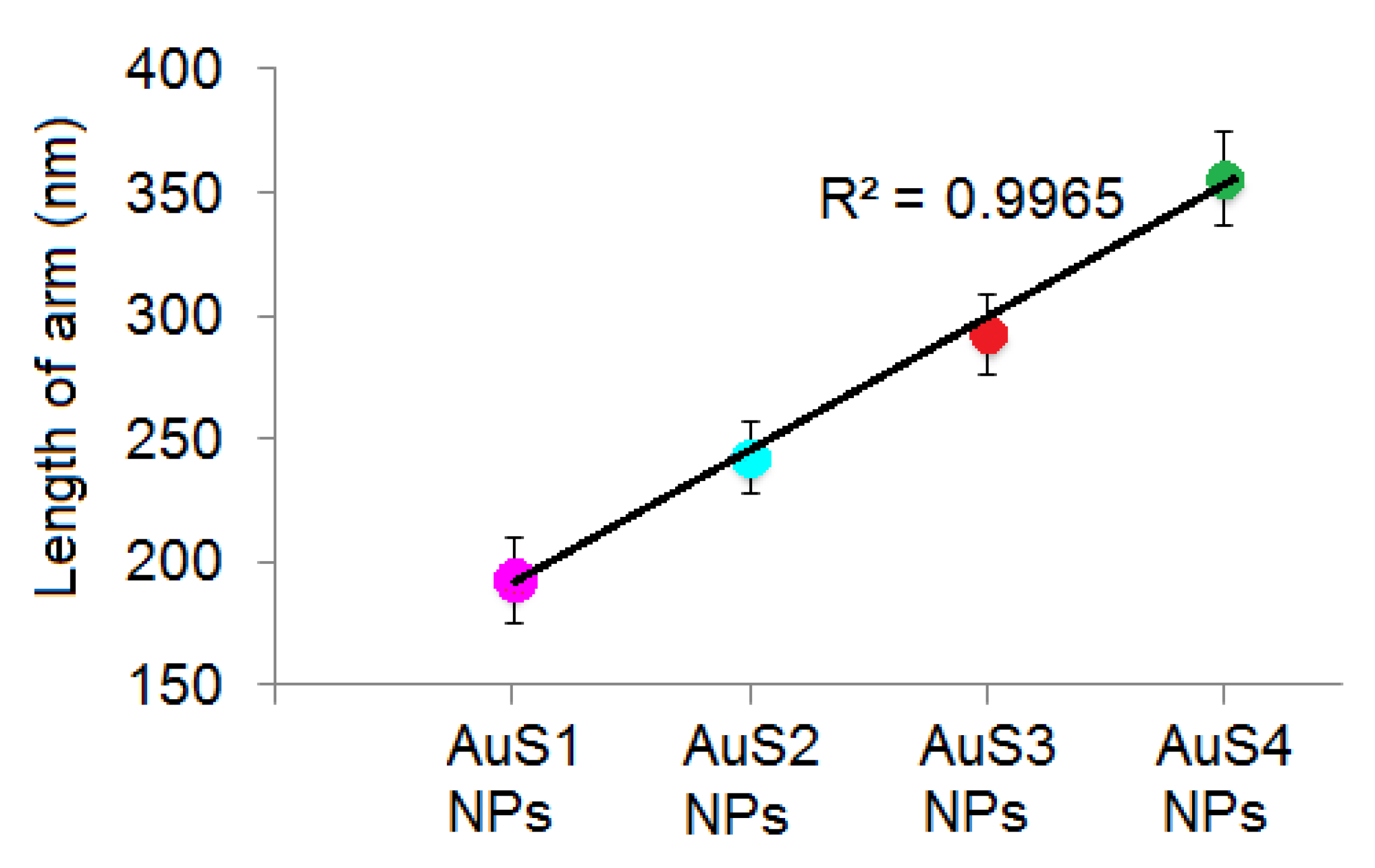
| Parameter (nm) | Samples | |||
|---|---|---|---|---|
| AuS1 NPs | AuS2 NPs | AuS3 NPs | AuS4 NPs | |
| a | 193.75 ± 17.11 | 243.75 ± 15.21 | 293.75 ± 16.76 | 356.27 ± 19.25 |
| b | 28.13 ± 4.21 | 19.38 ± 3.17 | 26.25 ± 4.58 | 44.38 ± 6.32 |
| c | 3.75 ± 1.02 | 5.63 ± 2.12 | 5.00 ± 1.87 | 6.25 ± 2.09 |
| b/c | 7.50 ± 2.28 | 3.44 ± 2.75 | 5.25 ± 3.01 | 7.10 ± 3.02 |
| d | 146.38 ± 4.27 | 143.75 ± 3.77 | 140.63 ± 5.08 | 146.21 ± 5.27 |
| Lattice Parameter (Å) | Samples | |||
| AuS1 NPs | AuS2 NPs | AuS3 NPs | AuS4 NPs | |
| 4.0740 (6) | 4.0763 (3) | 4.077 (1) | 4.0784 (2) | |
| η (%) | Samples | |||
|---|---|---|---|---|
| AuS1 NPs | AuS2 NPs | AuS3 NPs | AuS4 NPs | |
| η650 | 38 | 40 | 39 | 44 |
| η808 | 33 | 32 | 33 | 34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Depciuch, J.; Stec, M.; Maximenko, A.; Pawlyta, M.; Baran, J.; Parlinska-Wojtan, M. Control of Arms of Au Stars Size and its Dependent Cytotoxicity and Photosensitizer Effects in Photothermal Anticancer Therapy. Int. J. Mol. Sci. 2019, 20, 5011. https://doi.org/10.3390/ijms20205011
Depciuch J, Stec M, Maximenko A, Pawlyta M, Baran J, Parlinska-Wojtan M. Control of Arms of Au Stars Size and its Dependent Cytotoxicity and Photosensitizer Effects in Photothermal Anticancer Therapy. International Journal of Molecular Sciences. 2019; 20(20):5011. https://doi.org/10.3390/ijms20205011
Chicago/Turabian StyleDepciuch, Joanna, Malgorzata Stec, Alexey Maximenko, Miroslawa Pawlyta, Jarek Baran, and Magdalena Parlinska-Wojtan. 2019. "Control of Arms of Au Stars Size and its Dependent Cytotoxicity and Photosensitizer Effects in Photothermal Anticancer Therapy" International Journal of Molecular Sciences 20, no. 20: 5011. https://doi.org/10.3390/ijms20205011
APA StyleDepciuch, J., Stec, M., Maximenko, A., Pawlyta, M., Baran, J., & Parlinska-Wojtan, M. (2019). Control of Arms of Au Stars Size and its Dependent Cytotoxicity and Photosensitizer Effects in Photothermal Anticancer Therapy. International Journal of Molecular Sciences, 20(20), 5011. https://doi.org/10.3390/ijms20205011






