Solid Nanocrystals of Rebamipide Promote Recovery from Indomethacin-Induced Gastrointestinal Bleeding
Abstract
1. Introduction
2. Results
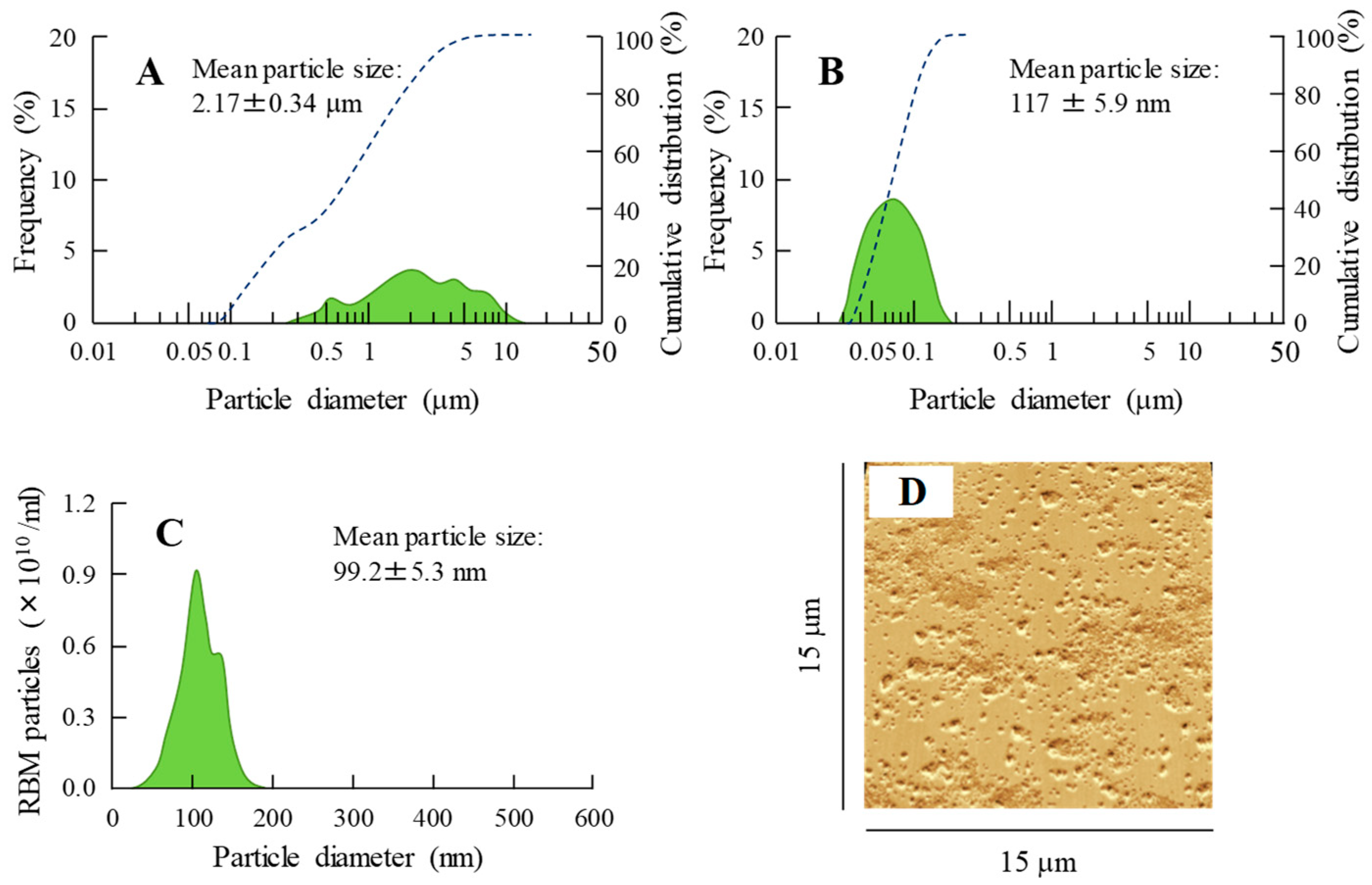
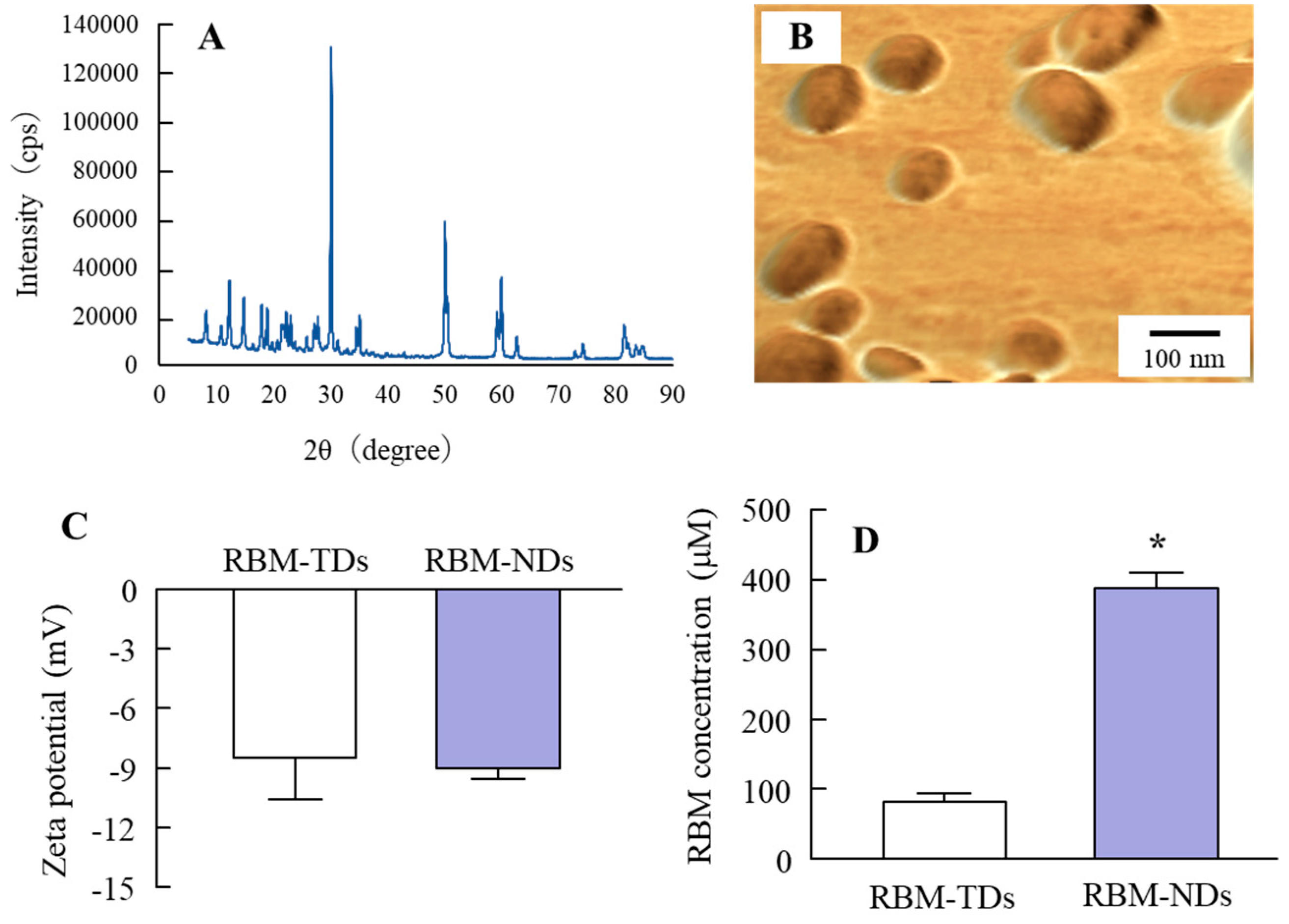
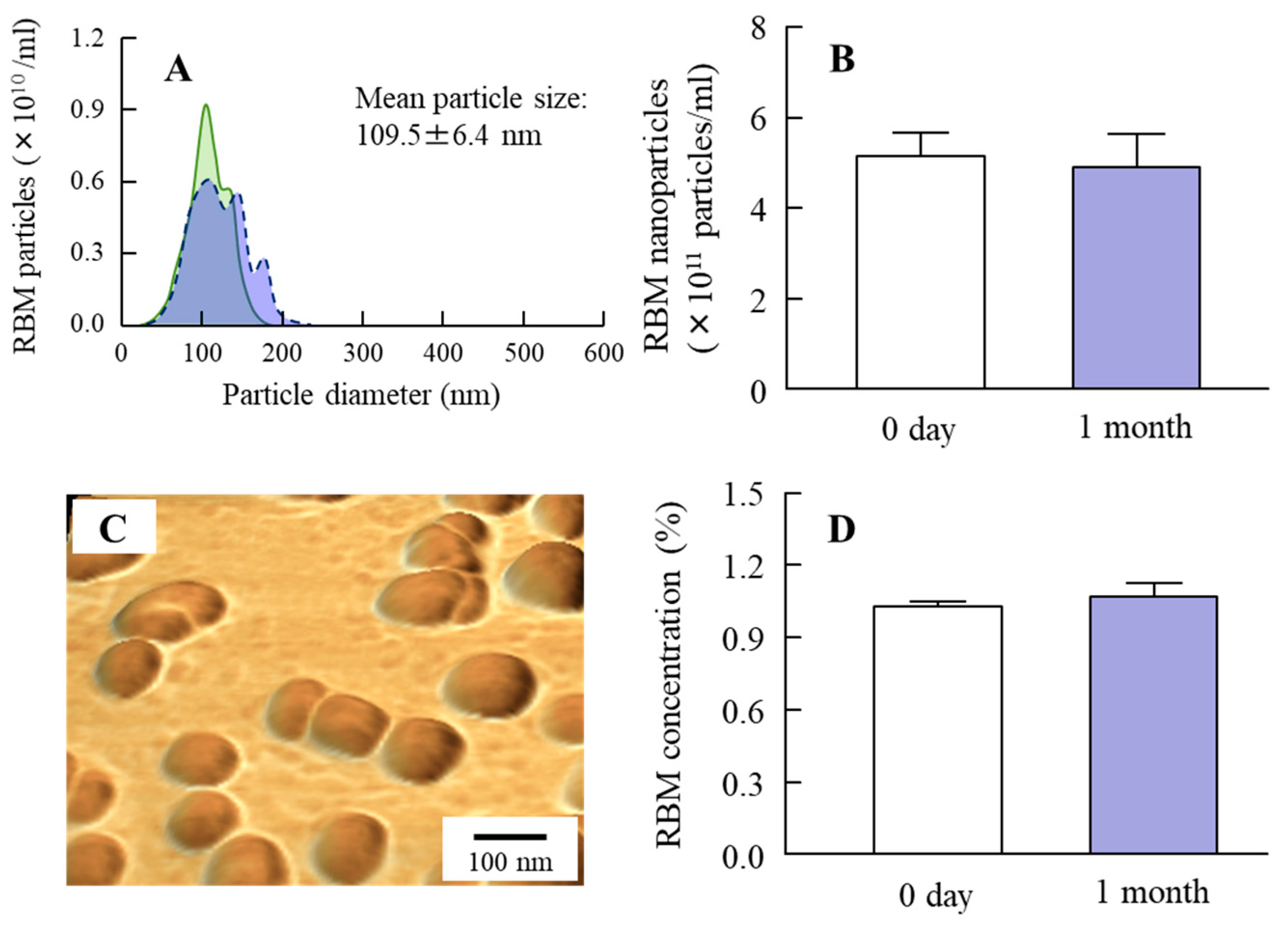
2.1. Development of RBM-NDs
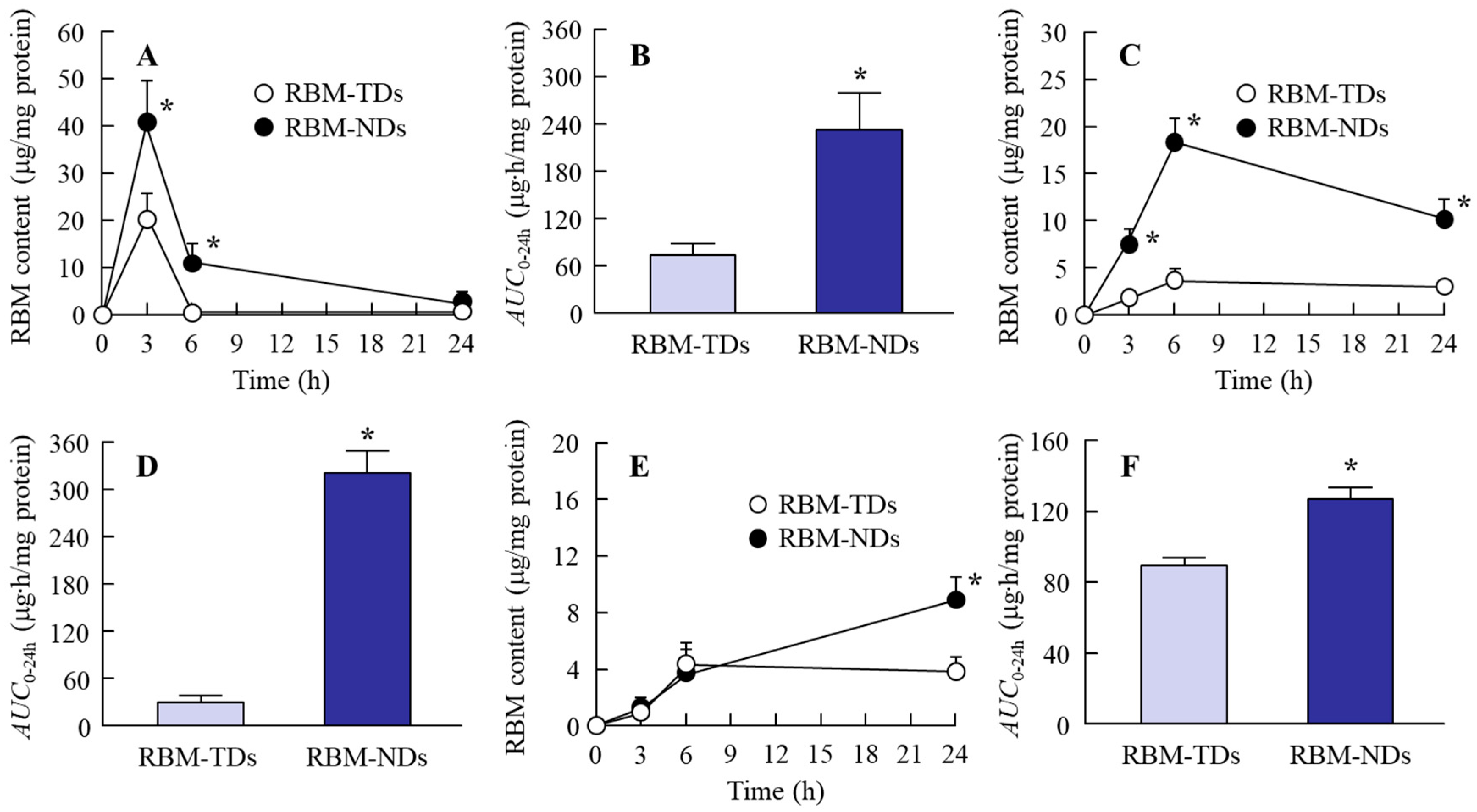
2.2. Drug Retention in GI Mucosa after the Oral Administration of RBM-NDs
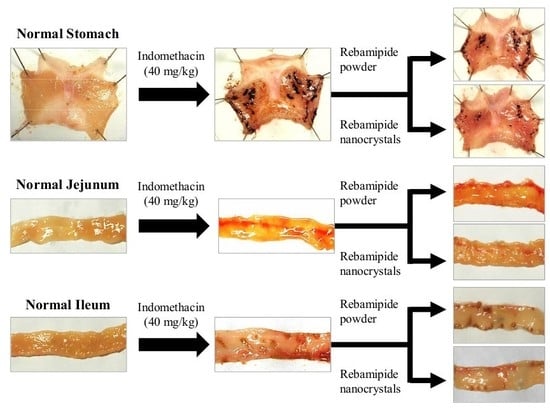
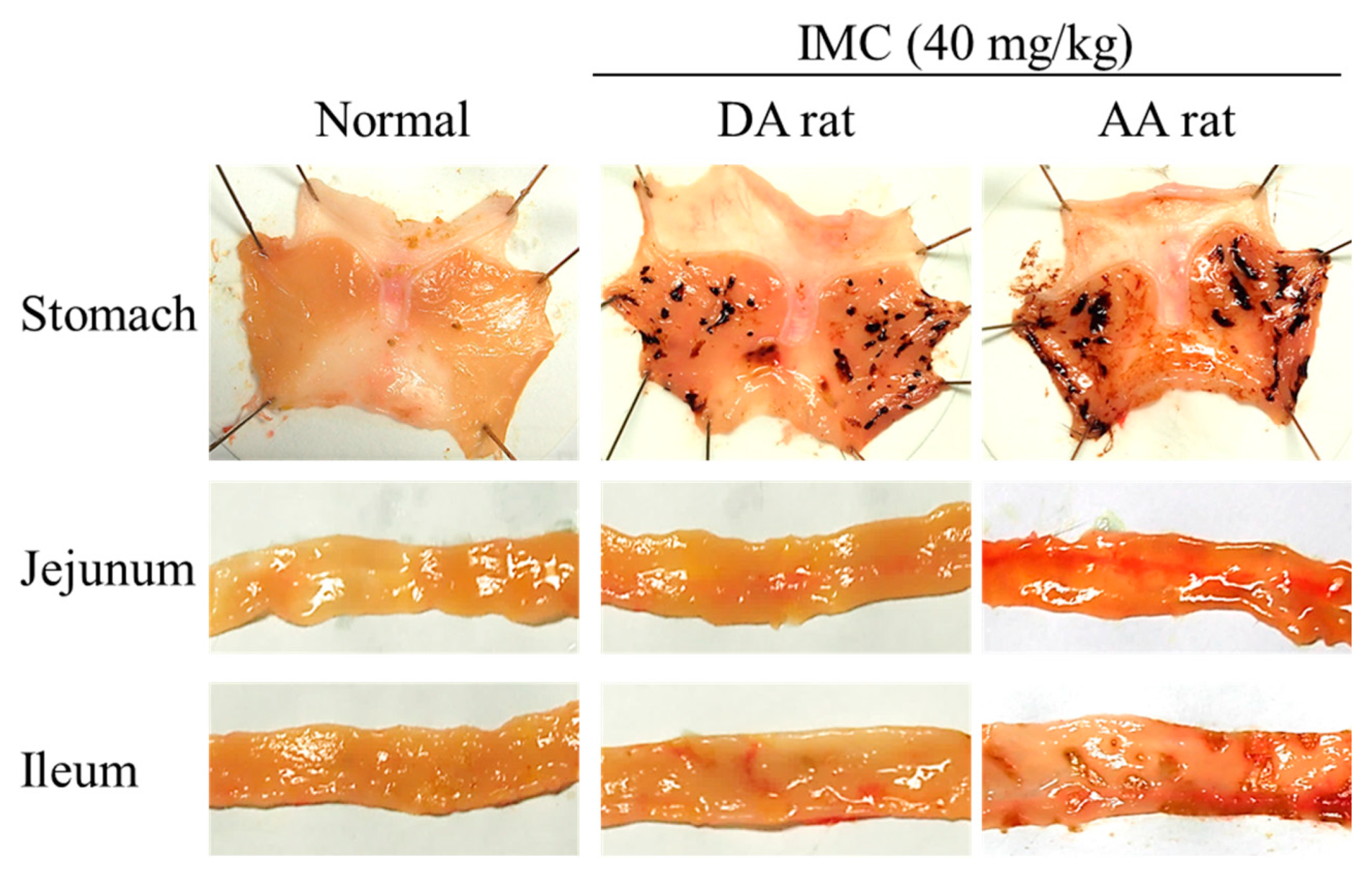
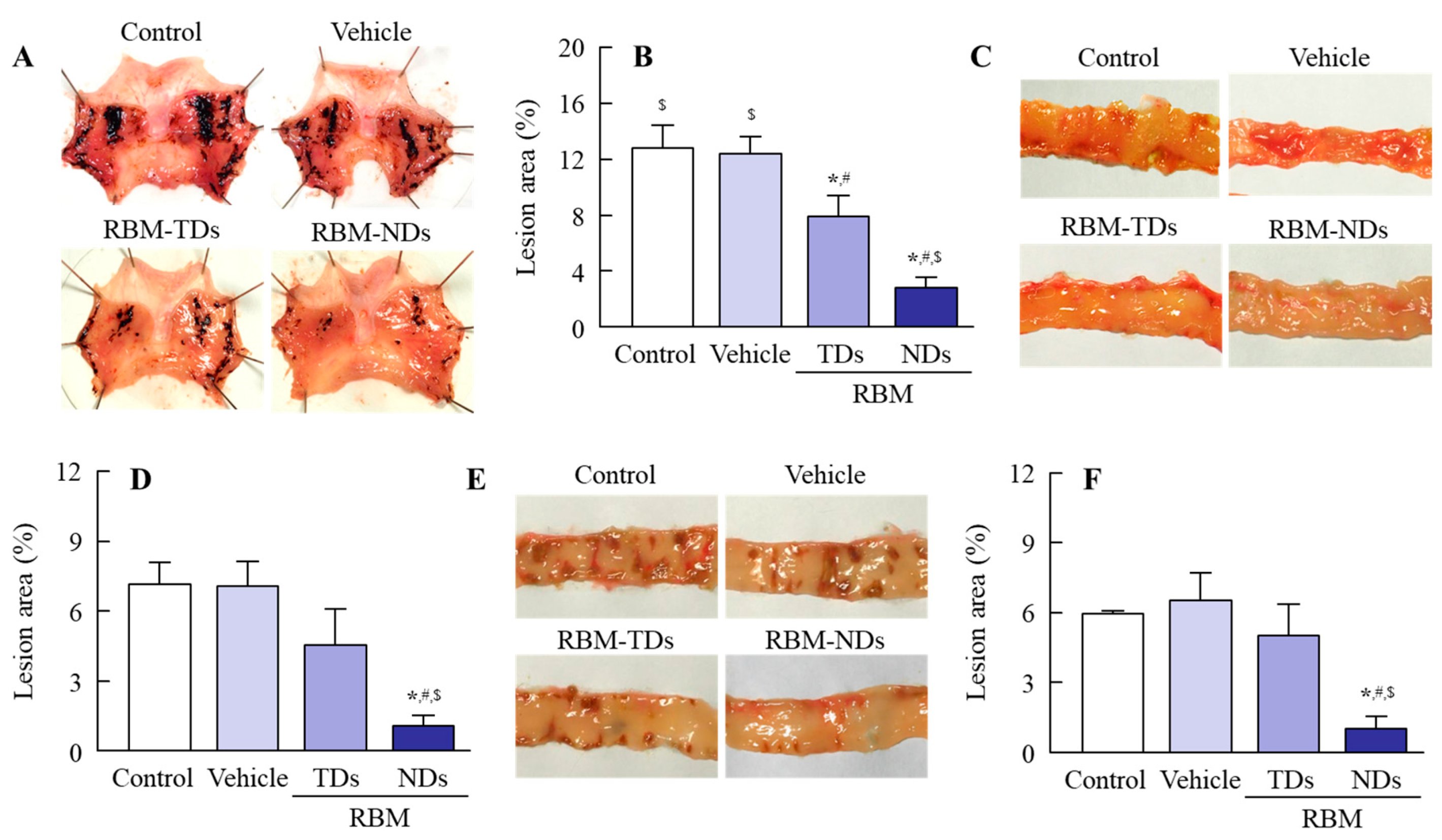
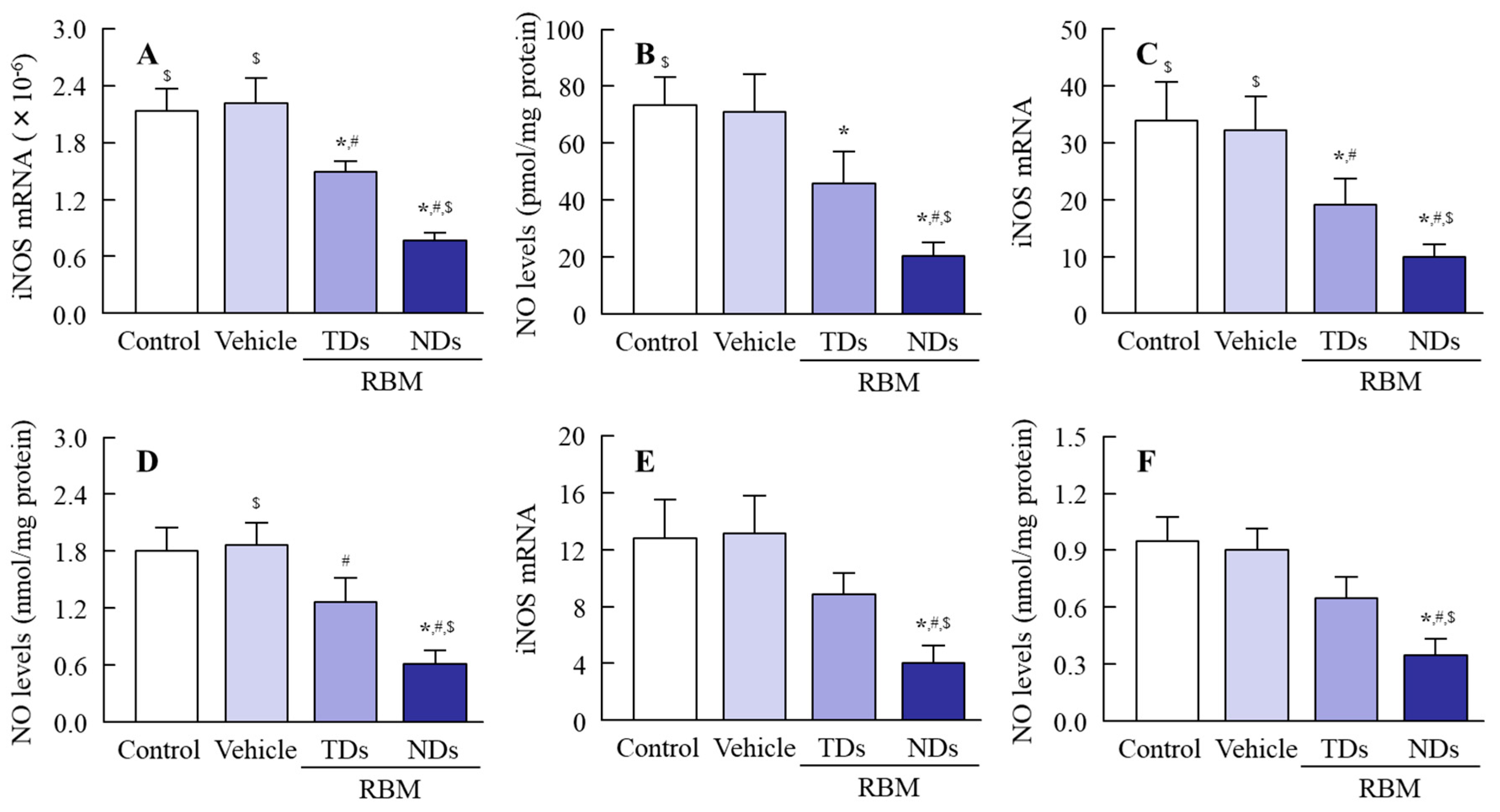
2.3. Therapeutic Effect of RBM-NDs on IMC-Induced GI Bleeding
3. Discussion
4. Materials and Methods
4.1. Drug and Chemicals
4.2. Experimental Animals
4.3. Design of RBM Solid Nanocrystals
4.4. Powder X-ray Diffraction (XRD)
4.5. Characterization in RBM Solid Nanocrystals
4.6. Model of IMC-Induced GI Injuries
4.7. Therapeutic Effect of RBM in Model Rats with IMC-Induced GI Injuries
4.8. RBM Contents in the Gastric, Jejunal, and Ileal Mucosa after Oral Administration of RBM
4.9. Real Time Polymerase Chain Reaction (PCR)
4.10. Measurement of NO Levels
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA rat | adjuvant-induced arthritis rat |
| AFM | atomic force microscope |
| AUC | area under the drug concentration-time curve |
| BCS | Biopharmaceutical Classification System |
| DA rat | dark agouti rat |
| GAPDH | glyceraldehyde-3-phosophate dehydrogenase |
| GI | gastrointestinal |
| HPβCD | 2-hydroxypropyl-β-cyclodextrin |
| IL | interleukin |
| IMC | indomethacin |
| iNOS | inducible nitric oxide synthase |
| MC | methylcellulose |
| NO | nitric oxide |
| NSAID | non-steroidal anti-inflammatory drug |
| PCR | polymerase chain reaction |
| RA | rheumatoid arthritis |
| RBM | rebamipide |
| RBM-NDs | dispersions containing rebamipide solid nanocrystals |
| RBM-TDs | dispersions consisting of traditional rebamipide |
| RT | reverse transcription |
| S.E. | standard error |
| SPM | scanning probe microscope |
| XRD | X-ray diffraction |
References
- Fries, J.F.; Williams, C.A.; Bloch, D.A.; Michel, B.A. Non-steroidal anti-inflammatory drug-associated gastropathy: Incidence and risk factor models. Am. J. Med. 1991, 91, 213–222. [Google Scholar] [CrossRef]
- Smalley, W.E.; Ray, W.A.; Daugherty, J.R.; Griffin, M.R. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am. J. Epidemiol. 1995, 141, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Ogawa, Y.; Kanatsu, K.; Okayama, M.; Watanabe, T.; Arakawa, T.; Takeuchi, K. Ulcerogenic influence of selective cyclooxygenase-2 inhibitors in the rat stomach with adjuvant-induced arthritis. J. Pharmacol. Exp. Ther. 2002, 303, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Satoh, H. NSAID-induced small intestinal damage-roles of various pathogenic factors. Digestion 2015, 91, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Fukuhata, T.; Ito, Y.; Usui, S.; Hirano, K. Involvement of interleukin 18 in indomethacin-induced lesions of the gastric mucosa in adjuvant-induced arthritis rat. Toxicology 2009, 255, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Tanino, T.; Ito, Y. Excessive Interleukin 18 Relate the Aggravation of Indomethacin-Induced Intestinal Ulcerogenic Lesions in Adjuvant-Induced Arthritis Rat. Biol. Pharm. Bull. 2015, 38, 1580–1590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fries, J.F.; Miller, S.R.; Spitz, P.W.; Williams, C.A.; Hubert, H.B.; Bloch, D.A. Toward an epidemiology of gastropathy associated with non-steroidal antiinflammatory drug use. Gastroenterology 1989, 96, 647–655. [Google Scholar] [CrossRef]
- Kato, S.; Ito, Y.; Nishio, H.; Aoi, Y.; Amagase, K.; Takeuchi, K. Increased susceptibility of small intestine to NSAID-provoked ulceration in rats with adjuvant-induced arthritis: Involvement of enhanced expression of TLR4. Life Sci. 2007, 81, 1309–1316. [Google Scholar] [CrossRef]
- Urashima, H.; Takeji, Y.; Okamoto, T.; Fujisawa, S.; Shinohara, H. Rebamipide increases mucin-like substance contents and periodic acid Schiff reagent-positive cells density in normal rabbits. J. Ocul. Pharmacol. Ther. 2012, 28, 264–270. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T. Rebamipide: A gastrointestinal protective drug with pleiotropic activities. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 261–270. [Google Scholar] [CrossRef]
- Tanaka, H.; Fukuda, K.; Ishida, W.; Harada, Y.; Sumi, T.; Fukushima, A. Rebamipide increases barrier function and attenuates TNFalpha-induced barrier disruption and cytokine expression in human corneal epithelial cells. Br. J. Ophthalmol. 2013, 97, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Ichikawa, T.; Okada, S.; Ogawa, M.; Koike, T.; Ohara, S.; Shimosegawa, T. Rebamipide, a cytoprotective drug, increases gastric mucus secretion in human: Evaluations with endoscopic gastrin test. Dig. Dis. Sci. 2009, 54, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Nanke, Y.; Kobashigawa, T.; Yago, T.; Kawamoto, M.; Yamanaka, H.; Kotake, S. Rebamipide, an Amino Acid Analog of 2(1H)-Quinolinone, Inhibits the Formation of Human Osteoclasts. Biomed. Res. Int. 2016, 6824719. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kim, J.I.; Kim, J.K.; Han, J.Y.; Park, S.H.; Choi, K.Y.; Chung, I.S. Preventive effects of Rebamipide on nsaid-induced gastric mucosal injury and reduction of gastric mucosal blood flow in healthy volunteers. Dig. Dis. Sci. 2007, 52, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Banan, A.; Fitzpatrick, L.; Zhang, Y.; Keshavarzian, A. Opc-compounds prevent oxidant-induced carbonylation and depolymerization of the f-actin cytoskeleton and intestinal barrier hyperpermeability. Free Radic. Biol. Med. 2001, 30, 287–298. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Naito, Y.; Tanigawa, T.; Kondo, M. Free radical scavenging activity of the novel anti-ulcer agent rebsamipide studied by electron spin resonance. Arzneimittelforschung 1993, 43, 363–366. [Google Scholar] [PubMed]
- Murakami, K.; Okajima, K.; Uchiba, M.; Harada, N.; Johno, M.; Okabe, H.; Takatsuki, K. Rebamipide attenuates indomethacin-induced gastric mucosal lesion formation by inhibiting activation of leukocytes in rats. Dig. Dis. Sci. 1997, 42, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Kobayashi, K.; Yoshikawa, T.; Tarnawski, A. Rebamipide: Overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig. Dis. Sci. 1998, 43, 5S–13S. [Google Scholar]
- Zhang, S.; Qing, Q.; Bai, Y.; Mao, H.; Zhu, W.; Chen, Q.; Zhang, Y.; Chen, Y. Rebamipide helps defend against nonsteroidal anti-inflammatory drugs induced gastroenteropathy: A systematic review and meta-analysis. Dig. Dis Sci. 2013, 58, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Vong, L.B.; Chonpathompikunlert, P.; Yoshitomi, T.; Matsui, H.; Nagasaki, Y. Suppression of NSAID-induced small intestinal inflammation by orally administered redox nanoparticles. Biomaterials 2013, 34, 8393–8400. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Watanabe, A.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal permeability of estradiol using combination of PLGA nanoparticles system and iontophoresis. Colloids Surf. B Biointerfaces 2012, 97, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Terashima, H.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal delivery of indomethacin using combination of PLGA nanoparticles and iontophoresis in vivo. Colloids Surf. B Biointerfaces 2012, 92, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Terashima, H.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal delivery of indomethacin-loaded PLGA nanoparticles by iontophoresis. Colloids Surf. B Biointerfaces 2011, 88, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Yoshioka, T.; Lucarelli, M.; Hwang, L.H.; Langer, R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm. Res. 1991, 8, 713–720. [Google Scholar] [CrossRef]
- Nagai, N.; Iwamae, A.; Tanimoto, S.; Yoshioka, C.; Ito, Y. Pharmacokinetics and Antiinflammatory Effect of a Novel Gel System Containing Ketoprofen Solid Nanoparticles. Biol. Pharm. Bull. 2015, 38, 1918–1924. [Google Scholar] [CrossRef]
- Nagai, N.; Yoshioka, C.; Ito, Y.; Funakami, Y.; Nishikawa, H.; Kawabata, A. Intravenous Administration of Cilostazol Nanoparticles Ameliorates Acute Ischemic Stroke in a Cerebral Ischemia/Reperfusion-Induced Injury Model. Int. J. Mol. Sci. 2015, 16, 29329–29344. [Google Scholar] [CrossRef]
- Nagai, N.; Deguchi, S.; Otake, H.; Hiramatsu, N.; Yamamoto, N. Therapeutic Effect of Cilostazol Ophthalmic Nanodispersions on Retinal Dysfunction in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 1971. [Google Scholar] [CrossRef]
- Nagai, N.; Ono, H.; Hashino, M.; Ito, Y.; Okamoto, N.; Shimomura, Y. Improved corneal toxicity and permeability of tranilast by the preparation of ophthalmic formulations containing its nanoparticles. J. Oleo Sci. 2014, 63, 177–186. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y. Effect of solid nanoparticle of indomethacin on therapy for rheumatoid arthritis in adjuvant-induced arthritis rat. Biol. Pharm. Bull. 2014, 37, 1109–1118. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef]
- Yoshioka, C.; Ito, Y.; Nagai, N. An oral formulation of cilostazol nanoparticles enhances intestinal drug absorption in rats. Exp. Ther. Med. 2018, 15, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Xiu, Z.; Shen, H.; Tian, Y.; Xia, L.; Lu, J. Serum and synovial fluid levels of tumor necrosis factor-like ligand 1A and decoy receptor 3 in rheumatoid arthritis. Cytokine 2015, 72, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Asenso, J.; Yang, X.D.; Yu, J.; Zhou, P.; Wang, C.; Wei, W. Plant-based anti-inflammatory agents: Progress from Africa and China. Clin. Anti-Inflamm. Anti-Allerg Drugs 2015, 2, 52–66. [Google Scholar]
- Billingham, M.E. Models of arthritis and the search for anti-arthritic drugs. Pharmacol. Ther. 1983, 21, 389–428. [Google Scholar] [CrossRef]
- Sakuma, S.; Nishigaki, F.; Magari, K.; Ogawa, T.; Miyata, S.; Ohkubo, Y.; Goto, T. FK506 is superior to methotrexate in therapeutic effects on advanced stage of rat adjuvant-induced arthritis. Inflamm. Res. 2001, 50, 509–514. [Google Scholar] [CrossRef]
- Kato, S.; Takeuchi, K. Alteration of gastric ulcerogenic and healing responses in rats with adjuvant-induced arthritis. Jpn. J. Pharmacol. 2002, 89, 1–6. [Google Scholar] [CrossRef]
- Hiratsuka, T.; Futagami, S.; Shindo, T.; Hamamoto, T.; Ueki, N.; Suzuki, K.; Shinji, Y.; Kusunoki, M.; Shinoki, K.; Wada, K.; et al. Rebamipide reduces indomethacin-induced gastric injury in mice via down-regulation of ICAM-1 expression. Dig. Dis. Sci. 2005, 50, S84–S89. [Google Scholar] [CrossRef]
- Kleine, A.; Kluge, S.; Peskar, B.M. Stimulation of prostaglandin biosynthesis mediates gastroprotective effect of Rebamipide in rats. Dig. Dis. Sci. 1993, 38, 1441–1449. [Google Scholar] [CrossRef]
- Ishihara, K.; Komuro, Y.; Nishiyama, N.; Yamasaki, K.; Hotta, K. Effect of Rebamipide on mucus secretion by endogenous prostaglandin-independent mechanism in rat gastric mucosa. Arzneimittelforschung 1992, 42, 1462–1466. [Google Scholar] [PubMed]
- Imaeda, H.; Fujimoto, T.; Takahashi, K.; Kasumi, E.; Fujiyama, Y.; Andoh, A. Terminal-restriction fragment length polymorphism (T-RFLP) analysis for changes in the gut microbiota Profiles of indomethacin- and rebamipide-treated mice. Digestion 2012, 86, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, H.; Ogawa, Y.; Kanatsu, K.; Tanaka, A.; Kato, S.; Takeuchi, K. Protective effect of Rebamipide on indomethacin-induced intestinal damage in rats. J. Gastroenterol. Hepatol. 2001, 16, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Fukuoka, Y.; Deguchi, S.; Otake, H.; Tanino, T.; Nagai, N. Energy-Dependent Endocytosis is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine. Int. J. Mol. Sci. 2019, 20, 476. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Sakamoto, R.; Yamamoto, S.; Deguchi, S.; Otake, H.; Tanino, T. Solid Nanocrystals of Rebamipide Promote Recovery from Indomethacin-Induced Gastrointestinal Bleeding. Int. J. Mol. Sci. 2019, 20, 4990. https://doi.org/10.3390/ijms20204990
Nagai N, Sakamoto R, Yamamoto S, Deguchi S, Otake H, Tanino T. Solid Nanocrystals of Rebamipide Promote Recovery from Indomethacin-Induced Gastrointestinal Bleeding. International Journal of Molecular Sciences. 2019; 20(20):4990. https://doi.org/10.3390/ijms20204990
Chicago/Turabian StyleNagai, Noriaki, Ryusuke Sakamoto, Seiji Yamamoto, Saori Deguchi, Hiroko Otake, and Tadatoshi Tanino. 2019. "Solid Nanocrystals of Rebamipide Promote Recovery from Indomethacin-Induced Gastrointestinal Bleeding" International Journal of Molecular Sciences 20, no. 20: 4990. https://doi.org/10.3390/ijms20204990
APA StyleNagai, N., Sakamoto, R., Yamamoto, S., Deguchi, S., Otake, H., & Tanino, T. (2019). Solid Nanocrystals of Rebamipide Promote Recovery from Indomethacin-Induced Gastrointestinal Bleeding. International Journal of Molecular Sciences, 20(20), 4990. https://doi.org/10.3390/ijms20204990