Hydrogel-Mediated DOX⋅HCl/PTX Delivery System for Breast Cancer Therapy
Abstract
1. Introduction
2. Results
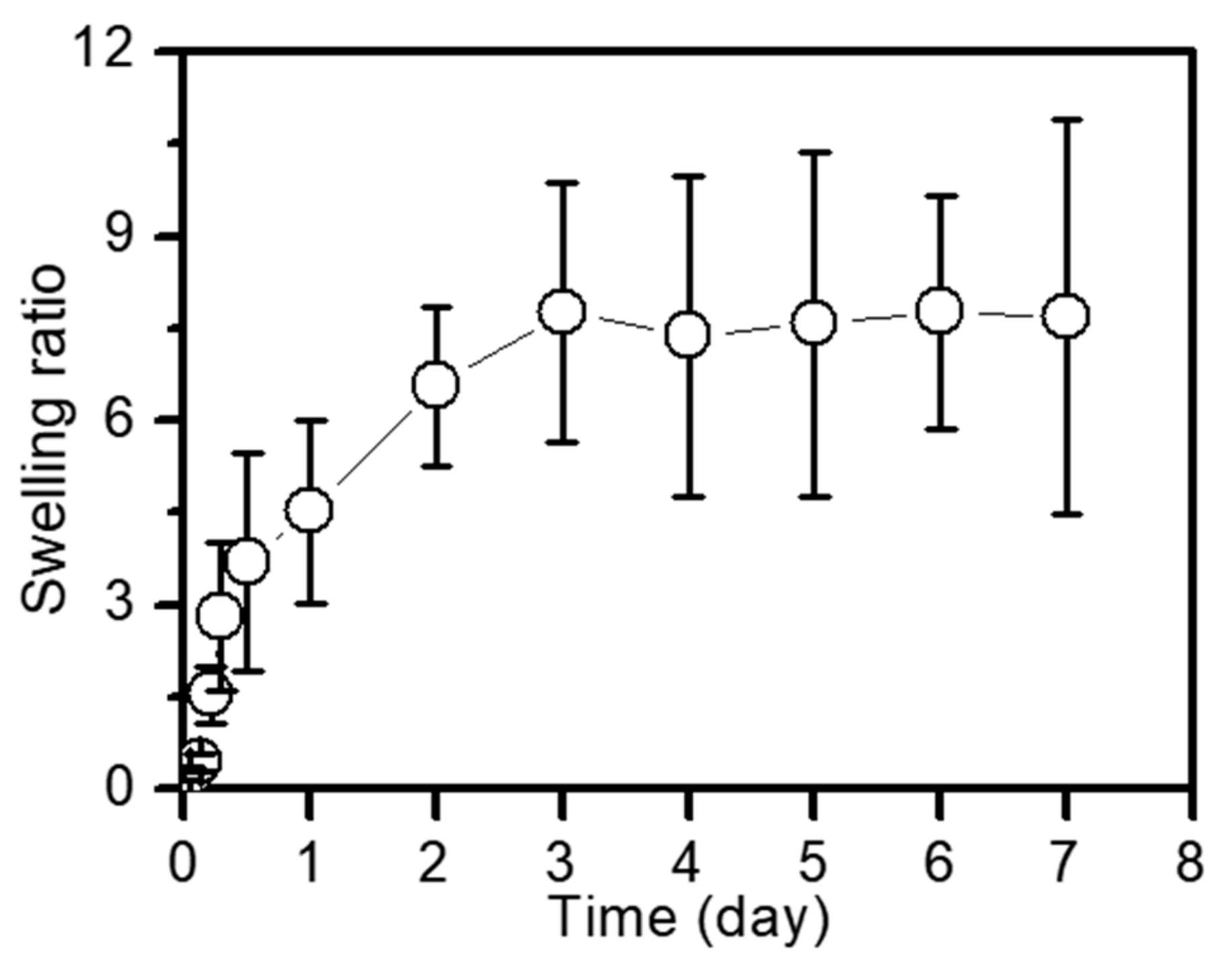
2.1. Preparation and Swelling Ratio of DOX⋅HCl/PTX-loaded GC Hydrogel (GDCP)
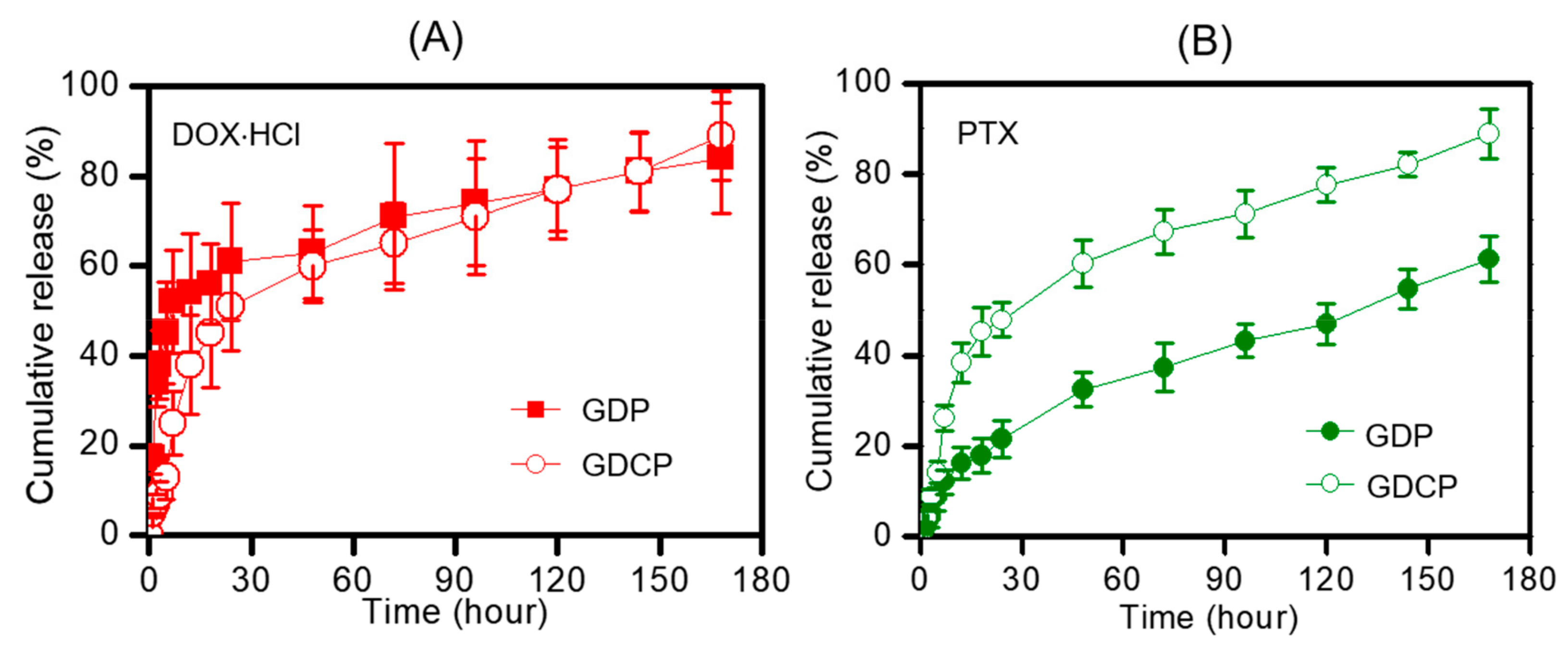
2.2. Release Behavior of DOX⋅HCl and CD/PTX
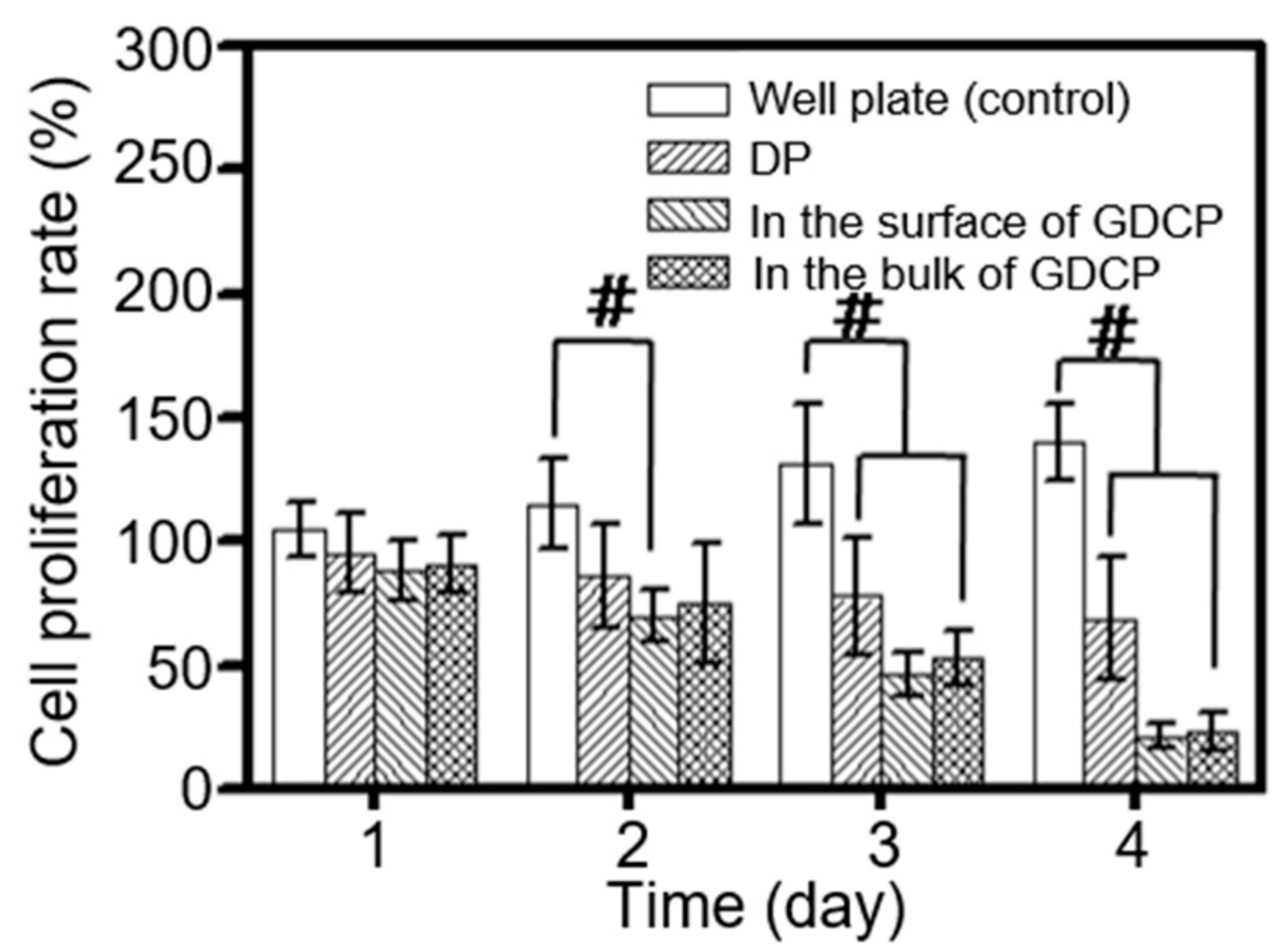
2.3. In Vitro Anticancer Effect
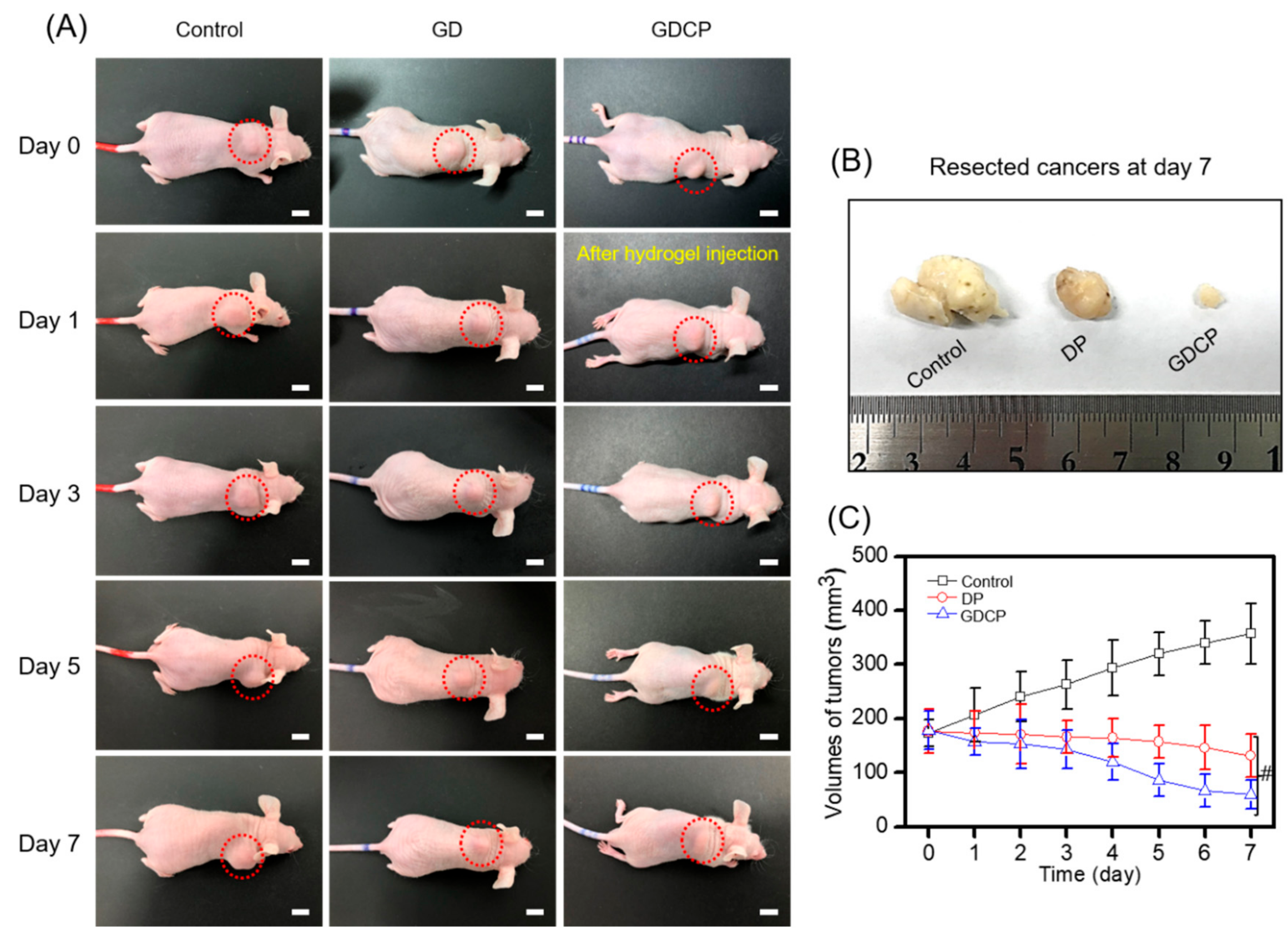
2.4. In Vivo Anticancer Effect
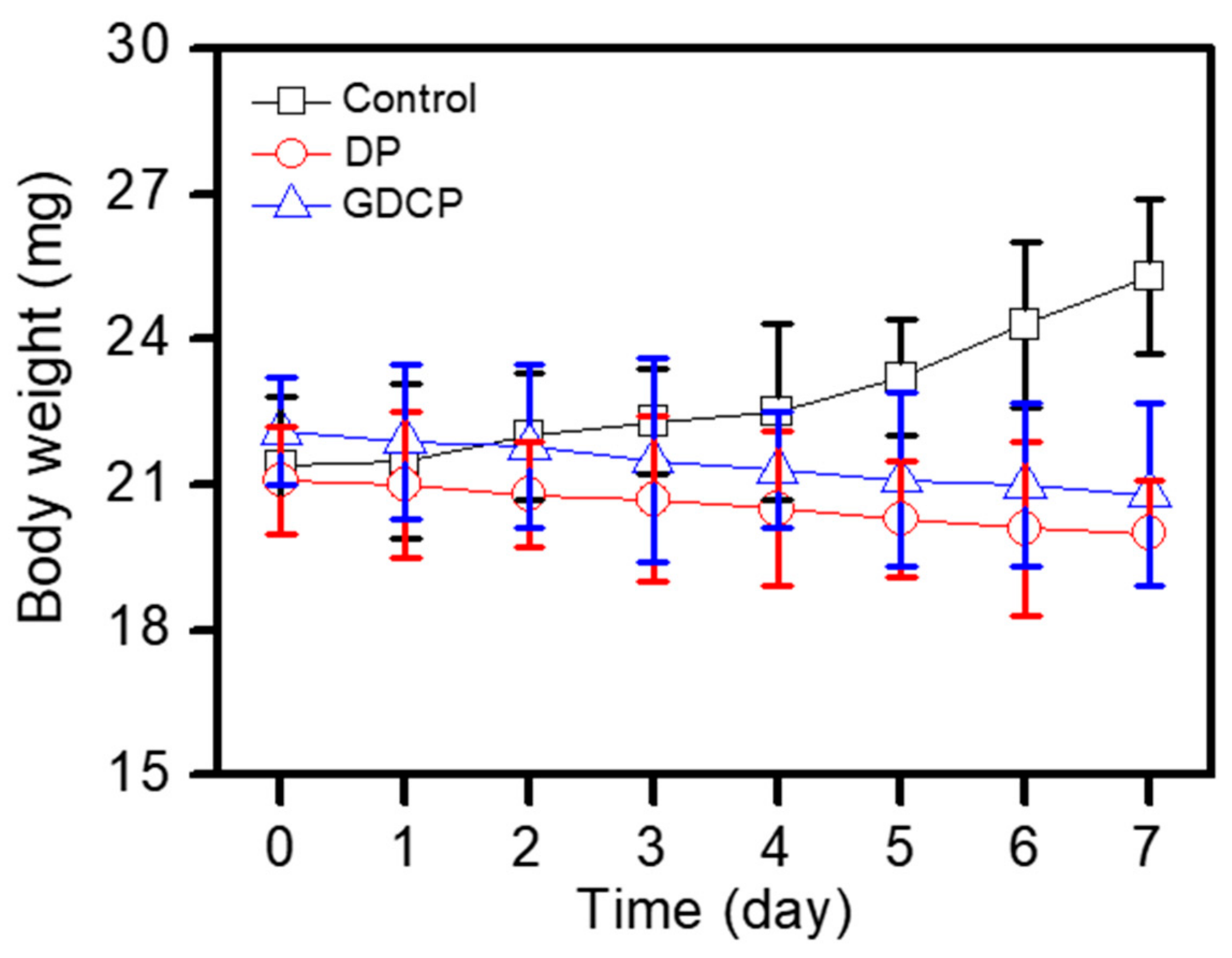
2.5. Systemic Toxicity
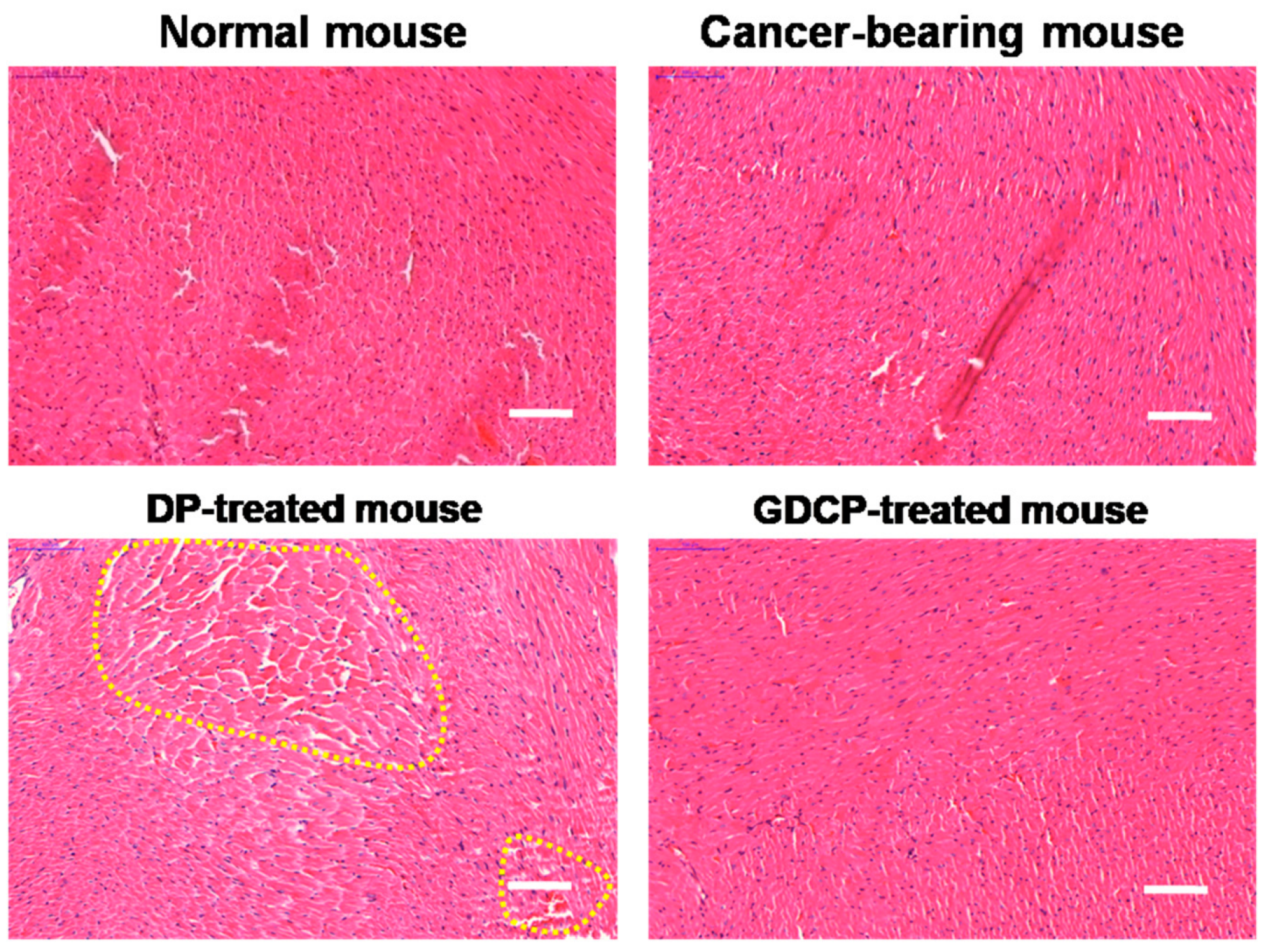
2.6. Cardiotoxicity
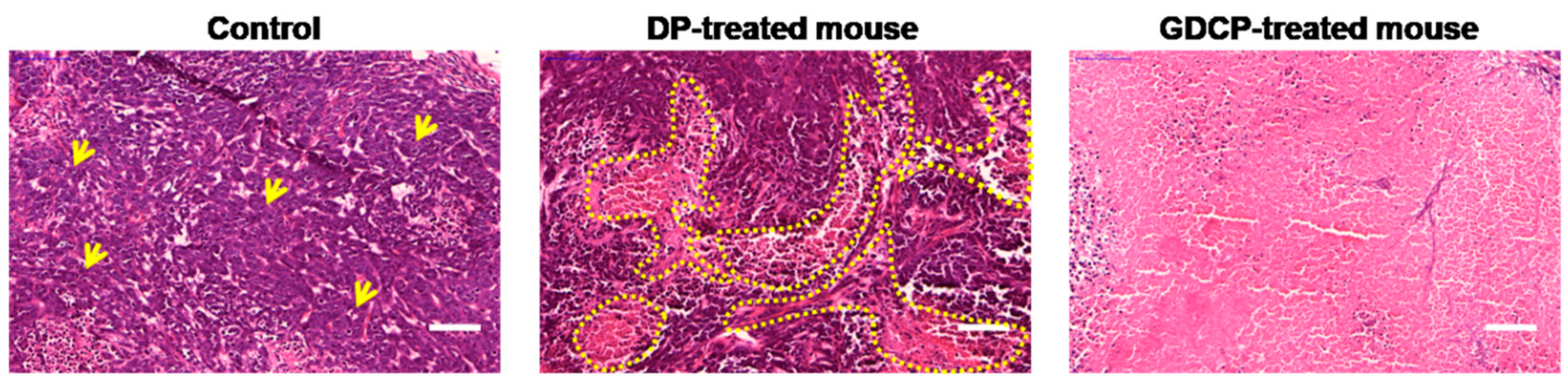
2.7. Histological Evaluations
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. β-CD and PTX Complex (CD/PTX)
4.3. Preparation of Methacrylated GC (GM-GC)
4.4. Preparation of Injectable GDCP Hydrogel
4.5. Release Test of DOX⋅HCl and PTX in GDP and GDCP
4.6. In Vitro Cell Prolferation Rate Measurement
4.7. Establishment of MCF-7 Tumor-Bearing Mouse Model
4.8. Administration of DP and GDCP
4.9. Histological Evaluation
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sopik, V.; Narod, S.A. The relationship between tumour size, nodal status and distant metastases: On the origins of breast cancer. Breast Cancer Res. Treat. 2018, 170, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Kayl, A.E.; Meyers, C.A. Side-effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr. Opin. Obstet. Gynecol. 2006, 18, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Sponchioni, M.; Morbidelli, M.; Moscatelli, D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: The checkpoints on the road from the synthesis to clinical translation. Nanoscale 2018, 10, 22701–22719. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Yoon, S.-J.; Kim, S.Y.; Lee, D.-W.; Um, S.; Hyun, H.; Hong, S.O.; Yang, D.H. A local drug delivery system based on visible light-cured glycol chitosan and doxorubicin⋅hydrochloride for thyroid cancer treatment in vitro and in vivo. Drug Deliv. 2018, 25, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Park, M.H.; Lim, W.; Kim, S.Y.; Jo, D.; Jung, J.S.; Jo, G.; Um, S.; Lee, D.-W.; Yang, D.H. Injectable visible light-cured glycol chitosan hydrogels with controlled release of anticancer drugs for local cancer therapy in vivo: A feasible study. Artf. Cells Nanomed. Biotech. 2018, 46, 874–882. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Jo, G.; Kim, S.Y.; Chun, H.J.; Yang, D.H. Photo-cured glycol chitosan hydrogel for ovarian cancer drug delivery. Mar. Drugs 2019, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.L.; Smith, L.; Tomlinson, D.C. Multidrug-resistant breast cancer: Current perspectives. Breast Cancer (Dove Med Press) 2014, 6, 1–13. [Google Scholar] [PubMed]
- Pérez-Tomás, R. Multidrug resistance: Retrospect and prospects in anti-cancer drug treatment. Curr. Med. Chem. 2006, 13, 1859–1876. [Google Scholar] [CrossRef]
- Lee, J.H.; Nan, A. Combination drug delivery approaches in metastatic breast cancer. J. Drug Deliv. 2012, 2012, 915375. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; Cazenave, L.A.; Onehower, R.C. Taxol: A novel investigational antimicrotubule agent. J. Natl. Cancer Inst. 1990, 82, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.K.; Mimnaugh, E.G.; Rajagopalan, S.; Myers, C.E. Adriamycin activation and oxygen free radical formation in human breast tumor cells: Protective role of glutathione peroxidase in Adriamycin resistance. Cancer Res. 1989, 49, 3844–3848. [Google Scholar]
- Pfitzer, L.; Moser, C.; Gegenfurtner, F.; Arner, A.; Foerster, F.; Atzberger, C.; Zisis, T.; Kubisch-Dohmen, R.; Busse, J.; Smith, R.; et al. Targeting actin inhibits repair of doxorubicin-induced DNA damage: A novel therapeutic approach for combination therapy. Cell Death Dis. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Allen, J.D.; Brinkhuis, R.F.; van Deemter, L.; Wijnholds, J.; Schinkel, A.H. Extensive contribution of the multidrug transporters P-Glycoprotein and Mrp1 to basal drug resistance. Cancer Res. 2000, 60, 5761–5766. [Google Scholar] [PubMed]
- Cole, S.P.; Sparks, K.E.; Fraser, K.; Loe, D.W.; Grant, C.E.; Wilson, G.M.; Deeley, R.G. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994, 54, 5902–5910. [Google Scholar]
- Gehl, J.; Boesgaard, M.; Paaske, T.; Vittrup Jensen, B.; Dombernowsky, P. Combined doxorubicin and paclitaxel in advanced breast cancer: Effective and cardiotoxic. Ann. Oncol. 1996, 7, 687–693. [Google Scholar] [CrossRef]
- Gianni, L.; Vigano, L.; Locatelli, A.; Capri, G.; Giani, A.; Tarenzi, E.; Bonadonna, G. Human pharmacokinetic characterization and in vitro study of the interaction between doxorubicin and paclitaxel in patients with breast cancer. J. Clin. Oncol. 1997, 15, 1906–1915. [Google Scholar] [CrossRef]
- Kim, S.W.; Bae, Y.H.; Okano, T. Hydrogels: Swelling, drug loading, and release. Pharm. Res. 1992, 9, 283–290. [Google Scholar] [CrossRef]
- Priyadarshini, K.; Keerthi Aparajitha, U. Paclitaxel Against Cancer: A Short Review. Med. Chem. 2012, 2, 7. [Google Scholar]
- Jordan, M.A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anticancer Agents 2002, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Takemura, G.; Fujiwara, H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 2007, 49, 330–352. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Q.; Ni, X.W.; Xu, H.L.; Zheng, L.; ZhuGe, D.L.; Chen, B.; Lu, C.T.; Yuan, J.J.; Zhao, Y.Z. Prevention of doxorubicin-induced cardiomyopathy using targeted MaFGF mediated by nanoparticles combined with ultrasound-targeted MB destruction. Int. J. Nanomed. 2017, 12, 7103–7119. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules (Basel Switzerland) 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.T.; Chen, C.H.; Chen, J.P. Intratumoral Delivery of Doxorubicin on Folate-Conjugated Graphene Oxide by In-Situ Forming Thermo-Sensitive Hydrogel for Breast Cancer Therapy. Nanomaterials (Basel Switzerland) 2017, 7, 388. [Google Scholar] [CrossRef]

| Abbreviation | Explanation |
|---|---|
| GDCP | DOX⋅HCl/PTX-complex β-CD-loaded GC hydrogel |
| GDP | DOX⋅HCl/PTX-loaded GC hydrogel |
| DP | DOX⋅HCl/PTX |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, H.; Yoo, Y.B.; Kim, S.Y.; Ko, H.S.; Chun, H.J.; Yang, D.H. Hydrogel-Mediated DOX⋅HCl/PTX Delivery System for Breast Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 4671. https://doi.org/10.3390/ijms20194671
Hyun H, Yoo YB, Kim SY, Ko HS, Chun HJ, Yang DH. Hydrogel-Mediated DOX⋅HCl/PTX Delivery System for Breast Cancer Therapy. International Journal of Molecular Sciences. 2019; 20(19):4671. https://doi.org/10.3390/ijms20194671
Chicago/Turabian StyleHyun, Hoon, Young Bum Yoo, So Yeon Kim, Hyun Sun Ko, Heung Jae Chun, and Dae Hyeok Yang. 2019. "Hydrogel-Mediated DOX⋅HCl/PTX Delivery System for Breast Cancer Therapy" International Journal of Molecular Sciences 20, no. 19: 4671. https://doi.org/10.3390/ijms20194671
APA StyleHyun, H., Yoo, Y. B., Kim, S. Y., Ko, H. S., Chun, H. J., & Yang, D. H. (2019). Hydrogel-Mediated DOX⋅HCl/PTX Delivery System for Breast Cancer Therapy. International Journal of Molecular Sciences, 20(19), 4671. https://doi.org/10.3390/ijms20194671





