The Crossroad of Ion Channels and Calmodulin in Disease
Abstract
1. Universal Calcium Signaling
2. Calmodulin Links Chemical and Electrical Signals
3. Cardiac Calmodulinopathies
4. Cardiac Ryanodine Receptors
5. Cardiac Voltage-Gated Calcium Channels
6. Cardiac Voltage-Gated Sodium Channels
7. Cardiac Voltage-Gated KV7.1 Potassium Channels
8. Neuronal channels
9. TrpV4 Channels
10. SK2 Channels
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BFNS | Benign familial neonatal seizures |
| BrS | Brugada syndrome |
| CaM | Calmodulin |
| CaMBD | CaM-binding domains |
| CaV | Voltage-gated Ca2+-channels |
| CDI | Ca2+-dependent inactivation |
| CDR | Ca2+-dependent regulator |
| CPVT | Catecholaminergic polymorphic ventricular tachycardia |
| DI–DIV | Homologous repeat domains |
| ECC | Excitation–contraction coupling |
| EE | Epileptic encephalopathy |
| GF | Gingival fibromatosis |
| hA | Helix A |
| hB | Helix B |
| hC | Helix C |
| hD | Helix D |
| hiPSC-CM | Human induced pluripotent stem cell-derived cardiomyocytes |
| hTW | TW helix |
| IGE | Idiopathic generalized epilepsy |
| IKs | Slow delayed rectifier potassium current |
| IVF | Idiopathic ventricular fibrillation |
| KV7 | Voltage-gated K+ channels 7 |
| LQTS | Long QT syndrome |
| NaV | Voltage-gated Na+-channels |
| NCX | Na+-Ca2+ exchanger |
| NSCaTE | N-terminal spatial Ca2+ transforming element |
| OS | Ohtahara syndrome |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| RyR | Ryanodine receptor ion channels |
| S1–S6 | Six transmembrane segments |
| SCD | Sudden cardiac death |
| SD | Skeletal dysplasia |
| SK | Small conductance calcium activated potassium channel |
| SQTS | Short QT syndromes |
| SR | Sarcoplasmic reticulum |
| TrpV4 | Transient receptor potential vanilloid subtype 4 |
References
- Hoeflich, K.P.; Ikura, M. Calmodulin in action: Diversity in target recognition and activation mechanisms. Cell 2002, 108, 739–742. [Google Scholar] [CrossRef]
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.Y. Cyclic 3’,5’-nucleotide phosphodiesterase. Demonstration of an activator. Biochem. Biophys. Res. Commun. 1970, 38, 533–538. [Google Scholar] [CrossRef]
- Kakiuchi, S.; Yamazaki, R. Calcium dependent phosphodiesterase activity and its activating factor (PAF) from brain studies on cyclic 3’,5’-nucleotide phosphodiesterase (3). Biochem. Biophys. Res. Commun. 1970, 41, 1104–1110. [Google Scholar] [CrossRef]
- Klee, C.B.; Crouch, T.H.; Richman, P.G. Calmodulin. Annu. Rev. Biochem. 1980, 49, 489–515. [Google Scholar] [CrossRef] [PubMed]
- Tidow, H.; Nissen, P. Structural diversity of calmodulin binding to its target sites. Febs J. 2013, 280, 5551–5565. [Google Scholar] [CrossRef] [PubMed]
- Preston, R.R.; Kink, J.A.; Hinrichsen, R.D.; Saimi, Y.; Kung, C. Calmodulin mutants and Ca2+-dependent channels in Paramecium. Annu. Rev. Physiol. 1991, 53, 309–319. [Google Scholar] [CrossRef]
- Kink, J.A.; Maley, M.E.; Preston, R.R.; Ling, K.Y.; Wallen-Friedman, M.A.; Saimi, Y.; Kung, C. Mutations in paramecium calmodulin indicate functional differences between the C-terminal and N-terminal lobes in vivo. Cell 1990, 62, 165–174. [Google Scholar] [CrossRef]
- Linse, S.; Helmersson, A.; Forsen, S. Calcium binding to calmodulin and its globular domains. J. Biol. Chem. 1991, 266, 8050–8054. [Google Scholar]
- Villarroel, A.; Taglialatela, M.; Bernardo-Seisdedos, G.; Alaimo, A.; Agirre, J.; Alberdi, A.; Gomis-Perez, C.; Soldovieri, M.V.; Ambrosino, P.; Malo, C.; et al. The ever changing moods of calmodulin: How structural plasticity entails transductional adaptability. J. Mol. Biol. 2014, 426, 2717–2735. [Google Scholar] [CrossRef]
- Jurado, L.A.; Chockalingam, P.S.; Jarrett, H.W. Apocalmodulin. Physiol. Rev. 1999, 79, 661–682. [Google Scholar] [CrossRef] [PubMed]
- Ikura, M.; Ames, J.B. Genetic polymorphism and protein conformational plasticity in the calmodulin superfamily: Two ways to promote multifunctionality. Proc. Natl. Acad. Sci. USA 2006, 103, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Rivard, A.F.; Bachinger, H.P.; Adelman, J.P. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature 2001, 410, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, A.; Alberdi, A.; Gomis-Perez, C.; Fernandez-Orth, J.; Bernardo-Seisdedos, G.; Malo, C.; Millet, O.; Areso, P.; Villarroel, A. Pivoting between Calmodulin Lobes Triggered by Calcium in the Kv7.2/Calmodulin Complex. PLoS ONE 2014, 9, e86711. [Google Scholar] [CrossRef] [PubMed]
- Sachyani, D.; Dvir, M.; Strulovich, R.; Tria, G.; Tobelaim, W.; Peretz, A.; Pongs, O.; Svergun, D.; Attali, B.; Hirsch, J.A. Structural Basis of a Kv7.1 Potassium Channel Gating Module: Studies of the Intracellular C-Terminal Domain in Complex with Calmodulin. Structure 2014, 22, 1582–1594. [Google Scholar] [CrossRef]
- Tobelaim, W.S.; Dvir, M.; Lebel, G.; Cui, M.; Buki, T.; Peretz, A.; Marom, M.; Haitin, Y.; Logothetis, D.E.; Hirsch, J.A.; et al. Ca2+-Calmodulin and PIP2 interactions at the proximal C-terminus of Kv7 channels. Channels 2017, 11, 686–695. [Google Scholar] [CrossRef]
- Lee, C.H.; MacKinnon, R. Activation mechanism of a human SK-calmodulin channel complex elucidated by cryo-EM structures. Science 2018, 360, 508–513. [Google Scholar] [CrossRef]
- Kung, C.; Preston, R.R.; Maley, M.E.; Ling, K.Y.; Kanabrocki, J.A.; Seavey, B.R.; Saimi, Y. In vivo Paramecium mutants show that calmodulin orchestrates membrane responses to stimuli. Cell Calcium 1992, 13, 413–425. [Google Scholar] [CrossRef]
- Nyegaard, M.; Overgaard, M.T.; Sondergaard, M.T.; Vranas, M.; Behr, E.R.; Hildebrandt, L.L.; Lund, J.; Hedley, P.L.; Camm, A.J.; Wettrell, G.; et al. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am. J. Hum. Genet. 2012, 91, 703–712. [Google Scholar] [CrossRef]
- Marshall, C.B.; Nishikawa, T.; Osawa, M.; Stathopulos, P.B.; Ikura, M. Calmodulin and STIM proteins: Two major calcium sensors in the cytoplasm and endoplasmic reticulum. Biochem. Biophys. Res. Commun. 2015, 460, 5–21. [Google Scholar] [CrossRef]
- Marsman, R.F.; Tan, H.L.; Bezzina, C.R. Genetics of sudden cardiac death caused by ventricular arrhythmias. Nat. Rev. Cardiol. 2014, 11, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.C.; Tristani-Firouzi, M. hERG potassium channels and cardiac arrhythmia. Nature 2006, 440, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Mohler, P.J.; Schott, J.J.; Gramolini, A.O.; Dilly, K.W.; Guatimosim, S.; DuBell, W.H.; Song, L.S.; Haurogne, K.; Kyndt, F.; Ali, M.E.; et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 2003, 421, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Vatta, M.; Faulkner, G. Cytoskeletal basis of ion channel function in cardiac muscle. Future. Cardiol. 2006, 2, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Marquardt, M.L.; Tester, D.J.; Sampson, K.J.; Ackerman, M.J.; Kass, R.S. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 20990–20995. [Google Scholar] [CrossRef] [PubMed]
- Crotti, L.; Johnson, C.N.; Graf, E.; De Ferrari, G.M.; Cuneo, B.F.; Ovadia, M.; Papagiannis, J.; Feldkamp, M.D.; Rathi, S.G.; et al. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation 2013, 127, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Zuhlke, R.D.; Pitt, G.S.; Deisseroth, K.; Tsien, R.W.; Reuter, H. Calmodulin supports both inactivation and facilitation of l-type calcium channels. Nature 1999, 399, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Colinas, O.; Gallego, M.; Setien, R.; Lopez-Lopez, J.R.; Perez-Garcia, M.T.; Casis, O. Differential modulation of Kv4.2 and Kv4.3 channels by calmodulin-dependent protein kinase II in rat cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1978–H1987. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Fernandez, D.; Ahyayauch, H.; Casis, E.; Casis, O. Reduced calmodulin expression accelerates transient outward potassium current inactivation in diabetic rat heart. Cell Physiol. Biochem. 2008, 22, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Howard, T.; Greer-Short, A.; Satroplus, T.; Patel, N.; Nassal, D.; Mohler, P.J.; Hund, T.J. CaMKII-dependent late Na+ current increases electrical dispersion and arrhythmia in ischemia-reperfusion. Am. J. Physiol Heart Circ. Physiol. 2018, 315, H794–H801. [Google Scholar] [CrossRef]
- Crotti, L.; Kotta, M.C. The role of genetics in primary ventricular fibrillation, inherited channelopathies and cardiomyopathies. Int. J. Cardiol. 2017, 237, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Kotta, M.C.; Sala, L.; Ghidoni, A.; Badone, B.; Ronchi, C.; Parati, G.; Zaza, A.; Crotti, L. Calmodulinopathy: A Novel, Life-Threatening Clinical Entity Affecting the Young. Front. Cardiovasc. Med. 2018, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Badone, B.; Ronchi, C.; Kotta, M.C.; Sala, L.; Ghidoni, A.; Crotti, L.; Zaza, A. Calmodulinopathy: Functional Effects of CALM Mutations and Their Relationship With Clinical Phenotypes. Front. Cardiovasc. Med. 2018, 5, 176. [Google Scholar] [CrossRef] [PubMed]
- George, A.L., Jr. Calmodulinopathy: A genetic trilogy. Heart Rhythm 2015, 12, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.H.; Brohus, M.; Nyegaard, M.; Overgaard, M.T. Human Calmodulin Mutations. Front. Mol. Neurosci. 2018, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Holt, C.; Lu, J.; Brohus, M.; Larsen, K.T.; Overgaard, M.T.; Wimmer, R.; Van, P.F. Arrhythmia mutations in calmodulin cause conformational changes that affect interactions with the cardiac voltage-gated calcium channel. Proc. Natl. Acad. Sci. USA 2018, 115, E10556–E10565. [Google Scholar] [CrossRef]
- Veeraraghavan, R.; Gyorke, S.; Radwanski, P.B. Neuronal sodium channels: Emerging components of the nano-machinery of cardiac calcium cycling. J. Physiol. 2017, 595, 3823–3834. [Google Scholar] [CrossRef] [PubMed]
- Niwa, N.; Nerbonne, J.M. Molecular determinants of cardiac transient outward potassium current (I(to)) expression and regulation. J. Mol. Cell Cardiol. 2010, 48, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, B.H.; Kim, H.J.; Jung, S.W.; Kim, H.S.; Shin, H.C.; Lee, J.H.; Kim, H.C.; Rhim, H.; Hwang, S.H. Ginseng gintonin activates the human cardiac delayed rectifier K+ channel: Involvement of Ca2+/calmodulin binding sites. Mol. Cells 2014, 37, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, B.; Chen-Izu, Y.; Jian, Z.; Shimkunas, R.; Izu, L.T.; Banyasz, T. KN-93 inhibits IKr in mammalian cardiomyocytes. J. Mol. Cell Cardiol. 2015, 89, 173–176. [Google Scholar] [CrossRef]
- Qu, Y.J.; Bondarenko, V.E.; Xie, C.; Wang, S.; Awayda, M.S.; Strauss, H.C.; Morales, M. W-7 modulates Kv4.3: Pore block and Ca2+-calmodulin inhibition. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2364–H2377. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Napolitano, C.; Memmi, M.; Colombi, B.; Drago, F.; Gasparini, M.; DeSimone, L.; Coltorti, F.; Bloise, R.; Keegan, R.; et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 2002, 106, 69–74. [Google Scholar] [CrossRef]
- Yano, M.; Yamamoto, T.; Ikeda, Y.; Matsuzaki, M. Mechanisms of Disease: Ryanodine receptor defects in heart failure and fatal arrhythmia. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Chopra, N.; Knollmann, B.C. Genetics of sudden cardiac death syndromes. Curr. Opin. Cardiol. 2011, 26, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Laver, D.R. Regulation of the RyR channel gating by Ca2+ and Mg2+. Biophys. Rev. 2018, 10, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yano, M.; Uchinoumi, H.; Hino, A.; Suetomi, T.; Ono, M.; Tateishi, H.; Oda, T.; Okuda, S.; Doi, M.; et al. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem. Biophys. Res. Commun. 2010, 394, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Takahashi, N.; Xu, L.; Smithies, O.; Meissner, G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca release channel. J. Clin. Invest. 2007, 117, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Chakraborty, A.; Huang, T.Q.; Xu, L.; Gomez, A.C.; Pasek, D.A.; Meissner, G. Cardiac hypertrophy associated with impaired regulation of cardiac ryanodine receptor by calmodulin and S100A1. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H86–H94. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.A.; Caldwell, J.L.; Kistamas, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.E.; Moreno, C.M.; Yuan, C.; Opitz-Araya, X.; Binder, M.D.; Navedo, M.F.; Santana, L.F. Graded Ca2+/calmodulin-dependent coupling of voltage-gated CaV1.2 channels. Elife 2015, 4, e05608. [Google Scholar] [CrossRef] [PubMed]
- Limpitikul, W.B.; Dick, I.E.; Joshi-Mukherjee, R.; Overgaard, M.T.; George, A.L., Jr.; Yue, D.T. Calmodulin Mutations Associated with Long QT Syndrome Prevent Inactivation of Cardiac l-type Ca Currents and Promote Proarrhythmic Behavior in Ventricular Myocytes. J. Mol. Cell Cardiol. 2014, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Waddell-Smith, K.E.; Skinner, J.R. Update on the Diagnosis and Management of Familial Long QT Syndrome. Heart Lung Circ. 2016, 25, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, M.; Sala, L.; Dreizehnter, L.; Crotti, L.; Sinnecker, D.; Mura, M.; Pane, L.S.; Altomare, C.; Torre, E.; Mostacciuolo, G.; et al. Elucidating arrhythmogenic mechanisms of long-QT syndrome CALM1-F142L mutation in patient-specific induced pluripotent stem cell-derived cardiomyocytes. Cardiovasc. Res. 2017, 113, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Makita, N.; Yagihara, N.; Crotti, L.; Johnson, C.N.; Beckmann, B.M.; Roh, M.S.; Shigemizu, D.; Lichtner, P.; Ishikawa, T.; Aiba, T.; et al. Novel calmodulin mutations associated with congenital arrhythmia susceptibility. Circ. Cardiovasc. Genet. 2014, 7, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.G.; Liang, H.Y.; Mori, M.X.; Yue, D.T. FRET two-hybrid mapping reveals function and location of l-type Ca2+ channel CaM preassociation. Neuron 2003, 39, 97–107. [Google Scholar] [CrossRef]
- Rhoads, A.R.; Friedberg, F. Sequence motifs for calmodulin recognition. Faseb J. 1997, 11, 331–340. [Google Scholar] [CrossRef]
- Li, Y.; Maleki, M.; Carruthers, N.J.; Stemmer, P.M.; Ngom, A.; Rueda, L. The predictive performance of short-linear motif features in the prediction of calmodulin-binding proteins. BMC Bioinform. 2018, 19, 410. [Google Scholar] [CrossRef]
- Mruk, K.; Farley, B.M.; Ritacco, A.W.; Kobertz, W.R. Calmodulation meta-analysis: Predicting calmodulin binding via canonical motif clustering. J. Gen. Physiol. 2014, 144, 105–114. [Google Scholar] [CrossRef]
- Yap, K.L.; Kim, J.; Truong, K.; Sherman, M.; Yuan, T.; Ikura, M. Calmodulin target database. J. Struct. Funct. Genom. 2000, 1, 8–14. [Google Scholar] [CrossRef]
- Hovey, L.; Fowler, C.A.; Mahling, R.; Lin, Z.; Miller, M.S.; Marx, D.C.; Yoder, J.B.; Kim, E.H.; Tefft, K.M.; Waite, B.C.; et al. Calcium triggers reversal of calmodulin on nested anti-parallel sites in the IQ motif of the neuronal voltage-dependent sodium channel NaV1.2. Biophys. Chem. 2017, 224, 1–19. [Google Scholar]
- Dick, I.E.; Tadross, M.R.; Liang, H.; Tay, L.H.; Yang, W.; Yue, D.T. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature 2008, 451, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Simms, B.A.; Souza, I.A.; Zamponi, G.W. Effect of the Brugada syndrome mutation A39V on calmodulin regulation of Cav1.2 channels. Mol. Brain 2014, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Brugada, P.; Brugada, J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. J. Am. Coll. Cardiol. 1992, 20, 1391–1396. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Pollevick, G.D.; Cordeiro, J.M.; Casis, O.; Sanguinetti, M.C.; Aizawa, Y.; Guerchicoff, A.; Pfeiffer, R.; Oliva, A.; Wollnik, B.; et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation 2007, 115, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Gabelli, S.B.; Yoder, J.B.; Tomaselli, G.F.; Amzel, L.M. Calmodulin and Ca2+ control of voltage gated Na+ channels. Channels 2016, 10, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, C.; Marx, S.O.; Pitt, G.S. Calmodulin limits pathogenic Na+ channel persistent current. J. Gen. Physiol. 2017, 149, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Ben-Johny, M.; Yang, P.S.; Niu, J.; Yang, W.; Joshi-Mukherjee, R.; Yue, D.T. Conservation of Ca2+/calmodulin regulation across Na+ and Ca2+ channels. Cell 2014, 157, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Kapplinger, J.D.; Giudicessi, J.R.; Ye, D.; Tester, D.J.; Callis, T.E.; Valdivia, C.R.; Makielski, J.C.; Wilde, A.A.; Ackerman, M.J. Enhanced Classification of Brugada Syndrome-Associated and Long-QT Syndrome-Associated Genetic Variants in the SCN5A-Encoded NaV1.5 Cardiac Sodium Channel. Circ. Cardiovasc. Genet. 2015, 8, 582–595. [Google Scholar] [CrossRef]
- Winkel, B.G.; Larsen, M.K.; Berge, K.E.; Leren, T.P.; Nissen, P.H.; Olesen, M.S.; Hollegaard, M.V.; Jespersen, T.; Yuan, L.; Nielsen, N.; et al. The prevalence of mutations in KCNQ1, KCNH2, and SCN5A in an unselected national cohort of young sudden unexplained death cases. J. Cardiovasc. Electrophysiol. 2012, 23, 1092–1098. [Google Scholar] [CrossRef]
- Abdelsayed, M.; Baruteau, A.E.; Gibbs, K.; Sanatani, S.; Krahn, A.D.; Probst, V.; Ruben, P.C. Differential calcium sensitivity in NaV1.5 mixed syndrome mutants. J. Physiol. 2017, 595, 6165–6186. [Google Scholar] [CrossRef]
- Chagot, B.; Chazin, W.J. Solution NMR structure of Apo-calmodulin in complex with the IQ motif of human cardiac sodium channel NaV1.5. J. Mol. Biol. 2011, 406, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Potet, F.; Thompson, M.K.; Kroncke, B.M.; Glazer, A.M.; Voehler, M.W.; Knollmann, B.C.; George, A.L., Jr.; Chazin, W.J. A Mechanism of Calmodulin Modulation of the Human Cardiac Sodium Channel. Structure 2018, 26, 683–694. [Google Scholar] [CrossRef]
- Hedley, P.L.; Jorgensen, P.; Schlamowitz, S.; Wangari, R.; Moolman-Smook, J.; Brink, P.A.; Kanters, J.K.; Corfield, V.A.; Christiansen, M. The genetic basis of long QT and short QT syndromes: A mutation update. Hum. Mutat. 2009, 30, 1486–1511. [Google Scholar] [CrossRef] [PubMed]
- Kapplinger, J.D.; Tester, D.J.; Alders, M.; Benito, B.; Berthet, M.; Brugada, J.; Brugada, P.; Fressart, V.; Guerchicoff, A.; Harris-Kerr, C.; et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm 2010, 7, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, I.; Baroudi, G.; Berthet, M.; Barde, I.; Chalvidan, T.; Denjoy, I.; Guicheney, P.; Chahine, M. Electrophysiological characterization of SCN5A mutations causing long QT (E1784K) and Brugada (R1512W and R1432G) syndromes. Cardiovasc. Res. 2000, 46, 55–65. [Google Scholar] [CrossRef]
- Yokoi, H.; Makita, N.; Sasaki, K.; Takagi, Y.; Okumura, Y.; Nishino, T.; Makiyama, T.; Kitabatake, A.; Horie, M.; Watanabe, I.; et al. Double SCN5A mutation underlying asymptomatic Brugada syndrome. Heart Rhythm. 2005, 2, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Gardill, B.R.; Rivera-Acevedo, R.E.; Tung, C.C.; Okon, M.; McIntosh, L.P.; Van Petegem, F. The voltage-gated sodium channel EF-hands form an interaction with the III-IV linker that is disturbed by disease-causing mutations. Sci. Rep. 2018, 8, 4483. [Google Scholar] [CrossRef] [PubMed]
- Olesen, M.S.; Yuan, L.; Liang, B.; Holst, A.G.; Nielsen, N.; Nielsen, J.B.; Hedley, P.L.; Christiansen, M.; Olesen, S.P.; Haunso, S.; et al. High prevalence of long QT syndrome-associated SCN5A variants in patients with early-onset lone atrial fibrillation. Circ. Cardiovasc. Genet. 2012, 5, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, Z.; Zhou, Q.; Shen, H.; Wu, X.; Huang, X.; Chen, J.; Zhang, J.; Zhu, X.; Lei, J.; et al. Structure of the human voltage-gated sodium channel Nav1.4 in complex with beta1. Science 2018, 362, eaau2486. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhou, Q.; Pan, X.; Li, Z.; Wu, J.; Yan, N. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science 2017, 355, eaal4326. [Google Scholar] [CrossRef]
- Kim, J.; Ghosh, S.; Liu, H.; Tateyama, M.; Kass, R.S.; Pitt, G.S. Calmodulin mediates Ca2+ sensitivity of sodium channels. J. Biol. Chem. 2004, 279, 45004–45012. [Google Scholar] [CrossRef] [PubMed]
- Gabelli, S.B.; Boto, A.; Kuhns, V.H.; Bianchet, M.A.; Farinelli, F.; Aripirala, S.; Yoder, J.; Jakoncic, J.; Tomaselli, G.F.; Amzel, L.M. Regulation of the NaV1.5 cytoplasmic domain by calmodulin. Nat. Commun. 2014, 5, 5126. [Google Scholar] [CrossRef] [PubMed]
- Clatot, J.; Hoshi, M.; Wan, X.; Liu, H.; Jain, A.; Shinlapawittayatorn, K.; Marionneau, C.; Ficker, E.; Ha, T.; Deschenes, I. Voltage-gated sodium channels assemble and gate as dimers. Nat. Commun. 2017, 8, 2077. [Google Scholar] [CrossRef]
- Yus-Nájera, E.; Santana-Castro, I.; Villarroel, A. The identification and characterization of a noncontinuous calmodulin-binding site in noninactivating voltage-dependent KCNQ potassium channels. J. Biol. Chem. 2002, 277, 28545–28553. [Google Scholar] [CrossRef]
- Ghosh, S.; Nunziato, D.A.; Pitt, G.S. KCNQ1 assembly and function is blocked by long-QT syndrome mutations that disrupt interaction with calmodulin. Circ. Res. 2006, 98, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Shamgar, L.; Ma, L.J.; Schmitt, N.; Haitin, Y.; Peretz, A.; Wiener, R.; Hirsch, J.; Pongs, O.; Attali, B. Calmodulin is essential for cardiac IKs channel gating and assembly—Impaired function in long-QT mutations. Circ. Res. 2006, 98, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; MacKinnon, R. Cryo-EM Structure of a KCNQ1/CaM Complex Reveals Insights into Congenital Long QT Syndrome. Cell 2017, 169, 1042–1050. [Google Scholar] [CrossRef]
- Schmitt, N.; Calloe, K.; Nielsen, N.H.; Buschmann, M.; Speckmann, E.J.; Schulze-Bahr, E.; Schwarz, M. The novel C-terminal KCNQ1 mutation M520R alters protein trafficking. Biochem. Biophys. Res. Commun. 2007, 358, 304–310. [Google Scholar] [CrossRef]
- Tobelaim, W.S.; Dvir, M.; Lebel, G.; Cui, M.; Buki, T.; Peretz, A.; Marom, M.; Haitin, Y.; Logothetis, D.E.; Hirsch, J.A.; et al. Competition of calcified calmodulin N lobe and PIP2 to an LQT mutation site in Kv7.1 channel. Proc. Natl. Acad. Sci. USA 2017, 114, E869–E878. [Google Scholar] [CrossRef]
- Chang, A.; Abderemane-Ali, F.; Hura, G.L.; Rossen, N.D.; Gate, R.E.; Minor, D.L., Jr. A Calmodulin C-Lobe Ca2+-Dependent Switch Governs Kv7 Channel Function. Neuron 2018, 97, 836–852. [Google Scholar] [CrossRef]
- Bernardo-Seisdedos, G.; Nunez, E.; Gomis, C.; Malo, C.; Villarroel, A.; Millet, O. Structural basis and energy landscape for the Ca2+ gating and calmodulation of the Kv7.2 K+ channel. Proc. Natl. Acad. Sci. USA 2018, 115, 2395–2400. [Google Scholar] [CrossRef]
- Tommiska, J.; Kansakoski, J.; Skibsbye, L.; Vaaralahti, K.; Liu, X.; Lodge, E.J.; Tang, C.; Yuan, L.; Fagerholm, R.; Kanters, J.K.; et al. Two missense mutations in KCNQ1 cause pituitary hormone deficiency and maternally inherited gingival fibromatosis. Nat. Commun. 2017, 8, 1289. [Google Scholar] [CrossRef]
- Larsen, L.A.; Fosdal, I.; Andersen, P.S.; Kanters, J.K.; Vuust, J.; Wettrell, G.; Christiansen, M. Recessive Romano-Ward syndrome associated with compound heterozygosity for two mutations in the KVLQT1 gene. Eur. J. Hum. Genet. 1999, 7, 724–728. [Google Scholar] [CrossRef]
- Antunez-Arguelles, E.; Rojo-Dominguez, A.; Arregui-Mena, A.L.; Jacobo-Albavera, L.; Marquez, M.F.; Iturralde-Torres, P.; Villarreal-Molina, M.T. Compound heterozygous KCNQ1 mutations (A300T/P535T) in a child with sudden unexplained death: Insights into possible molecular mechanisms based on protein modeling. Gene 2017, 627, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zaydman, M.A.; Wu, D.; Shi, J.; Guan, M.; Virgin-Downey, B.; Cui, J. KCNE1 enhances phosphatidylinositol 4,5-bisphosphate (PIP2) sensitivity of IKs to modulate channel activity. Proc. Natl. Acad. Sci. USA 2011, 108, 9095–9100. [Google Scholar] [CrossRef]
- Kang, S.; Xu, M.; Cooper, E.C.; Hoshi, N. Channel anchored protein kinase CK2 and protein phosphatase 1 reciprocally regulate KCNQ2-containing M-channels via phosphorylation of calmodulin. J. Biol. Chem. 2014, 289, 11536–11544. [Google Scholar] [CrossRef] [PubMed]
- Gomis-Perez, C.; Soldovieri, M.V.; Malo, C.; Ambrosino, P.; Taglialatela, M.; Areso, P.; Villarroel, A. Differential Regulation of PI(4,5)P2 Sensitivity of Kv7.2 and Kv7.3 Channels by Calmodulin. Front. Mol. Neurosci. 2017, 10, 117. [Google Scholar] [CrossRef]
- Haug, K.; Hallmann, K.; Rebstock, J.; Dullinger, J.; Muth, S.; Haverkamp, F.; Pfeiffer, H.; Rau, B.; Elger, C.E.; Propping, P.; et al. The voltage-gated sodium channel gene SCN2A and idiopathic generalized epilepsy. Epilepsy Res. 2001, 47, 243–246. [Google Scholar] [CrossRef]
- Wang, C.; Chung, B.C.; Yan, H.; Wang, H.G.; Lee, S.Y.; Pitt, G.S. Structural analyses of Ca2+/CaM interaction with NaV channel C-termini reveal mechanisms of calcium-dependent regulation. Nat. Commun. 2014, 5, 4896. [Google Scholar] [CrossRef] [PubMed]
- Biervert, C.; Schroeder, B.C.; Kubisch, C.; Berkovic, S.F.; Propping, P.; Jentsch, T.J.; Steinlein, O.K. A potassium channel mutation in neonatal human epilepsy. Science 1998, 279, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Charlier, C.; Stauffer, D.; DuPont, B.R.; Leach, R.J.; Melis, R.; Ronen, G.M.; Bjerre, I.; Quattlebaum, T.; Murphy, J.V.; et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat. Genet. 1998, 18, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Singh, N.A.; Ryan, S.G.; Lewis, T.B.; Reus, B.E.; Leach, R.J.; Leppert, M. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat. Genet. 1998, 18, 53–55. [Google Scholar] [CrossRef]
- Kato, M.; Yamagata, T.; Kubota, M.; Arai, H.; Yamashita, S.; Nakagawa, T.; Fujii, T.; Sugai, K.; Imai, K.; Uster, T.; et al. Clinical spectrum of early onset epileptic encephalopathies caused by KCNQ2 mutation. Epilepsia 2013, 54, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Weckhuysen, S.; Mandelstam, S.; Suls, A.; Audenaert, D.; Deconinck, T.; Claes, L.R.; Deprez, L.; Smets, K.; Hristova, D.; Yordanova, I.; et al. KCNQ2 encephalopathy: Emerging phenotype of a neonatal epileptic encephalopathy. Ann. Neurol. 2012, 71, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, A.; Etxeberria, A.; Gomez-Posada, J.C.; Gomis-Perez, C.; Fernandez-Orth, J.; Malo, C.; Villarroel, A. Lack of correlation between surface expression and currents in epileptogenic AB-calmodulin binding domain Kv7.2 potassium channel mutants. Channels 2018, 12, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Zhang, J.; Pang, W.; Wang, S.; Lee, K.Y.; Cavaretta, J.P.; Walters, J.; Procko, E.; Tsai, N.P.; Chung, H.J. Reduced axonal surface expression and phosphoinositide sensitivity in Kv7 channels disrupts their function to inhibit neuronal excitability in Kcnq2 epileptic encephalopathy. Neurobiol. Dis. 2018, 118, 76–93. [Google Scholar] [CrossRef]
- Bellini, G.; Miceli, F.; Soldovieri, M.V.; Miraglia del, G.E.; Coppola, G.; Taglialatela, M. KCNQ2-Related Disorders. 2010. Available online: http://www.ncbi.nlm.nih.gov/books/NBK32534/ (accessed on 1 December 2018).
- Etxeberria, A.; Aivar, P.; Rodriguez-Alfaro, J.A.; Alaimo, A.; Villace, P.; Gomez-Posada, J.C.; Areso, P.; Villarroel, A. Calmodulin regulates the trafficking of KCNQ2 potassium channels. Faseb J. 2008, 22, 1135–1143. [Google Scholar] [CrossRef]
- Ambrosino, P.; Alaimo, A.; Bartollino, S.; Manocchio, L.; De, M.M.; Mosca, I.; Gomis-Perez, C.; Alberdi, A.; Scambia, G.; Lesca, G.; et al. Epilepsy-causing mutations in Kv7.2 C-terminus affect binding and functional modulation by calmodulin. Biochim. Biophys. Acta 2015, 1852, 1856–1866. [Google Scholar] [CrossRef]
- Alaimo, A.; Gomez-Posada, J.C.; Aivar, P.; Etxeberria, A.; Rodriguez-Alfaro, J.A.; Areso, P.; Villarroel, A. Calmodulin activation limits the rate of KCNQ2 K+ channel exit from the endoplasmic reticulum. J. Biol. Chem. 2009, 284, 20668–20675. [Google Scholar] [CrossRef]
- Cavaretta, J.P.; Sherer, K.R.; Lee, K.Y.; Kim, E.H.; Issema, R.S.; Chung, H.J. Polarized axonal surface expression of neuronal KCNQ potassium channels is regulated by calmodulin interaction with KCNQ2 subunit. PLoS ONE 2014, 9, e103655. [Google Scholar] [CrossRef]
- Volkers, L.; Rook, M.B.; Das, J.H.; Verbeek, N.E.; Groenewegen, W.A.; van Kempen, M.J.; Lindhout, D.; Koeleman, B.P. Functional analysis of novel KCNQ2 mutations found in patients with Benign Familial Neonatal Convulsions. Neurosci. Lett. 2009, 462, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Borgatti, R.; Zucca, C.; Cavallini, A.; Ferrario, M.; Panzeri, C.; Castaldo, P.; Soldovieri, M.V.; Baschirotto, C.; Bresolin, N.; Dalla Bernardina, B.; et al. A novel mutation in KCNQ2 associated with BFNC, drug resistant epilepsy, and mental retardation. Neurology 2004, 63, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Weckhuysen, S.; Ivanovic, V.; Hendrickx, R.; van Coster, R.; Hjalgrim, H.; Moller, R.S.; Gronborg, S.; Schoonjans, A.S.; Ceulemans, B.; Heavin, S.B.; et al. Extending the KCNQ2 encephalopathy spectrum: Clinical and neuroimaging findings in 17 patients. Neurology 2013, 81, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, A.; Gomis-Perez, C.; Bernardo-Seisdedos, G.; Alaimo, A.; Malo, C.; Aldaregia, J.; Lopez-Robles, C.; Areso, P.; Butz, E.; Wahl-Schott, C.; et al. Uncoupling PIP2-calmodulin regulation of Kv7.2 channels by an assembly de-stabilizing epileptogenic mutation. J. Cell Sci. 2015, 128, 4014–4023. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, A.; Nunez, E.; Aivar, P.; Fernandez-Orth, J.; Gomis-Perez, C.; Bernardo-Seisdedos, G.; Malo, C.; Villarroel, A. Calmodulin confers calcium sensitivity to the stability of the distal intracellular assembly domain of Kv7.2 channels. Sci. Rep. 2017, 7, 13425. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Jan, Y.N.; Jan, L.Y. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc. Natl. Acad. Sci. USA 2006, 103, 8870–8875. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J. Role of calmodulin in neuronal Kv7/KCNQ potassium channels and epilepsy. Front. Biol. 2014, 9, 205–215. [Google Scholar] [CrossRef]
- Cruzblanca, H.; Koh, D.S.; Hille, B. Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proc. Natl. Acad. Sci. USA 1998, 95, 7151–7156. [Google Scholar] [CrossRef] [PubMed]
- Selyanko, A.A.; Brown, D.A. Intracellular calcium directly inhibits potassium M channels in excised membrane patches from rat sympathetic neurons. Neuron 1996, 16, 151–162. [Google Scholar] [CrossRef]
- Alaimo, A.; Alberdi, A.; Gomis-Perez, C.; Fernandez-Orth, J.; Gomez-Posada, J.C.; Areso, P.; Villarroel, A. Cooperativity between calmodulin-binding sites in Kv7.2 channels. J. Cell Sci. 2013, 126, 244–253. [Google Scholar] [CrossRef]
- Strulovich, R.; Tobelaim, W.S.; Attali, B.; Hirsch, J.A. Structural Insights into the M-Channel Proximal C-Terminus/Calmodulin Complex. Biochemistry 2016, 55, 5353–5365. [Google Scholar] [CrossRef] [PubMed]
- Soldovieri, M.V.; Boutry-Kryza, N.; Milh, M.; Doummar, D.; Heron, B.; Bourel, E.; Ambrosino, P.; Miceli, F.; De, M.M.; Dorison, N.; et al. Novel KCNQ2 and KCNQ3 mutations in a large cohort of families with benign neonatal epilepsy: First evidence for an altered channel regulation by syntaxin-1A. Hum. Mutat. 2014, 35, 356–367. [Google Scholar] [CrossRef]
- Gomis-Perez, C.; Alaimo, A.; Fernandez-Orth, J.; Alberdi, A.; Aivar-Mateo, P.; Bernardo-Seisdedos, G.; Malo, C.; Areso, P.; Felipe, A.; Villarroel, A. Unconventional calmodulin anchoring site within the AB module of Kv7.2 channels. J. Cell Sci. 2015, 128, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Strotmann, R.; Schultz, G.; Plant, T.D. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J. Biol. Chem. 2003, 278, 26541–26549. [Google Scholar] [CrossRef] [PubMed]
- Loukin, S.H.; Teng, J.; Kung, C. A channelopathy mechanism revealed by direct calmodulin activation of TrpV4. Proc. Natl. Acad. Sci. USA 2015, 112, 9400–9405. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Ko, J.S.; Ai, T.; Tsai, W.C.; Chen, Z.; Rubart, M.; Vatta, M.; Everett, T.H.; George, A.L., Jr.; Chen, P.S. Arrhythmogenic calmodulin mutations impede activation of small-conductance calcium-activated potassium current. Heart Rhythm 2016, 13, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
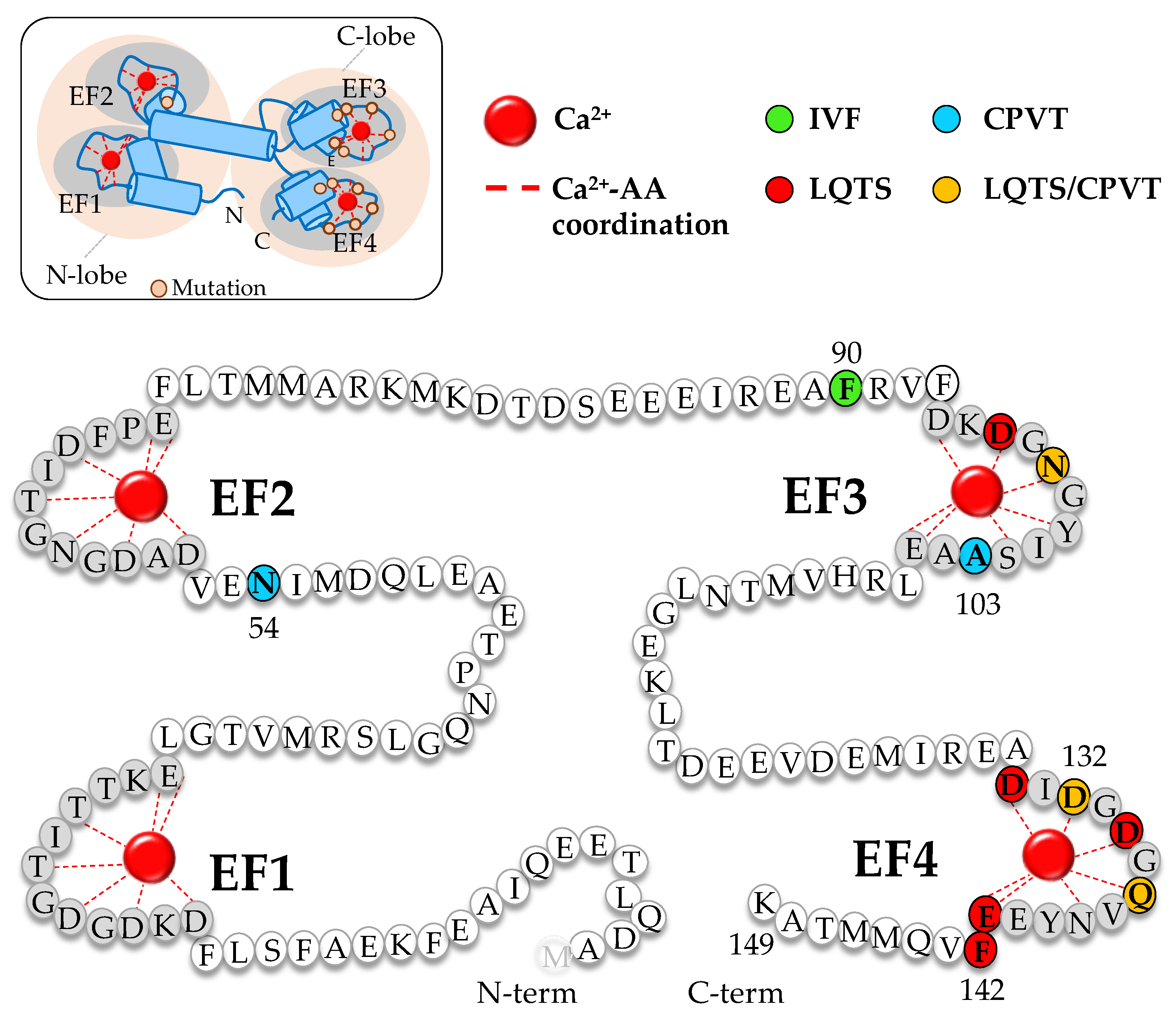
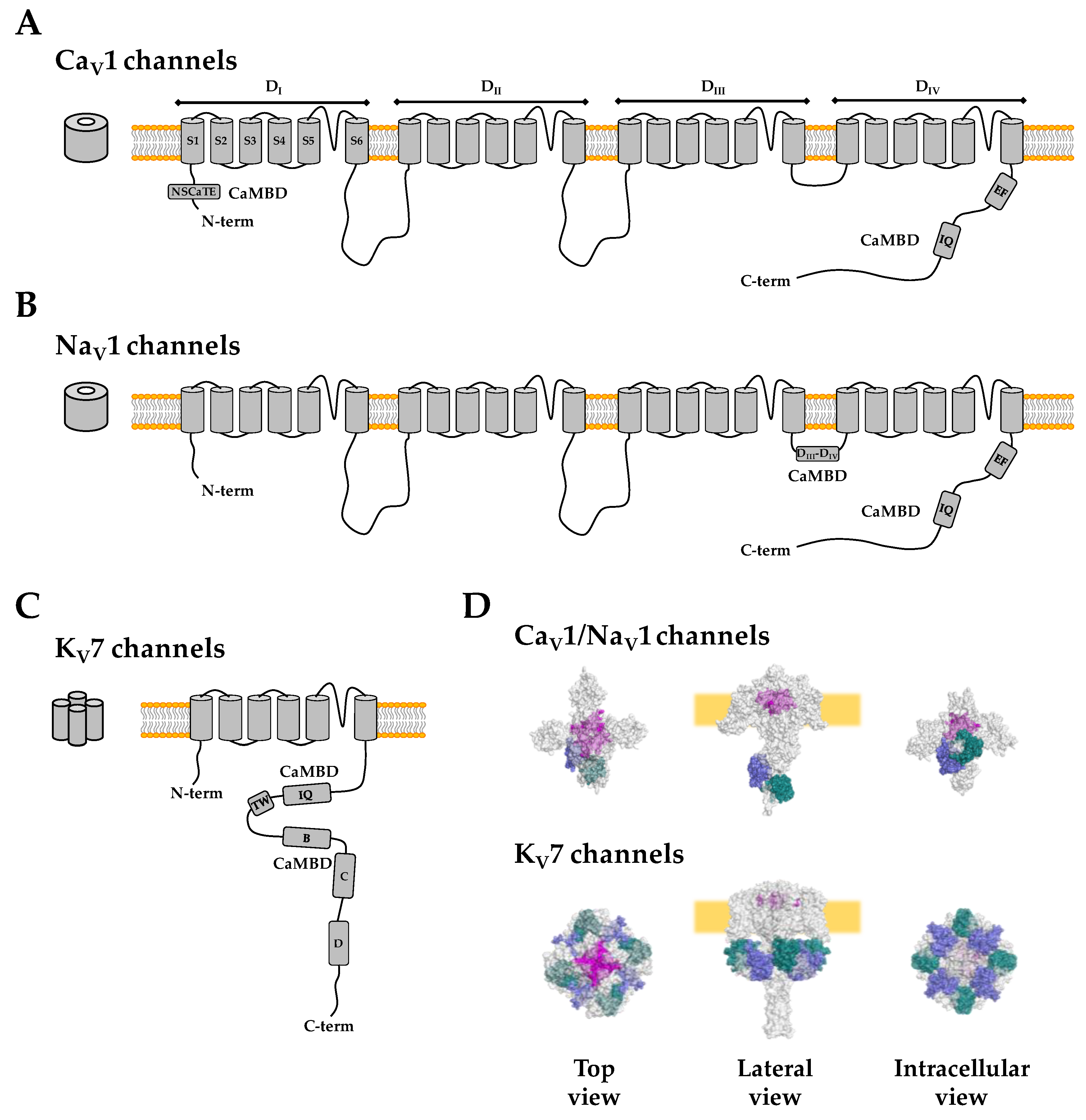
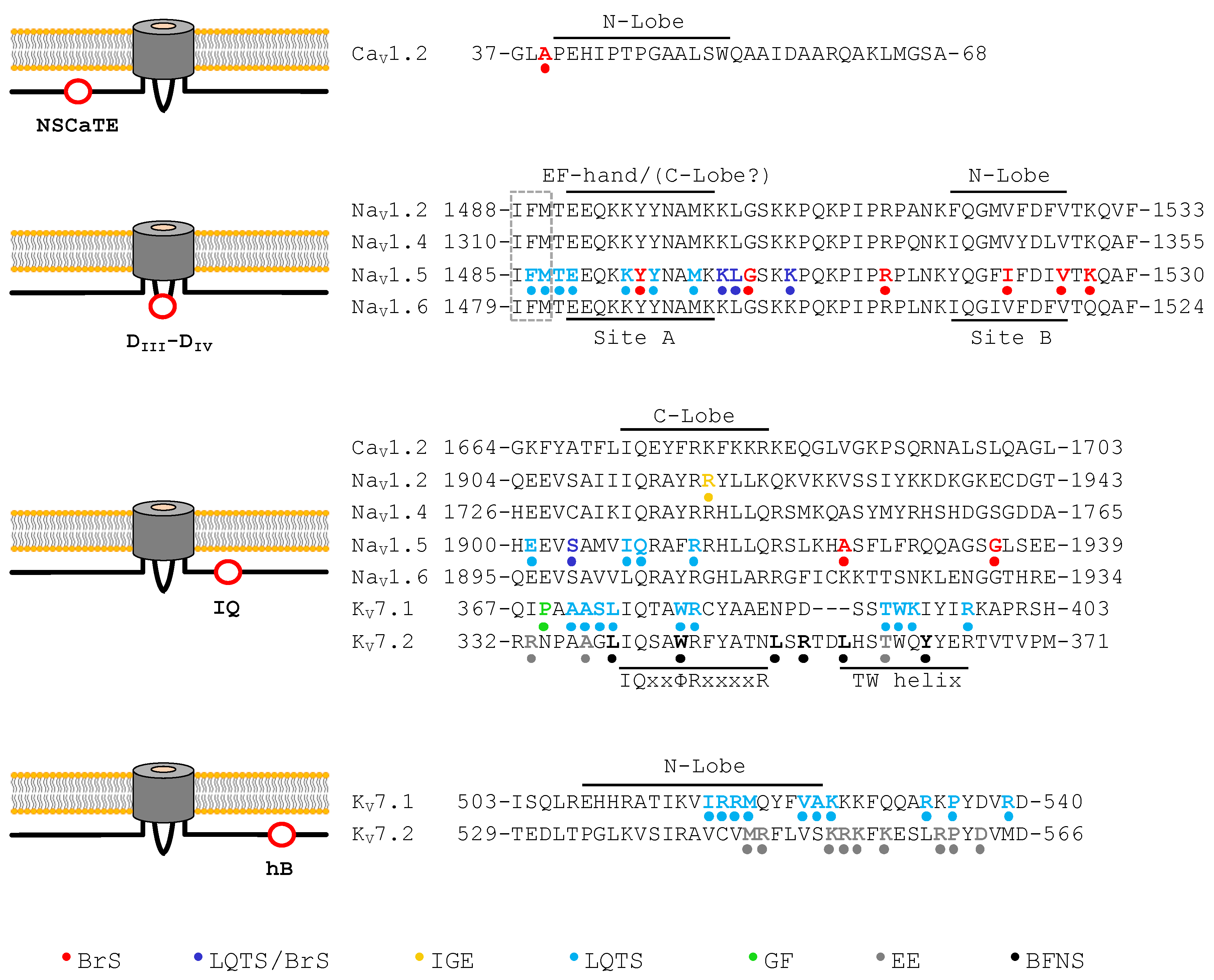
| NaV1.5 Mutation | Location | Interacting Site | Disease | Reference |
|---|---|---|---|---|
| F1486L | IFM motif | / | LQT3 | [73] |
| M1487L | IFM motif | / | LQT3 | [74] |
| T1488R | A DIII–DIV linker | N-lobe | LQT3 | [74] |
| E1489D | A DIII–DIV linker | N-lobe | LQT3 | [74] |
| K1493R | A DIII–DIV linker | N-lobe | LQT3 | [74] |
| Y1494N | A DIII–DIV linker | N-lobe | BrS | [72] |
| Y1495S | A DIII–DIV linker | N-lobe | LQT3 | [73] |
| M1498T | A DIII–DIV linker | N-lobe | LQT3 | [73] |
| L1501V | A DIII–DIV linker | / | LQT3/BrS | [72] |
| G1502S | A DIII–DIV linker | / | BrS | [73] |
| K1505N | DIII–DIV linker | / | LQT3/BrS | [74] |
| R1512W | DIII–DIV linker | / | BrS | [75] |
| I1521K | B DIII–DIV linker | EF hand/C-lobe | BrS | [72] |
| V1525M | B DIII–DIV linker | EF hand/C-lobe | BrS | [72] |
| K1527R | DIII–DIV linker | / | BrS | [76] |
| V1777M | Pre-EF hands | / | LQT3 | [73] |
| T1779M | Pre-EF hands | / | LQT3/BrS | [73] |
| E1784K | EF hands | B DIII–DIV linker | LQT3 | [73] |
| L1786Q | EF hands | B DIII–DIV linker | LQT3/BrS | [73] |
| S1787N | EF hands | B DIII–DIV linker | LQT3 | [73] |
| D1790G | EF hands | B DIII–DIV linker | LQT3/BrS | [66] |
| Y1795InsD | EF hands | B DIII–DIV linker | LQT3/BrS | [77] |
| Y1795C | EF hands | B DIII–DIV linker | LQT3 | [66] |
| D1819N | EF hands | B DIII–DIV linker | LQT3 | [73] |
| L1825P | EF hands | B DIII–DIV linker | LQT3 | [77] |
| R1826H | EF hands | B DIII–DIV linker | LQT3 | [77] |
| Q1832E | EF hands | B DIII–DIV linker | BrS | [77] |
| D1839G | EF hands | B DIII–DIV linker | LQT3 | [73] |
| H1849R | EF hands | B DIII–DIV linker | LQT3 | [73] |
| C1850S | EF hands | B DIII–DIV linker | BrS | [73] |
| V1861I | EF hands | B DIII–DIV linker | BrS | [73] |
| K1872N | EF hands | B DIII–DIV linker | BrS | [73] |
| M1875T | Pre-IQ motif | / | LQT3 | [73] |
| R1897W | Pre-IQ motif | / | LQT3 | [78] |
| E1901Q | Pre-IQ motif | / | LQT3 | [66] |
| S1904L | Pre-IQ motif | / | LQT3/BrS | [74] |
| Q1909R | IQ motif | C-lobe | LQT3 | [66] |
| R1913H | IQ motif | C-lobe | LQT3 | [66] |
| A1924T | Post-IQ motif | / | BrS | [71] |
| G1935S | Post IQ motif | / | BrS | [74] |
| KV7.1 Mutation | Location | Interacting Site | Disease | Reference |
|---|---|---|---|---|
| P369L | hA | C-lobe | GF | [92] |
| A371T | hA | C-lobe | LQT1 | [73] |
| A372D | hA | C-lobe | LQT1 | [73] |
| S373P | hA | C-lobe | LQT1 | [73] |
| L374H | hA | C-lobe | LQT1 | [73] |
| W379S | hA | C-lobe | LQT1 | [73] |
| R380S | hA | C-lobe | LQT1 | [73] |
| S389Y | hTW | / | LQT1 | [88] |
| T391I | hTW | / | LQT1 | [73] |
| W392R | hTW | / | LQT1 | [73] |
| K393M | hTW | / | LQT1 | [73] |
| K393N | hTW | / | LQT1 | [73] |
| R397N | hTW | / | LQT1 | [73] |
| P448R | hTW-hB linker | / | LQT1 | [73] |
| R451Q | hTW-hB linker | / | LQT1 | [73] |
| R452W | hTW-hB linker | / | LQT1 | [73] |
| G460S | hTW-hB linker | / | LQT1 | [73] |
| I517T | hB | N-lobe | LQT1 | [73] |
| R518G | hB | N-lobe | LQT1 | [73] |
| R518P | hB | N-lobe | LQT1 | [73] |
| R519C | hB | N-lobe | LQT1 | [73] |
| M520R | hB | N-lobe | LQT1 | [73] |
| V524G | hB | N-lobe | LQT1 | [73] |
| A525T | hB | N-lobe | LQT1 | [93] |
| K526E | hB | N-lobe | LQT1 | [89] |
| R533W | Post-hB | / | LQT1 | [94] |
| P535T | Post-hB | / | LQT1 | [73] |
| R539W | Post-hB | / | LQT1 | [85] |
| NaV1.2 Mutation | Location | Interacting Site | Disease | Reference |
|---|---|---|---|---|
| H1853R | Pre-IQ motif | / | OS | [66] |
| R1918H | IQ motif | C-lobe | IGE | [66] |
| KV7.2 Mutation | Location | Interacting Site | Location | Reference | |
|---|---|---|---|---|---|
| Isoform 1 | Isoform 3 | ||||
| R333Q | R333Q | Pre-hA | / | EE | [105] |
| R333W | R333W | Pre-hA | / | EE | [106] |
| A337G | A337G | hA | C-lobe | EE | [107] |
| L339R | L339R | hA | C-lobe | BFNS | [108,109,110,111] |
| W344R | W344R | hA | C-lobe | BFNS | [109] |
| L351F | L351F | hA-hTW linker | / | BFNS | [109] |
| L351V | L351V | hA-hTW linker | / | BFNS | [109] |
| R353G | R353G | hA-hTW linker | / | BFNS | [108,109,110,111] |
| L356V | L356V | hTW | / | BFNS | [107] |
| T359K | T359K | hTW | / | EE | [112] |
| Y362C | Y362C | hTW | / | BFNS | [109] |
| M546V | M518V | hB | N-lobe | EE | [106,109] |
| R547W | R519W | hB | N-lobe | EE | [107] |
| K552T | K524T | hB | N-lobe | EE | [107] |
| R553Q | R525Q | hB | N-lobe | EE | [107] |
| K554N | K526N | hB | N-lobe | EE | [105,106,113] |
| K556E | K528E | hB | N-lobe | EE | [107] |
| R560W | R532W | hB | N-lobe | EE | [106] |
| P561L | P533L | hB | N-lobe | EE | [107] |
| D563N | D535N | hB | N-lobe | EE | [107] |
| R581Q | R553Q | hB | N-lobe | EE | [106,109] |
| R581G | R553G | hB | N-lobe | EE | [114] |
| L637R | L609R | hD | N-lobe | BFNS | [115,116] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urrutia, J.; Aguado, A.; Muguruza-Montero, A.; Núñez, E.; Malo, C.; Casis, O.; Villarroel, A. The Crossroad of Ion Channels and Calmodulin in Disease. Int. J. Mol. Sci. 2019, 20, 400. https://doi.org/10.3390/ijms20020400
Urrutia J, Aguado A, Muguruza-Montero A, Núñez E, Malo C, Casis O, Villarroel A. The Crossroad of Ion Channels and Calmodulin in Disease. International Journal of Molecular Sciences. 2019; 20(2):400. https://doi.org/10.3390/ijms20020400
Chicago/Turabian StyleUrrutia, Janire, Alejandra Aguado, Arantza Muguruza-Montero, Eider Núñez, Covadonga Malo, Oscar Casis, and Alvaro Villarroel. 2019. "The Crossroad of Ion Channels and Calmodulin in Disease" International Journal of Molecular Sciences 20, no. 2: 400. https://doi.org/10.3390/ijms20020400
APA StyleUrrutia, J., Aguado, A., Muguruza-Montero, A., Núñez, E., Malo, C., Casis, O., & Villarroel, A. (2019). The Crossroad of Ion Channels and Calmodulin in Disease. International Journal of Molecular Sciences, 20(2), 400. https://doi.org/10.3390/ijms20020400





