Lipid Headgroup Charge and Acyl Chain Composition Modulate Closure of Bacterial β-Barrel Channels
Abstract
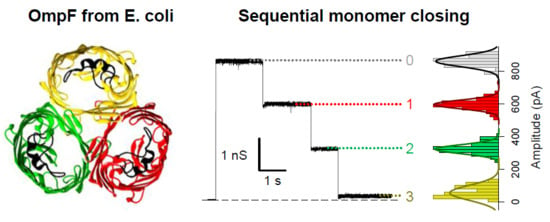
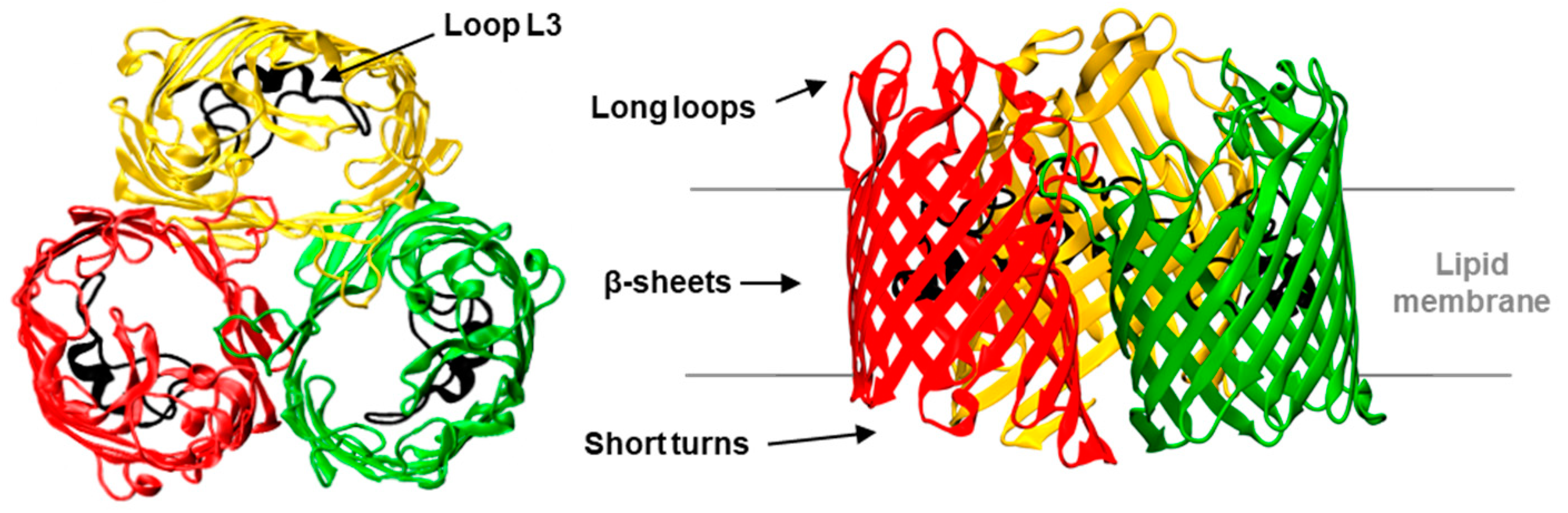
1. Introduction
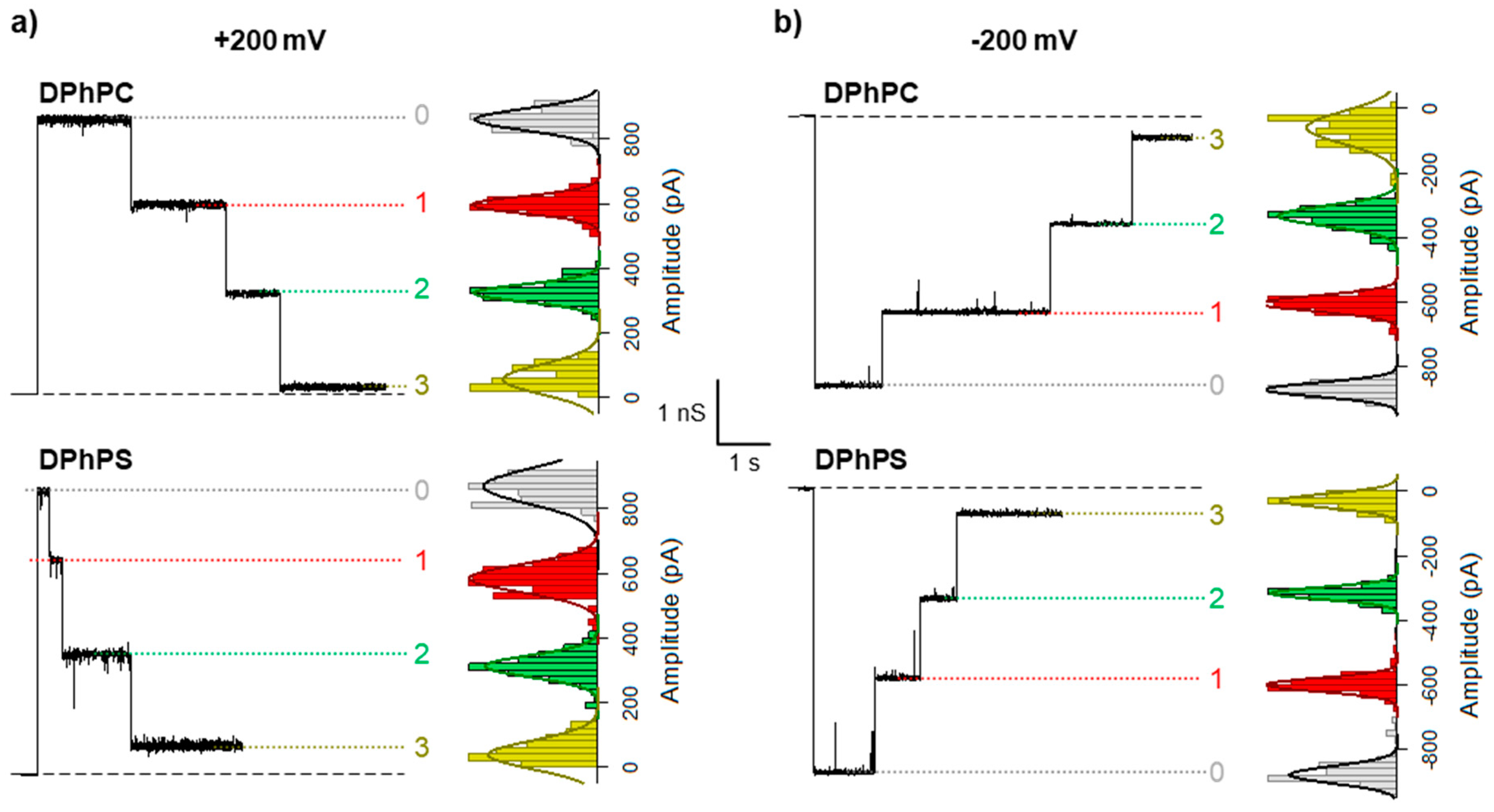
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aidley, D.J.; Stanfield, P.R. Ion Channels: Molecules in Action, 1st ed.Cambridge University Press: New York, NY, USA, 1996; ISBN 0521498821. [Google Scholar]
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2001; ISBN 9780878933211. [Google Scholar]
- Zhou, H.-X.; McCammon, J.A. The gates of ion channels and enzymes. Trends Biochem. Sci. 2010, 35, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 2008, 9, 323–332. [Google Scholar] [CrossRef]
- Nestorovich, E.M.; Bezrukov, S.M. Voltage-induced “gating” of bacterial porin as reversible protein denaturation. In Proceedings of the SPIE 5467, Fluctuations and Noise in Biological, Biophysical, and Biomedical Systems II, Gran Canaria Island, Spain, 26–28 May 2004; Abbott, D., Bezrukov, S.M., Der, A., Sanchez, A., Eds.; 2004; p. 42. [Google Scholar]
- Galdiero, S.; Galdiero, M.; Pedone, C. beta-Barrel membrane bacterial proteins: Structure, function, assembly and interaction with lipids. Curr. Protein Pept. Sci. 2007, 8, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.W.; Feil, S.C. Pore-forming protein toxins: From structure to function. Prog. Biophys. Mol. Biol. 2005, 88, 91–142. [Google Scholar] [CrossRef] [PubMed]
- Nestorovich, E.M.; Bezrukov, S.M. Obstructing toxin pathways by targeted pore blockage. Chem. Rev. 2012, 112, 6388–6430. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Teijido, O.; Hoogerheide, D.P.; Rostovtseva, T.K.; Berezhkovskii, A.M.; Bezrukov, S.M. Conductance hysteresis in the voltage-dependent anion channel. Eur. Biophys. J. 2015, 44, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rathke, A.; Fahie, M.A.; Chisholm, C.; Liang, J.; Chen, M. Mechanism of OmpG pH-Dependent Gating from Loop Ensemble and Single Channel Studies. J. Am. Chem. Soc. 2018, 140, 1105–1115. [Google Scholar] [CrossRef]
- Teijido, O.; Ujwal, R.; Hillerdal, C.O.; Kullman, L.; Rostovtseva, T.K.; Abramson, J. Affixing N-terminal α-helix to the wall of the voltage-dependent anion channel does not prevent its voltage gating. J. Biol. Chem. 2012, 287, 11437–11445. [Google Scholar] [CrossRef] [PubMed]
- Rostovtseva, T.K.; Kazemi, N.; Weinrich, M.; Bezrukov, S.M. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. J. Biol. Chem. 2006, 281, 37496–37506. [Google Scholar] [CrossRef]
- Liko, I.; Degiacomi, M.T.; Lee, S.; Newport, T.D.; Gault, J.; Reading, E.; Hopper, J.T.S.; Housden, N.G.; White, P.; Colledge, M.; et al. Lipid binding attenuates channel closure of the outer membrane protein OmpF. Proc. Natl. Acad. Sci. 2018, 115, 6691–6696. [Google Scholar] [CrossRef]
- Bot, C.T.; Prodan, C. Quantifying the membrane potential during E. coli growth stages. Biophys. Chem. 2010, 146, 133–137. [Google Scholar] [CrossRef]
- Foster, J.W. Escherichia coli acid resistance: Tales of an amateur acidophile. Nat. Rev. Microbiol. 2004, 2, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed]
- Montal, M.; Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Cowan, S.W.; Schirmer, T.; Rummel, G.; Steiert, M.; Ghosh, R.; Pauptit, R.A.; Jansonius, J.N.; Rosenbusch, J.P. Crystal structures explain functional properties of two E. coli porins. Nature 1992, 358, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.J.; Engel, A. Voltage and pH-induced channel closure of porin OmpF visualized by atomic force microscopy. J. Mol. Biol. 1999, 285, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Baslé, A.; Qutub, R.; Mehrazin, M.; Wibbenmeyer, J.; Delcour, A.H. Deletions of single extracellular loops affect pH sensitivity, but not voltage dependence, of the Escherichia coli porin OmpF. Protein Eng. Des. Sel. 2004, 17, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Todt, J.C.; Rocque, W.J.; McGroarty, E.J. Effects of pH on bacterial porin function. Biochemistry 1992, 31, 10471–10478. [Google Scholar] [CrossRef]
- Wager, B.; Baslé, A.; Delcour, A.H. Disulfide bond tethering of extracellular loops does not affect the closure of OmpF porin at acidic pH. Proteins 2010, 78, 2886–2894. [Google Scholar]
- Samartzidou, H.; Delcour, A.H.E. coli PhoE porin has an opposite voltage-dependence to the homologous OmpF. EMBO J. 1998, 17, 93–100. [Google Scholar] [CrossRef]
- Robertson, K.M.; Tieleman, D.P. Molecular basis of voltage gating of OmpF porin. Biochem. Cell Biol. 2002, 80, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, G.; Mobasheri, H.; Armstrong, G.A.; Lea, E.J.; Lakey, J.H. Voltage-gating of Escherichia coli porin: A cystine-scanning mutagenesis study of loop 3. J. Mol. Biol. 1998, 275, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Saint, N.; Lou, K.L.; Widmer, C.; Luckey, M.; Schirmer, T.; Rosenbusch, J.P. Structural and functional characterization of OmpF porin mutants selected for larger pore size. II. Functional characterization. J. Biol. Chem. 1996, 271, 20676–20680. [Google Scholar] [CrossRef] [PubMed]
- Saint, N.; Prilipov, A.; Hardmeyer, A.; Lou, K.L.; Schirmer, T.; Rosenbusch, J.P. Replacement of the sole histidinyl residue in OmpF porin from E. coli by threonine (H21T) does not affect channel structure and function. Biochem. Biophys. Res. Commun. 1996, 223, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, P.; Saint, N.; Phale, P.; Eppens, E.F.; Prilipov, A.; van Boxtel, R.; Rosenbusch, J.P.; Tommassen, J. Voltage sensing in the PhoE and OmpF outer membrane porins of Escherichia coli: Role of charged residues. J. Mol. Biol. 1997, 269, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Miedema, H.; Vrouenraets, M.; Wierenga, J.; Eisenberg, B.; Schirmer, T.; Baslé, A.; Meijberg, W. Conductance and selectivity fluctuations in D127 mutants of the bacterial porin OmpF. Eur. Biophys. J. 2006, 36, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Nestorovich, E.M.; Rostovtseva, T.K.; Bezrukov, S.M. Residue ionization and ion transport through OmpF channels. Biophys. J. 2003, 85, 3718–3729. [Google Scholar] [CrossRef]
- Alcaraz, A.; Nestorovich, E.M.; Aguilella-Arzo, M.; Aguilella, V.M.; Bezrukov, S.M. Salting out the ionic selectivity of a wide channel: The asymmetry of OmpF. Biophys. J. 2004, 87, 943–957. [Google Scholar] [CrossRef]
- Tristram-Nagle, S.; Kim, D.J.; Akhunzada, N.; Kuerka, N.; Mathai, J.C.; Katsaras, J.; Zeidel, M.; Nagle, J.F. Structure and water permeability of fully hydrated diphytanoylPC. Chem. Phys. Lipids 2010, 163, 630–637. [Google Scholar] [CrossRef]
- Pustovoit, M.A.; Berezhkovskii, A.M.; Bezrukov, S.M. Analytical theory of hysteresis in ion channels: Two-state model. J. Chem. Phys. 2006, 125, 1–8. [Google Scholar] [CrossRef]
- Banerjee, K. Dynamic memory of a single voltage-gated potassium ion channel: A stochastic nonequilibrium thermodynamic analysis. J. Chem. Phys. 2015, 142, 185101. [Google Scholar] [CrossRef] [PubMed]
- Sigworth, F.J.; Sine, S.M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys. J. 1987, 52, 1047–1054. [Google Scholar] [CrossRef]
- Petrache, H.I. 5.2 Lipid Bilayer Structure. In Comprehensive Biophysics; Egelman, E.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 5, pp. 3–15. ISBN 9780080957180. [Google Scholar]
- Nestorovich, E.M.; Danelon, C.; Winterhalter, M.; Bezrukov, S.M. Designed to penetrate: Time-resolved interaction of single antibiotic molecules with bacterial pores. Proc. Natl. Acad. Sci. USA 2002, 99, 9789–9794. [Google Scholar] [CrossRef] [PubMed]
- Duneau, J.P.; Khao, J.; Sturgis, J.N. Lipid perturbation by membrane proteins and the lipophobic effect. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.L.; Fleming, P.J.; Yeom, M.S.; Widmalm, G.; Klauda, J.B.; Fleming, K.G.; Im, W.E. coli outer membrane and interactions with OmpLA. Biophys. J. 2014, 106, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, A.H.; East, J.M.; Lee, A.G. Selectivity in lipid binding to the bacterial outer membrane protein OmpF. Biophys. J. 2000, 79, 2066–2074. [Google Scholar] [CrossRef]
- Ranjan Srivastava, S.; Zadafiya, P.; Mahalakshmi, R. Hydrophobic mismatch modulates stability and plasticity of human mitochondrial VDAC2. Biophys. J. 2018, 115, 2386–2394. [Google Scholar] [CrossRef]
- Mlayeh, L.; Krammer, E.-M.M.; Léonetti, M.; Prévost, M.; Homblé, F. The mitochondrial VDAC of bean seeds recruits phosphatidylethanolamine lipids for its proper functioning. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 786–794. [Google Scholar] [CrossRef]
- McIntosh, T.J.; Simon, S.A. Roles of Bilayer Material Properties in Function and Distribution of Membrane Proteins. Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 177–198. [Google Scholar] [CrossRef]
- Lundbaek, J.A.; Collingwood, S.A.; Ingólfsson, H.I.; Kapoor, R.; Andersen, O.S. Lipid bilayer regulation of membrane protein function: Gramicidin channels as molecular force probes. J. R. Soc. Interface 2010, 7, 373–395. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Gurnev, P.A.; Chen, M.-Y.; Bezrukov, S.M. Membrane lipid composition regulates tubulin interaction with mitochondrial voltage-dependent anion channel. J. Biol. Chem. 2012, 287, 29589–29598. [Google Scholar] [CrossRef] [PubMed]
- Aguilella, V.M.; Verdiá-Báguena, C.; Alcaraz, A. Lipid charge regulation of non-specific biological ion channels. Phys. Chem. Chem. Phys. 2014, 16, 3881–3893. [Google Scholar] [PubMed]
- D’Avanzo, N. Lipid Regulation of Sodium Channels. In Current Topics in Membranes; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 78, pp. 353–407. ISBN 9780128053867. [Google Scholar]
- Alcaraz, A.; López, M.L.; Queralt-Martín, M.; Aguilella, V.M. Ion Transport in Confined Geometries below the Nanoscale: Access Resistance Dominates Protein Channel Conductance in Diluted Solutions. ACS Nano 2017, 11, 10392–10400. [Google Scholar] [CrossRef] [PubMed]
- Queralt-Martín, M.; López, M.L.; Aguilella-Arzo, M.; Aguilella, V.M.; Alcaraz, A. Scaling Behavior of Ionic Transport in Membrane Nanochannels. Nano Lett. 2018, 18, 6604–6610. [Google Scholar] [CrossRef] [PubMed]
- Queralt-Martín, M.; Verdiá-Báguena, C.; Aguilella, V.M.; Alcaraz, A. Electrostatic Interactions Drive the Nonsteric Directional Block of OmpF Channel by La3+. Langmuir 2013, 29, 15320–15327. [Google Scholar] [CrossRef] [PubMed]
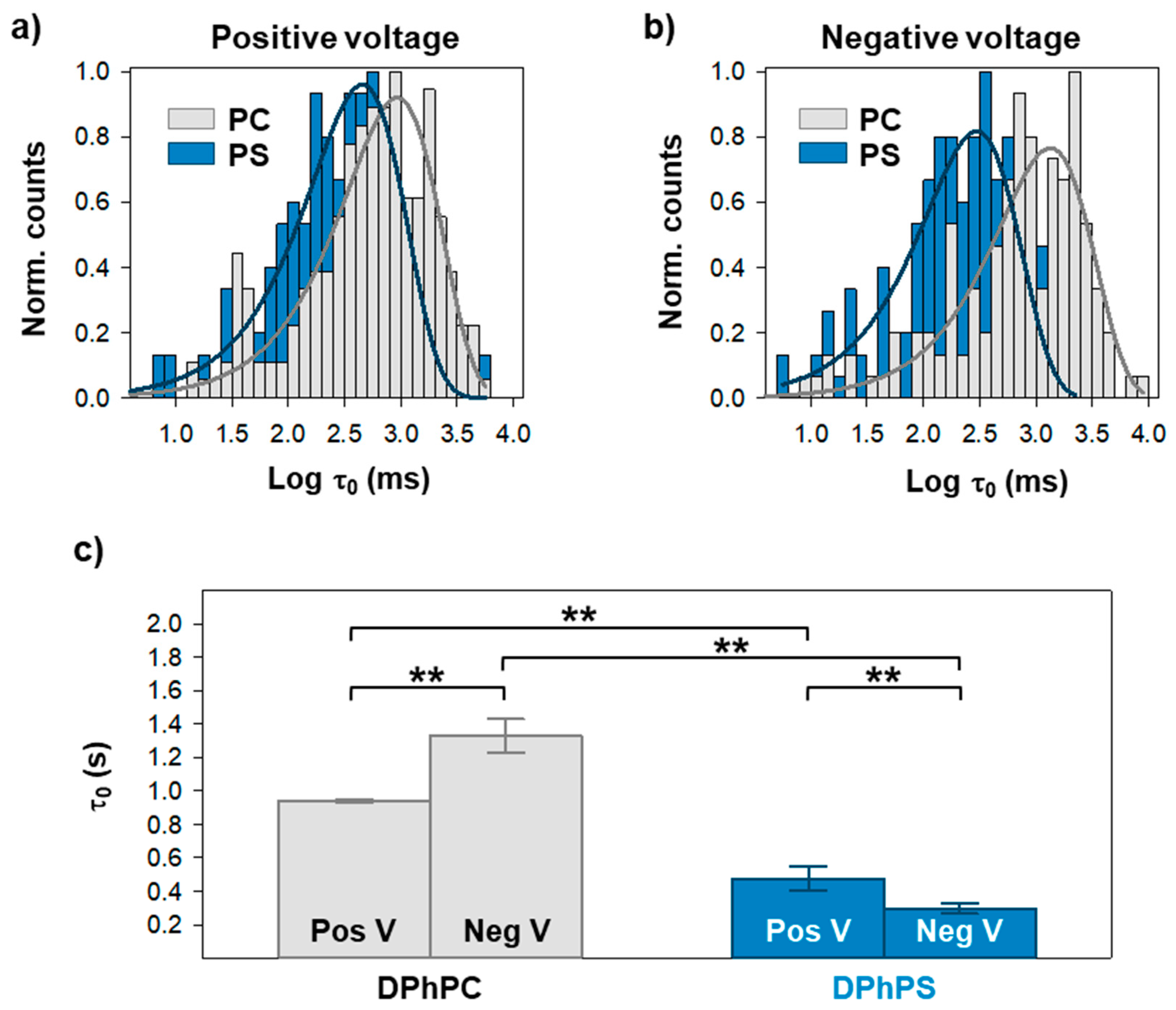

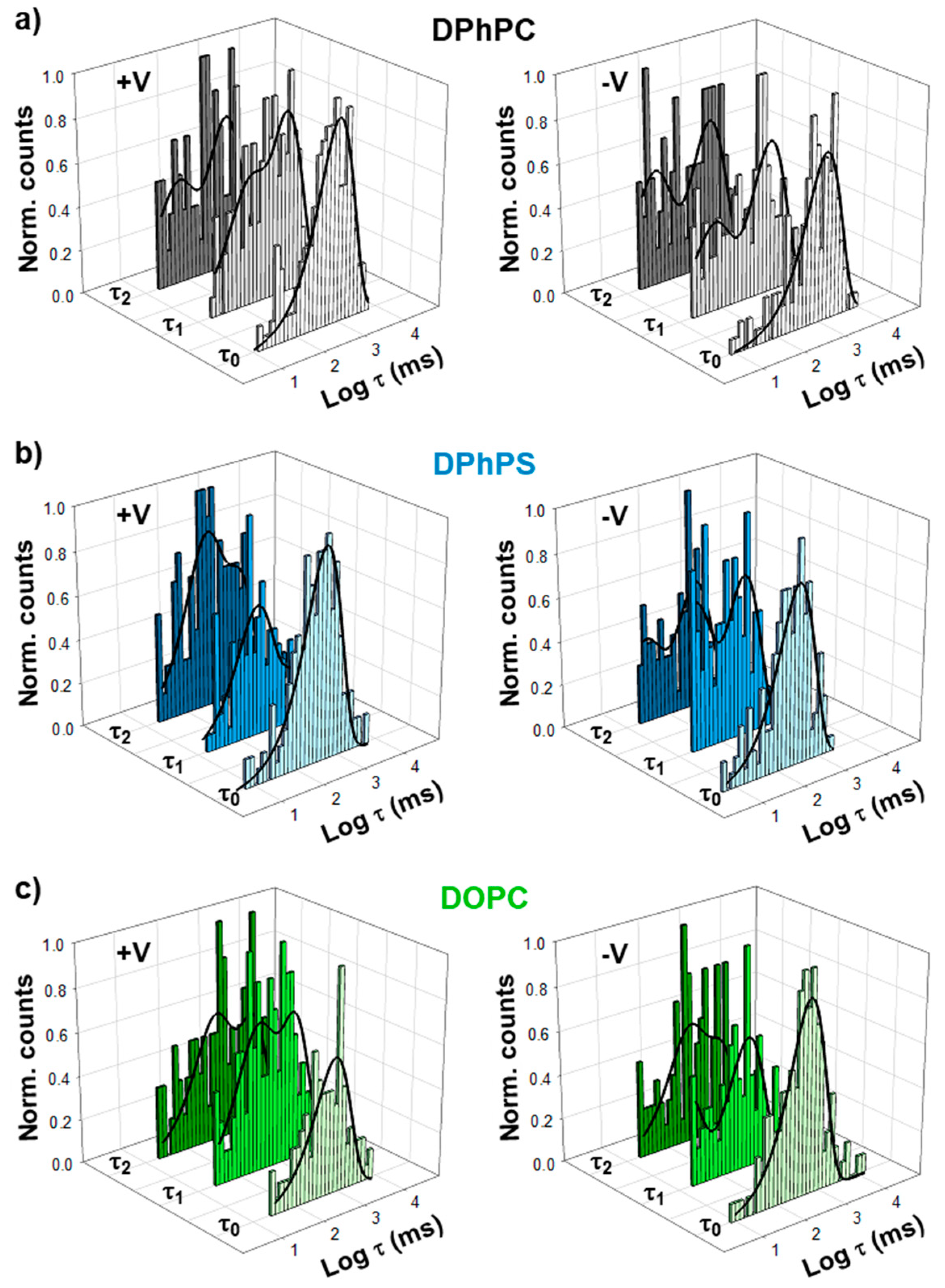
| Positive V | τ0 (ms) | τ1 (1) (ms) | τ1 (2) (ms) | τ2 (1) (ms) | τ2 (2) (ms) |
| DPhPC | 942.0 ± 10.9 | 34.2 ± 51.7 (0.31) | 944.3 ± 295.5 (0.69) | 42.1 ± 11.4 (0.34) | 868.4 ± 156.2 (0.66) |
| DPhPS | 476.3 ± 73.5 | 189.4 ± 45.2 (0.67) | 1246.1 ± 322.9 (0.33) | 222.6 ± 9.6 (0.49) | 1662.8 ± 93.3 (0.51) |
| DOPC | 856.0 ± 98.0 | 56.4 ± 95.0 (0.41) | 1099.4 ± 529.9 (0.59) | 360.6 ± 91.6(0.44) | 3394.4 ± 502.5 (0.56) |
| Negative V | τ0 (ms) | τ1 (1) (ms) | τ1 (2) (ms) | τ2 (1) (ms) | τ2 (2) (ms) |
| DPhPC | 1329.8 ± 100.2 | 14.3 ± 26.8 (0.33) | 933.4 ± 397.1 (0.67) | 11.0 ± 19.8 (0.40) | 889.5 ± 342.6 (0.60) |
| DPhPS | 296.9 ± 31.0 | 6.0 ± 8.6 (0.43) | 314.5 ± 114.9 (0.57) | 336.1 ± 102.6 (0.35) | 245.9 ± 316.6 (0.65) |
| DOPC | 554.0 ± 15.2 | 69.0 ± 116.2 (0.41) | 439.4 ± 25.6 (0.59) | 391.1 ± 188.2 (0.46) | 6169.0 ± 7397.1 (0.54) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perini, D.A.; Alcaraz, A.; Queralt-Martín, M. Lipid Headgroup Charge and Acyl Chain Composition Modulate Closure of Bacterial β-Barrel Channels. Int. J. Mol. Sci. 2019, 20, 674. https://doi.org/10.3390/ijms20030674
Perini DA, Alcaraz A, Queralt-Martín M. Lipid Headgroup Charge and Acyl Chain Composition Modulate Closure of Bacterial β-Barrel Channels. International Journal of Molecular Sciences. 2019; 20(3):674. https://doi.org/10.3390/ijms20030674
Chicago/Turabian StylePerini, D. Aurora, Antonio Alcaraz, and María Queralt-Martín. 2019. "Lipid Headgroup Charge and Acyl Chain Composition Modulate Closure of Bacterial β-Barrel Channels" International Journal of Molecular Sciences 20, no. 3: 674. https://doi.org/10.3390/ijms20030674
APA StylePerini, D. A., Alcaraz, A., & Queralt-Martín, M. (2019). Lipid Headgroup Charge and Acyl Chain Composition Modulate Closure of Bacterial β-Barrel Channels. International Journal of Molecular Sciences, 20(3), 674. https://doi.org/10.3390/ijms20030674