Investigation of the Prognostic Role of Carbonic Anhydrase 9 (CAIX) of the Cellular mRNA/Protein Level or Soluble CAIX Protein in Patients with Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Results
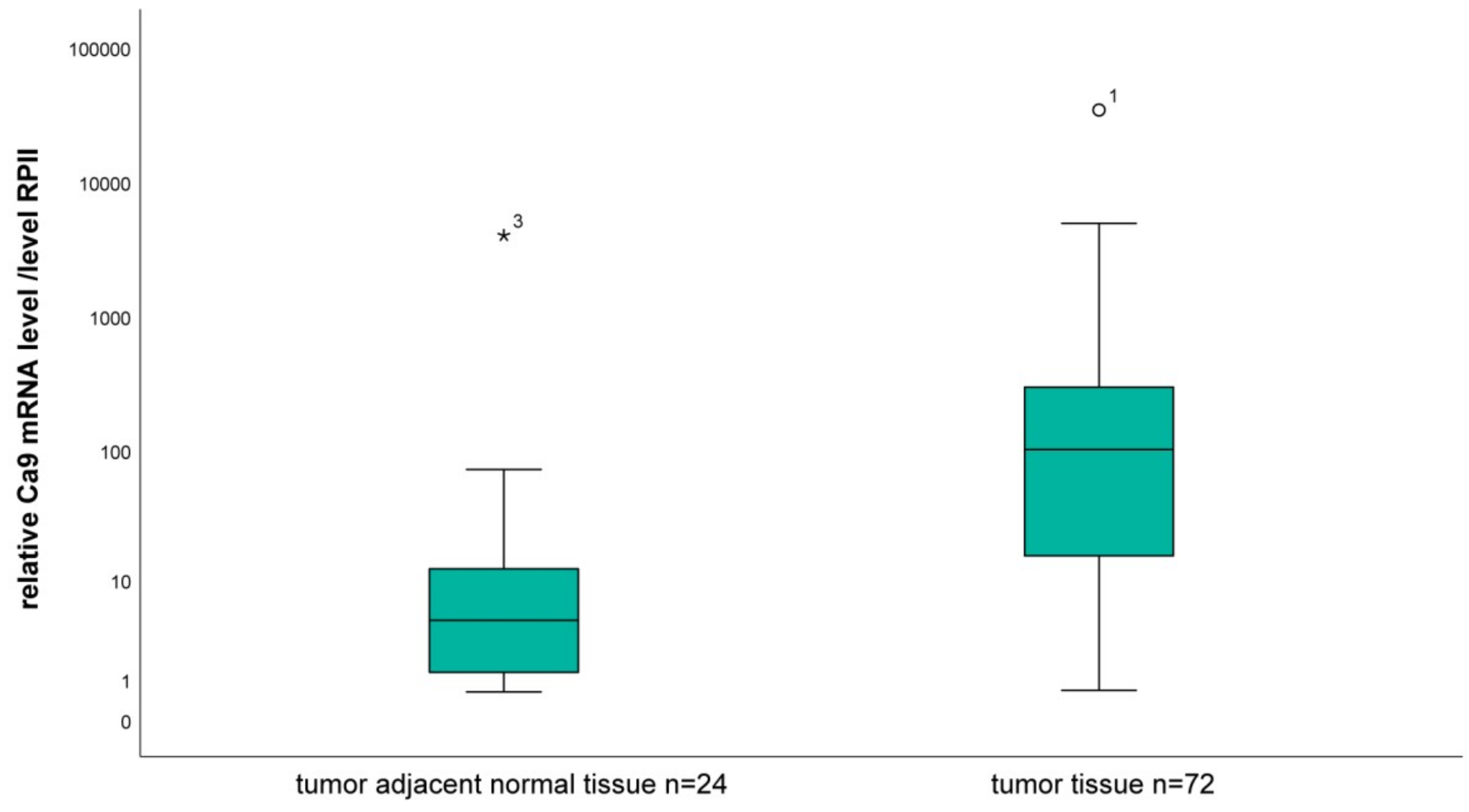
2.1. Analysis of the mRNA Level of CA9 in Tumor Tissue Compared to Tumor-Associated Normal Tissue
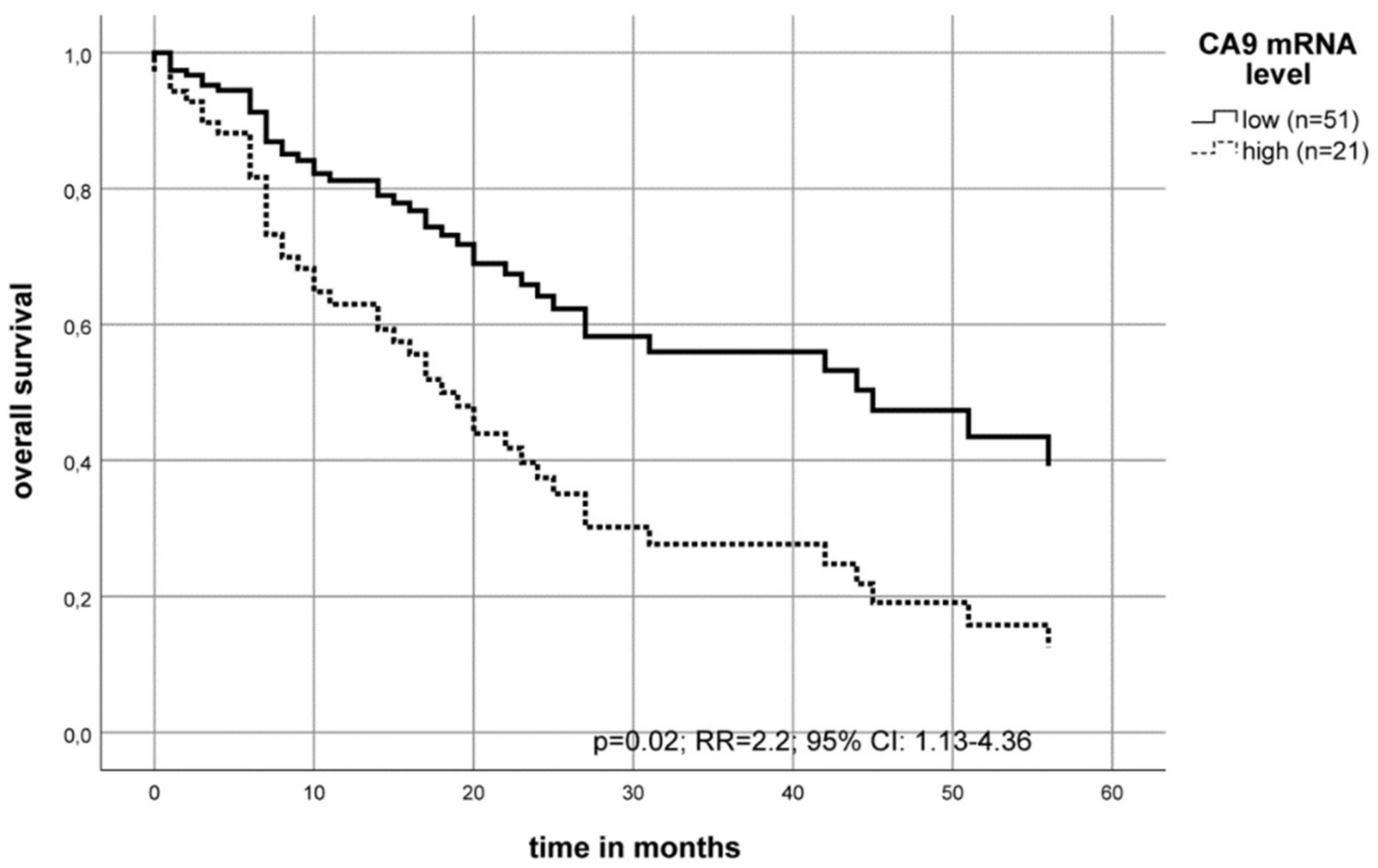
2.2. Association of the CA9 mRNA Level with the Survival of OSCC Patients
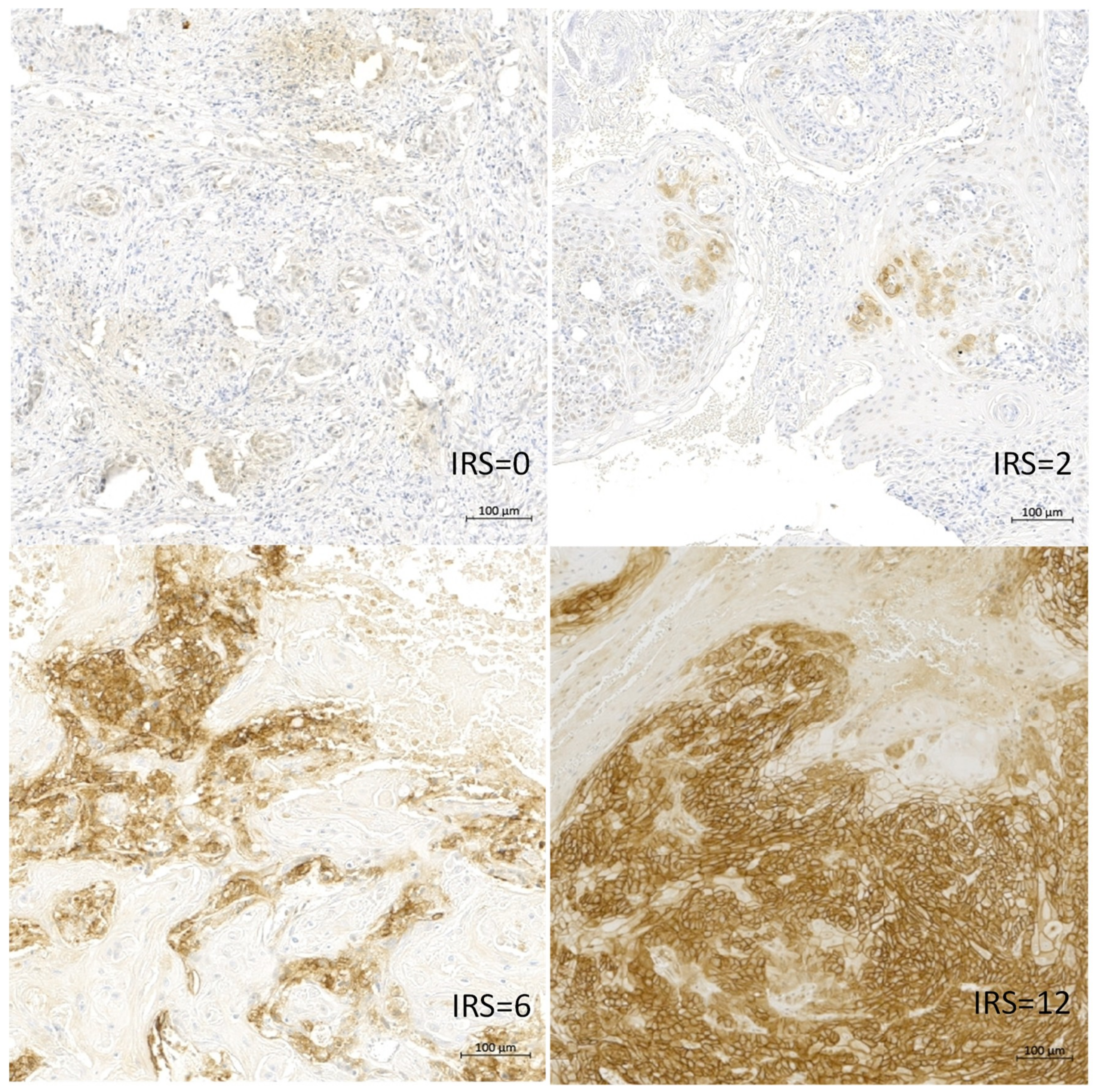
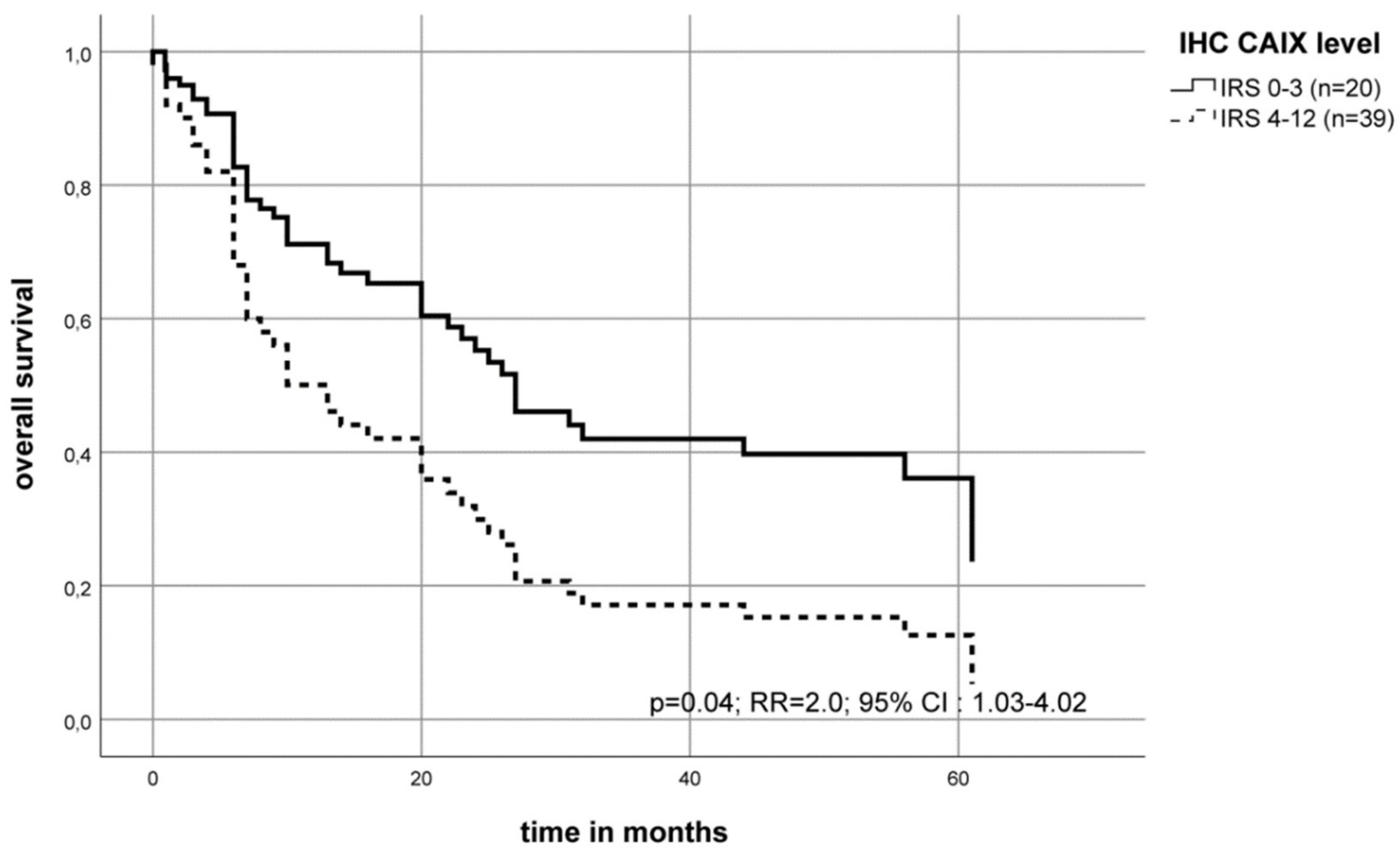
2.3. Association of the CAIX Protein Level with the Survival of OSCC Patients
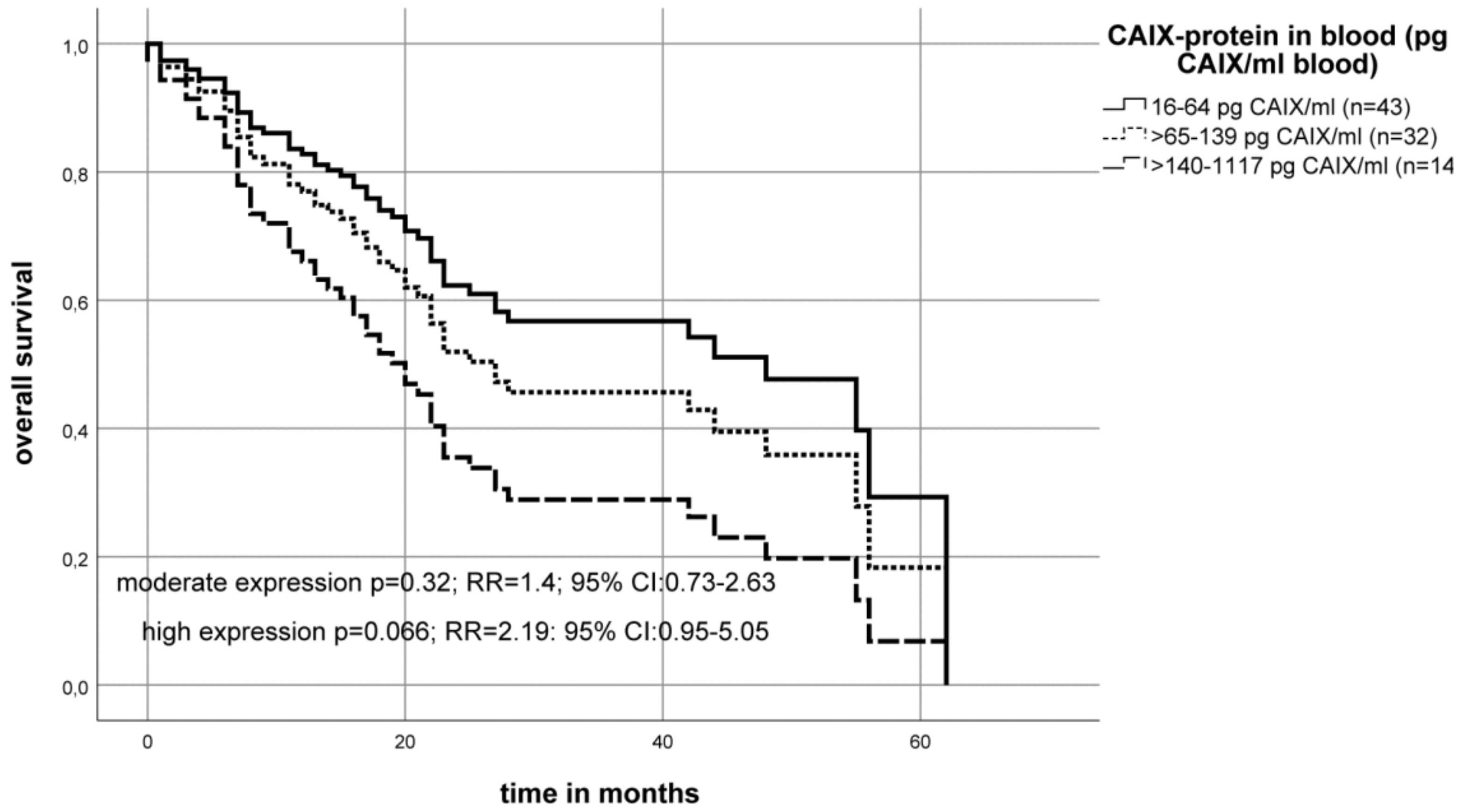
2.4. Association of the CAIX Protein Level in the Blood Serum of OSCC Patients with Survival
2.5. Correlation of the CA9 mRNA, CAIX Protein Expression and CAIX Protein Level in the Blood
2.6. Correlation of CA9 mRNA, CAIX Protein Expression and CAIX Protein Levels in the Blood Serum with Other Tumor-Related Markers
3. Discussion
4. Materials and Methods
4.1. Tissue Samples, Histomorphological Data and Study Approval
4.2. RNA-Isolation
4.3. Quantitative RT-PCR
4.4. Immunohistochemistry
4.5. ELISA
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Listl, S.; Jansen, L.; Stenzinger, A.; Freier, K.; Emrich, K.; Holleczek, B.; Katalinic, A.; Gondos, A.; Brenner, H. Survival of patients with oral cavity cancer in Germany. PloS ONE 2013, 8, e53415. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, H. Prognostic relevance of molecular markers of oral cancer--a review. Int. J. Oral Maxillofac. Surg. 2003, 32, 233–245. [Google Scholar] [CrossRef]
- Lothaire, P.; de Azambuja, E.; Dequanter, D.; Lalami, Y.; Sotiriou, C.; Andry, G.; Castro, G.; Awada, A. Molecular markers of head and neck squamous cell carcinoma: Promising signs in need of prospective evaluation. Head Neck 2006, 28, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.R.; Ribeiro-Silva, A. Prognostic significance of immunohistochemical biomarkers in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2011, 40, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Oliveira, A.K.; Costa, R.A.P.; de Rossi, T.; Paes Leme, A.F. Prognostic biomarkers in oral squamous cell carcinoma: A systematic review. Oral Oncol. 2017, 72, 38–47. [Google Scholar] [CrossRef]
- Blatt, S.; Krüger, M.; Ziebart, T.; Sagheb, K.; Schiegnitz, E.; Goetze, E.; Al-Nawas, B.; Pabst, A.M. Biomarkers in diagnosis and therapy of oral squamous cell carcinoma: A review of the literature. J. Craniomaxillofac. Surg. 2017, 45, 722–730. [Google Scholar] [CrossRef]
- Feng, Z.; Bethmann, D.; Kappler, M.; Ballesteros-Merino, C.; Eckert, A.; Bell, R.B.; Cheng, A.; Bui, T.; Leidner, R.; Urba, W.J.; et al. Multiparametric immune profiling in HPV- oral squamous cell cancer. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Stasikowska-Kanicka, O.; Wągrowska-Danilewicz, M.; Danilewicz, M. CD8+ and CD163+ infiltrating cells and PD-L1 immunoexpression in oral leukoplakia and oral carcinoma. APMIS 2018, 126, 732–738. [Google Scholar] [CrossRef]
- Shimizu, S.; Hiratsuka, H.; Koike, K.; Tsuchihashi, K.; Sonoda, T.; Ogi, K.; Miyakawa, A.; Kobayashi, J.; Kaneko, T.; Igarashi, T.; et al. Tumor-infiltrating CD8+ T-cell density is an independent prognostic marker for oral squamous cell carcinoma. Cancer Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zou, H.; Wang, J.; Shen, J.; Liu, H. A Three Long Noncoding RNA-Based Signature for Oral Squamous Cell Carcinoma Prognosis Prediction. DNA Cell Biol. 2018, 37, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Bala, S. Emerging role of non-coding RNA in oral cancer. Cell. Signal. 2018, 42, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, J.; Pan, L.; Yin, X.; Wang, Q.; Chen, H. Diagnostic and prognostic value of serum miR-99a expression in oral squamous cell carcinoma. Cancer Biomark. 2018, 23, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Srivastava, A.N.; Sharma, R.; Mateen, S.; Shukla, B.; Singh, A.; Chandel, S. Circulating MicroRNA-21 Expression as a Novel Serum Biomarker for Oral Sub-Mucous Fibrosis and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2018, 19, 1053–1057. [Google Scholar] [PubMed]
- Gai, C.; Camussi, F.; Broccoletti, R.; Gambino, A.; Cabras, M.; Molinaro, L.; Carossa, S.; Camussi, G.; Arduino, P.G. Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma. BMC Cancer 2018, 18, 439. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-A.; Weng, S.-L.; Yang, S.-F.; Chou, C.-H.; Huang, W.-C.; Tu, S.-J.; Chang, T.-H.; Huang, C.-N.; Jong, Y.-J.; Huang, H.-D. A Three-MicroRNA Signature as a Potential Biomarker for the Early Detection of Oral Cancer. Int. J. Mol. Sci. 2018, 19, 758. [Google Scholar] [CrossRef] [PubMed]
- Greither, T.; Vorwerk, F.; Kappler, M.; Bache, M.; Taubert, H.; Kuhnt, T.; Hey, J.; Eckert, A.W. Salivary miR-93 and miR-200a as post-radiotherapy biomarkers in head and neck squamous cell carcinoma. Oncol. Rep. 2017, 38, 1268–1275. [Google Scholar] [CrossRef]
- Tandon, D.; Dewangan, J.; Srivastava, S.; Garg, V.K.; Rath, S.K. miRNA genetic variants: As potential diagnostic biomarkers for oral cancer. Pathol. Res. Pract. 2018, 214, 281–289. [Google Scholar] [CrossRef]
- Sridharan, G.; Ramani, P.; Patankar, S. Serum metabolomics in oral leukoplakia and oral squamous cell carcinoma. J. Cancer Res. Ther. 2017, 13, 556–561. [Google Scholar] [CrossRef]
- Lin, L.-H.; Chang, K.-W.; Kao, S.-Y.; Cheng, H.-W.; Liu, C.-J. Increased Plasma Circulating Cell-Free DNA Could Be a Potential Marker for Oral Cancer. Int. J. Mol. Sci. 2018, 19, 3303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zou, H.; Zhang, J.; Wang, J.; Liu, H. An integrated methylation and gene expression microarray analysis reveals significant prognostic biomarkers in oral squamous cell carcinoma. Oncol. Rep. 2018, 40, 2637–2647. [Google Scholar] [CrossRef] [PubMed]
- Carnielli, C.M.; Macedo, C.C.S.; de Rossi, T.; Granato, D.C.; Rivera, C.; Domingues, R.R.; Pauletti, B.A.; Yokoo, S.; Heberle, H.; Busso-Lopes, A.F.; et al. Combining discovery and targeted proteomics reveals a prognostic signature in oral cancer. Nat. Commun. 2018, 9, 3598. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yuan, Y.; Zhou, Y.; Zhang, D.; Zhang, L.; Zeng, X.; Ji, N.; Zhou, M.; Liang, X.; Chen, Y.; et al. Screening diagnostic biomarkers of OSCC via an LCM-based proteomic approach. Oncol. Rep. 2018, 40, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.W.; Schutze, A.; Lautner, M.H.W.; Taubert, H.; Schubert, J.; Bilkenroth, U. HIF-1alpha is a prognostic marker in oral squamous cell carcinomas. Int. J. Biol. Marker. 2010, 25, 87–92. [Google Scholar] [CrossRef]
- Kappler, M.; Taubert, H.; Schubert, J.; Vordermark, D.; Eckert, A.W. The real face of HIF1α in the tumor process. Cell Cycle 2012, 11, 3932–3936. [Google Scholar] [CrossRef] [PubMed]
- Kappler, M.; Pabst, U.; Rot, S.; Taubert, H.; Wichmann, H.; Schubert, J.; Bache, M.; Weinholdt, C.; Immel, U.-D.; Grosse, I.; et al. Normoxic accumulation of HIF1α is associated with glutaminolysis. Clin. Oral Investig. 2017, 21, 211–224. [Google Scholar] [CrossRef]
- Vordermark, D.; Kaffer, A.; Riedl, S.; Katzer, A.; Flentje, M. Characterization of carbonic anhydrase IX (CA IX) as an endogenous marker of chronic hypoxia in live human tumor cells. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1197–1207. [Google Scholar] [CrossRef]
- Kessler, J.; Hahnel, A.; Wichmann, H.; Rot, S.; Kappler, M.; Bache, M.; Vordermark, D. HIF-1α inhibition by siRNA or chetomin in human malignant glioma cells: Effects on hypoxic radioresistance and monitoring via CA9 expression. BMC Cancer 2010, 10, 605. [Google Scholar] [CrossRef]
- Sedlakova, O.; Svastova, E.; Takacova, M.; Kopacek, J.; Pastorek, J.; Pastorekova, S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front. Physiol. 2014, 4, 400. [Google Scholar] [CrossRef]
- van Kuijk, S.J.A.; Yaromina, A.; Houben, R.; Niemans, R.; Lambin, P.; Dubois, L.J. Prognostic Significance of Carbonic Anhydrase IX Expression in Cancer Patients: A Meta-Analysis. Front. Oncol. 2016, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Brockton, N.T.; Lohavanichbutr, P.; Enwere, E.K.; Upton, M.P.; Kornaga, E.N.; Nakoneshny, S.C.; Bose, P.; Chen, C.; Dort, J.C. Impact of tumoral carbonic anhydrase IX and Ki-67 expression on survival in oral squamous cell carcinoma patients. Oncol. Lett. 2017, 14, 5434–5442. [Google Scholar] [CrossRef]
- Lin, C.-W.; Yang, W.-E.; Lee, W.-J.; Hua, K.-T.; Hsieh, F.-K.; Hsiao, M.; Chen, C.-C.; Chow, J.-M.; Chen, M.-K.; Yang, S.-F.; et al. Lipocalin 2 prevents oral cancer metastasis through carbonic anhydrase IX inhibition and is associated with favourable prognosis. Carcinogenesis 2016, 37, 712–722. [Google Scholar] [CrossRef]
- Brockton, N.T.; Klimowicz, A.C.; Bose, P.; Petrillo, S.K.; Konno, M.; Rudmik, L.; Dean, M.; Nakoneshny, S.C.; Matthews, T.W.; Chandarana, S.; et al. High stromal carbonic anhydrase IX expression is associated with nodal metastasis and decreased survival in patients with surgically-treated oral cavity squamous cell carcinoma. Oral Oncol. 2012, 48, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-S.; Lin, C.-W.; Hsieh, Y.-H.; Chien, M.-H.; Chuang, C.-Y.; Yang, S.-F. Overexpression of carbonic anhydrase IX induces cell motility by activating matrix metalloproteinase-9 in human oral squamous cell carcinoma cells. Oncotarget 2017, 8, 83088–83099. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-S.; Lin, C.-W.; Chuang, C.-Y.; Su, S.-C.; Lin, S.-H.; Yang, S.-F. Carbonic anhydrase IX overexpression regulates the migration and progression in oral squamous cell carcinoma. Tumor. Biol. 2015, 36, 9517–9524. [Google Scholar] [CrossRef]
- Peterle, G.T.; Maia, L.L.; Trivilin, L.O.; de Oliveira, M.M.; Dos Santos, J.G.; Mendes, S.O.; Stur, E.; Agostini, L.P.; Rocha, L.A.; Moysés, R.A.; et al. PAI-1, CAIX, and VEGFA expressions as prognosis markers in oral squamous cell carcinoma. J. Oral Pathol. Med. 2018, 47, 566–574. [Google Scholar] [CrossRef]
- Betof, A.S.; Rabbani, Z.N.; Hardee, M.E.; Kim, S.J.; Broadwater, G.; Bentley, R.C.; Snyder, S.A.; Vujaskovic, Z.; Oosterwijk, E.; Harris, L.N.; et al. Carbonic anhydrase IX is a predictive marker of doxorubicin resistance in early-stage breast cancer independent of HER2 and TOP2A amplification. Br. J. Cancer 2012, 106, 916–922. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Bentzen, S.M.; Giatromanolaki, A.; Wilson, G.D.; Daley, F.M.; Saunders, M.I.; Dische, S.; Sivridis, E.; Harris, A.L. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J. Clin. Oncol. 2006, 24, 727–735. [Google Scholar] [CrossRef]
- Ilie, M.; Mazure, N.M.; Hofman, V.; Ammadi, R.E.; Ortholan, C.; Bonnetaud, C.; Havet, K.; Venissac, N.; Mograbi, B.; Mouroux, J.; et al. High levels of carbonic anhydrase IX in tumour tissue and plasma are biomarkers of poor prognostic in patients with non-small cell lung cancer. Br. J. Cancer 2010, 102, 1627–1635. [Google Scholar] [CrossRef]
- Måseide, K.; Kandel, R.A.; Bell, R.S.; Catton, C.N.; O’Sullivan, B.; Wunder, J.S.; Pintilie, M.; Hedley, D.; Hill, R.P. Carbonic anhydrase IX as a marker for poor prognosis in soft tissue sarcoma. Clin. Cancer Res. 2004, 10, 4464–4471. [Google Scholar] [CrossRef]
- Srivastava, S.; Thakkar, B.; Yeoh, K.G.; Ho, K.Y.; Teh, M.; Soong, R.; Salto-Tellez, M. Expression of proteins associated with hypoxia and Wnt pathway activation is of prognostic significance in hepatocellular carcinoma. Virchows Arch. 2015, 466, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Alterio, V.; Di Fiore, A.; Ambrosio, K.; d’ Carta, F.; Monti, S.M.; de Simone, G. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: Three for the price of one. Med. Res. Rev. 2018, 38, 1799–1836. [Google Scholar] [CrossRef] [PubMed]
- Kappler, M.; Taubert, H.; Holzhausen, H.-J.; Reddemann, R.; Rot, S.; Becker, A.; Kuhnt, T.; Dellas, K.; Dunst, J.; Vordermark, D.; et al. Immunohistochemical Detection of HIF-1α and CAIX in Advanced Head-and-Neck Cancer. Strahlenther. Onkol. 2008, 184, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.W.; Kappler, M.; Schubert, J.; Taubert, H. Correlation of expression of hypoxia-related proteins with prognosis in oral squamous cell carcinoma patients. Oral Maxillofac. Surg. 2012, 16, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Tilakaratne, W.M.; Nissanka-Jayasuriya, E.H. Value of HIF-1α as an independent prognostic indicator in oral squamous cell carcinoma. Expert Rev. Mol. Diagn. 2011, 11, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, S.; Wang, L.; Yuan, X.; Dong, Q.; Zhang, D.; Wang, X. Clinical and prognostic significance of HIF-1α overexpression in oral squamous cell carcinoma: A meta-analysis. World J. Surg. Oncol. 2017, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Moroz, E.; Carlin, S.; Dyomina, K.; Burke, S.; Thaler, H.T.; Blasberg, R.; Serganova, I. Real-time imaging of HIF-1alpha stabilization and degradation. PloS ONE 2009, 4, e5077. [Google Scholar] [CrossRef]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sayáns, M.; Supuran, C.T.; Pastorekova, S.; Suárez-Peñaranda, J.M.; Pilar, G.-D.; Barros-Angueira, F.; Gándara-Rey, J.M.; García-García, A. The role of carbonic anhydrase IX in hypoxia control in OSCC. J. Oral Pathol. Med. 2013, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T. Carbonic Anhydrase IX Shedding Through Exosomes in Renal Carcinoma Cells. Glycobiol. Insight. 2009, 1, 3–12. [Google Scholar] [CrossRef][Green Version]
- Peng, X.-H.; Karna, P.; Cao, Z.; Jiang, B.-H.; Zhou, M.; Yang, L. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J. Biol. Chem. 2006, 281, 25903–25914. [Google Scholar] [CrossRef] [PubMed]
- Rot, S.; Taubert, H.; Bache, M.; Greither, T.; Würl, P.; Holzhausen, H.-J.; Eckert, A.; Vordermark, D.; Kappler, M. Low HIF-1α and low EGFR mRNA Expression Significantly Associate with Poor Survival in Soft Tissue Sarcoma Patients; the Proteins React Differently. Int. J. Mol. Sci. 2018, 19, 3842. [Google Scholar] [CrossRef] [PubMed]
- Torres López, M.; Pérez Sayáns, M.; Chamorro Petronacci, C.; Barros Angueira, F.; Gándara Vila, P.; Lorenzo Pouso, A.; García García, A. Determination and diagnostic value of CA9 mRNA in peripheral blood of patients with oral leukoplakia. J. Enzym. Inhib. Med. Chem. 2018, 33, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.W.; Lautner, M.H.W.; Schütze, A.; Bolte, K.; Bache, M.; Kappler, M.; Schubert, J.; Taubert, H.; Bilkenroth, U. Co-expression of Hif1alpha and CAIX is associated with poor prognosis in oral squamous cell carcinoma patients. J. Oral Pathol. Med. 2010, 39, 313–317. [Google Scholar] [PubMed]
- Roh, J.-L.; Cho, K.-J.; Kwon, G.Y.; Ryu, C.H.; Chang, H.W.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. The prognostic value of hypoxia markers in T2-staged oral tongue cancer. Oral Oncol. 2009, 45, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Chafe, S.C.; Dedhar, S. Carving out its niche: A role for carbonic anhydrase IX in pre-metastatic niche development. Oncoimmunology 2015, 4, e1048955. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tafreshi, N.K.; Lloyd, M.C.; Bui, M.M.; Gillies, R.J.; Morse, D.L. Carbonic anhydrase IX as an imaging and therapeutic target for tumors and metastases. Sub.-Cell. Biochem. 2014, 75, 221–254. [Google Scholar]
- Parks, S.K.; Chiche, J.; Pouyssegur, J. pH control mechanisms of tumor survival and growth. J. Cell. Physiol. 2011, 226, 299–308. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Bellot, G.; Pouysségur, J. Hypoxia and energetic tumour metabolism. Curr. Opin. Genet. Dev. 2011, 21, 67–72. [Google Scholar] [CrossRef]
- Benej, M.; Pastorekova, S.; Pastorek, J. Carbonic anhydrase IX: Regulation and role in cancer. Sub.-Cell. Biochem. 2014, 75, 199–219. [Google Scholar]
- Mboge, M.Y.; Mahon, B.P.; McKenna, R.; Frost, S.C. Carbonic Anhydrases: Role in pH Control and Cancer. Metabolites 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Giaccia, A.J. Hypoxic control of metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Kappler, M.; Kotrba, J.; Kaune, T.; Bache, M.; Rot, S.; Bethmann, D.; Wichmann, H.; Güttler, A.; Bilkenroth, U.; Horter, S.; et al. P4HA1: A single-gene surrogate of hypoxia signatures in oral squamous cell carcinoma patients. Clin. Transl. Radia. Oncol. 2017, 5, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Swenson, E.R. Hypoxia and Its Acid-Base Consequences: From Mountains to Malignancy. Adv. Exp. Med. Biol. 2016, 903, 301–323. [Google Scholar] [PubMed]
- Eckert, A.W.; Wickenhauser, C.; Salins, P.C.; Kappler, M.; Bukur, J.; Seliger, B. Clinical relevance of the tumor microenvironment and immune escape of oral squamous cell carcinoma. J. Transl. Med. 2016, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.C.; Swayampakula, M.; Dedhar, S. Coordinated Regulation of Metabolic Transporters and Migration/Invasion by Carbonic Anhydrase IX. Metabolites 2018, 8, 20. [Google Scholar] [CrossRef]
- Swietach, P.; Hulikova, A.; Vaughan-Jones, R.D.; Harris, A.L. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene 2010, 29, 6509–6521. [Google Scholar] [CrossRef]
- Kappler, M.; Taubert, H.; Eckert, A.W. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 2011, 365, 1845–6. [Google Scholar]
- Nocentini, A.; Supuran, C.T. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: A patent review (2008-2018). Expert Opin. Ther. Patents 2018, 28, 729–740. [Google Scholar] [CrossRef]
- Zatovicova, M.; Sedlakova, O.; Svastova, E.; Ohradanova, A.; Ciampor, F.; Arribas, J.; Pastorek, J.; Pastorekova, S. Ectodomain shedding of the hypoxia-induced carbonic anhydrase IX is a metalloprotease-dependent process regulated by TACE/ADAM17. Br. J. Cancer 2005, 93, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Greither, T.; Würl, P.; Grochola, L.; Bond, G.; Bache, M.; Kappler, M.; Lautenschläger, C.; Holzhausen, H.-J.; Wach, S.; Eckert, A.W.; et al. Expression of microRNA 210 associates with poor survival and age of tumor onset of soft-tissue sarcoma patients. Int. J. Cancer 2012, 130, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Der Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]


| Category | Number of Cases (CA9) | Low CA9 mRNA Level (0.63–246) | High CA9 mRNA Level (246.1–33779) | Number of Cases (CAIX) | Low CAIX Protein Level (IRS 0–6) | High CAIX Protein Level (IRS 8–12) |
|---|---|---|---|---|---|---|
| Total | 72 | 51 | 21 | 158 | 134 | 24 |
| Gender | p = 1.00 | p = 0.29 | ||||
| men | 58 | 41 | 17 | 123 | 102 | 21 |
| women | 14 | 10 | 4 | 35 | 32 | 3 |
| T-stage | p = 0.22 | p = 0.03 * | ||||
| I | 12 | 10 | 2 | 37 | 35 | 2 |
| II | 23 | 18 | 5 | 43 | 37 | 6 |
| III | 7 | 3 | 4 | 19 | 16 | 3 |
| IV | 30 | 20 | 10 | 59 | 46 | 13 |
| N-stage | p = 0.60 | p = 0.16 | ||||
| N0 | 28 | 21 | 7 | 72 | 64 | 8 |
| N1-3 | 44 | 30 | 14 | 86 | 70 | 16 |
| Grade | p = 0.62 | p = 0.54 | ||||
| 1 | 9 | 8 | 1 | 16 | 15 | 1 |
| 2 | 52 | 35 | 17 | 93 | 78 | 15 |
| 3 | 10 | 7 | 3 | 49 | 41 | 8 |
| x | 1 | 1 | 0 | |||
| Category | Number of Cases | Low Soluble CAIX Protein Level | High Soluble CAIX Protein Level | Very High Soluble CAIX Protein Level |
|---|---|---|---|---|
| Total | 89 | 43 | 32 | 14 |
| Gender | p = 0.71 | |||
| men | 67 | 31 | 26 | 10 |
| women | 22 | 12 | 6 | 4 |
| T-stage | p = 0.10 | |||
| I | 19 | 12 | 6 | 1 |
| II | 30 | 15 | 7 | 8 |
| III | 13 | 8 | 5 | 0 |
| IV | 27 | 8 | 14 | 5 |
| N-stage | p = 0.70 | |||
| N0 | 38 | 20 | 12 | 6 |
| N1-3 | 51 | 23 | 20 | 8 |
| Grading | p = 1.00 | |||
| 1 | 12 | 5 | 4 | 3 |
| 2 | 65 | 33 | 24 | 8 |
| 3 | 12 | 5 | 4 | 3 |
| rs | P-value | n | |
|---|---|---|---|
| Ca9 mRNA in TC (n = 72) correlates with levels of | |||
| hypoxic markers | |||
| CAIX protein in TC | 0.611 | <0.001 | 37 |
| miR-210 in TC | 0.331 | 0.005 | 70 |
| VEGFa mRNA in TC | 0.472 | <0.001 | 72 |
| Glut1 mRNA in TC | 0.367 | 0.002 | 72 |
| GAPDH mRNA in TC | −0.369 | 0.001 | 72 |
| structure proteins | |||
| CDH mRNA in TC | 0.197 | 0.10 | 70 |
| KRT13 mRNA in TC | 0.230 | 0.001 | 69 |
| EMT and stem cell markers | |||
| ZEB2-mRNA in TC | −0.233 | 0.052 | 70 |
| Survivin-mRNA in TC | 0.371 | 0.001 | 72 |
| CAIX protein in TC (n = 158) correlates with levels of | |||
| hypoxic markers | |||
| CA9 mRNA in TC | 0.611 | <0.001 | 37 |
| HIF1α protein in TC | 0.234 | 0.015 | 107 |
| miR-210 in TC | 0.054 | 0.75 | 36 |
| CAIX protein in blood serum (n = 89) correlates with levels of | |||
| hypoxic markers | |||
| CA9 mRNA in TC | 0.115 | 0.487 | 39 |
| CAIX protein in TC | 0.432 | 0.11 | 15 |
| miR-210 in TC | −0.060 | 0.724 | 37 |
| miR-210 in serum | 0.457 | 0.037 | 21 |
| other markers | |||
| Her2 mRNA in TC | −0.339 | 0.040 | 37 |
| Her2 protein in TC | 0.263 | 0.204 | 25 |
| Her2 protein in serum | 0.300 | 0.064 | 39 |
| EGFR mRNA in TC | −0.325 | 0.050 | 37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckert, A.W.; Horter, S.; Bethmann, D.; Kotrba, J.; Kaune, T.; Rot, S.; Bache, M.; Bilkenroth, U.; Reich, W.; Greither, T.; et al. Investigation of the Prognostic Role of Carbonic Anhydrase 9 (CAIX) of the Cellular mRNA/Protein Level or Soluble CAIX Protein in Patients with Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 375. https://doi.org/10.3390/ijms20020375
Eckert AW, Horter S, Bethmann D, Kotrba J, Kaune T, Rot S, Bache M, Bilkenroth U, Reich W, Greither T, et al. Investigation of the Prognostic Role of Carbonic Anhydrase 9 (CAIX) of the Cellular mRNA/Protein Level or Soluble CAIX Protein in Patients with Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2019; 20(2):375. https://doi.org/10.3390/ijms20020375
Chicago/Turabian StyleEckert, Alexander W., Susanne Horter, Daniel Bethmann, Johanna Kotrba, Tom Kaune, Swetlana Rot, Matthias Bache, Udo Bilkenroth, Waldemar Reich, Thomas Greither, and et al. 2019. "Investigation of the Prognostic Role of Carbonic Anhydrase 9 (CAIX) of the Cellular mRNA/Protein Level or Soluble CAIX Protein in Patients with Oral Squamous Cell Carcinoma" International Journal of Molecular Sciences 20, no. 2: 375. https://doi.org/10.3390/ijms20020375
APA StyleEckert, A. W., Horter, S., Bethmann, D., Kotrba, J., Kaune, T., Rot, S., Bache, M., Bilkenroth, U., Reich, W., Greither, T., Wickenhauser, C., Vordermark, D., Taubert, H., & Kappler, M. (2019). Investigation of the Prognostic Role of Carbonic Anhydrase 9 (CAIX) of the Cellular mRNA/Protein Level or Soluble CAIX Protein in Patients with Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences, 20(2), 375. https://doi.org/10.3390/ijms20020375







