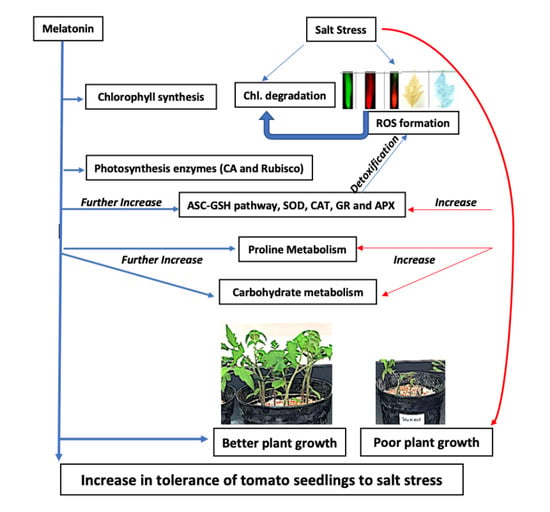
Exogenous Melatonin Counteracts NaCl-Induced Damage by Regulating the Antioxidant System, Proline and Carbohydrates Metabolism in Tomato Seedlings
Abstract
1. Introduction
2. Results
2.1. Effect of Melatonin on Growth Attributes of Tomato Seedlings under NaCl Stress
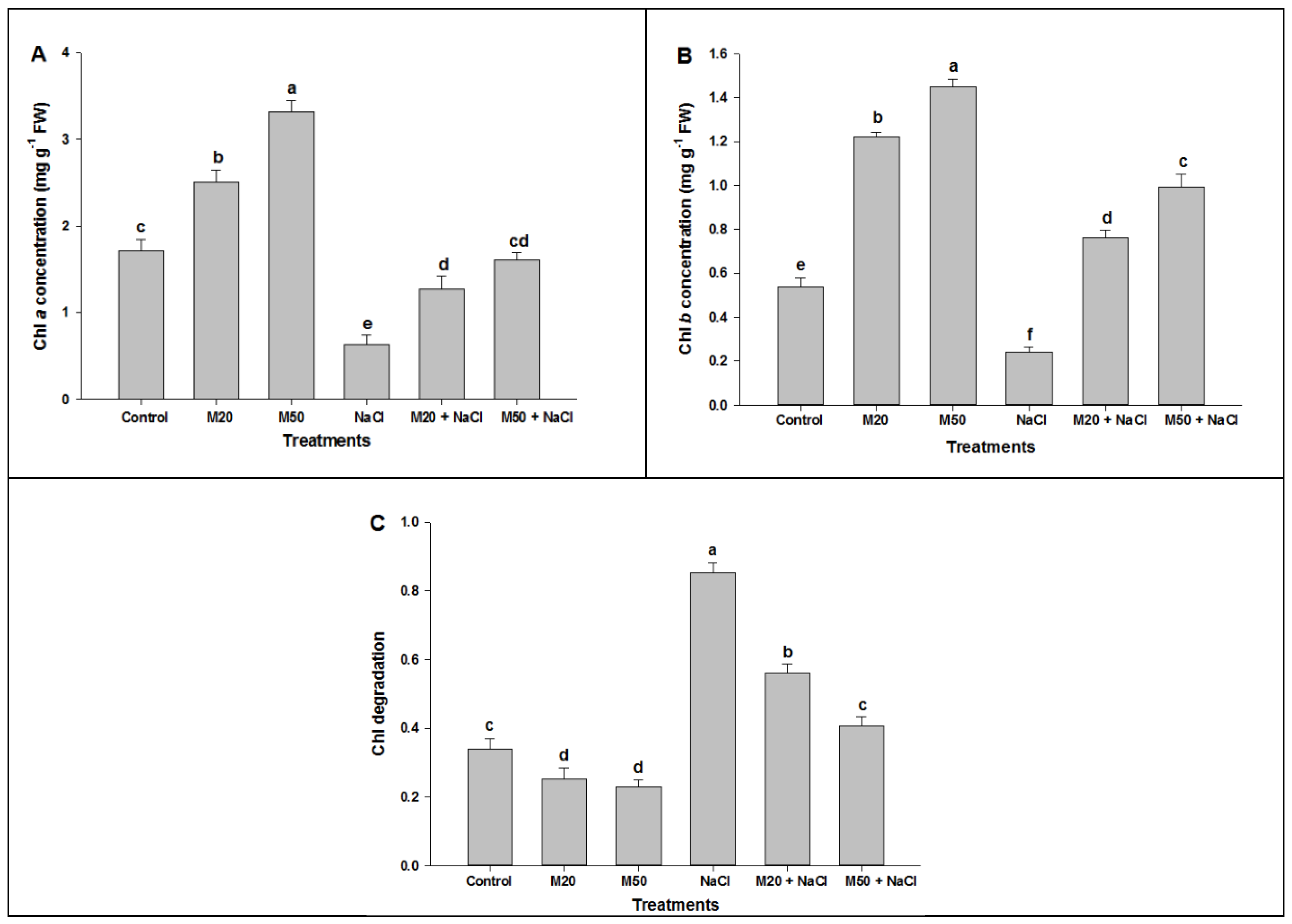
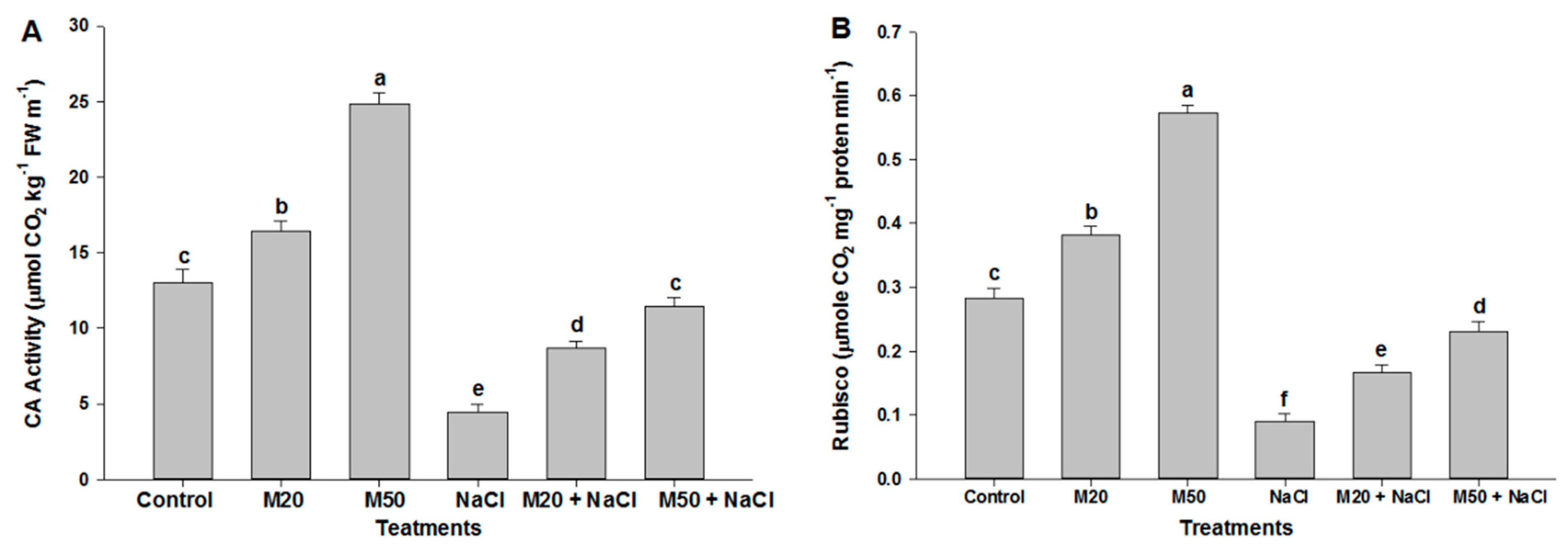
2.2. Effect of Melatonin on Chl Content, and Activities of CA and Rubisco under NaCl Stress
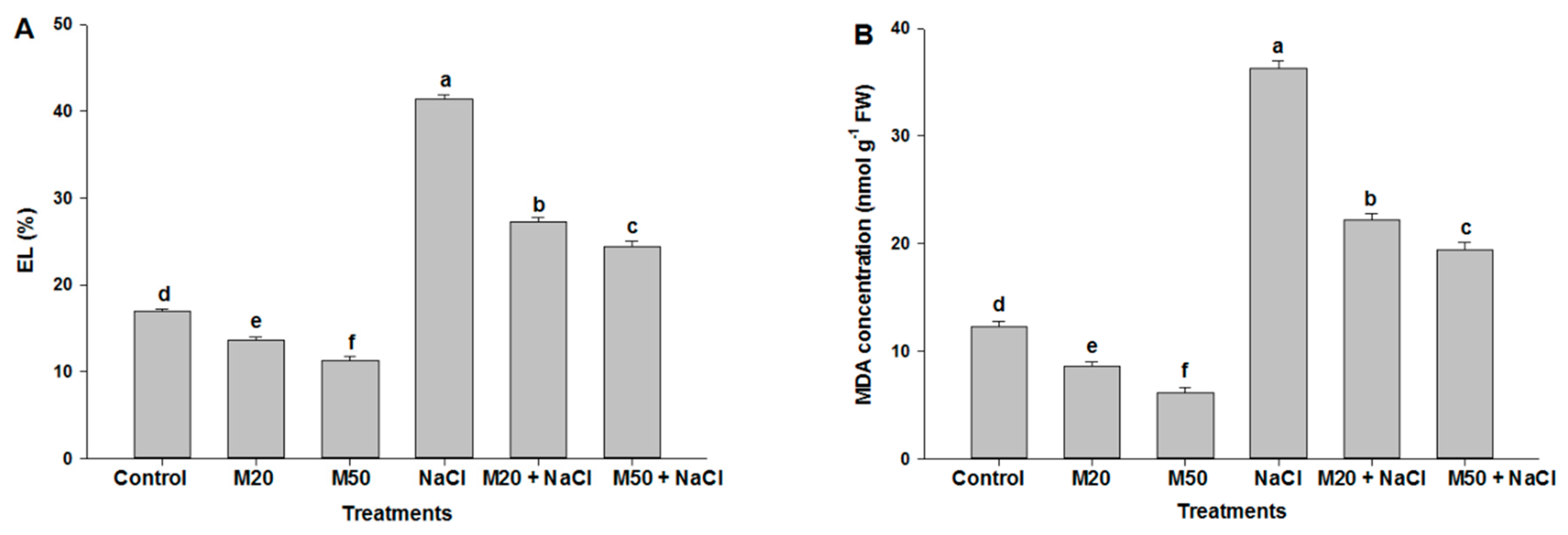
2.3. Effect of M on Electrolyte Leakage (EL) and Malondialdehyde (MDA) Concentration under NaCl Stress
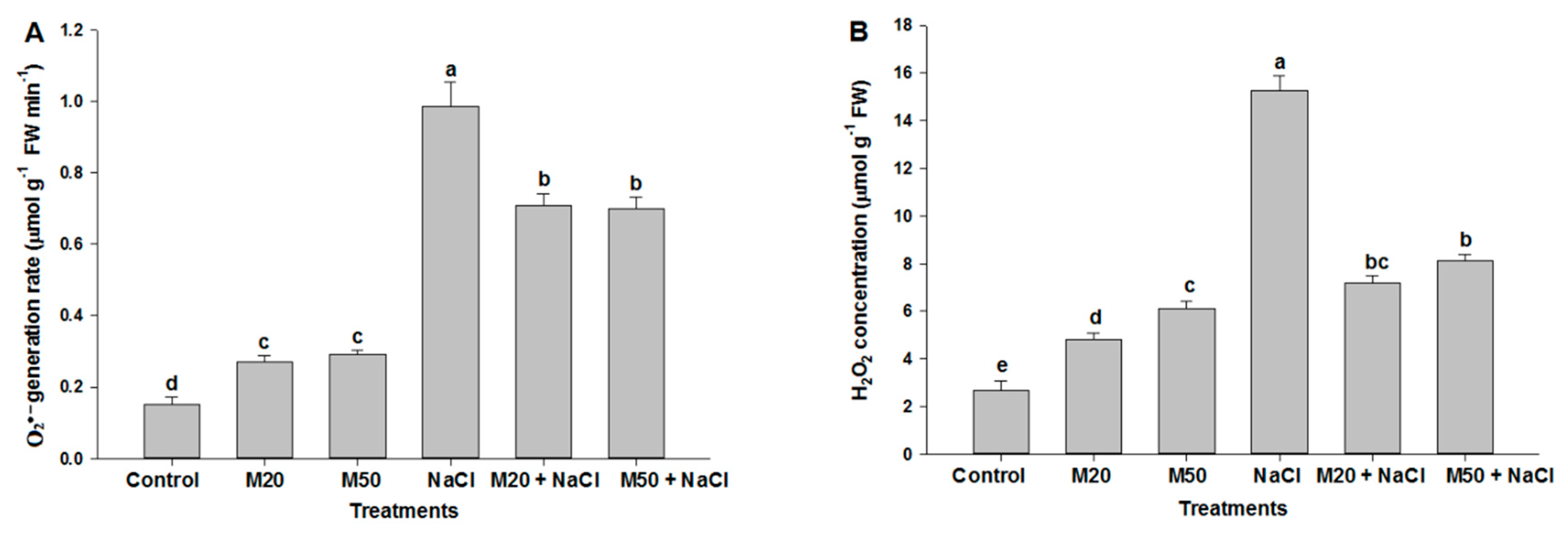
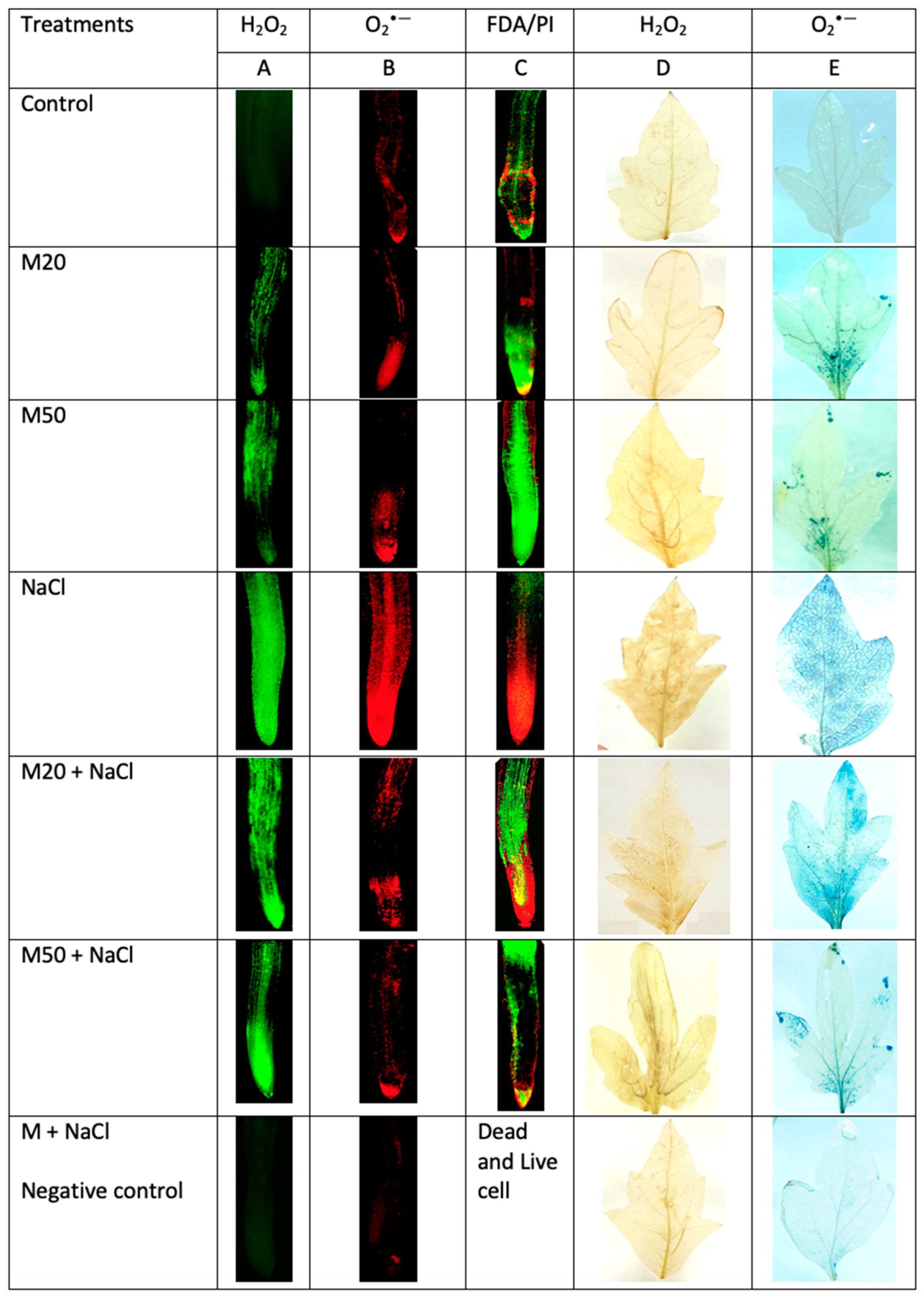
2.4. Effect of Melatonin on Hydrogen Peroxide (H2O2) and Superoxide (O2•−) Concentration under NaCl Stress
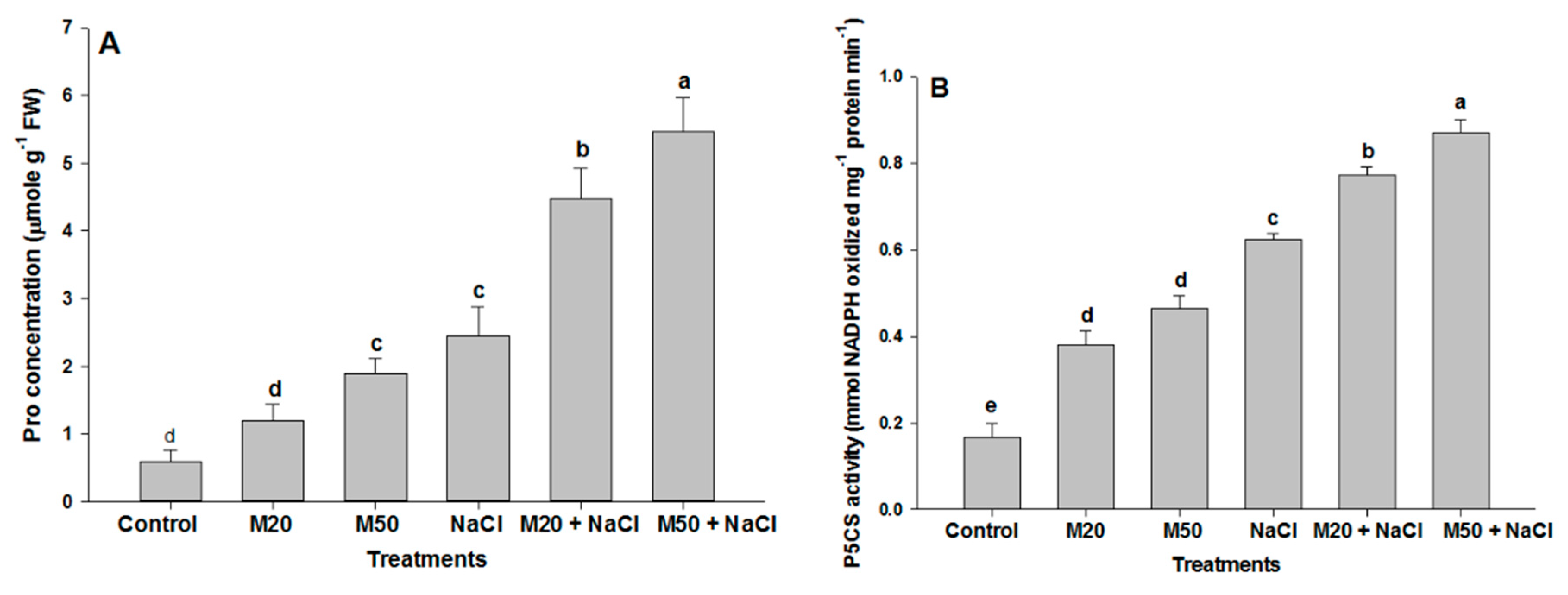
2.5. Effect of Melatonin on Proline (Pro) Content, Δ1-Pyrroline-5-Carboxylate Synthetase (P5CS) Activity and Total Soluble Carbohydrates (TSC) Content under NaCl Stress
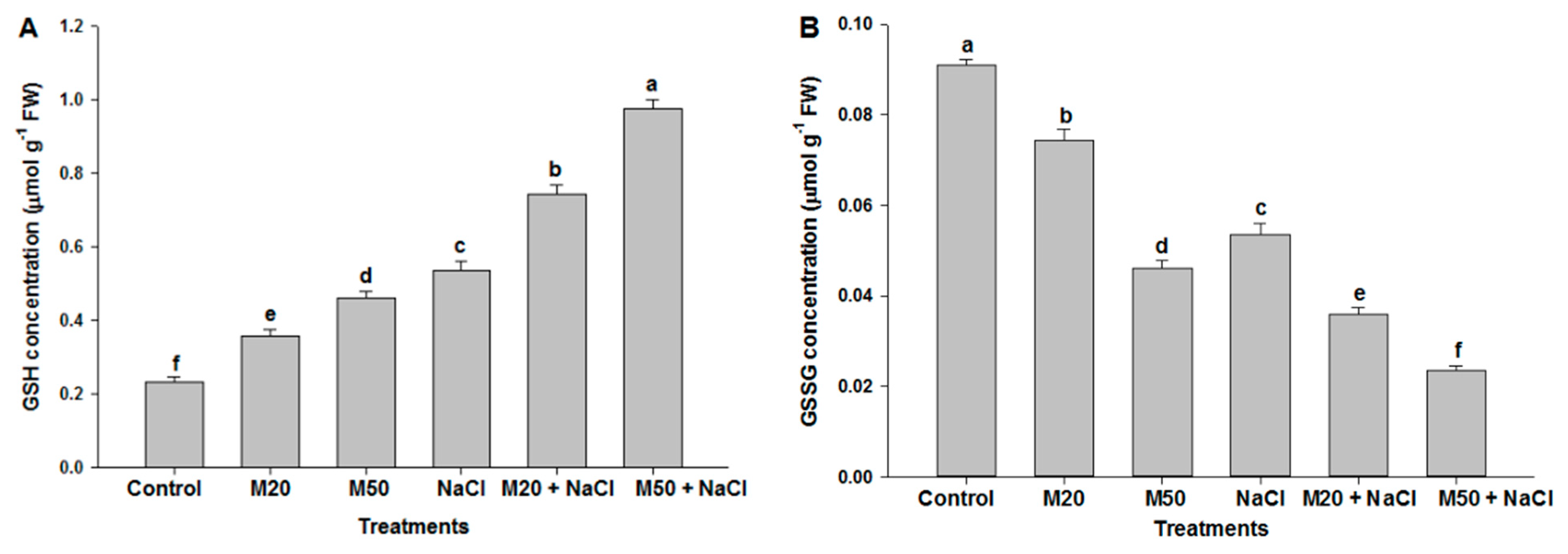
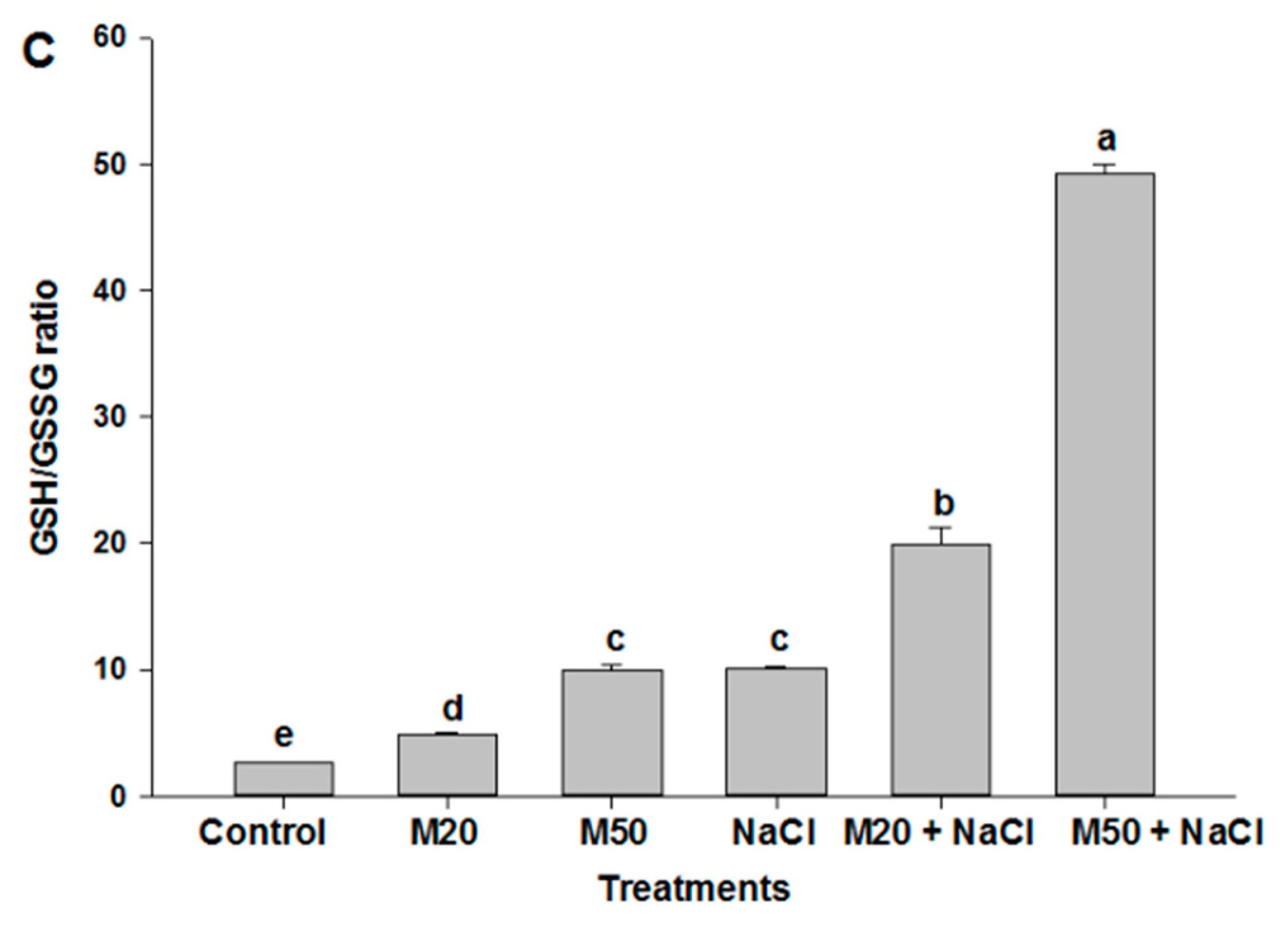
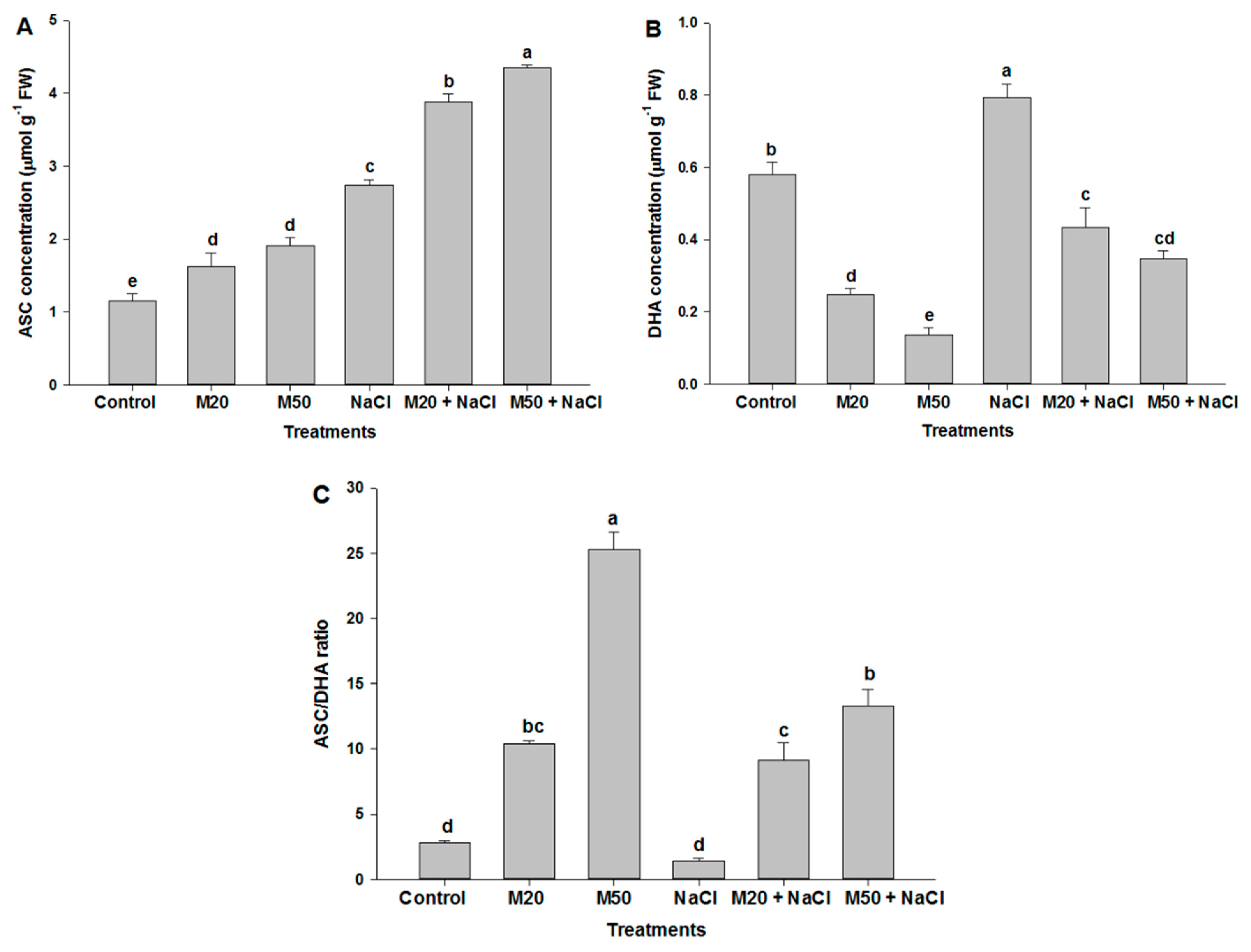
2.6. Effect of Melatonin on Non-Enzymatic Antioxidants under NaCl Stress
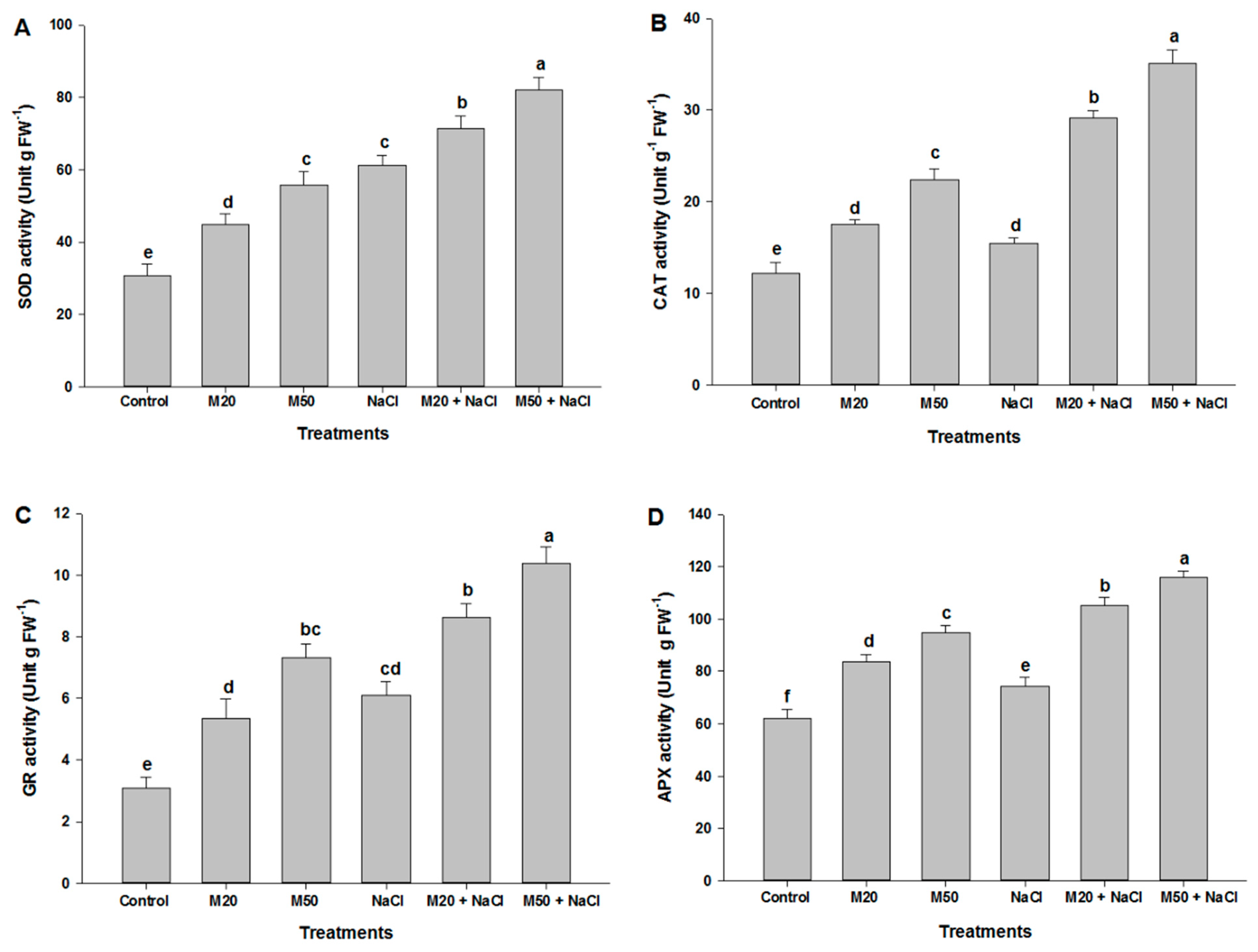
2.7. Effect of Melatonin on the Activities of Antioxidant Enzymes under NaCl Stress
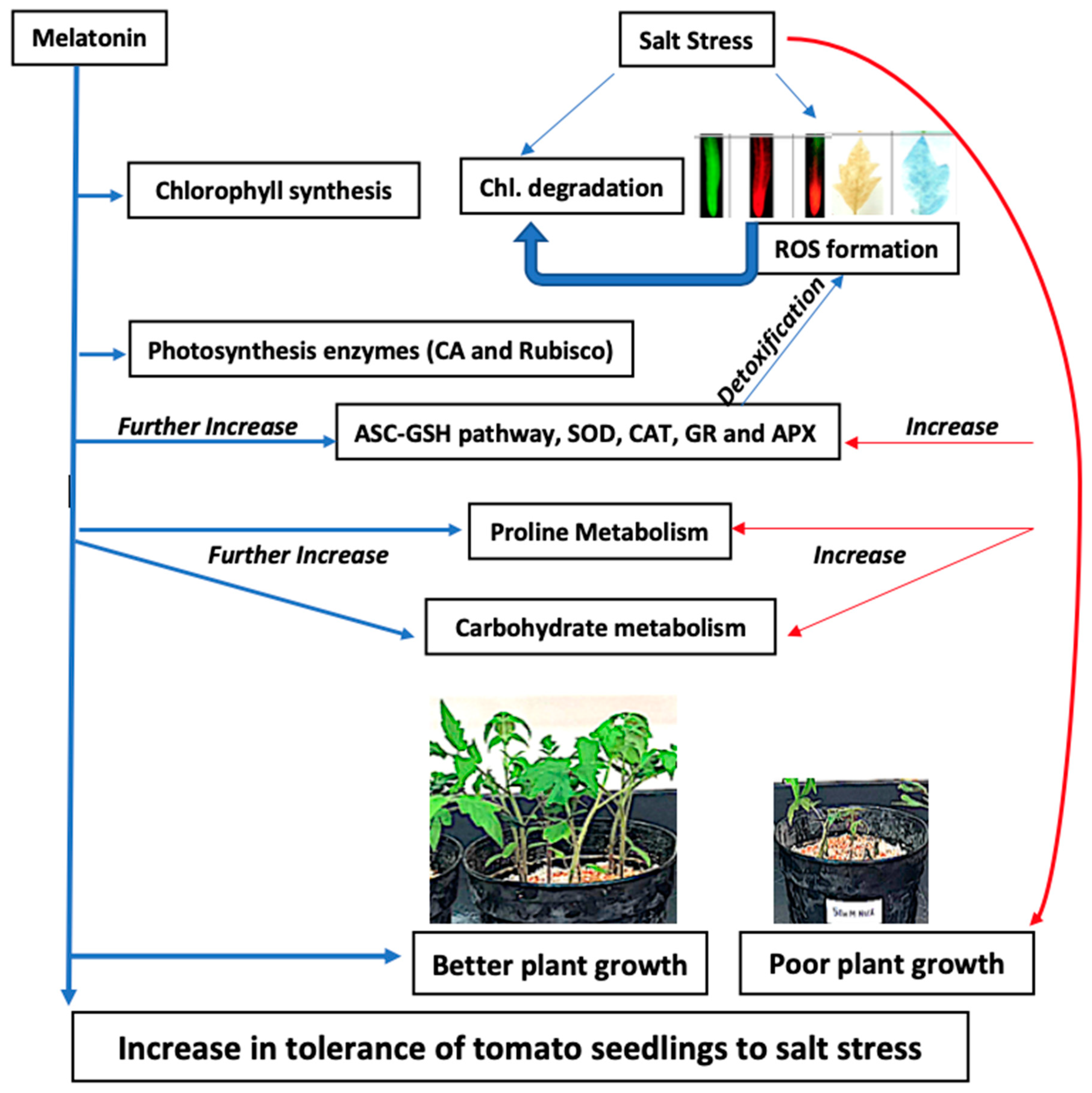
3. Discussion
3.1. Exogenous Melatonin Enhances Growth by Stimulating Photosynthetic Enzymes, and Proline and Carbohydrate Metabolism under Salinity and Non-Salinity Conditions
3.2. Exogenous Melatonin Regulates Antioxidant System under Salinity
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
4.2. Morphological Characteristics of Tomato Seedlings Determination of Tomato Seedlings
4.3. Physiological and Biochemical Characteristics Analysis of Tomato Seedlings
4.3.1. Photosynthetic Pigments
4.3.2. Photosynthetic Enzymes
4.3.3. Cell Membrane Stability, Lipid Peroxidation, and ROS Determination and Detection
4.3.4. Determination of Pro Content and Its Metabolizing Enzyme P5CS and Total Soluble Carbohydrates
4.3.5. Determination of Antioxidants and Antioxidant Enzymes Assay
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Carbonic anhydrase |
| Rubisco | ribulose-1,5-bisphosphate carboxylase/oxygenase |
| Pro | Proline |
| TSC | Total soluble carbohydrates |
| MDA | Malondialdehyde |
| Chl | Chlorophyll |
| EL | Electrolyte leakage |
| GSH | Reduced glutathione |
| GSSG | Oxidized |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| APX | Ascorbate peroxidase |
| ASC | Ascorbate |
| DHA | Dehydroascorbate reductase |
References
- Zandalinas, S.I.; Mittler, R.; Balfagon, D.; Arbona, V.; Gomez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Alamri, S.A.; Al-Khaishany, M.Y.; Al-Qutami, M.A.; Ali, H.M.; AL-Rabiah, H.; Kalaji, H.M. Exogenous application of nitric oxide and spermidine reduces the negative effects of salt stress on tomato. Hortic. Environ. Biotechnol. 2017, 58, 537–547. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Sakran, A.M.; Basalah, M.O.; Ali, H.M. Effect of calcium and potassium on antioxidant system of Vicia faba L. Under cadmium stress. Int. J. Mol. Sci. 2012, 13, 6604–6619. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Cirillo, C.; De Micco, V.; Arena, C.; De Pascale, S.; Rouphael, Y. Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis Willd. trained to different canopy shapes. Agric. Water Manag. 2019, 212, 12–22. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Mohammad, F.; Khan, M.N. Morphological and physio-biochemical characterization of Brassica juncea L. Czern. & Coss. genotypes under salt stress. J. Plant Interact. 2009, 4, 67–80. [Google Scholar]
- Siddiqui, M.H.; Mohammad, F.; Khan, M.N.; Al-Whaibi, M.H.; Bahkali, A.H.A. Nitrogen in Relation to Photosynthetic Capacity and Accumulation of Osmoprotectant and Nutrients in Brassica Genotypes Grown Under Salt Stress. Agric. Sci. China 2010, 9, 671–680. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin-A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.H.; Huang, B.; Ding, C.B.; Zhang, Z.W.; Chen, Y.E.; Hu, C.; Zhou, L.J.; Huang, Y.; Liao, J.Q.; Yuan, S.; et al. Effects of Melatonin on Anti-oxidative Systems and Photosystem II in Cold-Stressed Rice Seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.; Bolborea, M. Molecular pathways involved in seasonal body weight and reproductive responses governed by melatonin. J. Pineal Res. 2012, 52, 376–388. [Google Scholar] [CrossRef]
- Hardeland, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.R.; Gonzalez-Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. Melatonin feedback on clock genes: A theory involving the proteasome. J. Pineal Res. 2015, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by High-Performance Liquid Chromatography-Mass Spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtanikaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current Status and Future Perspectives in Plant Science. Front. Plant Sci. 2015, 6, 1230. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Campbell, S.S.B.; Saxena, P.K. The role of serotonin and melatonin in plant morphogenesis: Regulation of auxin-induced root organogenesis in in vitro-cultured explants of St. John’s wort (Hypericum perforatum L.). In Vitro Cell. Dev.-Plant 2001, 37, 786–793. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to Stress Combination in Tomato Plants: New Insights in the Protective Role of Melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef]
- Erland, L.A.; Murch, S.J.; Reiter, R.J.; Saxena, P.K. A new balancing act: The many roles of melatonin and serotonin in plant growth and development. Plant Signal. Behav. 2015, 10, e1096469. [Google Scholar] [CrossRef]
- Byeon, Y.; Park, S.; Kim, Y.S.; Park, D.H.; Lee, S.; Back, K. Light-regulated melatonin biosynthesis in rice during the senescence process in detached leaves. J. Pineal Res. 2012, 53, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Gao, T.; Liang, B.; Zhao, Q.; Ma, F.; Li, C. Effects of Exogenous Melatonin on Methyl Viologen-Mediated Oxidative Stress in Apple Leaf. Int. J. Mol. Sci. 2018, 19, 316. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.Q.; Zhang, H.J.; Cao, Y.Y.; Weeda, S.; Ren, S.X.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant. Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Shi, H.T.; Tan, D.X.; Reiter, R.J.; Ye, T.T.; Yang, F.; Chan, Z.L. Melatonin induces class A1 heat-shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 2015, 58, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, R.; Meng, J.; Li, Z.; Wu, Y.; Tao, J. Ameliorative effects of melatonin on dark-induced leaf senescence in gardenia (Gardenia jasminoides Ellis): Leaf morphology, anatomy, physiology and transcriptome. Sci. Rep. 2017, 7, 10423. [Google Scholar] [CrossRef]
- Byeon, Y.; Park, S.; Kim, Y.S.; Back, K. Microarray analysis of genes differentially expressed in melatonin-rich transgenic rice expressing a sheep serotonin N-acetyltransferase. J. Pineal Res. 2013, 55, 357–363. [Google Scholar]
- Byeon, Y.; Back, K. An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J. Pineal Res. 2014, 56, 408–414. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef]
- Zhao, H.B.; Su, T.; Huo, L.Q.; Wei, H.B.; Jiang, Y.; Xu, L.F.; Ma, F.W. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Z.; Zheng, G.Y.; Li, W.Z.; Wang, Y.Q.; Hu, B.; Wang, H.R.; Wu, H.K.; Qian, Y.W.; Zhu, X.G.; Tan, D.X.; et al. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.Q.; Hu, C.; Zhang, Z.W.; Yuan, S.; et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plantarum. 2018, 164, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Afreen, F.; Zobayed, S.M.A.; Kozai, T. Melatonin in Glycyrrhiza uralensis: Response of plant roots to spectral quality of light and UV-B radiation. J. Pineal Res. 2006, 41, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Fischer, T.W.; Kleszczynski, K.; Hardkop, L.H.; Kruse, N.; Zillikens, D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J. Pineal Res. 2013, 54, 303–312. [Google Scholar] [CrossRef]
- Marta, B.; Szafranska, K.; Posmyk, M.M. Exogenous Melatonin Improves Antioxidant Defense in Cucumber Seeds (Cucumis sativus L.) Germinated under Chilling Stress. Front. Plant. Sci 2016, 7, 575. [Google Scholar] [CrossRef] [PubMed]
- Weeda, S.; Zhang, N.; Zhao, X.L.; Ndip, G.; Guo, Y.D.; Buck, G.A.; Fu, C.G.; Ren, S.X. Arabidopsis Transcriptome Analysis Reveals Key Roles of Melatonin in Plant Defense Systems. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Lee, H.Y.; Byeon, Y.; Tan, D.X.; Reiter, R.J.; Back, K. Arabidopsis serotonin N-acetyltransferase knockout mutant plants exhibit decreased melatonin and salicylic acid levels resulting in susceptibility to an avirulent pathogen. J. Pineal Res. 2015, 58, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Benazzouk, S.; Djazouli, Z.E.; Lutts, S. Assessment of the preventive effect of vermicompost on salinity resistance in tomato (Solanum lycopersicum cv. Ailsa Craig). Acta Physiol. Plant. 2018, 40, 121. [Google Scholar] [CrossRef]
- Cuartero, J.; Bolarin, M.C.; Asins, M.J.; Moreno, V. Increasing salt tolerance in the tomato. J. Exp. Bot. 2006, 57, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Saxena, P.K. Melatonin: A potential regulator of plant growth and development? In Vitro Cell. Dev.-Plant. 2002, 38, 531–536. [Google Scholar] [CrossRef]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin acts as a growth-stimulating compound in some monocot species. J. Pineal Res. 2005, 39, 137–142. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, W.B.; Reiter, R.J.; Wei, W.; Wang, B.M. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef]
- Simlat, M.; Ptak, A.; Skrzypek, E.; Warchol, M.; Moranska, E.; Piorkowska, E. Melatonin significantly influences seed germination and seedling growth of Stevia rebaudiana Bertoni. PeerJ 2018, 6, e5009. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Mohammad, F.; Khan, M.N.; Khan, M.M.A. Cumulative effect of soil and foliar application of nitrogen, phosphorus, and sulfur on growth, physico-biochemical parameters, yield attributes, and fatty acid composition in oil of erucic acid-free rapeseed-mustard genotypes. J. Plant Nutr. 2008, 31, 1284–1298. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium × Prunus cerasus). Plant Physiol. Biochem. 2012, 61, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.Y.; Tsai, T.T.; Shih, C.N. Photosynthetic gas exchange and chlorophyll a fluorescence of three wild soybean species in response to NaCl treatments. Photosynthetica 2003, 41, 415–419. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Khan, M.N.; Mohammad, F.; Khan, M.M.A. Role of nitrogen and gibberellin (GA(3)) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop Sci. 2008, 194, 214–224. [Google Scholar] [CrossRef]
- Lin, Y.H.; Pan, K.Y.; Hung, C.H.; Huang, H.E.; Chen, C.L.; Feng, T.Y.; Huang, L.F. Overexpression of Ferredoxin, PETF, Enhances Tolerance to Heat Stress in Chlamydomonas reinhardtii. Int. J. Mol. Sci. 2013, 14, 20913–20929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Yang, S.J.; Chen, Y.Y. Effects of melatonin on photosynthetic performance and antioxidants in melon during cold and recovery. Biol. Plantarum. 2017, 61, 571–578. [Google Scholar] [CrossRef]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Zhou, X.T.; Zhao, H.L.; Cao, K.; Hu, L.P.; Du, T.H.; Baluska, F.; Zou, Z.R. Beneficial Roles of Melatonin on Redox Regulation of Photosynthetic Electron Transport and Synthesis of D1 Protein in Tomato Seedlings under Salt Stress. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef]
- Badger, M.R.; Price, G.D. The Role of Carbonic-Anhydrase in Photosynthesis. Annu. Rev. Plant Phys. 1994, 45, 369–392. [Google Scholar] [CrossRef]
- DiMario, R.J.; Clayton, H.; Mukherjee, A.; Ludwig, M.; Moroney, J.V. Plant Carbonic Anhydrases: Structures, Locations, Evolution, and Physiological Roles. Mol. Plant 2017, 10, 30–46. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Physiol. 2006, 53, 257–277. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.B.; Liu, T.Y.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Sako, K.; Matsui, A.; Suzuki, Y.; Mostofa, M.G.; Van Ha, C.; Tanaka, M.; Tran, L.S.P.; Habu, Y.; Seki, M. Ethanol Enhances High-Salinity Stress Tolerance by Detoxifying Reactive Oxygen Species in Arabidopsis thaliana and Rice. Front. Plant. Sci. 2017, 8, 1001. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Hasan, M.K.; Liu, C.X.; Pan, Y.T.; Ahammed, G.J.; Qi, Z.Y.; Zhou, J. Melatonin alleviates low-sulfur stress by promoting sulfur homeostasis in tomato plants. Sci. Rep. 2018, 8, 10182. [Google Scholar] [CrossRef]
- Rhodes, D.; Hanson, A.D. Quaternary Ammonium and Tertiary Sulfonium Compounds in Higher-Plants. Annu. Rev. Plant Phys. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Mohammad, F.; Khan, M.M.; Al-Whaibi, M.H. Cumulative effect of nitrogen and sulphur on Brassica juncea L. genotypes under NaCl stress. Protoplasma 2012, 249, 139–153. [Google Scholar] [CrossRef]
- Carillo, P. GABA Shunt in Durum Wheat. Front. Plant. Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Ferchichi, S.; Hessini, K.; Dell’Aversana, E.; D’Amelia, L.; Woodrow, P.; Ciarmiello, L.F.; Fuggi, A.; Carillo, P. Hordeum vulgare and Hordeum maritimum respond to extended salinity stress displaying different temporal accumulation pattern of metabolites. Funct. Plant Biol. 2018, 45, 1096–1109. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant. Physiology, 5th ed.; Sinauer Associates: Sunderland, VT, USA, 2010; p. 782. [Google Scholar]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Sakran, A.M.; Ali, H.M.; Basalah, M.O.; Faisal, M.; Alatar, A.; Al-Amri, A.A. Calcium-Induced Amelioration of Boron Toxicity in Radish. J. Plant Growth Regul. 2013, 32, 61–71. [Google Scholar] [CrossRef]
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Khan, M.N.A.; Alakeel, K.A. Nitric oxide-mediated cross-talk of proline and heat shock proteins induce thermotolerance in Vicia faba L. Environ. Exp. Bot. 2018. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Reactive Oxygen Species and Antioxidant Enzymes Involved in Plant Tolerance to Stress; IntechOpen Limited: London, UK, 2016; pp. 463–480. [Google Scholar]
- Arbona, V.; Manzi, M.; Zandalinas, S.I.; Vives-Peris, V.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Physiological, Metabolic, and Molecular Responses of Plants to Abiotic Stress; Springer International Publishing: Basel, Switzerland, 2017; Volume 2, pp. 1–35. [Google Scholar]
- Arnao, M.B.; Hernandez-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- May, J.M. Ascorbate function and metabolism in the human erythrocyte. Front. Biosci 1998, 3, 1–10. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahammed, G.J.; Yin, L.L.; Shi, K.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A Reappraisal of the Use of Dmso for the Extraction and Determination of Chlorophylls-a and Chlorophylls-B in Lichens and Higher-Plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Ronen, R.; Galun, M. Pigment Extraction from Lichens with Dimethylsulfoxide (Dmso) and Estimation of Chlorophyll Degradation. Environ. Exp. Bot. 1984, 24, 239–245. [Google Scholar] [CrossRef]
- Dwivedi, R.S.; Randhawa, N.S. Evaluation of a Rapid Test for Hidden Hunger of Zinc in Plants. Plant Soil 1974, 40, 445–451. [Google Scholar] [CrossRef]
- Usuda, H. The Activation State of Ribulose 1,5-Bisphosphate Carboxylase in Maize Leaves in Dark and Light. Plant Cell Physiol. 1985, 26, 1455–1463. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J. Exp. Bot. 1995, 46, 1843–1852. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts.I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants–Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Formation of Hydrogen-Peroxide by Isolated Cell-Walls from Horseradish (Armoracia-Lapathifolia Gilib). Planta 1976, 130, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Serrano, M.; Romero-Puertas, M.C.; Pazmino, D.M.; Testillano, P.S.; Risueno, M.C.; del Rio, L.A.; Sandalio, L.M. Cellular Response of Pea Plants to Cadmium Toxicity: Cross Talk between Reactive Oxygen Species, Nitric Oxide, and Calcium. Plant. Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Hou, J.; Qian, J.; Ao, Y.H.; Lu, J.; Li, L. Salicylic acid involved in the regulation of nutrient elements uptake and oxidative stress in Vallisneria natans (Lour.) Hara under Pb stress. Chemosphere 2011, 84, 136–142. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 2013, 22, 959–973. [Google Scholar] [CrossRef]
- Fan, G.; Liu, D.; Lin, Q. Fluorescein diacetate and propidium iodide FDA-PI double staining detect the viability of Microcystis sp. after ultrasonic irradiation. J. Food Agric. Environ. 2013, 11, 2419–2421. [Google Scholar]
- Jones, K.; Kim, D.W.; Park, J.S.; Khang, C.H. Live-cell fluorescence imaging to investigate the dynamics of plant cell death during infection by the rice blast fungus Magnaporthe oryzae. BMC Plant Biol. 2016, 16. [Google Scholar] [CrossRef]
- Bates, L.S.; Walden, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1972, 39, 205–207. [Google Scholar] [CrossRef]
- Sumithra, K.; Jutur, P.P.; Carmel, B.D.; Reddy, A.R. Salinity-induced changes in two cultivars of Vigna radiata: Responses of antioxidative and proline metabolism. Plant. Growth Regul. 2006, 50, 11–22. [Google Scholar] [CrossRef]
- Charest, C.; Phan, C.T. Cold-Acclimation of Wheat (Triticum-Aestivum)–Properties of Enzymes Involved in Proline Metabolism. Physiol. Plantarum. 1990, 80, 159–168. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for Superoxide-Dismutase Activity - Some Large Consequences of Minor Changes in Conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen-Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach-Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Takahama, U.; Oniki, T. Regulation of Peroxidase-Dependent Oxidation of Phenolics in the Apoplast of Spinach Leaves by Ascorbate. Plant Cell Physiol. 1992, 33, 379–387. [Google Scholar]
- Turcsányi, E.; Lyons, T.; Plochl, M.; Barnes, J. Does ascorbate in the mesophyll cell walls form the first line of defence against ozone? Testing the concept using broad bean (Vicia faba L.). J. Exp. Bot. 2000, 51, 901–910. [Google Scholar] [CrossRef]
- Yu, C.W.; Murphy, T.M.; Lin, C.H. Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955–963. [Google Scholar] [CrossRef]
- Paradiso, A.; Berardino, R.; de Pinto, M.C.; Sanita di Toppi, L.; Storelli, M.M.; Tommasi, F.; De Gara, L. Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol. 2008, 49, 362–374. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Parameters | |||
|---|---|---|---|---|
| SL (cm) | RL (cm) | Shoot FW (g) | Root FW (g) | |
| Control | 20.32 ± 1.19 c | 6.87 ± 0.34 c | 1.38 ± 0.093 b | 0.061 ± 0.006 d |
| M20 | 24.66 ± 0.90 b | 9.12 ± 0.17 b | 1.76 ± 0.093 a | 0.129 ± 0.003 b |
| M50 | 28.29 ± 0.70 a | 12.58 ± 0.28 a | 1.95 ± 0.078 a | 0.156 ± 0.005 a |
| NaCl | 13.21 ± 0.59 e | 4.42 ± 0.14 e | 0.71 ± 0.067 d | 0.042 ± 0.006 e |
| M20 + NaCl | 16.36 ± 0.55 d | 5.58 ± 0.28 d | 1.08 ± 0.038 c | 0.081 ± 0.005 c |
| M50 + NaCl | 18.22 ± 0.64 cd | 6.55 ± 0.11 c | 1.34 ± 0.091 b | 0.093 ± 0.004 c |
| Treatments | Parameters | ||
|---|---|---|---|
| Shoot DW (mg) | Root DW (mg) | LA (cm2) | |
| Control | 92.67 ± 2.33 c | 12.03 ± 0.58 c | 33.08 ± 0.53 c |
| M20 | 108.33 ± 2.60 b | 24.19 ± 0.61 b | 36.43 ± 0.91 b |
| M50 | 119.67 ± 2.02 a | 27.00 ± 0.58 a | 45.01 ± 0.58 a |
| NaCl | 45.33 ± 3.18 f | 6.33 ± 0.67 e | 10.55 ± 0.63 f |
| M20 + NaCl | 68.00 ± 1.73 e | 9.67 ± 0.67 d | 19.83 ± 0.77 e |
| M50 + NaCl | 78.67 ± 1.85 d | 12.67 ± 0.67 c | 22.75 ± 1.25 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, M.H.; Alamri, S.; Al-Khaishany, M.Y.; Khan, M.N.; Al-Amri, A.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A. Exogenous Melatonin Counteracts NaCl-Induced Damage by Regulating the Antioxidant System, Proline and Carbohydrates Metabolism in Tomato Seedlings. Int. J. Mol. Sci. 2019, 20, 353. https://doi.org/10.3390/ijms20020353
Siddiqui MH, Alamri S, Al-Khaishany MY, Khan MN, Al-Amri A, Ali HM, Alaraidh IA, Alsahli AA. Exogenous Melatonin Counteracts NaCl-Induced Damage by Regulating the Antioxidant System, Proline and Carbohydrates Metabolism in Tomato Seedlings. International Journal of Molecular Sciences. 2019; 20(2):353. https://doi.org/10.3390/ijms20020353
Chicago/Turabian StyleSiddiqui, Manzer H., Saud Alamri, Mutahhar Y. Al-Khaishany, M. Nasir Khan, Abdullah Al-Amri, Hayssam M. Ali, Ibrahim A. Alaraidh, and Abdulaziz A. Alsahli. 2019. "Exogenous Melatonin Counteracts NaCl-Induced Damage by Regulating the Antioxidant System, Proline and Carbohydrates Metabolism in Tomato Seedlings" International Journal of Molecular Sciences 20, no. 2: 353. https://doi.org/10.3390/ijms20020353
APA StyleSiddiqui, M. H., Alamri, S., Al-Khaishany, M. Y., Khan, M. N., Al-Amri, A., Ali, H. M., Alaraidh, I. A., & Alsahli, A. A. (2019). Exogenous Melatonin Counteracts NaCl-Induced Damage by Regulating the Antioxidant System, Proline and Carbohydrates Metabolism in Tomato Seedlings. International Journal of Molecular Sciences, 20(2), 353. https://doi.org/10.3390/ijms20020353