Natural Killer Cells and Current Applications of Chimeric Antigen Receptor-Modified NK-92 Cells in Tumor Immunotherapy
Abstract
1. Introduction
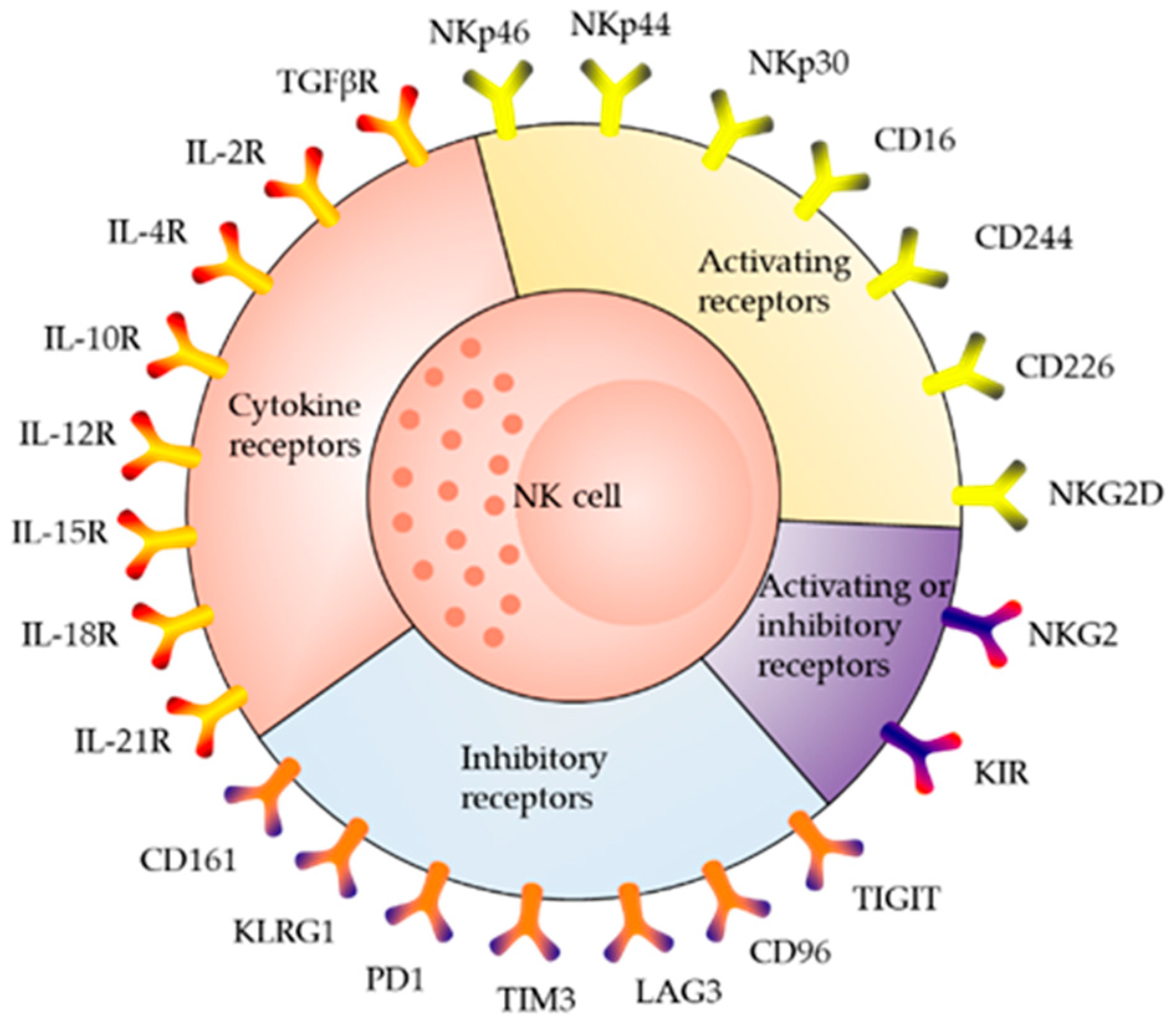
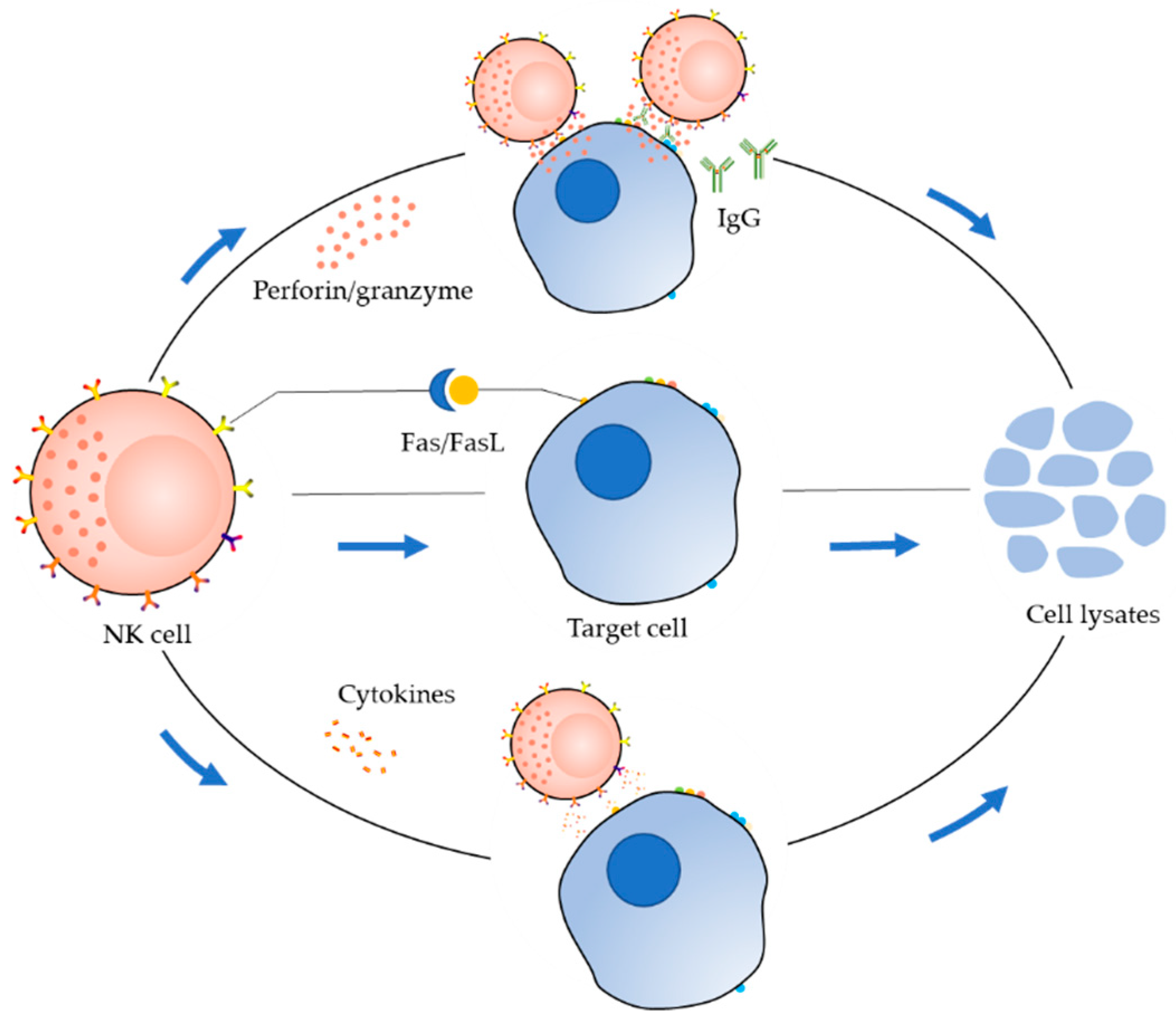
2. Receptor Distribution and Killing Mechanism of NK Cells
3. Currently Known NK Cell Lines
3.1. NK3.3 Cells
3.2. YT Cells
3.3. NKL Cells
3.4. HANK1 Cells
3.5. NK-YS Cells
3.6. KHYG-1 Cells
3.7. SNK-6 and SNT-8 Cells
3.8. IMC-1 Cells
3.9. NK-92 Cells
4. Progress in the Application of NK-92 Cells
5. Structure of CARs and Their Applications in NK-92 Cells
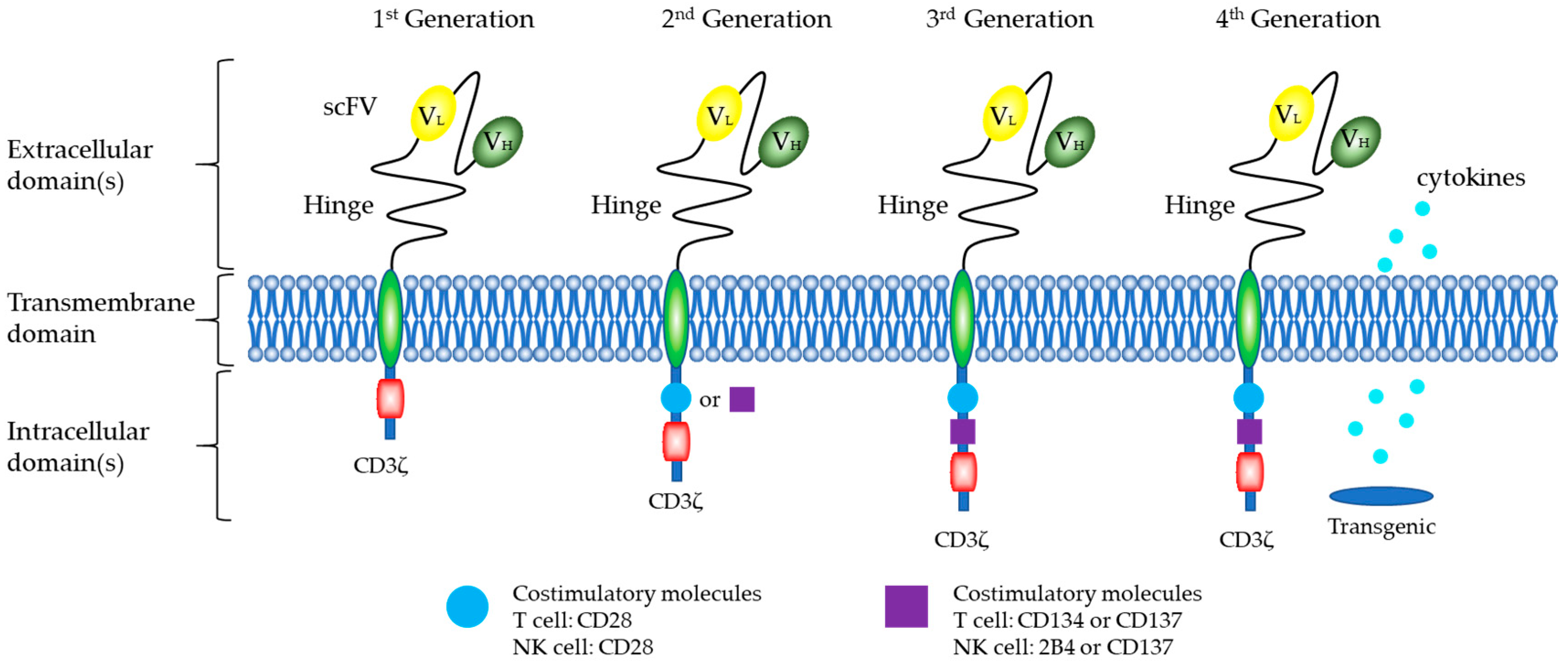
5.1. Structure of CARs
CARs of NK Cells
5.2. Preclinical Studies of CAR-NK-92 Cells
5.3. Ongoing Clinical Trials
5.4. Advantages of CAR-NK-92 Cells
5.5. Challenges and Coping Strategies
5.5.1. Tumor-Producing and Potential Epstein-Barr (EB) Virus Susceptibility
5.5.2. NK-92 Cells Have A Short Life Cycle after Irradiation
5.5.3. Defects in the Transfected Vector
5.5.4. Off-Target Effects
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | Antibody dependent cell mediated cytotoxicity |
| CAR | Chimeric antigen receptor |
| cGMP | Current good manufacturing practice |
| EB | Epstein-Barr |
| FDA | Food and Drug Administration |
| GVHD | Graft-versus-host disease |
| HER2 | Human epidermal growth factor receptor 2 |
| IL-2 | Interleukin-2 |
| ITAMs | Immunoreceptor tyrosine-based activation motif |
| KIRs | Killer immunoglobulin-like receptor |
| LGLs | Large granular lymphocytes |
| MHC | Major histocompatibility complex |
| NK | Natural killer |
| PB | Peripheral blood |
| scFv | Single-chain variable fragment |
| TCR | T-cell receptor |
| TNF | Tumor necrosis factor |
References
- Fang, F.; Xiao, W.; Tian, Z. NK cell-based immunotherapy for cancer. Semin. Immunol. 2017, 31, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Hazenberg, M.D.; Spits, H. Human innate lymphoid cells. Blood 2014, 124, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Rouce, R.; Liu, E.; Shpall, E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol. Ther. 2017, 25, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.S.; Hasegawa, J. Natural killer cell biology: An update and future directions. J. Allergy Clin. Immun. 2013, 132, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Cerwenka, A.; Lanier, L.L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 2016, 16, 112–123. [Google Scholar] [CrossRef]
- Hammer, Q.; Ruckert, T.; Romagnani, C. Natural killer cell specificity for viral infections. Nat. Immunol. 2018, 19, 800–808. [Google Scholar] [CrossRef]
- Mehta, R.S.; Rezvani, K. Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer. Front. Immunol. 2018, 9, 283. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Handgretinger, R.; Lang, P.; André, M.C. Exploitation of natural killer cells for the treatment of acute leukemia. Blood 2016, 127, 3341–3349. [Google Scholar] [CrossRef]
- Romagnani, C.; Juelke, K.; Falco, M.; Morandi, B.; D’Agostino, A.; Costa, R.; Ratto, G.; Forte, G.; Carrega, P.; Lui, G.; et al. CD56brightCD16- Killer Ig-Like Receptor- NK Cells Display Longer Telomeres and Acquire Features of CD56dim NK Cells upon Activation. J. Immunol. 2007, 178, 4947–4955. [Google Scholar] [CrossRef] [PubMed]
- Fehniger, T.A.; Cooper, M.A.; Nuovo, G.J.; Cella, M.; Facchetti, F.; Colonna, M.; Caligiuri, M.A. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: A potential new link between adaptive and innate immunity. Blood 2003, 101, 3052–3057. [Google Scholar] [CrossRef] [PubMed]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Romain, G.; Senyukov, V.; Rey-Villamizar, N.; Merouane, A.; Kelton, W.; Liadi, I.; Mahendra, A.; Charab, W.; Georgiou, G.; Roysam, B.; et al. Antibody Fc engineering improves frequency and promotes kinetic boosting of serial killing mediated by NK cells. Blood 2014, 124, 3241–3249. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, J. Reformation in chimeric antigen receptor based cancer immunotherapy: Redirecting natural killer cell. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 200–215. [Google Scholar] [CrossRef] [PubMed]
- De Maria, A.; Bozzano, F.; Cantoni, C.; Moretta, L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc. Natl. Acad. Sci. USA 2011, 108, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.V.; Gunturi, A.; Salcedo, M.; Schatzle, J.D.; Lai, W.C.; Kurepa, Z.; Pitcher, L.; Seaman, M.S.; Lemonnier, F.A.; Bennett, M.; et al. Cutting edge: Expression of functional CD94/NKG2A inhibitory receptors on fetal NK1.1+Ly-49- cells: A possible mechanism of tolerance during NK cell development. J. Immunol. 1999, 162, 6976–6980. [Google Scholar]
- Kumar, S. Natural killer cell cytotoxicity and its regulation by inhibitory receptors. Immunology 2018, 154, 383–393. [Google Scholar] [CrossRef]
- Wajant, H. The Fas Signaling Pathway: More Than a Paradigm. Science 2002, 296, 1635–1636. [Google Scholar] [CrossRef] [PubMed]
- Waring, P.; Mullbacher, A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol. Cell Biol. 1999, 77, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Kornbluth, J.; Flomenberg, N.; Dupont, B. Cell surface phenotype of a cloned line of human natural killer cells. J. Immunol. 1982, 129, 2831–2837. [Google Scholar] [PubMed]
- Le Bouteiller, P.; Barakonyi, A.; Giustiniani, J.; Lenfant, F.; Marie-Cardine, A.; Aguerre-Girr, M.; Rabot, M.; Hilgert, I.; Mami-Chouaib, F.; Tabiasco, J.; et al. Engagement of CD160 receptor by HLA-C is a triggering mechanism used by circulating natural killer (NK) cells to mediate cytotoxicity. Proc. Natl. Acad. Sci. USA 2002, 99, 16963–16968. [Google Scholar] [CrossRef] [PubMed]
- Umehara, H.; Huang, J.Y.; Kono, T.; Tabassam, F.H.; Okazaki, T.; Bloom, E.T.; Domae, N. Involvement of protein tyrosine kinase p72syk and phosphatidylinositol 3-kinase in CD2-mediated granular exocytosis in the natural killer cell line, NK3.3. J. Immunol. 1997, 159, 1200–1207. [Google Scholar] [PubMed]
- Mahle, N.H.; Radcliff, G.; Sevilla, C.L.; Kornbluth, J.; Callewaert, D.M. Kinetics of cellular cytotoxicity mediated by a cloned human natural killer cell line. Immunobiology 1989, 179, 230–243. [Google Scholar] [CrossRef]
- Yodoi, J.; Teshigawara, K.; Nikaido, T.; Fukui, K.; Noma, T.; Honjo, T.; Takigawa, M.; Sasaki, M.; Minato, N.; Tsudo, M.; et al. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J. Immunol. 1985, 134, 1623–1630. [Google Scholar]
- Yoneda, N.; Tatsumi, E.; Kawano, S.; Teshigawara, K.; Oka, T.; Fukuda, M.; Yamaguchi, N. Detection of Epstein-Barr virus genome in natural-killer-like cell line, YT. Leukemia 1992, 6, 136–141. [Google Scholar]
- Chen, X.; Allan, D.; Krzewski, K.; Ge, B.; Kopcow, H.; Strominger, J.L. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc. Natl. Acad. Sci. USA 2006, 103, 10346–10351. [Google Scholar] [CrossRef]
- Robertson, M.J.; Cochran, K.J.; Cameron, C.; Le, J.M.; Tantravahi, R.; Ritz, J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp. Hematol. 1996, 24, 406–415. [Google Scholar]
- Kagami, Y.; Nakamura, S.; Suzuki, R.; Iida, S.; Yatabe, Y.; Okada, Y.; Kobayashi, T.; Tsurumi, T.; Seto, M.; Ogura, M.; et al. Establishment of an IL-2-dependent cell line derived from ‘nasal-type’ NK/T-cell lymphoma of CD2+, sCD3-, CD3epsilon+, CD56+ phenotype and associated with the Epstein-Barr virus. Br. J. Haematol. 1998, 103, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiyama, J.; Yoshino, T.; Mori, M.; Kondoh, E.; Oka, T.; Akagi, T.; Hiraki, A.; Nakayama, H.; Shibuya, A.; Ma, Y.; et al. Characterization of a novel human natural killer-cell line (NK-YS) established from natural killer cell lymphoma/leukemia associated with Epstein-Barr virus infection. Blood 1998, 92, 1374–1383. [Google Scholar]
- Yagita, M.; Huang, C.L.; Umehara, H.; Matsuo, Y.; Tabata, R.; Miyake, M.; Konaka, Y.; Takatsuki, K. A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia 2000, 14, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Suck, G.; Branch, D.R.; Smyth, M.J.; Miller, R.G.; Vergidis, J.; Fahim, S.; Keating, A. KHYG-1, a model for the study of enhanced natural killer cell cytotoxicity. Exp. Hematol. 2005, 33, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Konno, A.; Kimura, N.; Zhang, Y.; Kimura, M.; Demachi, A.; Sekine, T.; Yamamoto, K.; Shimizu, N. Characterization of novel natural killer (NK)-cell and gammadelta T-cell lines established from primary lesions of nasal T/NK-cell lymphomas associated with the Epstein-Barr virus. Blood 2001, 97, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Whalen, M.; Bankhurst, A.; Sever, C.E.; Doshi, R.; Hardekopf, D.; Montgomery, K.; Willman, C.L. A new human natural killer leukemia cell line, IMC-1. A complex chromosomal rearrangement defined by spectral karyotyping: Functional and cytogenetic characterization. Leukemia Res. 2004, 28, 275–284. [Google Scholar] [CrossRef]
- Gong, J.H.; Maki, G.; Klingemann, H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994, 8, 652–658. [Google Scholar] [PubMed]
- Tam, Y.K.; Maki, G.; Miyagawa, B.; Hennemann, B.; Tonn, T.; Klingemann, H.G. Characterization of genetically altered, interleukin 2-independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum. Gene Ther. 1999, 10, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Tam, Y.K.; Miyagawa, B.; Ho, V.C.; Klingemann, H.G. Immunotherapy of malignant melanoma in a SCID mouse model using the highly cytotoxic natural killer cell line NK-92. J. Hematother. 1999, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Klingemann, H.G.; Miyagawa, B. Purging of malignant cells from blood after short ex vivo incubation with NK-92 cells. Blood 1996, 87, 4913–4914. [Google Scholar]
- Isobe, Y.; Sugimoto, K.; Yang, L.; Tamayose, K.; Egashira, M.; Kaneko, T.; Takada, K.; Oshimi, K. Epstein-Barr virus infection of human natural killer cell lines and peripheral blood natural killer cells. Cancer Res. 2004, 64, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Maki, G.; Klingemann, H.G.; Martinson, J.A.; Tam, Y.K. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J. Hematother. Stem Cell. Res. 2001, 10, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Boyiadzis, M.; Agha, M.; Redner, R.L.; Sehgal, A.; Im, A.; Hou, J.; Farah, R.; Dorritie, K.A.; Raptis, A.; Lim, S.H.; et al. Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy 2017, 19, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Tonn, T.; Schwabe, D.; Klingemann, H.G.; Becker, S.; Esser, R.; Koehl, U.; Suttorp, M.; Seifried, E.; Ottmann, O.G.; Bug, G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013, 15, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Meagher, R.; Swearingen, M.; Myint, H.; Rich, E.; Martinson, J.; Klingemann, H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: A phase I trial. Cytotherapy 2008, 10, 625–632. [Google Scholar] [CrossRef]
- Williams, B.A.; Law, A.D.; Routy, B.; DenHollander, N.; Gupta, V.; Wang, X.H.; Chaboureau, A.; Viswanathan, S.; Keating, A. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 2017, 8, 89256–89268. [Google Scholar] [CrossRef]
- Tonn, T.; Becker, S.; Esser, R.; Schwabe, D.; Seifried, E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J. Hematother. Stem Cell. Res. 2001, 10, 535–544. [Google Scholar] [CrossRef]
- Yan, Y.; Steinherz, P.; Klingemann, H.G.; Dennig, D.; Childs, B.H.; McGuirk, J.; O’Reilly, R.J. Antileukemia activity of a natural killer cell line against human leukemias. Clin. Cancer Res. 1998, 4, 2859–2868. [Google Scholar]
- Swift, B.E.; Williams, B.A.; Kosaka, Y.; Wang, X.H.; Medin, J.A.; Viswanathan, S.; Martinez-Lopez, J.; Keating, A. Natural killer cell lines preferentially kill clonogenic multiple myeloma cells and decrease myeloma engraftment in a bioluminescent xenograft mouse model. Haematologica 2012, 97, 1020–1028. [Google Scholar] [CrossRef][Green Version]
- Konstantinidis, K.V.; Alici, E.; Aints, A.; Christensson, B.; Ljunggren, H.G.; Dilber, M.S. Targeting IL-2 to the endoplasmic reticulum confines autocrine growth stimulation to NK-92 cells. Exp. Hematol. 2005, 33, 159–164. [Google Scholar] [CrossRef]
- Tam, Y.K.; Martinson, J.A.; Doligosa, K.; Klingemann, H.G. Ex vivo expansion of the highly cytotoxic human natural killer-92 cell-line under current good manufacturing practice conditions for clinical adoptive cellular immunotherapy. Cytotherapy 2003, 5, 259–272. [Google Scholar] [PubMed]
- Klingemann, H.; Boissel, L.; Toneguzzo, F. Natural Killer Cells for Immunotherapy—Advantages of the NK-92 Cell Line over Blood NK Cells. Front. Immunol. 2016, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Pegram, H.J.; Smith, E.L.; Rafiq, S.; Brentjens, R.J. CAR therapy for hematological cancers: Can success seen in the treatment of B-cell acute lymphoblastic leukemia be applied to other hematological malignancies? Immunotherapy 2015, 7, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Hwang, W.T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.; et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015, 7, 139r–303r. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014, 6, 224r–225r. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Sredni, B.; Longo, D.L. Cancer immunotherapy: Are we there yet? Semin. Cancer Biol. 2012, 22, 1–2. [Google Scholar] [CrossRef]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011, 365, 725–733. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Wilson, W.H. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010, 116, 4099–4102. [Google Scholar] [CrossRef]
- Brentjens, R.J.; Davila, M.L.; Riviere, I.; Park, J.; Wang, X.; Cowell, L.G.; Bartido, S.; Stefanski, J.; Taylor, C.; Olszewska, M.; et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013, 5, 138r–177r. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Second CAR T-cell Therapy. Cancer Discov. 2018, 8, 5–6. [CrossRef] [PubMed]
- First-ever CAR T-cell therapy approved in U.S. Cancer Discov. 2017. [CrossRef]
- Wang, Z.; Wu, Z.; Liu, Y.; Han, W. New development in CAR-T cell therapy. J. Hematol. Oncol. 2017, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, E.; Yang, Y.; Qin, H.; Chien, C.D.; Kochenderfer, J.N.; Fry, T.J. Murine allogeneic CD19 CAR T cells harbor potent antileukemic activity but have the potential to mediate lethal GVHD. Blood 2016, 127, 1361–1370. [Google Scholar] [CrossRef]
- Zhang, C.; Oberoi, P.; Oelsner, S.; Waldmann, A.; Lindner, A.; Tonn, T.; Wels, W.S. Chimeric Antigen Receptor-Engineered NK-92 Cells: An Off-the-Shelf Cellular Therapeutic for Targeted Elimination of Cancer Cells and Induction of Protective Antitumor Immunity. Front. Immunol. 2017, 8, 533. [Google Scholar] [CrossRef]
- Fan, M.; Li, M.; Gao, L.; Geng, S.; Wang, J.; Wang, Y.; Yan, Z.; Yu, L. Chimeric antigen receptors for adoptive T cell therapy in acute myeloid leukemia. J. Hematol. Oncol. 2017, 10, 151. [Google Scholar] [CrossRef]
- Jensen, M.C.; Riddell, S.R. Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunol. Rev. 2014, 257, 127–144. [Google Scholar] [CrossRef]
- Wang, J.; Jensen, M.; Lin, Y.; Sui, X.; Chen, E.; Lindgren, C.G.; Till, B.; Raubitschek, A.; Forman, S.J.; Qian, X.; et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum. Gene Ther. 2007, 18, 712–725. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. TRUCKs: The fourth generation of CARs. Expert Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Kopecky, C.; Hombach, A.A.; Abken, H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011, 71, 5697–5706. [Google Scholar] [CrossRef] [PubMed]
- Love, P.E.; Hayes, S.M. ITAM-mediated signaling by the T-cell antigen receptor. Cold Spring Harb. Perspect. Biol. 2010, 2, a2485. [Google Scholar] [CrossRef] [PubMed]
- Arnon, T.I.; Markel, G.; Mandelboim, O. Tumor and viral recognition by natural killer cells receptors. Semin. Cancer Biol. 2006, 16, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Tassev, D.V.; Cheng, M.; Cheung, N.K. Retargeting NK92 cells using an HLA-A2-restricted, EBNA3C-specific chimeric antigen receptor. Cancer Gene Ther. 2012, 19, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, S.; Ho, E.L.; Cella, M.; Yokoyama, W.M.; Colonna, M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat. Immunol. 2002, 3, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. The choices of a natural killer. Nat. Immunol. 2003, 4, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Connolly, J.; Shimasaki, N.; Mimura, K.; Kono, K.; Campana, D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013, 73, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- McNerney, M.E.; Lee, K.; Kumar, V. 2B4 (CD244) is a non-MHC binding receptor with multiple functions on natural killer cells and CD8+ T cells. Mol. Immunol. 2005, 42, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Altvater, B.; Landmeier, S.; Pscherer, S.; Temme, J.; Schweer, K.; Kailayangiri, S.; Campana, D.; Juergens, H.; Pule, M.; Rossig, C. 2B4 (CD244) Signaling by Recombinant Antigen-specific Chimeric Receptors Costimulates Natural Killer Cell Activation to Leukemia and Neuroblastoma Cells. Clin. Cancer Res. 2009, 15, 4857–4866. [Google Scholar] [CrossRef] [PubMed]
- Uherek, C.; Tonn, T.; Uherek, B.; Becker, S.; Schnierle, B.; Klingemann, H.G.; Wels, W. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood 2002, 100, 1265–1273. [Google Scholar] [PubMed]
- Romanski, A.; Uherek, C.; Bug, G.; Seifried, E.; Klingemann, H.; Wels, W.S.; Ottmann, O.G.; Tonn, T. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J. Cell. Mol. Med. 2016, 20, 1287–1294. [Google Scholar] [CrossRef]
- Oelsner, S.; Friede, M.E.; Zhang, C.; Wagner, J.; Badura, S.; Bader, P.; Ullrich, E.; Ottmann, O.G.; Klingemann, H.; Tonn, T.; et al. Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy 2017, 19, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Boissel, L.; Betancur, M.; Wels, W.S.; Tuncer, H.; Klingemann, H. Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells. Leukemia Res. 2009, 33, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Boissel, L.; Betancur, M.; Lu, W.; Krause, D.; Van Etten, R.; Wels, W.; Klingemann, H. Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology 2014, 2, e26527. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, W.; Shang, P.; Zhang, H.; Fu, W.; Ye, F.; Zeng, T.; Huang, H.; Zhang, X.; Sun, W.; et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol. Oncol. 2014, 8, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, J.; Chu, J.; Zhang, L.; Zhang, J.; Chen, C.; Chen, L.; Wang, Y.; Wang, H.; Yi, L.; et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget 2016, 7, 27764. [Google Scholar] [CrossRef]
- Alkins, R.; Burgess, A.; Kerbel, R.; Wels, W.S.; Hynynen, K. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro-Oncology 2016, 18, 974–981. [Google Scholar] [CrossRef]
- Alkins, R.; Burgess, A.; Ganguly, M.; Francia, G.; Kerbel, R.; Wels, W.S.; Hynynen, K. Focused Ultrasound Delivers Targeted Immune Cells to Metastatic Brain Tumors. Cancer Res. 2013, 73, 1892–1899. [Google Scholar] [CrossRef]
- Sahm, C.; Schonfeld, K.; Wels, W.S. Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer Immunol. Immunother. 2012, 61, 1451–1461. [Google Scholar] [CrossRef]
- Daldrup-Link, H.E.; Meier, R.; Rudelius, M.; Piontek, G.; Piert, M.; Metz, S.; Settles, M.; Uherek, C.; Wels, W.; Schlegel, J.R.; et al. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur. Radiol. 2005, 15, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Piert, M.; Piontek, G.; Rudelius, M.; Oostendorp, R.A.; Senekowitsch-Schmidtke, R.; Henning, T.D.; Wels, W.S.; Uherek, C.; Rummeny, E.J.; et al. Tracking of [18F]FDG-labeled natural killer cells to HER2/neu-positive tumors. Nucl. Med. Biol. 2008, 35, 579–588. [Google Scholar] [CrossRef]
- Schonfeld, K.; Sahm, C.; Zhang, C.; Naundorf, S.; Brendel, C.; Odendahl, M.; Nowakowska, P.; Bonig, H.; Kohl, U.; Kloess, S.; et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol. Ther. 2015, 23, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, B.; Sun, T.; Lin, L.; Hu, Y.; Deng, M.; Yang, J.; Liu, T.; Li, J.; Sun, S.; et al. Specific growth inhibition of ErbB2expressing human breast cancer cells by genetically modified NK92 cells. Oncol. Rep. 2015, 33, 95–102. [Google Scholar] [PubMed]
- Seidel, D.; Shibina, A.; Siebert, N.; Wels, W.S.; Reynolds, C.P.; Huebener, N.; Lode, H.N. Disialoganglioside-specific human natural killer cells are effective against drug-resistant neuroblastoma. Cancer Immunol. Immunother. 2015, 64, 621–634. [Google Scholar] [CrossRef]
- Esser, R.; Müller, T.; Stefes, D.; Kloess, S.; Seidel, D.; Gillies, S.D.; Aperlo-Iffland, C.; Huston, J.S.; Uherek, C.; Schönfeld, K.; et al. NK cells engineered to express a GD2-specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J. Cell. Mol. Med. 2012, 16, 569–581. [Google Scholar] [CrossRef]
- Binyamin, L.; Alpaugh, R.K.; Hughes, T.L.; Lutz, C.T.; Campbell, K.S.; Weiner, L.M. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J. Immunol. 2008, 180, 6392–6401. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23, 181–192. [Google Scholar] [CrossRef]
- Martín-Antonio, B.; Suñe, G.; Perez-Amill, L.; Castella, M.; Urbano-Ispizua, A. Natural Killer Cells: Angels and Devils for Immunotherapy. Int J. Mol. Sci. 2017, 18, 1868. [Google Scholar] [CrossRef]
- Tang, X.; Yang, L.; Li, Z.; Nalin, A.P.; Dai, H.; Xu, T.; Yin, J.; You, F.; Zhu, M.; Shen, W.; et al. First-in-man clinical trial of CAR NK-92 cells: Safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer Res. 2018, 8, 1083. [Google Scholar]
- Müller, T.; Uherek, C.; Maki, G.; Chow, K.U.; Schimpf, A.; Klingemann, H.; Tonn, T.; Wels, W.S. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol. Immunother. 2008, 57, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Tavri, S.; Jha, P.; Meier, R.; Henning, T.D.; Muller, T.; Hostetter, D.; Knopp, C.; Johansson, M.; Reinhart, V.; Boddington, S.; et al. Optical imaging of cellular immunotherapy against prostate cancer. Mol. Imaging 2009, 8, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Golovko, D.; Tavri, S.; Henning, T.D.; Knopp, C.; Piontek, G.; Rudelius, M.; Heinrich, P.; Wels, W.S.; Daldrup-Link, H. Depicting adoptive immunotherapy for prostate cancer in an animal model with magnetic resonance imaging. Magn. Reson. Med. 2011, 65, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, R.; Zhu, X.; Wang, L.; Ma, J.; Han, H.; Wang, X.; Zhang, G.; He, W.; Wang, W.; et al. Retargeting NK-92 for anti-melanoma activity by a TCR-like single-domain antibody. Immunol. Cell Biol. 2013, 91, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Deng, Y.; Benson, D.M.; He, S.; Hughes, T.; Zhang, J.; Peng, Y.; Mao, H.; Yi, L.; Ghoshal, K.; et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 2014, 28, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chu, J.; Keung, C.W.; Zhang, J.; Wang, Y.; Cohen, J.B.; Victor, A.; Meisen, W.H.; Kim, S.H.; Grandi, P.; et al. CAR-Engineered NK Cells Targeting Wild-Type EGFR and EGFRvIII Enhance Killing of Glioblastoma and Patient-Derived Glioblastoma Stem Cells. Sci. Rep. 2015, 5, 11483. [Google Scholar] [CrossRef] [PubMed]
- Genssler, S.; Burger, M.C.; Zhang, C.; Oelsner, S.; Mildenberger, I.; Wagner, M.; Steinbach, J.P.; Wels, W.S. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology 2016, 5, e1119354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Burger, M.C.; Jennewein, L.; Genßler, S.; Schönfeld, K.; Zeiner, P.; Hattingen, E.; Harter, P.N.; Mittelbronn, M.; Tonn, T.; et al. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. J. Natl. Cancer Inst. 2016, 108, djv375. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Wada, M.; Firor, A.E.; Pinz, K.G.; Jares, A.; Liu, H.; Salman, H.; Golightly, M.; Lan, F.; Jiang, X.; et al. Novel anti-CD3 chimeric antigen receptor targeting of aggressive T cell malignancies. Oncotarget 2016, 7, 56219. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Wada, M.; Pinz, K.G.; Liu, H.; Lin, K.W.; Jares, A.; Firor, A.E.; Shuai, X.; Salman, H.; Golightly, M.; et al. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia 2017, 31, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Domogala, A.; Madrigal, J.A.; Saudemont, A. Natural Killer Cell Immunotherapy: From Bench to Bedside. Front. Immunol. 2015, 6, 264. [Google Scholar] [CrossRef] [PubMed]
- Glienke, W.; Esser, R.; Priesner, C.; Suerth, J.D.; Schambach, A.; Wels, W.S.; Grez, M.; Kloess, S.; Arseniev, L.; Koehl, U. Advantages and applications of CAR-expressing natural killer cells. Front. Pharmacol. 2015, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.L.; Puig-Saus, C.; Yu, R.; Shifrut, E.; Carnevale, J.; Li, P.J.; Hiatt, J.; Saco, J.; Krystofinski, P.; Li, H.; et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 2018, 559, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Yu, Y.P.; Zuo, Z.H.; Nelson, J.B.; Michalopoulos, G.K.; Monga, S.; Liu, S.; Tseng, G.; Luo, J.H. Targeting genomic rearrangements in tumor cells through Cas9-mediated insertion of a suicide gene. Nat. Biotechnol. 2017, 35, 543–550. [Google Scholar] [CrossRef] [PubMed]
| Cell Line | Year | Disease Diagnosis | Patient | Doubling Time | Viral Status | Cytokine | Primary Reference |
|---|---|---|---|---|---|---|---|
| NK3.3 | 1982 | NR | NR | NR | EBV− | IL-2-dependent | [23] |
| YT | 1983 | Acute lymphoblastic lymphoma (with thymoma) | 15-year-old male | 40–50 h | EBV+ | Independent of IL-2 | [27] |
| NKL | 1996 | NK-LGLL | 63-year-old male | 24–48 h | NR | IL-2-dependent | [30,41] |
| HANK1 | 1998 | Nasal-like NK/T-cell lymphoma | 46-year-old female | 3 day | EBV+ | IL-2-dependent | [31] |
| NK-YS | 1996 | NK cell lymphoma, Nasal angiocentric, Leukemic state with systemic skin infiltration | 19-year-old female | 48 h | EBV+ | IL-2-dependent | [32] |
| KHYG-1 | 1997 | Aggressive NK leukemia | 45-year-old female | 24–48 h | EBV− | IL-2-dependent | [33] |
| SNK-6 | 1998 | Nasal NK/T-cell lymphoma | 62-year-old male | NR | EBV+ | IL-2-dependent | [35] |
| SNT-8 | 1998 | Nasal NK/T-cell lymphoma | 48-year-old female | NR | EBV+ | IL-2-dependent | [35] |
| IMC-1 | 2004 | Aggressive NK cell leukemia | 42-year-old male | 24–36 h | EBV− | IL-2-dependent | [36] |
| NK-92 | 1992 | LGL-NHL | 50-year-old male | 24 h | EBV− | IL-2-dependent; Growth stimulation:IL-7 | [37] |
| Cancer Type | Antigen Targeted | Hinge | TM | Intracellular Signal Domain | Genetic Modification Method | Effector Cell | Year | References |
|---|---|---|---|---|---|---|---|---|
| Multiple myeloma | CD138 | CD8 | CD3ζ | CD3ζ | lentiviral vector | NK-92MI | 2014 | [86] |
| B-cell malignancies | CD19 | CD8 | NR | CD3ζ | Retrovirus | NK-92 | 2016 | [82] |
| B-cell malignancies | CD19 | CD8 | CD28 | CD3ζ | Lentiviral | NK-92 | 2017 | [83] |
| CLL | CD19 | CD8 | CD3ζ | CD3ζ | Electroporation | NK-92 | 2009 | [84] |
| ALL CLL | CD19 CD20 | NR | NR | CD3ζ | Lentivirus | NK-92 | 2014 | [85] |
| B-cell malignancies | CD20 | CD8 | CD3ζ | CD3ζ | Retroviral | NK-92 | 2008 | [101] |
| Prostate cancer | EpCAM | CD8 | CD3ζ | CD3ζ | Retrovirus | NK-92 | 2009 | [102] |
| Prostate cancer | EpCAM | CD8 | CD3ζ | CD3ζ | Retrovirus | NK-92 | 2011 | [103] |
| Neuroblastoma | GD2 | CD8 | CD3ζ | CD3ζ | Retrovirus | NK-92 | 2012 | [96] |
| Neuroblastoma | GD2 | CD8 | CD3ζ | CD3ζ | Retrovirus | NK-92 | 2015 | [95] |
| Melanoma | GPA7 | NR | HLA-A2 | CD3ζ | Electroporation | NK-92MI | 2013 | [104] |
| Brain metastasis | HER2 | CD8α | CD3ζ | CD3ζ | Retrovirus | NK-92 | 2016 | [88] |
| Brain metastasis | HER2 | CD8 | CD3ζ | CD3ζ | Retrovirus | NK-92 | 2013 | [89] |
| Breast cancer | HER2 | CD8 | CD3ζ | CD3ζ | Retrovirus | NK-92 | 2005 | [91] |
| Breast cancer | HER2 | CD8 | CD3ζ | CD3ζ | Retrovirus | NK-92 | 2008 | [92] |
| Breast/ovarian cancer | HER2 | CD8 | CD3ζ | CD3ζ | Retrovirus | NK-92 | 2002 | [81] |
| Breast cancer, Ovarian cancer, Melanoma Renal cell carcinoma | HER2 | CD8 | CD3ζ | CD3ζ | Lentiviral | NK-92 | 2015 | [93] |
| Ovarian cancer Mesothelin-expressing tumors | Mesothelin | CD8 | NKG2D | CD3ζ | Transposon plasmids | NK-92 | 2018 | [98] |
| Cancer Type | Antigen Targeted | Hinge | TM | Intracellular Signal Domain | Genetic Modification Method | Effector Cell | Year | References |
|---|---|---|---|---|---|---|---|---|
| B-cell malignancies | CD19 | CD8 | CD28 | CD28-CD3ζ CD137-CD3ζ | Lentiviral | NK-92 | 2017 | [83] |
| Multiple myeloma | CS1 | NR | NR | CD28-CD3ζ | Lentivirus | NK-92 | 2014 | [105] |
| EBV+ cells | EBNA3C | NR | NR | CD137-CD3ζ | Retrovirus | NK-92MI | 2012 | [75] |
| Glioblastoma | EGFR EGFRvIII | NR | CD28 | CD28-CD3ζ | Lentivirus | NK-92 and NKL | 2015 | [106] |
| Brain metastasis | EGFR | NR | NR | CD28-CD3ζ | Lentivirus | NK-92 | 2016 | [87] |
| Glioblastoma | EGFR EGFRvIII | CD8 | CD28 | CD28-CD3ζ | Lentivirus | NK-92 | 2015 | [107] |
| Breast cancer | EpCAM | CD8 | CD28 | CD28-CD3ζ | Lentivirus | NK-92 | 2012 | [90] |
| Breast cancer Renal cell carcinoma Ovarian carcinoma Melanoma | HER2 | CD8 | CD28 CD137 | CD28-CD3ζ CD137-CD3ζ | Lentiviral | NK-92 | 2015 | [93] |
| Glioblastoma | HER2 | CD8 | CD28 | CD28-CD3ζ | Lentiviral | NK-92 | 2016 | [108] |
| Breast cancer | HER2 | CD8 | CD28 | CD28-CD3ζ | Electroporation | NK-92 | 2015 | [94] |
| Ovarian cancer Mesothelin-expressing tumors | Mesothelin | CD8 | CD16 | 2B4-CD3ζ | Transposon plasmids | NK-92 | 2018 | [98] |
| Ovarian cancer mesothelin-expressing tumors | Mesothelin | CD8 | NKp44 | DAP10-CD3ζ 2B4-CD3ζ | Transposon plasmids | NK-92 | 2018 | [98] |
| Ovarian cancer Mesothelin-expressing tumors | Mesothelin | CD8 | NKG2D | 2B4-CD3ζ CD137-CD3ζ | Transposon plasmids | NK-92 | 2018 | [98] |
| Ovarian cancer Mesothelin-expressing tumors | Mesothelin | CD8 | CD28 | CD28-CD137-CD3ζ | Transposon plasmids | NK-92 | 2018 | [98] |
| Ovarian cancer Mesothelin-expressing tumors | Mesothelin | CD8 | NKG2D | 2B4-DAP12-CD3ζ 2B4-DAP10-CD3ζ CD137-2B4-CD3ζ | Transposon plasmids | NK-92 | 2018 | [98] |
| Aggressive T cell malignancies | CD3 | CD8 | CD8 | CD28-CD137-CD3ζ | Lentivirus | NK-92 | 2016 | [109] |
| Aggressive T-cell malignancies | CD5 | CD8 | CD8 | CD28-CD137-CD3ζ | Lentivirus | NK-92 | 2017 | [110] |
| NCT Number | NK Cell Source | Target Antigen | Disease | Phase | Estimated Enrollment | Age | Location | References |
|---|---|---|---|---|---|---|---|---|
| NCT02742727 | NK-92 | CD7 | Acute Myeloid Leukemia;Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; T-cell Prolymphocytic Leukemia; T-cell Large Granular Lymphocytic Leukemia; Peripheral T-cell Lymphoma, NOS; Angioimmunoblastic T-cell Lymphoma Extranodal NK/T-cell Lymphoma, Nasal Type; Enteropathy-type Intestinal T-cell Lymphoma; Hepatosplenic T-cell Lymphoma | Phase 1 Phase 2 | 10 participants | 18 Years and older (Adult, Older Adult) | China | NR |
| NCT02892695 | NK-92 | CD19 | Acute Lymphocytic Leukemia; Chronic Lymphocytic Leukemia; Follicular Lymphoma; Mantle Cell Lymphoma; B-cell Prolymphocytic Leukemia; Diffuse Large Cell Lymphoma; | Phase 1 Phase 2 | 10 participants | 3 Years to 80 Years (Child, Adult, Older Adult) | China | NR |
| NCT02944162 | NK-92 | CD33 | Acute Myelogenous Leukemia; Acute Myeloid Leukemia; Acute Myeloid Leukemia with Maturation; Acute Myeloid Leukemia Without Maturation; ANLL | Phase 1 Phase 2 | 10 participants | 3 Years to 80 Years (Child, Adult, Older Adult) | China | [100] |
| NCT03383978 | NK-92 | HER2 | Glioblastoma | Phase 1 | 30 participants | 18 Years and older (Adult, Older Adult) | Germany | NR |
| NCT03656705 | NK-92 | NR | Non-small Cell Lung Cancer | Phase 1 | 5 participants | 18 Years to 75 Years (Adult, Older Adult) | China | NR |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zheng, H.; Diao, Y. Natural Killer Cells and Current Applications of Chimeric Antigen Receptor-Modified NK-92 Cells in Tumor Immunotherapy. Int. J. Mol. Sci. 2019, 20, 317. https://doi.org/10.3390/ijms20020317
Zhang J, Zheng H, Diao Y. Natural Killer Cells and Current Applications of Chimeric Antigen Receptor-Modified NK-92 Cells in Tumor Immunotherapy. International Journal of Molecular Sciences. 2019; 20(2):317. https://doi.org/10.3390/ijms20020317
Chicago/Turabian StyleZhang, Jianguang, Huifang Zheng, and Yong Diao. 2019. "Natural Killer Cells and Current Applications of Chimeric Antigen Receptor-Modified NK-92 Cells in Tumor Immunotherapy" International Journal of Molecular Sciences 20, no. 2: 317. https://doi.org/10.3390/ijms20020317
APA StyleZhang, J., Zheng, H., & Diao, Y. (2019). Natural Killer Cells and Current Applications of Chimeric Antigen Receptor-Modified NK-92 Cells in Tumor Immunotherapy. International Journal of Molecular Sciences, 20(2), 317. https://doi.org/10.3390/ijms20020317




