The Antitumor Effect of Lipophilic Bisphosphonate BPH1222 in Melanoma Models: The Role of the PI3K/Akt Pathway and the Small G Protein Rheb
Abstract
1. Introduction
2. Results
2.1. BPH Decreased More Effectively Both Short-Term Cell Viability and Clonogenic Potential in Most Cell Lines than ZA
2.2. Cell Cycle Distribution and Apoptosis Induction upon Treatment with the Bisphosphonates
2.3. Effect of the Prenylation Inhibitors on Erk, Akt, S6, and Rheb Activation
2.4. Efficacy of BPH to Inhibit Cell Migration Depends on the Baseline Motility of the Cells
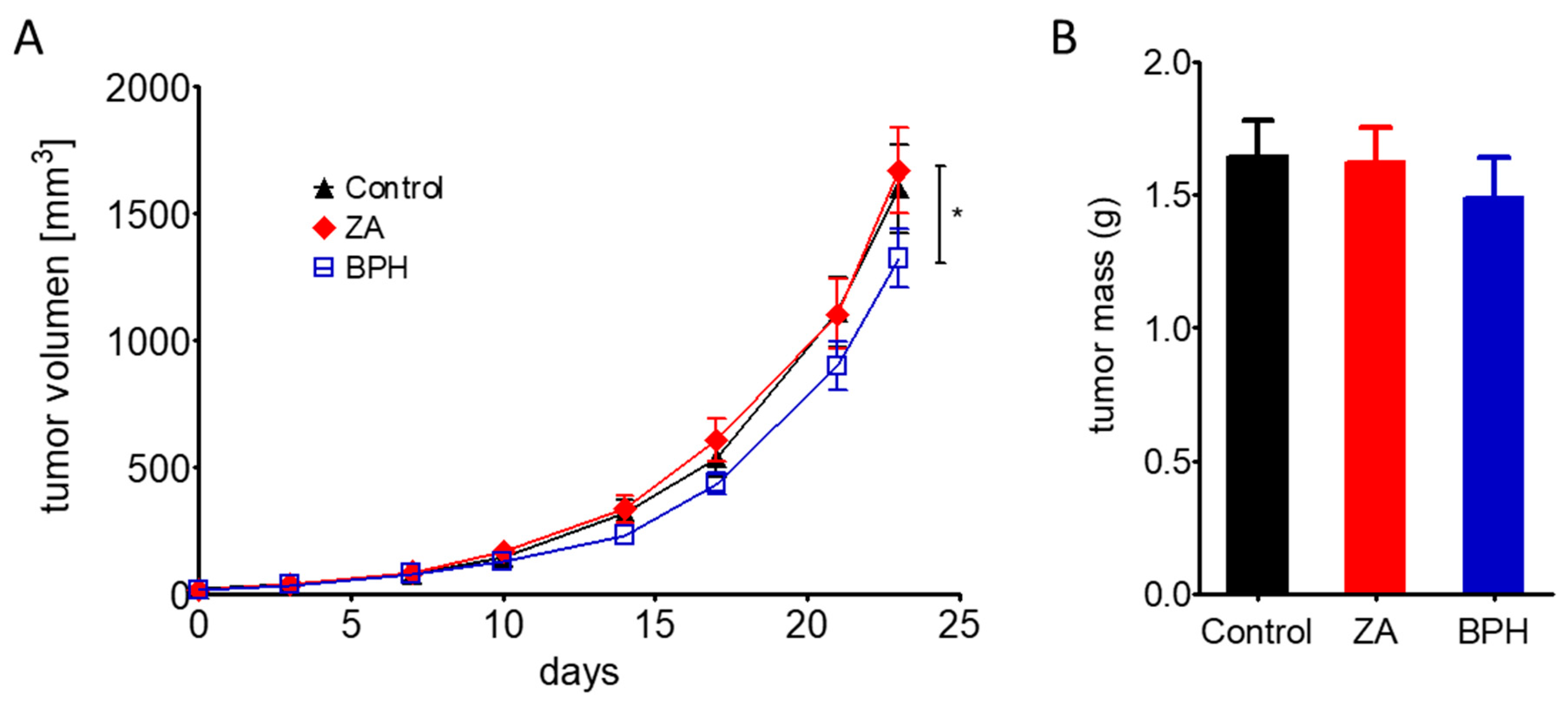
2.5. Efficacy of ZA and BPH on Three-Dimensional Spheroid and In Vivo Xenograft Models
3. Discussion
4. Material and Methods
4.1. Cell Lines and Reagents
4.2. Sulforhodamine B Assay
4.3. Clonogenic Assay
4.4. Time-Lapse Video Microscopy
4.5. Immunoblot Analysis
4.6. Cell Cycle Analysis
4.7. 3-Dimensional Spheroid Assay
4.8. In Vivo Experiment
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perera, E.; Gnaneswaran, N.; Jennens, R.; Sinclair, R. Malignant Melanoma. Healthcare 2014, 2, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, V.A.; Stratigos, A.J.; Flaherty, K.T.; Tsao, H. Melanoma: New insights and new therapies. J. Invest. Dermatol. 2012, 132, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Vera, J. Modelling of Protein Kinase Signaling Pathways in Melanoma and Other Cancers. Cancers 2019, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Schittek, B.; Busch, S.; Garbe, C.; Smalley, K.; Satyamoorthy, K.; Li, G.; Herlyn, M. The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front. Biosci. 2005, 10, 2986–3001. [Google Scholar] [CrossRef]
- Zuo, Q.; Liu, J.; Huang, L.; Qin, Y.; Hawley, T.; Seo, C.; Merlino, G.; Yu, Y. AXL/AKT axis mediated-resistance to BRAF inhibitor depends on PTEN status in melanoma. Oncogene 2018, 37, 3275–3289. [Google Scholar] [CrossRef]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the undruggable RAS: Mission possible? Nat. Rev. Drug. Discov. 2014, 13, 828–851. [Google Scholar] [CrossRef]
- Muñoz-Couselo, E.; Adelantado, E.Z.; Ortiz, C.; García, J.S.; Perez-Garcia, J. NRAS-mutant melanoma: Current challenges and future prospect. Onco. Targets Ther. 2017, 10, 3941–3947. [Google Scholar] [CrossRef]
- Mandala, M.; Merelli, B.; Massi, D. Nras in melanoma: Targeting the undruggable target. Crit. Rev. Oncol. Hematol. 2014, 92, 107–122. [Google Scholar] [CrossRef]
- Domingues, B.; Lopes, J.M.; Soares, P.; Pópulo, H. Melanoma treatment in review. Immunotargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef]
- Johnpulle, R.A.; Johnson, D.B.; Sosman, J.A. Molecular Targeted Therapy Approaches for BRAF Wild-Type Melanoma. Curr. Oncol. Rep. 2016, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Amin, D.; Cornell, S.A.; Gustafson, S.K.; Needle, S.J.; Ullrich, J.W.; Bilder, G.E.; Perrone, M.H. Bisphosphonates used for the treatment of bone disorders inhibit squalene synthase and cholesterol biosynthesis. J. Lipid Res. 1992, 33, 1657–1663. [Google Scholar] [PubMed]
- Green, J.R.; Clezardin, P. Mechanisms of bisphosphonate effects on osteoclasts, tumor cell growth, and metastasis. Am. J. Clin. Oncol. 2002, 25, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Itoh, T.; Satou, T.; Imano, M.; Komai, M.; Ogawa, N.; Mukai, J.; Nishida, S. Nitrogen-containing bisphosphonates induce apoptosis of hematopoietic tumor cells via inhibition of Ras signaling pathways and Bim-mediated activation of the intrinsic apoptotic pathway. Biochem. Pharmacol. 2013, 85, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Fragni, M.; Bonini, S.A.; Bettinsoli, P.; Bodei, S.; Generali, D.; Bottini, A.; Spano, P.F.; Memo, M.; Sigala, S. The miR-21/PTEN/Akt signaling pathway is involved in the anti-tumoral effects of zoledronic acid in human breast cancer cell lines. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, M.S.; Lee, K.H.; Koh, J.S.; Jung, W.G.; Kong, C.B. Zoledronic acid is an effective radiosensitizer in the treatment of osteosarcoma. Oncotarget 2016, 7, 70869–70880. [Google Scholar] [CrossRef] [PubMed]
- Hanker, A.B.; Mitin, N.; Wilder, R.S.; Henske, E.P.; Tamanoi, F.; Cox, A.D.; Der, C.J. Differential requirement of CAAX-mediated posttranslational processing for Rheb localization and signaling. Oncogene 2010, 29, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, P.N. Glucocorticoid osteoporosis. Curr. Pharm. Des. 2002, 8, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Dougados, M. Treatment of patients with Paget’s disease of bone. Drugs 1999, 58, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Goldvaser, H.; Amir, E. Role of Bisphosphonates in Breast Cancer Therapy. Curr. Treat. Option. Oncol. 2019, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Kozyrakis, D.; Paridis, D.; Perikleous, S.; Malizos, K.; Zarkadas, A.; Tsagkalis, A. The Current Role of Osteoclast Inhibitors in Patients with Prostate Cancer. Adv. Urol. 2018, 2018, 1525832. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, M.; Maas, M.; Rausch, S.; Walz, S.; Bedke, J.; Stenzl, A. Current concepts and trends in the treatment of bone metastases in patients with advanced prostate cancer. Asian J. Androl. 2019, 21, 12–18. [Google Scholar]
- Denoyelle, C.; Hong, L.; Vannier, J.P.; Soria, J.; Soria, C. New insights into the actions of bisphosphonate zoledronic acid in breast cancer cells by dual RhoA-dependent and -independent effects. Br. J. Cancer 2003, 88, 1631–1640. [Google Scholar] [CrossRef]
- Yamagishi, S.-I.; Abe, R.; Inagaki, Y.; Nakamura, K.; Sugawara, H.; Inokuma, D.; Nakamura, H.; Shimizu, T.; Takeuchi, M.; Yoshimura, A.; et al. Minodronate, a newly developed nitrogen-containing bisphosphonate, suppresses melanoma growth and improves survival in nude mice by blocking vascular endothelial growth factor signaling. Am. J. Pathol. 2004, 165, 1865–1874. [Google Scholar] [CrossRef]
- Santini, D.; Schiavon, G.; Angeletti, S.; Vincenzi, B.; Gasparro, S.; Grilli, C.; La Cesa, A.; Virzi, V.; Leoni, V.; Budillon, A.; et al. Last generation of amino-bisphosphonates (N-BPs) and cancer angio-genesis: A new role for these drugs? Recent Pat. Anticancer Drug Discov. 2006, 1, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Garay, T.; Kenessey, I.; Molnar, E.; Juhasz, E.; Reti, A.; Laszlo, V.; Rozsas, A.; Dobos, J.; Dome, B.; Berger, W.; et al. Prenylation inhibition-induced cell death in melanoma: Reduced sensitivity in BRAF mutant/PTEN wild-type melanoma cells. PLoS ONE 2015, 10, e0117021. [Google Scholar] [CrossRef] [PubMed]
- Forsea, A.M.; Muller, C.; Riebeling, C.; Orfanos, C.E.; Geilen, C.C. Nitrogen-containing bisphosphonates inhibit cell cycle progression in human melanoma cells. Br. J. Cancer 2004, 91, 803–810. [Google Scholar] [CrossRef]
- Hirbe, A.C.; Roelofs, A.J.; Floyd, D.H.; Deng, H.; Becker, S.N.; Lanigan, L.G.; Apicelli, A.J.; Xu, Z.; Prior, J.L.; Eagleton, M.C.; et al. The bisphosphonate zoledronic acid decreases tumor growth in bone in mice with defective osteoclasts. Bone 2009, 44, 908–916. [Google Scholar] [CrossRef]
- Weiss, H.M.; Pfaar, U.; Schweitzer, A.; Wiegand, H.; Skerjanec, A.; Schran, H. Biodistribution and plasma protein binding of zoledronic acid. Drug. Metab. Dispos. 2008, 36, 2043–2049. [Google Scholar] [CrossRef]
- Nancollas, G.H.; Tang, R.; Phipps, R.J.; Henneman, Z.; Gulde, S.; Wu, W.; Mangood, A.; Russell, R.G.; Ebetino, F.H. Novel insights into actions of bisphosphonates on bone: Differences in interactions with hydroxyapatite. Bone 2006, 38, 617–627. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Y.L.; Xie, Y.; Zhu, W.; Guerra, F.; Shen, S.; Yeddula, N.; Fischer, W.; Low, W.; Zhou, X.; et al. A combination therapy for KRAS-driven lung adenocarcinomas using lipophilic bisphosphonates and rapamycin. Sci. Transl. Med. 2014, 6, 263ra161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, R.; Yin, F.; Hudock, M.P.; Guo, R.T.; Krysiak, K.; Mukherjee, S.; Gao, Y.G.; Robinson, H.; Song, Y.; et al. Lipophilic bisphosphonates as dual farnesyl/geranylgeranyl diphosphate synthase inhibitors: An X-ray and NMR investigation. J. Am. Chem. Soc. 2009, 131, 5153–5162. [Google Scholar] [CrossRef] [PubMed]
- Boulmier, A.; Feng, X.; Oms, O.; Mialane, P.; Riviere, E.; Shin, C.J.; Yao, J.; Kubo, T.; Furuta, T.; Oldfield, E.; et al. Anticancer activity of polyoxometalate-bisphosphonate complexes: Synthesis, characterization, in vitro and in vivo results. Inorg. Chem. 2017, 56, 7558–7565. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; McDonald, J.S.; Yun, S.; Schneider, P.A.; Peterson, K.L.; Flatten, K.S.; Loegering, D.A.; Oberg, A.L.; Riska, S.M.; Huang, S.; et al. Farnesyltransferase inhibitor tipifarnib inhibits Rheb prenylation and stabilizes Bax in acute myelogenous leukemia cells. Haematologica 2014, 99, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Finlay, G.A.; Malhowski, A.J.; Liu, Y.; Fanburg, B.L.; Kwiatkowski, D.J.; Toksoz, D. Selective inhibition of growth of tuberous sclerosis complex 2 null cells by atorvastatin is associated with impaired Rheb and Rho GTPase function and reduced mTOR/S6 kinase activity. Cancer Res. 2007, 67, 9878–9886. [Google Scholar] [CrossRef] [PubMed]
- Garay, T.; Juhasz, E.; Molnar, E.; Eisenbauer, M.; Czirok, A.; Dekan, B.; Laszlo, V.; Hoda, M.A.; Dome, B.; Timar, J.; et al. Cell migration or cytokinesis and proliferation?--revisiting the “go or grow” hypothesis in cancer cells in vitro. Exp. Cell Res. 2013, 319, 3094–3103. [Google Scholar] [CrossRef] [PubMed]
- Yuen, T.; Stachnik, A.; Iqbal, J.; Sgobba, M.; Gupta, Y.; Lu, P.; Colaianni, G.; Ji, Y.; Zhu, L.L.; Kim, S.M.; et al. Bisphosphonates inactivate human EGFRs to exert antitumor actions. Proc. Natl. Acad. Sci. USA 2014, 111, 17989–17994. [Google Scholar] [CrossRef] [PubMed]
- Malwal, S.R.; O’Dowd, B.; Feng, X.; Turhanen, P.; Shin, C.; Yao, J.; Kim, B.K.; Baig, N.; Zhou, T.; Bansal, S.; et al. Bisphosphonate-Generated ATP-Analogs Inhibit Cell Signaling Pathways. J. Am. Chem. Soc. 2018, 140, 7568–7578. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.M.; Romerdahl, C.A.; Trent, J.M.; Reisfeld, R.A. Suppression of spontaneous melanoma metastasis in scid mice with an antibody to the epidermal growth factor receptor. Cancer Res. 1991, 51, 2193–2198. [Google Scholar] [PubMed]
- Watson, I.R.; Li, L.; Cabeceiras, P.K.; Mahdavi, M.; Gutschner, T.; Genovese, G.; Wang, G.; Fang, Z.; Tepper, J.M.; Stemke-Hale, K.; et al. The RAC1 P29S hotspot mutation in melanoma confers resistance to pharmacological inhibition of RAF. Cancer Res. 2014, 74, 4845–4852. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, T.; Williams, P.J.; Ueda, A.; Tamura, D.; Yoneda, T. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res. 2004, 10, 4559–4567. [Google Scholar] [CrossRef] [PubMed]
- Boissier, S.; Ferreras, M.; Peyruchaud, O.; Magnetto, S.; Ebetino, F.H.; Colombel, M.; Delmas, P.; Delaisse, J.M.; Clezardin, P. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000, 60, 2949–2954. [Google Scholar] [PubMed]
- Beaumont, K.A.; Mohana-Kumaran, N.; Haass, N.K. Modeling Melanoma In Vitro and In Vivo. Healthcare 2014, 2, 27–46. [Google Scholar] [CrossRef]
- Chen, L.; Ding, Y.; Wang, Y.; Liu, X.; Babu, R.; Ravis, W.; Yan, W. Codelivery of zoledronic acid and doublestranded RNA from core-shell nanoparticles. Int. J. Nanomed. 2013, 8, 137–145. [Google Scholar]
- Pirker, C.; Holzmann, K.; Spiegl-Kreinecker, S.; Elbling, L.; Thallinger, C.; Pehamberger, H.; Micksche, M.; Berger, W. Chromosomal imbalances in primary and metastatic melanomas: Over-representation of essential telomerase genes. Melanoma Res. 2003, 13, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.K.; Postovit, L.M. Investigating the utility of human melanoma cell lines as tumour models. Oncotarget 2017, 8, 10498–10509. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef]
- Lee, J.T.; Li, L.; Brafford, P.A.; van den Eijnden, M.; Halloran, M.B.; Sproesser, K.; Haass, N.K.; Smalley, K.S.; Tsai, J.; Bollag, G.; et al. PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas. Pigment Cell Melanoma Res. 2010, 23, 820–827. [Google Scholar] [CrossRef]
- Bani, M.; Rak, J.; Adachi, D.; Wiltshire, R.M.; Trent, J.; Kerbel, R.; Ben-David, Y. Multiple Features of Advanced Melanoma Recapitulated in Tumorigenic Variants of Early Stage (Radial Growth Phase) Human Melanoma Cell Lines: Evidence for a Dominant Phenotype. Cancer Res. 1996, 56, 3075–3086. [Google Scholar]
- Dzhambazov, B.; Asparuhova, D.; Koleva, L.; Popov, N. Modal karyotype of continuous cell line A2058 (human metastatic melanoma) and its evolution in culture. Caryologia 2001, 54, 313–317. [Google Scholar] [CrossRef]
- Eriksson, J.; Le Joncour, V.; Nummela, P.; Jahkola, T.; Virolainen, S.; Laakkonen, P.; Saksela, O.; Holtta, E. Gene expression analyses of primary melanomas reveal CTHRC1 as an important player in melanoma progression. Oncotarget 2016, 7, 15065–15092. [Google Scholar] [CrossRef] [PubMed]
- Herlyn, M.; Balaban, G.; Bennicelli, J.; Guerry, D.t.; Halaban, R.; Herlyn, D.; Elder, D.E.; Maul, G.G.; Steplewski, Z.; Nowell, P.C.; et al. Primary melanoma cells of the vertical growth phase: Similarities to metastatic cells. J Natl. Cancer Inst. 1985, 74, 283–289. [Google Scholar] [PubMed]
- Posch, C.; Moslehi, H.; Feeney, L.; Green, G.A.; Ebaee, A.; Feichtenschlager, V.; Chong, K.; Peng, L.; Dimon, M.T.; Phillips, T.; et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 4015–4020. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, J. Mutation induction by high-density, 50-Hz magnetic fields in human MeWo cells exposed in the DNA synthesis phase. Int. J. Radiat. Biol. 1997, 71, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Birner, P.; Berghoff, A.S.; Dinhof, C.; Pirker, C.; Capper, D.; Schoppmann, S.F.; Petzelbauer, P.; von Deimling, A.; Berger, W.; Preusser, M. MAP kinase activity supported by BRAF (V600E) mutation rather than gene amplification is associated with ETV1 expression in melanoma brain metastases. Arch. Dermatol. Res. 2014, 306, 873–884. [Google Scholar] [CrossRef]
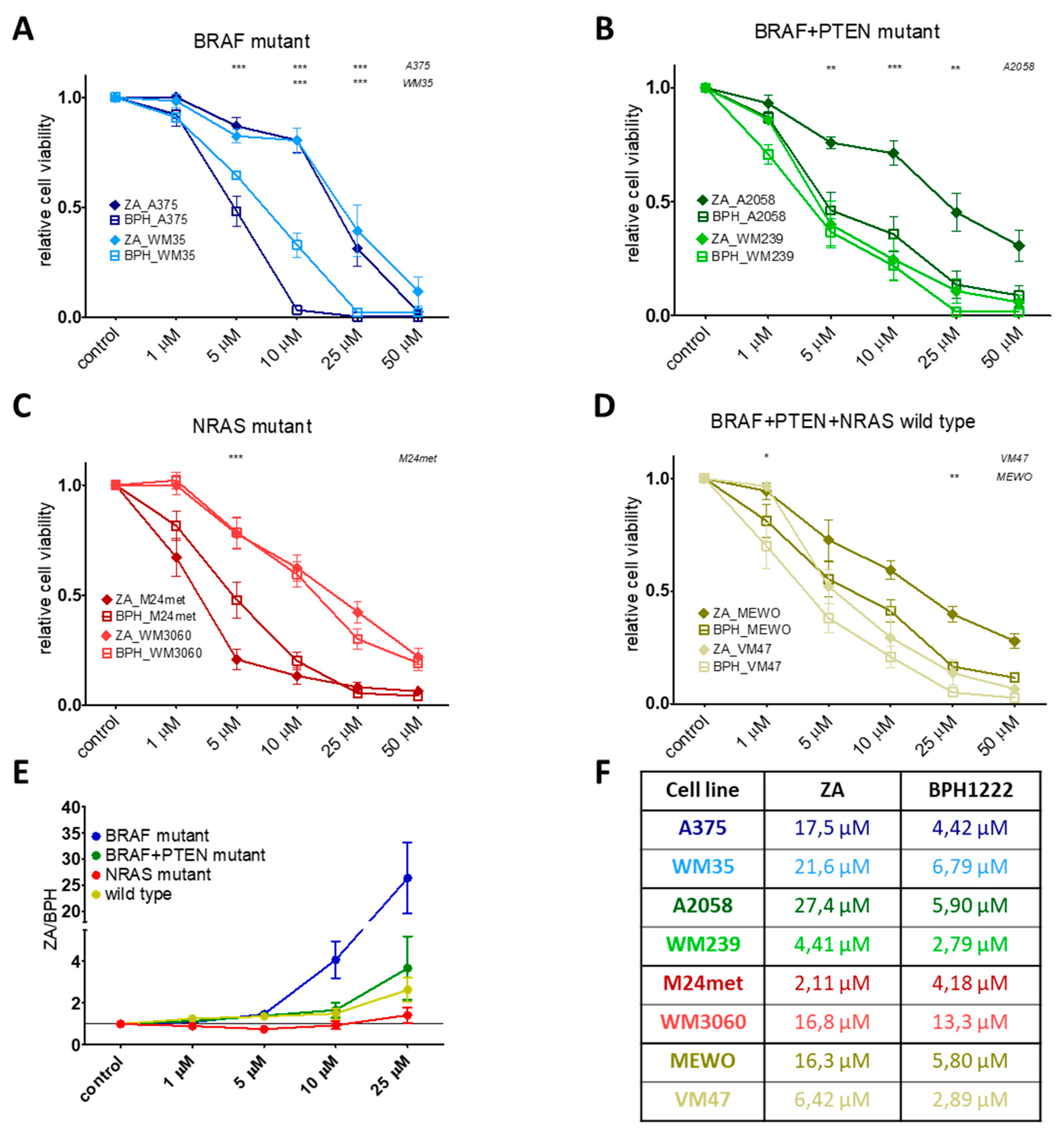

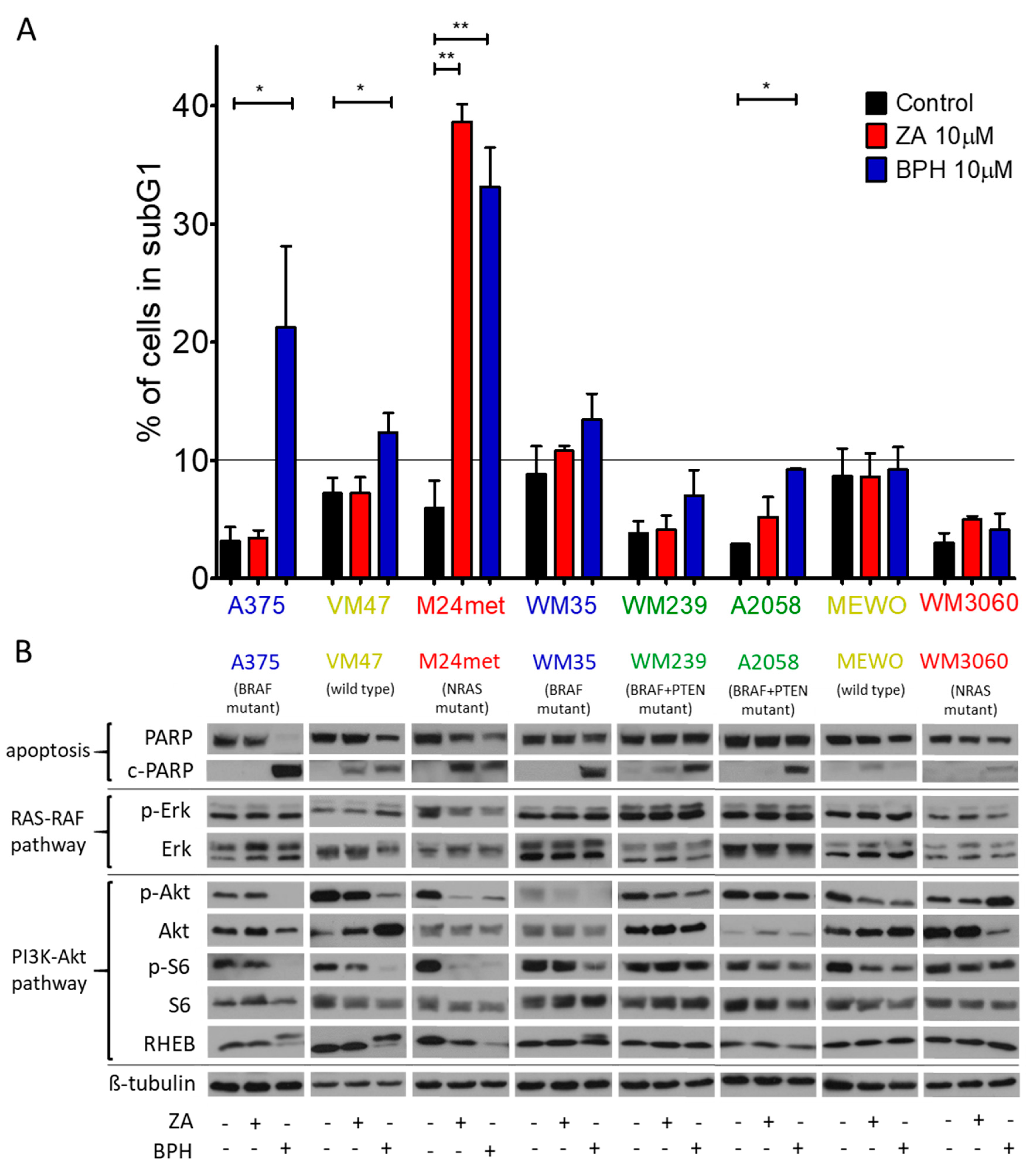
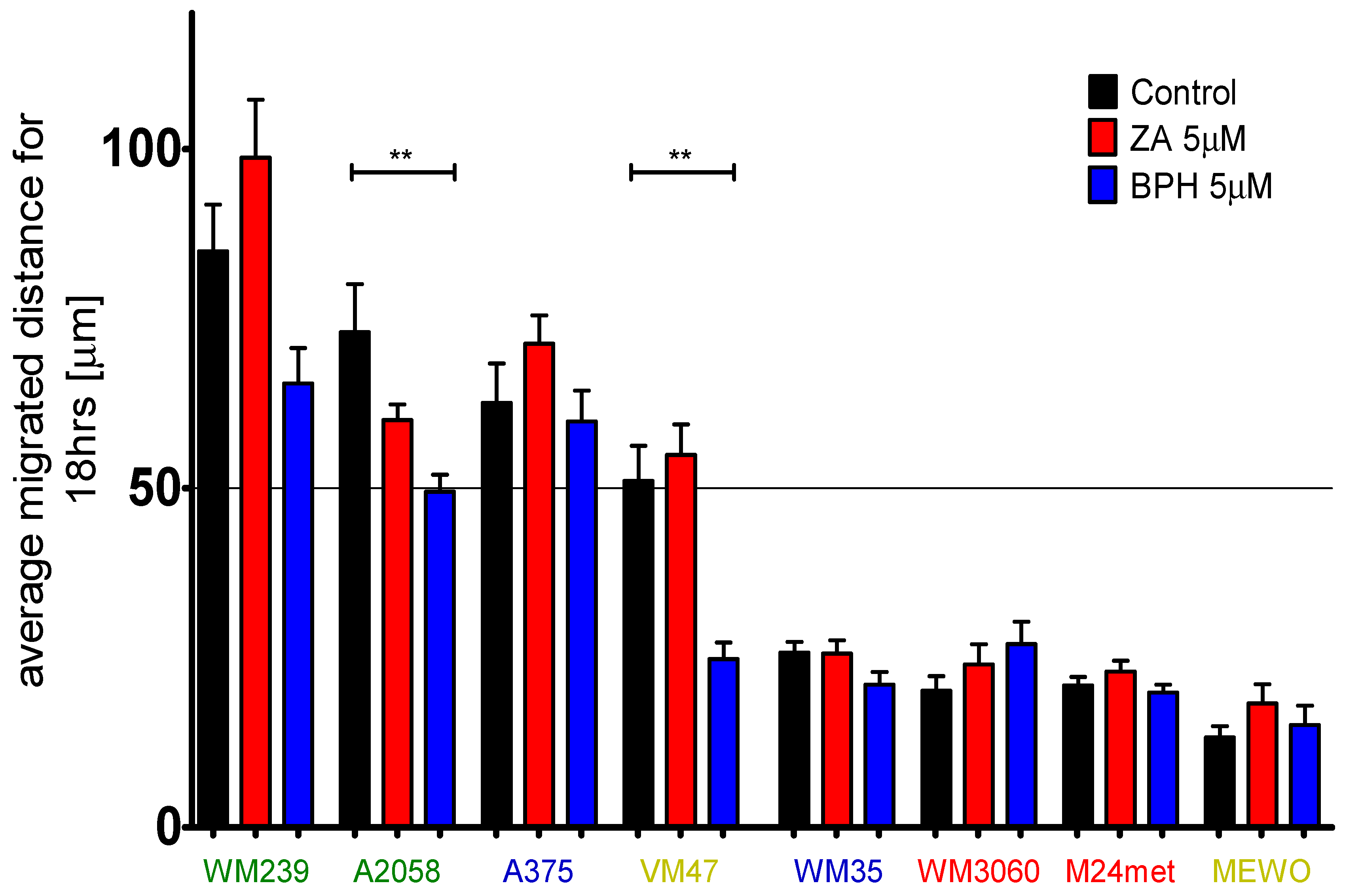
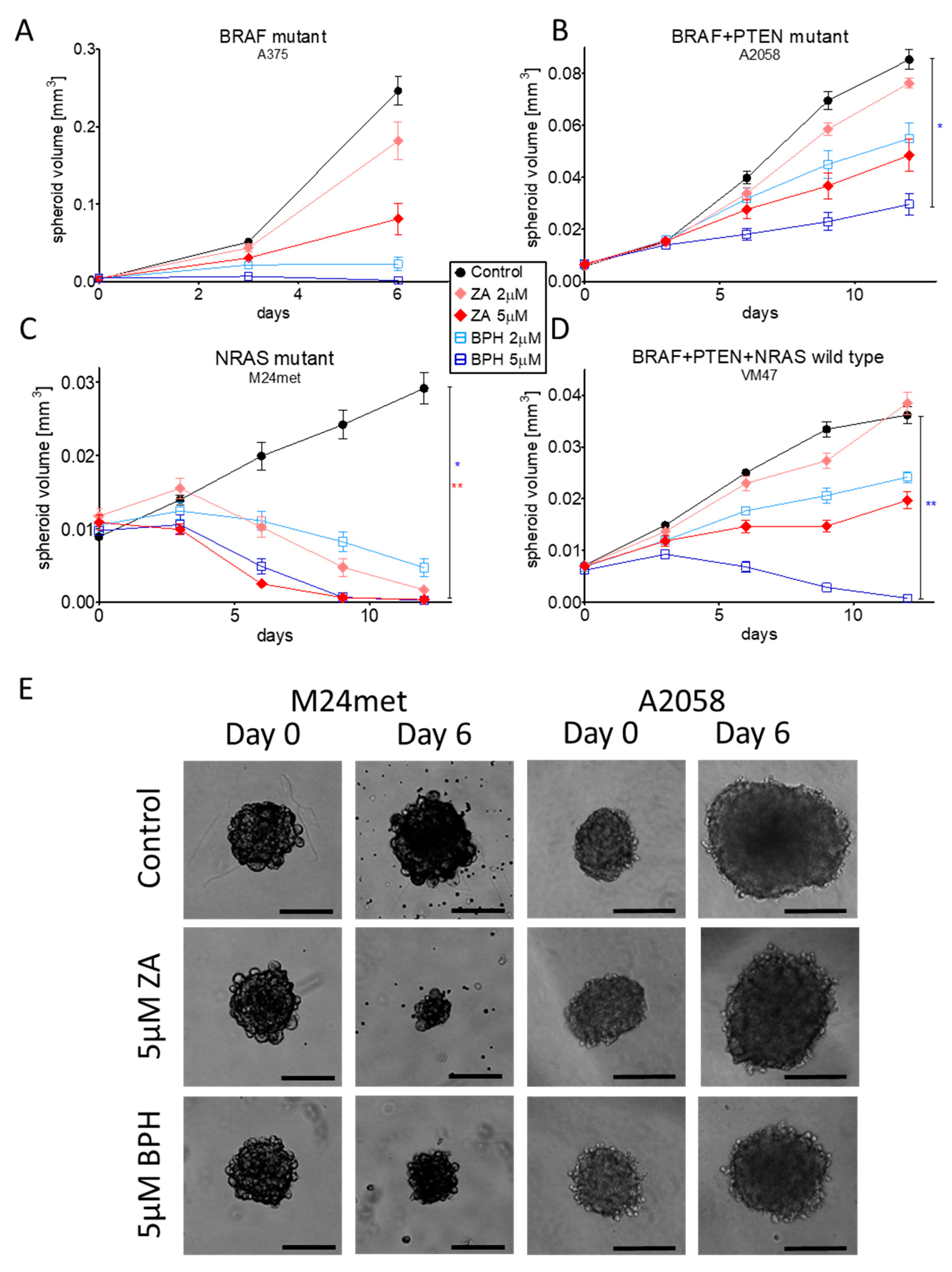
| Cell Line | BRAF | PTEN | NRAS | Karyotype | Reference |
|---|---|---|---|---|---|
| A375 | V600E | wt | wt | hypo triploid (62) | [46,47] |
| WM35 | V600E | wt | wt | near-triploid (68) | [26,48,49] |
| A2058 | V600E | null | wt | near-diploid (43-48) | [46,50] |
| WM239 | V600D | null | wt | near-diploid (43) | [26,51,52] |
| M24met | wt | wt | Q61R | hyper diploid (53-57) | [26,39] |
| WM3060 | wt | wt | Q61K | n.a. | [26,53] |
| MEWO | wt | wt | wt | near-diploid (43-46) | [46,54] |
| VM47 | wt | wt | wt | n.a. | [26,55] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rittler, D.; Baranyi, M.; Molnár, E.; Garay, T.; Jalsovszky, I.; Varga, I.K.; Hegedűs, L.; Aigner, C.; Tóvári, J.; Tímár, J.; et al. The Antitumor Effect of Lipophilic Bisphosphonate BPH1222 in Melanoma Models: The Role of the PI3K/Akt Pathway and the Small G Protein Rheb. Int. J. Mol. Sci. 2019, 20, 4917. https://doi.org/10.3390/ijms20194917
Rittler D, Baranyi M, Molnár E, Garay T, Jalsovszky I, Varga IK, Hegedűs L, Aigner C, Tóvári J, Tímár J, et al. The Antitumor Effect of Lipophilic Bisphosphonate BPH1222 in Melanoma Models: The Role of the PI3K/Akt Pathway and the Small G Protein Rheb. International Journal of Molecular Sciences. 2019; 20(19):4917. https://doi.org/10.3390/ijms20194917
Chicago/Turabian StyleRittler, Dominika, Marcell Baranyi, Eszter Molnár, Tamás Garay, István Jalsovszky, Imre Károly Varga, Luca Hegedűs, Clemens Aigner, József Tóvári, József Tímár, and et al. 2019. "The Antitumor Effect of Lipophilic Bisphosphonate BPH1222 in Melanoma Models: The Role of the PI3K/Akt Pathway and the Small G Protein Rheb" International Journal of Molecular Sciences 20, no. 19: 4917. https://doi.org/10.3390/ijms20194917
APA StyleRittler, D., Baranyi, M., Molnár, E., Garay, T., Jalsovszky, I., Varga, I. K., Hegedűs, L., Aigner, C., Tóvári, J., Tímár, J., & Hegedűs, B. (2019). The Antitumor Effect of Lipophilic Bisphosphonate BPH1222 in Melanoma Models: The Role of the PI3K/Akt Pathway and the Small G Protein Rheb. International Journal of Molecular Sciences, 20(19), 4917. https://doi.org/10.3390/ijms20194917





