Gene Expression Profiling Reveals Enhanced Defense Responses in an Invasive Weed Compared to Its Native Congener During Pathogenesis
Abstract
1. Introduction
2. Results
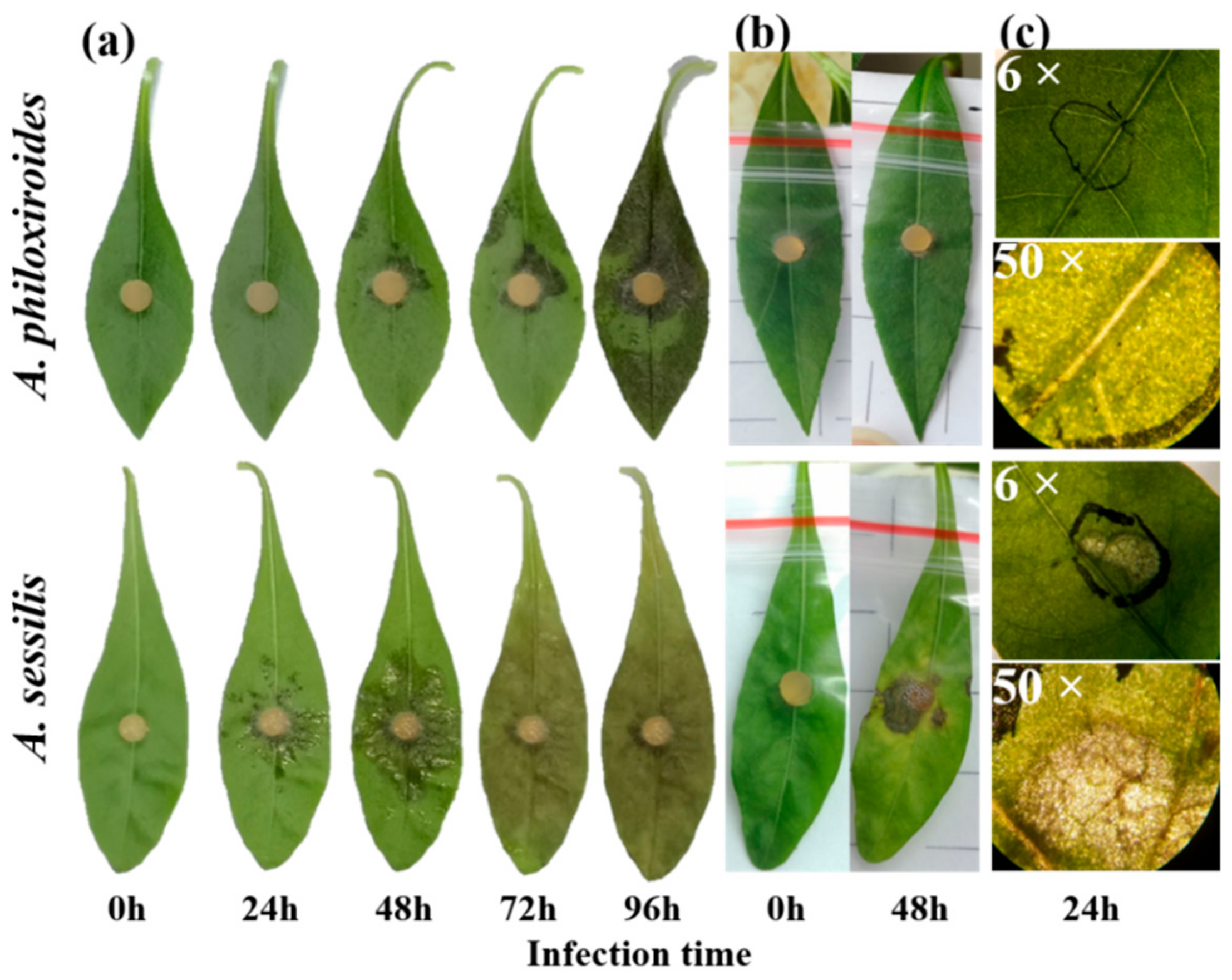
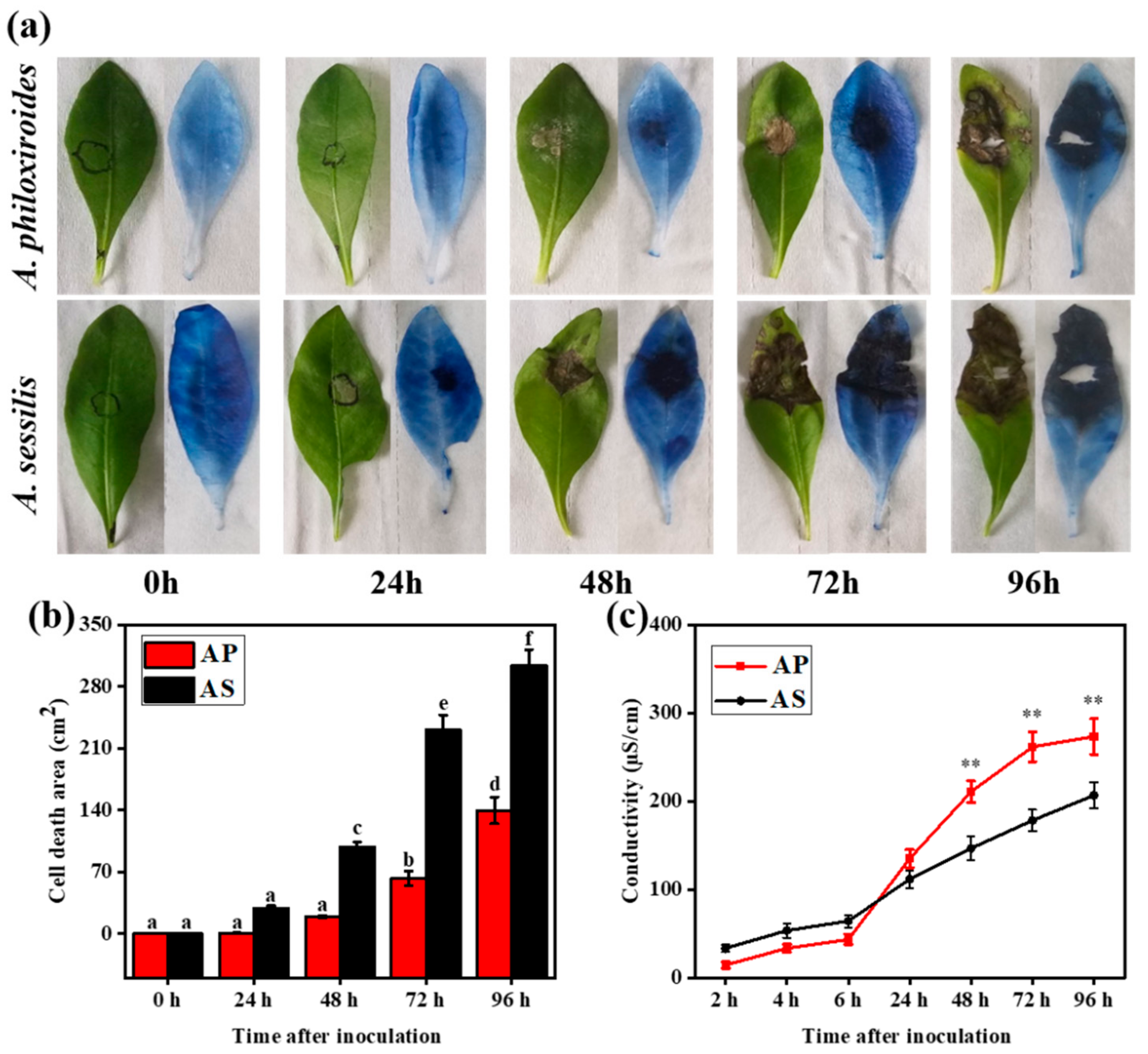
2.1. Disease Development in A. philoxeroides Was Delayed Compared with A. sessilis
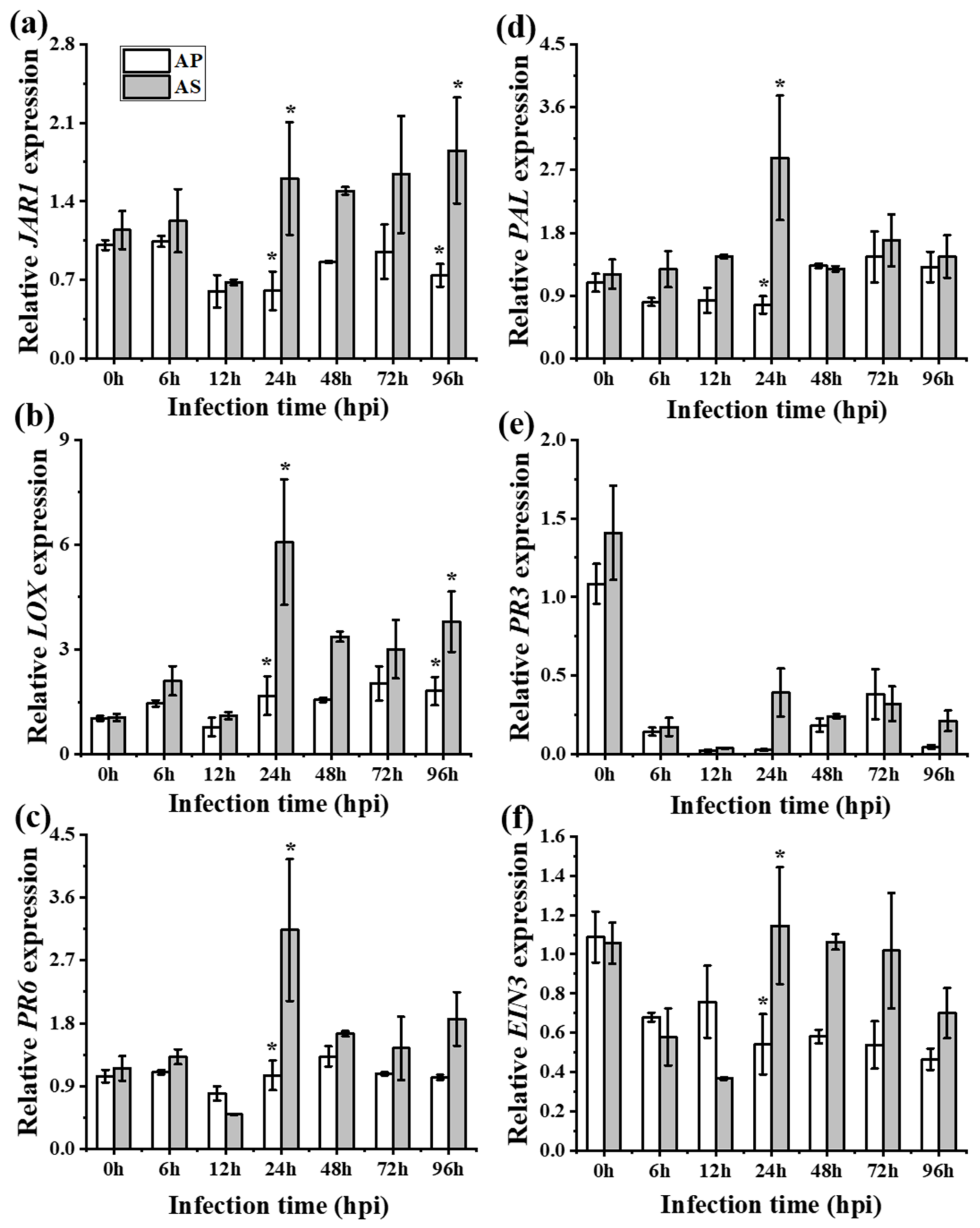
2.2. Expression Divergence of Defense Hormone Genes between A. philoxiroides and A. sessilis
2.3. Functional Analysis of Defense Hormone Gene Sequences between A. philoxiroides and A. sessilis
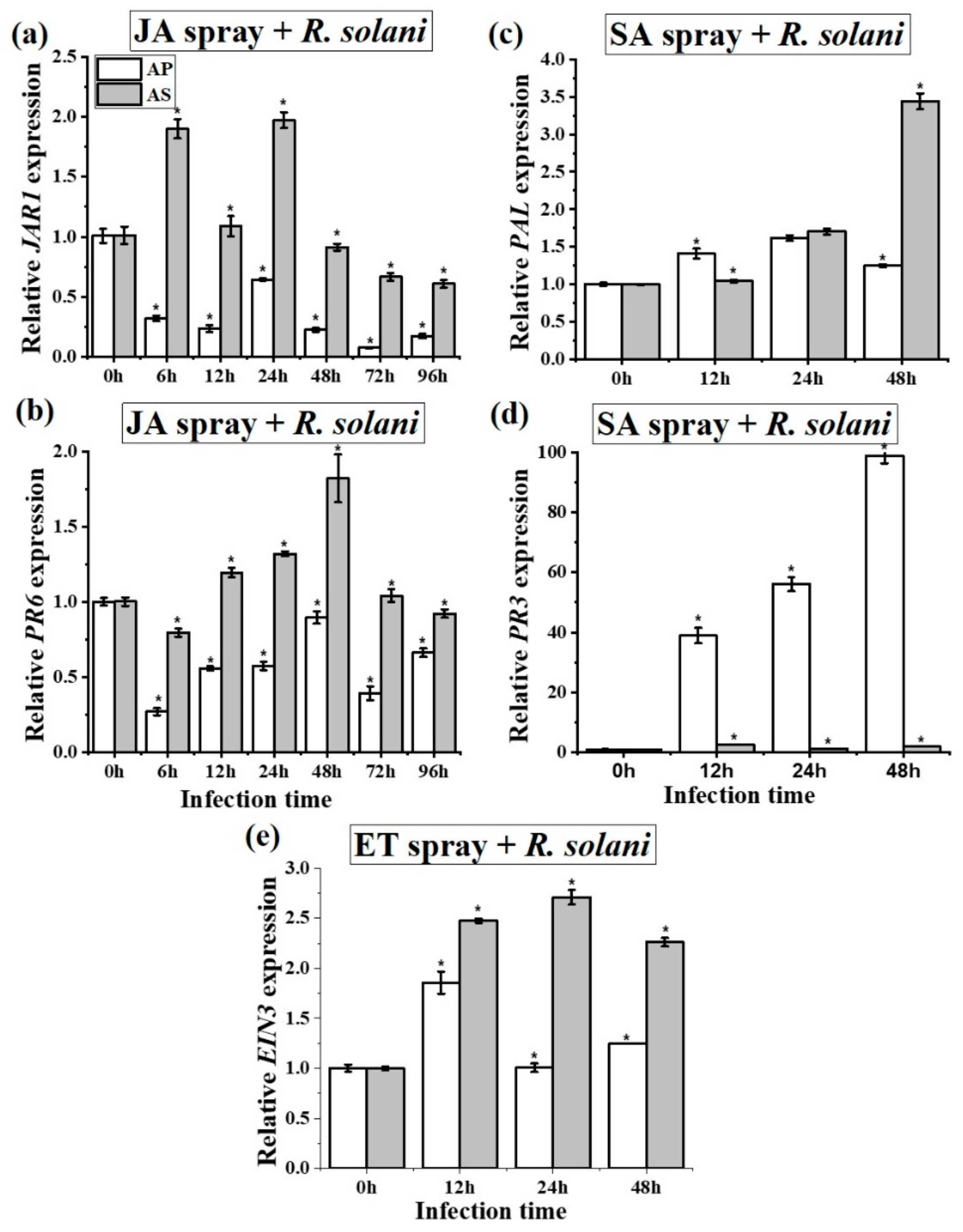
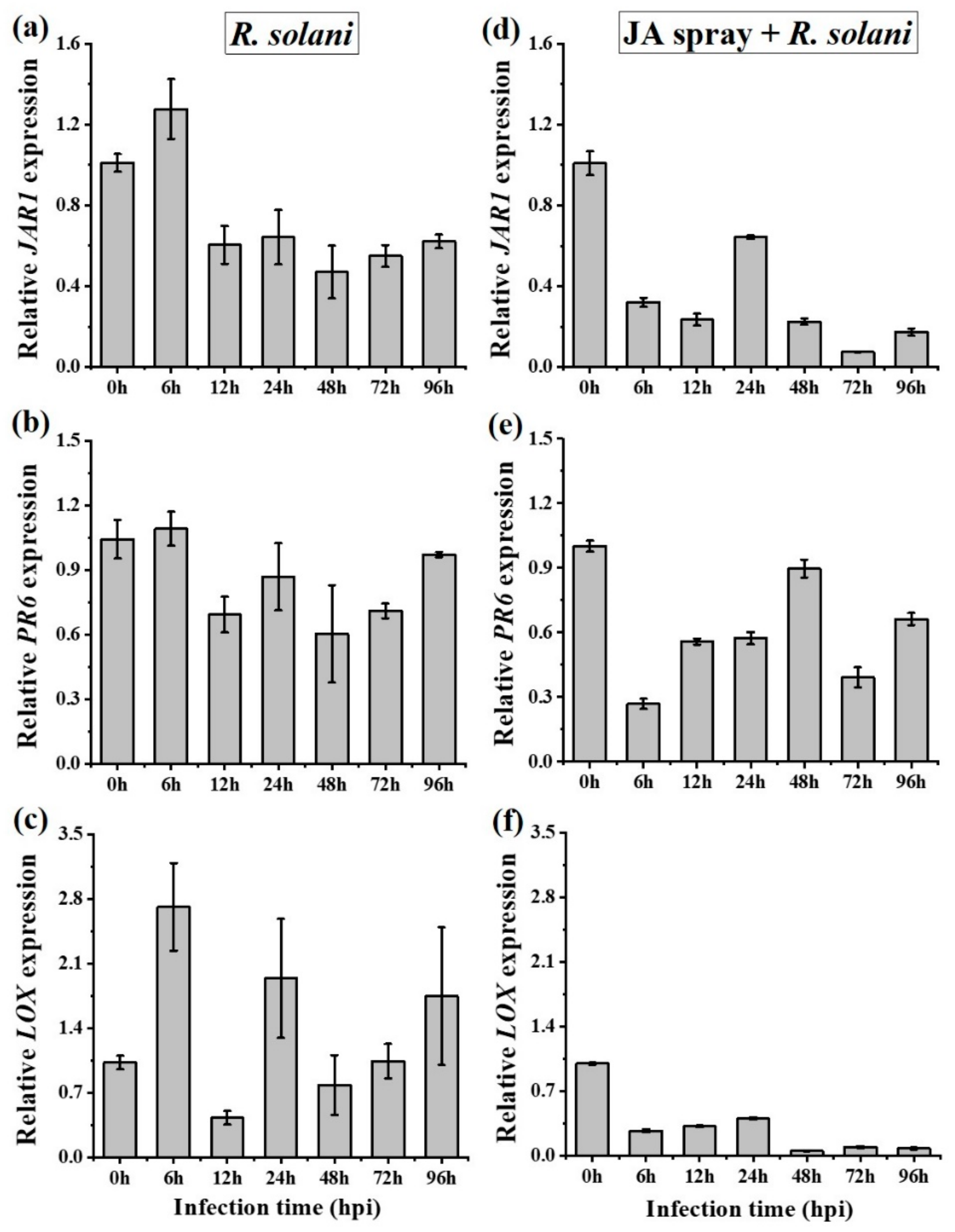
2.4. R. solani Suppresses Jasmonic Acid Signaling for Disease Promotion in A. philoxiroides
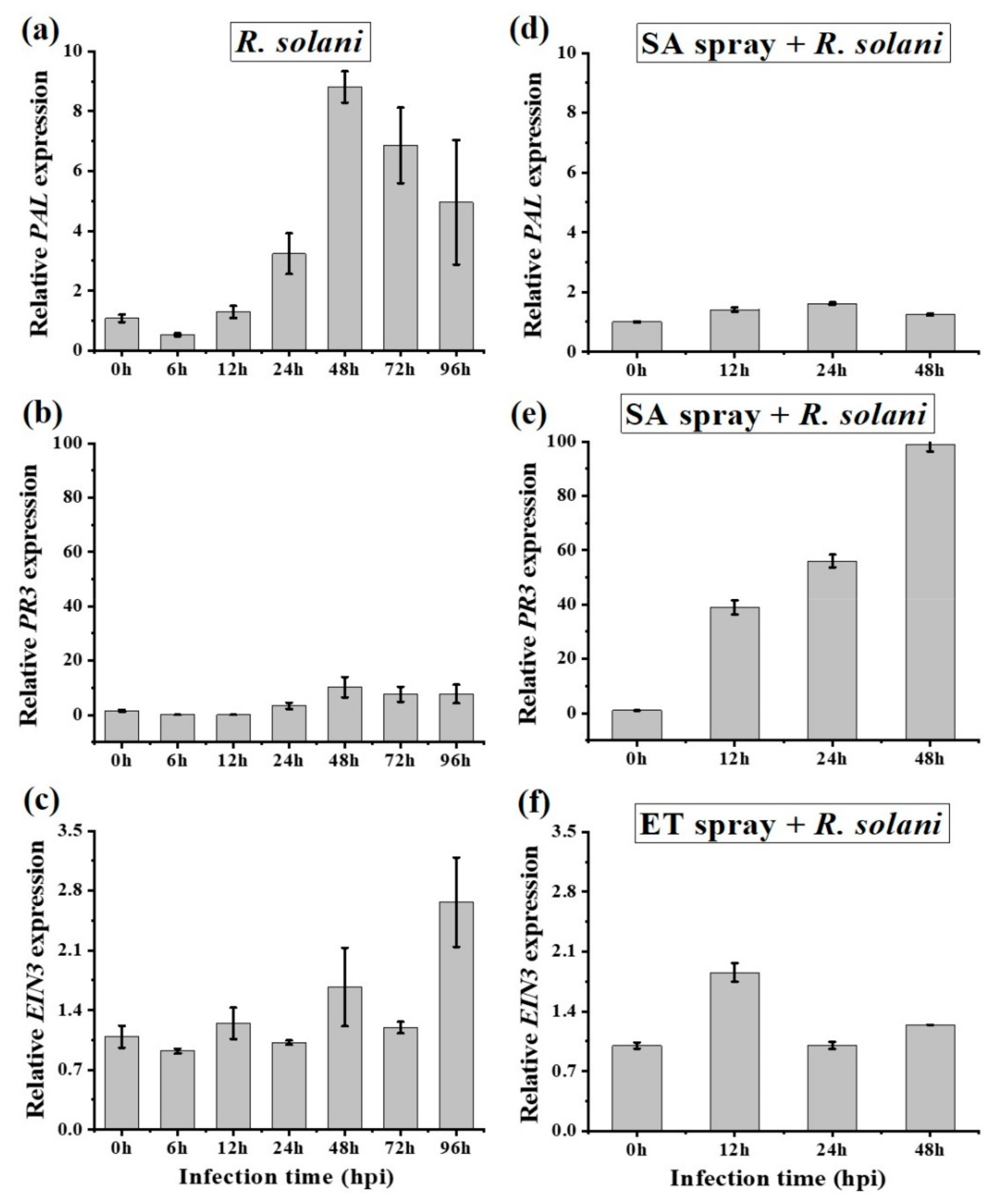
2.5. Salicylic Acid and Ethylene Signaling Enhances Disease Susceptibility in A. philoxiroides
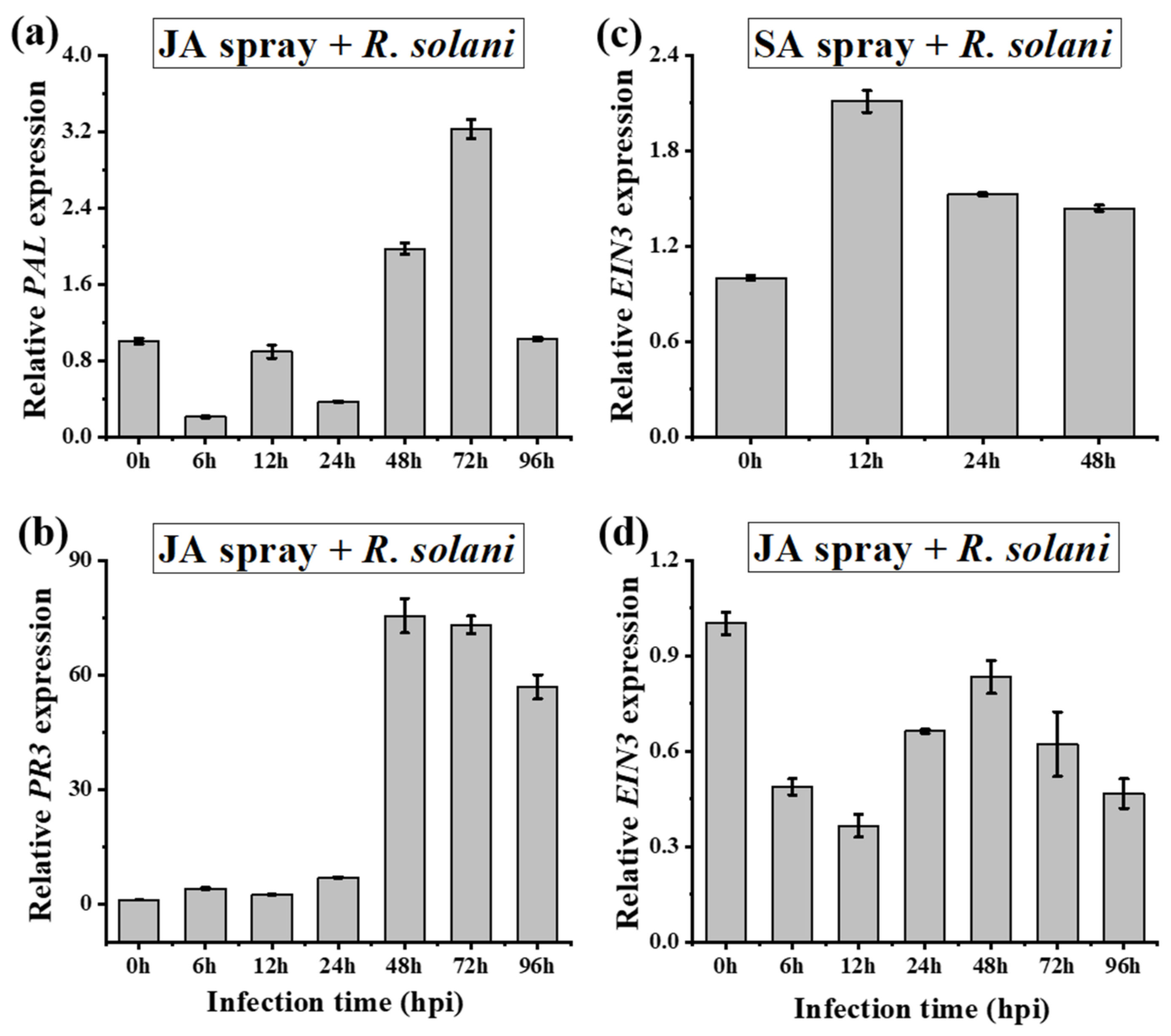
2.6. Signaling Cross-Talk between SA, JA and ET in A. philoxiroides during Interactions with R. solani
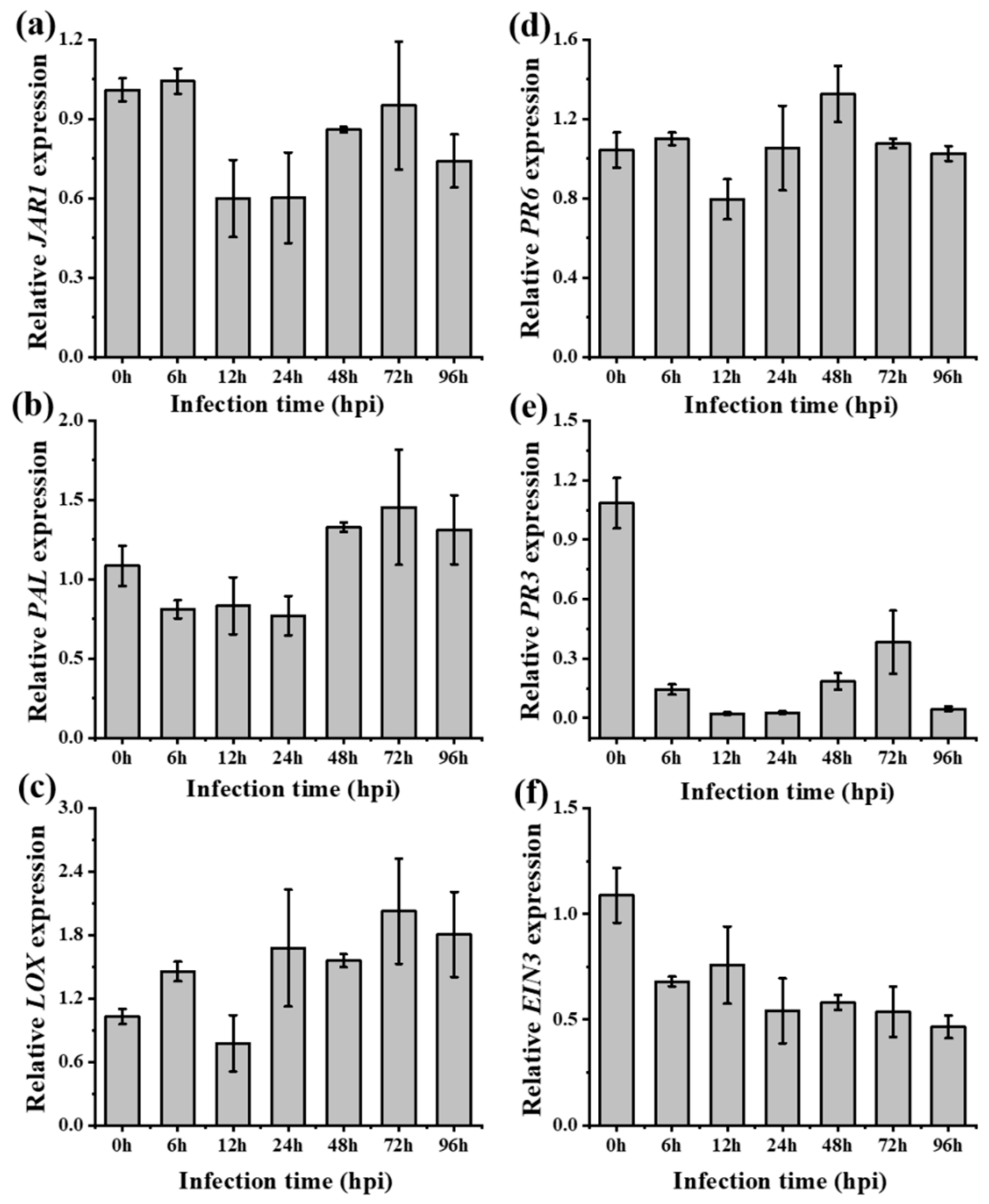
2.7. R. solani Induced Resistance Trade-Offs between SA and JA-Signaling in A. philoxiroides
3. Discussion
3.1. Differential Expression between Invasive A. philoxiroides and Native A. sessilis during Disease Development
3.2. Changes in Defense Hormone Gene Expression during Pathogenesis in A. philoxeroides
3.3. Hormonal Cross-Talk and Systemic Resistance in A. philoxeroides during R. solani Pathogenesis
4. Materials and Methods
4.1. Plant Species and Pathogens
4.2. Isolation of Putative Hormone-Responsive Genes for RT-qPCR
4.3. Plant Inoculations with R. solani for RT-qPCR
4.4. RT-qPCR Analysis
4.5. Hormone Content Quantification
4.6. Quantification of Plant Cell Death by Trypan Blue Staining and Ion Leakage Assay
4.7. Flanking Sequence Isolation and Bioinformatic Analyses
4.8. Data Availability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vila, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarosik, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pysek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.A.; Blumenthal, D.M.; Wilcove, D.S.; Ziska, L.H. Predicting plant invasions in an era of global change. Trends Ecol. Evol. 2010, 25, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Chown, S.L.; Hodgins, K.A.; Griffin, P.C.; Oakeshott, J.G.; Byrne, M.; Hoffmann, A.A. Biological invasions, climate change and genomics. Evol. Appl. 2015, 8, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Richardson, D.M. Invasive Species, Environmental Change and Management, and Health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Stewart, C.N.; Tranel, P.J.; Horvath, D.P.; Anderson, J.V.; Rieseberg, L.H.; Westwood, J.H.; Mallory-Smith, C.A.; Zapiola, M.L.; Dlugosch, K.M. Evolution of Weediness and Invasiveness: Charting the Course for Weed Genomics. Weed Sci. 2009, 57, 451–462. [Google Scholar] [CrossRef]
- Dlugosch, K.M.; Parker, I.M. Invading populations of an ornamental shrub show rapid life history evolution despite genetic bottlenecks. Ecol. Lett. 2008, 11, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.M.; Pyšek, P. Naturalization of introduced plants: Ecological drivers of biogeographical patterns. N. Phytol. 2012, 196, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Van Kleunen, M.; Dawson, W.; Schlaepfer, D.; Jeschke, J.M.; Fischer, M. Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol. Lett. 2010, 13, 947–958. [Google Scholar] [CrossRef]
- Engelkes, T.; Morriën, E.; Verhoeven, K.J.F.; Bezemer, T.M.; Biere, A.; Harvey, J.A.; McIntyre, L.M.; Tamis, W.L.M.; van der Putten, W.H. Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature 2008, 456, 946. [Google Scholar] [CrossRef]
- Cushman, J.H.; Lortie, C.J.; Christian, C.E. Native herbivores and plant facilitation mediate the performance and distribution of an invasive exotic grass. J. Ecol. 2011, 99, 524–531. [Google Scholar] [CrossRef]
- Fan, S.; Yu, H.; Dong, X.; Wang, L.; Chen, X.; Yu, D.; Liu, C. Invasive plant Alternanthera philoxeroides suffers more severe herbivory pressure than native competitors in recipient communities. Sci. Rep. 2016, 6, 36542. [Google Scholar] [CrossRef] [PubMed]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Blossey, B.; Notzold, R. Evolution of Increased Competitive Ability in Invasive Nonindigenous Plants: A Hypothesis. J. Ecol. 1995, 83, 887–889. [Google Scholar] [CrossRef]
- DeWalt, S.J.; Denslow, J.S.; Ickes, K. Natural-enemy Release Facilitates Habitat Expansion of The Invasive Tropical Shrub Clidemia Hirta. Ecology 2004, 85, 471–483. [Google Scholar] [CrossRef]
- Roy, B.A.; Hudson, K.; Visser, M.; Johnson, B.R. Grassland fires may favor native over introduced plants by reducing pathogen loads. Ecology 2014, 95, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Maron, J.L.; Marco, L. Evidence for the enemy release hypothesis in Hypericum perforatum. Oecologia 2005, 142, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Thelen, G.C.; Rodriguez, A.; Holben, W.E. Soil biota and exotic plant invasion. Nature 2004, 427, 731–733. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Callaway, R.M. Soil Biota Facilitate Exotic Acer Invasions in Europe and North America. Ecol. Appl. 2004, 14, 1737–1745. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Tytgat, T.; Van der Putten, W.H.; Clay, K. Virulence of soil-borne pathogens and invasion by Prunus serotina. N. Phytol. 2010, 186, 484–495. [Google Scholar] [CrossRef]
- Wolfe, L.M. Why alien invaders succeed: Support for the escape-from-enemy hypothesis. Am. Nat. 2002, 160, 705–711. [Google Scholar] [CrossRef]
- Parker, J.D.; Hay, M.E. Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecol. Lett. 2005, 8, 959–967. [Google Scholar] [CrossRef]
- Fan, S.; Yu, D.; Liu, C. The invasive plant Alternanthera philoxeroides was suppressed more intensively than its native congener by a native generalist: Implications for the biotic resistance hypothesis. PLoS ONE 2013, 8, e83619. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef]
- Lombardero, M.J.; Alonso-Rodríguez, M.; Roca-Posada, E.P. Tree insects and pathogens display opposite tendencies to attack native vs. non-native pines. For. Ecol. Manag. 2012, 281, 121–129. [Google Scholar] [CrossRef]
- Pearse, I.S.; Hipp, A.L. Phylogenetic and trait similarity to a native species predict herbivory on non-native oaks. Proc. Natl. Acad. Sci. USA 2009, 106, 18097–18102. [Google Scholar] [CrossRef] [PubMed]
- Knevel, I.C.; Lans, T.; Menting, F.B.; Hertling, U.M.; van der Putten, W.H. Release from native root herbivores and biotic resistance by soil pathogens in a new habitat both affect the alien Ammophila arenaria in South Africa. Oecologia 2004, 141, 502–510. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Power, A.G. Release of invasive plants from fungal and viral pathogens. Nature 2003, 421, 625–627. [Google Scholar] [CrossRef]
- Colautti, R.I.; Eckert, C.G.; Barrett, S.C.H. Evolutionary constraints on adaptive evolution during range expansion in an invasive plant. Proc. R. Soc. B Biol. Sci. 2010, 277, 1799–1806. [Google Scholar] [CrossRef]
- Guggisberg, A.; Lai, Z.; Huang, J.; Rieseberg, L.H. Transcriptome divergence between introduced and native populations of Canada thistle, Cirsium arvense. N. Phytol. 2013, 199, 595–608. [Google Scholar] [CrossRef]
- Hodgins, K.A.; Lai, Z.; Nurkowski, K.; Huang, J.; Rieseberg, L.H. The molecular basis of invasiveness: Differences in gene expression of native and introduced common ragweed (Ambrosia artemisiifolia) in stressful and benign environments. Mol. Ecol. 2013, 22, 2496–2510. [Google Scholar] [CrossRef]
- Lai, Z.; Kane, N.C.; Zou, Y.; Rieseberg, L.H. Natural variation in gene expression between wild and weedy populations of Helianthus annuus. Genetics 2008, 179, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.J.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M.P. Disease Resistance Mechanisms in Plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Robatzek, S.; Navarro, L.; Oakeley, E.J.; Jones, J.D.; Felix, G.; Boller, T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 2004, 428, 764–767. [Google Scholar] [CrossRef]
- Kaku, H.; Nishizawa, Y.; Ishii-Minami, N.; Akimoto-Tomiyama, C.; Dohmae, N.; Takio, K.; Minami, E.; Shibuya, N. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 11086–11091. [Google Scholar] [CrossRef] [PubMed]
- Fliegmann, J.; Mithofer, A.; Wanner, G.; Ebel, J. An ancient enzyme domain hidden in the putative beta-glucan elicitor receptor of soybean may play an active part in the perception of pathogen-associated molecular patterns during broad host resistance. J. Biol. Chem. 2004, 279, 1132–1140. [Google Scholar] [CrossRef]
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kourelis, J.; van der Hoorn, R.A.L. Defended to the Nines: 25 Years of Resistance Gene Cloning Identifies Nine Mechanisms for R Protein Function. Plant Cell 2018, 30, 285–299. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539. [Google Scholar] [CrossRef]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Han, G.Z. Origin and evolution of the plant immune system. N. Phytol. 2019, 222, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.-C.; Qi, S.-S.; Miao, S.-L.; Liu, Y.-T.; Tian, Y.-F.; Zhai, D.-L.; Huang, P.; Du, D.-L. Isolation of NBS-LRR RGAs from invasive Wedelia trilobata and the calculation of evolutionary rates to understand bioinvasion from a molecular evolution perspective. Biochem. Syst. Ecol. 2015, 61, 19–27. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Van Oosten, V.R.; Van Poecke, R.M.; Van Pelt, J.A.; Pozo, M.J.; Mueller, M.J.; Buchala, A.J.; Metraux, J.P.; Van Loon, L.C.; Dicke, M.; et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant Microbe Interact. MPMI 2005, 18, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Jegadeesan, S.; Beery, A.; Altahan, L.; Meir, S.; Pressman, E.; Firon, N. Ethylene production and signaling in tomato (Solanum lycopersicum) pollen grains is responsive to heat stress conditions. Plant Reprod. 2018, 31, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef]
- Mine, A.; Seyfferth, C.; Kracher, B.; Berens, M.L.; Becker, D.; Tsuda, K. The Defense Phytohormone Signaling Network Enables Rapid, High-Amplitude Transcriptional Reprogramming during Effector-Triggered Immunity. Plant Cell 2018, 30, 1199–1219. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Leon-Reyes, A.; Spoel, S.H.; De Lange, E.S.; Abe, H.; Kobayashi, M.; Tsuda, S.; Millenaar, F.F.; Welschen, R.A.; Ritsema, T.; Pieterse, C.M. Ethylene modulates the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling. Plant Physiol. 2009, 149, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Leon-Reyes, A.; Van der Does, D.; De Lange, E.S.; Delker, C.; Wasternack, C.; Van Wees, S.C.; Ritsema, T.; Pieterse, C.M. Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta 2010, 232, 1423–1432. [Google Scholar] [CrossRef]
- Niki, T.; Mitsuhara, I.; Seo, S.; Ohtsubo, N.; Ohashi, Y. Antagonistic Effect of Salicylic Acid and Jasmonic Acid on the Expression of Pathogenesis-Related (PR) Protein Genes in Wounded Mature Tobacco Leaves. Plant Cell Physiol. 1998, 39, 500–507. [Google Scholar] [CrossRef]
- El Oirdi, M.; El Rahman, T.A.; Rigano, L.; El Hadrami, A.; Rodriguez, M.C.; Daayf, F.; Vojnov, A.; Bouarab, K. Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. Plant Cell 2011, 23, 2405–2421. [Google Scholar] [CrossRef]
- Anderson, J.V.; Dogramaci, M.; Horvath, D.P.; Foley, M.E.; Chao, W.S.; Suttle, J.C.; Thimmapuram, J.; Hernandez, A.G.; Ali, S.; Mikel, M.A. Auxin and ABA act as central regulators of developmental networks associated with paradormancy in Canada thistle (Cirsium arvense). Funct. Integr. Genom. 2012, 12, 515–531. [Google Scholar] [CrossRef]
- Schooler, S.S. Alternanthera philoxeroides (Martius) Grisebach. In A Handbook of Global Freshwater Invasive Species; Francis, R.A., Ed.; Routlegde: Abingdon, UK, 2012. [Google Scholar]
- Buckingham, G.R. Biological Control of Alligatorweed, Alternanthera philoxeroides, the World’s First Aquatic Weed Success Story. Castanea 1996, 61, 232–243. [Google Scholar]
- Wang, B.; Li, W.; Wang, J. Genetic diversity of Alternanthera philoxeroides in China. Aquat. Bot. 2005, 81, 277–283. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, Y. Alien invasive plants in China: Risk assessment and spatial patterns. Biodivers. Conserv. 2010, 19, 3489–3497. [Google Scholar] [CrossRef]
- Wan, L.-Y. Clonal integration is beneficial for resource sharing in a creeping amphibian herb (Alteranthera philoxeroides). Folia Geobot. 2017, 52, 10–432. [Google Scholar] [CrossRef]
- Shen, J.; Shen, M.; Wang, X.; Lu, Y. Effect of environmental factors on shoot emergence and vegetative growth of alligatorweed (Alternanthera philoxcroides). Weed Sci. 2005, 53, 471–478. [Google Scholar] [CrossRef]
- Geng, Y.-P.; Pan, X.-Y.; Xu, C.-Y.; Zhang, W.-J.; Li, B.; Chen, J.-K.; Lu, B.-R.; Song, Z.-P. Phenotypic plasticity rather than locally adapted ecotypes allows the invasive alligator weed to colonize a wide range of habitats. Biol. Invasions 2007, 9, 245–256. [Google Scholar] [CrossRef]
- Gilbert, R.L.; Auld, B.A.; Hennecke, B.R. Leaf and stem spot of Alternanthera philoxeroides (alligatorweed) in Australia caused by Nimbya sp. Plant Pathol. 2005, 54, 585. [Google Scholar] [CrossRef]
- Pomella, A.W.V.; Barreto, R.W.; Charudattan, R. Nimbya alternantherae a potential biocontrol agent for alligatorweed, Alternanthera philoxeroides. BioControl 2007, 52, 271–288. [Google Scholar] [CrossRef]
- Tan, W.Z.; Li, Q.J.; Qing, L. Biological control of alligatorweed (Alternanthera philoxeroides) with a Fusarium sp. BioControl 2002, 47, 463–479. [Google Scholar] [CrossRef]
- Joyner, B.G.; Freeman, T.E. Pathogenicity of Rhizoctonia solani to aquatic plants. Phytopathology 1972, 63, 681–685. [Google Scholar] [CrossRef]
- Saveinai, R.; Baiswar, P.; Kumar, R.; Rajesh, T.; Behere, G.T. Pathogenicity of Rhizoctonia solani AG 1-IA on major weeds prevalent in rice and maize ecosystem in Meghalaya. Indian Phytopathol. 2017, 70, 91–97. [Google Scholar] [CrossRef]
- Prentis, P.J.; Wilson, J.R.; Dormontt, E.E.; Richardson, D.M.; Lowe, A.J. Adaptive evolution in invasive species. Trends Plant Sci. 2008, 13, 288–294. [Google Scholar] [CrossRef]
- Berens, M.L.; Berry, H.M.; Mine, A.; Argueso, C.T.; Tsuda, K. Evolution of Hormone Signaling Networks in Plant Defense. Annu. Rev. Phytopathol. 2017, 55, 401–425. [Google Scholar] [CrossRef]
- Mengiste, T. Plant immunity to necrotrophs. Annu. Rev. Phytopathol. 2012, 50, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, L.; Fu, C.; Wang, L.; Liu, H.; Cheng, Y.; Li, S.; Deng, Q.; Wang, S.; Zhu, J.; et al. Comparative Transcriptome Analyses of Gene Expression Changes Triggered by Rhizoctonia solani AG1 IA Infection in Resistant and Susceptible Rice Varieties. Front. Plant Sci. 2017, 8, 1422. [Google Scholar] [CrossRef] [PubMed]
- Melan, M.A.; Dong, X.; Endara, M.E.; Davis, K.R.; Ausubel, F.M.; Peterman, T.K. An Arabidopsis thaliana lipoxygenase gene can be induced by pathogens, abscisic acid, and methyl jasmonate. Plant Physiol. 1993, 101, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Beckers, G.J.; Spoel, S.H. Fine-Tuning Plant Defence Signalling: Salicylate versus Jasmonate. Plant Biol. 2006, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, A.M.; Argueso, C.T. No hormone to rule them all: Interactions of plant hormones during the responses of plants to pathogens. Semin. Cell Dev. Biol. 2016, 56, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Doares, S.H.; Narvaez-Vasquez, J.; Conconi, A.; Ryan, C.A. Salicylic Acid Inhibits Synthesis of Proteinase Inhibitors in Tomato Leaves Induced by Systemin and Jasmonic Acid. Plant Physiol. 1995, 108, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.W.; Ma, W. Phytohormone pathways as targets of pathogens to facilitate infection. Plant Mol. Biol. 2016, 91, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Han, X.; Kahmann, R. Microbial effectors target multiple steps in the salicylic acid production and signaling pathway. Front. Plant Sci. 2015, 6, 349. [Google Scholar] [CrossRef] [PubMed]
- Uppalapati, S.R.; Ishiga, Y.; Wangdi, T.; Kunkel, B.N.; Anand, A.; Mysore, K.S.; Bender, C.L. The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 2007, 20, 955–965. [Google Scholar] [CrossRef]
- Rahman, T.A.; Oirdi, M.E.; Gonzalez-Lamothe, R.; Bouarab, K. Necrotrophic pathogens use the salicylic acid signaling pathway to promote disease development in tomato. Mol. Plant Microbe Interact. MPMI 2012, 25, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Portieles, R.; Ochagavia, M.E.; Canales, E.; Silva, Y.; Chacon, O.; Hernandez, I.; Lopez, Y.; Rodriguez, M.; Terauchi, R.; Borroto, C.; et al. High-throughput SuperSAGE for gene expression analysis of Nicotiana tabacum-Rhizoctonia solani interaction. BMC Res. Notes 2017, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Derksen, H.; Rampitsch, C.; Daayf, F. Signaling cross-talk in plant disease resistance. Plant Sci. 2013, 207, 79–87. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Delaure, S.L.; De Bolle, M.F.; Cammue, B.P. The role of ethylene in host-pathogen interactions. Annu. Rev. Phytopathol. 2006, 44, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Penninckx, I.A.; Thomma, B.P.; Buchala, A.; Metraux, J.P.; Broekaert, W.F. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 1998, 10, 2103–2113. [Google Scholar] [CrossRef]
- Adie, B.A.; Perez-Perez, J.; Perez-Perez, M.M.; Godoy, M.; Sanchez-Serrano, J.J.; Schmelz, E.A.; Solano, R. ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 2007, 19, 1665–1681. [Google Scholar] [CrossRef]
- De Vos, M.; Van Zaanen, W.; Koornneef, A.; Korzelius, J.P.; Dicke, M.; Van Loon, L.C.; Pieterse, C.M.J. Herbivore-Induced Resistance against Microbial Pathogens in Arabidopsis. Plant Physiol. 2006, 142, 352–363. [Google Scholar] [CrossRef]
- Chen, H.; Xue, L.; Chintamanani, S.; Germain, H.; Lin, H.; Cui, H.; Cai, R.; Zuo, J.; Tang, X.; Li, X.; et al. ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 2009, 21, 2527–2540. [Google Scholar] [CrossRef]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Natl. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 2008, 3, 348–351. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Ton, J.; Van Pelt, J.A.; Van Loon, L.C.; Pieterse, C.M. Differential effectiveness of salicylate-dependent and jasmonate/ethylene-dependent induced resistance in Arabidopsis. Mol. Plant Microbe Interact. MPMI 2002, 15, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Ton, J. Long-distance signalling in plant defence. Trends Plant Sci. 2008, 13, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Julien, M.H.; Skarratt, B.; Maywald, G.F. Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. J. Aquat. Plant Manag. 1995, 33, 55–60. [Google Scholar]
- Weber, E.; Sun, S.-G.; Li, B. Invasive alien plants in China: Diversity and ecological insights. Biol. Invasions 2008, 10, 1411–1429. [Google Scholar] [CrossRef]
- Dai, Z.C.; Fu, W.; Qi, S.S.; Zhai, D.L.; Chen, S.C.; Wan, L.Y.; Huang, P.; Du, D.L. Different Responses of an Invasive Clonal Plant Wedelia trilobata and its Native Congener to Gibberellin: Implications for Biological Invasion. J. Chem. Ecol. 2016, 42, 85–94. [Google Scholar] [CrossRef]
- Paulitz, T.C.; Okubara, P.A.; Schillinger, W.F. First Report of Damping-Off of Canola Caused by Rhizoctonia solani AG 2-1 in Washington State. Plant Dis. 2006, 90, 829. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M. Trials of direct detection and identification of Rhizoctonia solani AG 1 and AG 2 subgroups using specifically primed PCR analysis. Mycoscience 2002, 43, 185–189. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- McWilliam, H.; Li, W.; Uludag, M.; Squizzato, S.; Park, Y.M.; Buso, N.; Cowley, A.P.; Lopez, R. Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res. 2013, 41, W597–W600. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Thornton, B.; Basu, C. Real-time PCR (qPCR) primer design using free online software. Biochem. Mol. Biol. Educ. A Bimon. Publ. Int. Union Biochem. Mol. Biol. 2011, 39, 145–154. [Google Scholar] [CrossRef] [PubMed]
- El Oirdi, M.; Bouarab, K. Plant signalling components EDS1 and SGT1 enhance disease caused by the necrotrophic pathogen Botrytis cinerea. N. Phytol. 2007, 175, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Han, X.; Chen, Y.; Wu, Q.; Wang, Y. Identification of appropriate reference genes for normalizing transcript expression by quantitative real-time PCR in Litsea cubeba. Mol. Genet. Genom. Mgg 2013, 288, 727–737. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Koch, E.; Slusarenko, A. Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 1990, 2, 437–445. [Google Scholar]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophoton. Int. 2004, 11, 36–42. [Google Scholar]
- Hatsugai, N.; Katagiri, F. Quantification of Plant Cell Death by Electrolyte Leakage Assay. Bio Protoc. 2018, 8, e2758. [Google Scholar] [CrossRef]
- Min, X.J.; Butler, G.; Storms, R.; Tsang, A. OrfPredictor: Predicting protein-coding regions in EST-derived sequences. Nucleic Acids Res. 2005, 33, W677–W680. [Google Scholar] [CrossRef]
- Huang, X.; Madan, A. CAP3: A DNA sequence assembly program. Genome Res. 1999, 9, 868–877. [Google Scholar] [CrossRef]
- Solovyev, V.; Kosarev, P.; Seledsov, I.; Vorobyev, D. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 2006, 7, S10.1–S12. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 4, D200–D203. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 2018, 173, 1454–1467. [Google Scholar] [CrossRef]

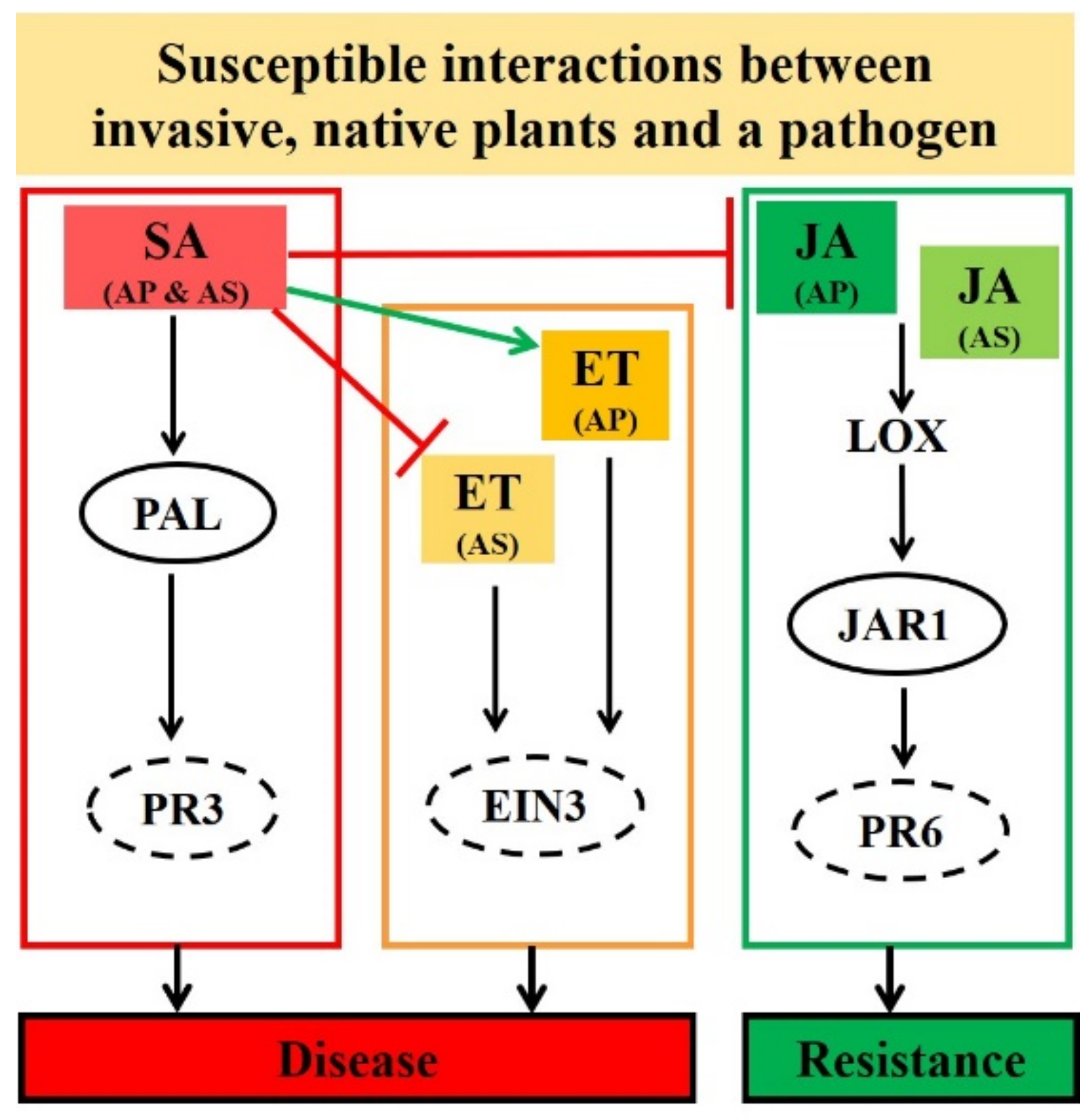
| Treatment | Alternanthera philoxeroides | Alternanthera sessilis |
|---|---|---|
| Rhizoctonia solani (Infected local leaves) | - JA was suppressed (JAR1 (Figure 3a) and PR6 (Figure 3b)) | - JA was partially suppressed (JAR1 (Figure 3a) and PR6 (Figure 3b)) |
| - SA was induced (PAL (Figure 3c) and PR3 (Figure 3d)) | - SA was induced (PAL (Figure 3c) and PR3 (Figure 3d)) | |
| - ET was induced (EIN3 (Figure 3e)) | - ET was suppressed (EIN3 (Figure 3e)) | |
| - SA may promote disease development by suppressing JA and by inducing ET (Figure 3) - Strong antagonistic cross-talk between SA and JA - Stronger antagonistic resistance cross-talk between SA and JA may be reason for the delayed disease development (Figure 1 and Figure 2) | - SA may promote disease development but by partially suppressing JA and by completely suppressing ET (Figure 3) - No strict or strong antagonistic cross-talk between SA and JA - Lack of stronger antagonistic resistance effect between SA and JA may be reason for the enhanced disease development (Figure 1 and Figure 2) | |
| Rhizoctonia solani (Systemic leaves) | - JA-JAR1 was suppressed (Figure 4a) - JA-LOX (Figure 4b) and PR6 (Figure 4c) was induced but lower than A. sessilis | - JA was induced (JAR1 (Figure 4a), LOX (Figure 4b) and PR6 (Figure 4c)) |
| - SA-PAL was induced from 48 hpi (Figure 4d) - SA-PR3 was suppressed at all time (Figure 4e) | - SA-PAL was induced at all the indicated time (Figure 4d) - SA-PR3 was completely suppressed (Figure 4e) | |
| - ET-EIN3 was suppressed all the time (Figure 4f) | - ET-EIN3 was partially suppressed (Figure 4f) | |
| - An antagonism between SA-PAL and JA-JAR1 was observed (Figure 4) | - No antagonism between SA-PAL and JA-JAR1 was observed (Figure 4) | |
| Rhizoctonia solani (Hormone contents) | - Endogenous contents of SA, JA and ET were higher. For example, at 96 hpi, SA, JA and ET contents were significantly higher than control samples (Figure S1) | - Endogenous contents of SA, JA and ET were comparatively lower. For example, at 96 hpi, SA, JA and ET contents were significantly higher than control samples (Figure S1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manoharan, B.; Qi, S.-S.; Dhandapani, V.; Chen, Q.; Rutherford, S.; Wan, J.S.; Jegadeesan, S.; Yang, H.-Y.; Li, Q.; Li, J.; et al. Gene Expression Profiling Reveals Enhanced Defense Responses in an Invasive Weed Compared to Its Native Congener During Pathogenesis. Int. J. Mol. Sci. 2019, 20, 4916. https://doi.org/10.3390/ijms20194916
Manoharan B, Qi S-S, Dhandapani V, Chen Q, Rutherford S, Wan JS, Jegadeesan S, Yang H-Y, Li Q, Li J, et al. Gene Expression Profiling Reveals Enhanced Defense Responses in an Invasive Weed Compared to Its Native Congener During Pathogenesis. International Journal of Molecular Sciences. 2019; 20(19):4916. https://doi.org/10.3390/ijms20194916
Chicago/Turabian StyleManoharan, Bharani, Shan-Shan Qi, Vignesh Dhandapani, Qi Chen, Susan Rutherford, Justin SH Wan, Sridharan Jegadeesan, Hong-Yu Yang, Qin Li, Jian Li, and et al. 2019. "Gene Expression Profiling Reveals Enhanced Defense Responses in an Invasive Weed Compared to Its Native Congener During Pathogenesis" International Journal of Molecular Sciences 20, no. 19: 4916. https://doi.org/10.3390/ijms20194916
APA StyleManoharan, B., Qi, S.-S., Dhandapani, V., Chen, Q., Rutherford, S., Wan, J. S., Jegadeesan, S., Yang, H.-Y., Li, Q., Li, J., Dai, Z.-C., & Du, D.-L. (2019). Gene Expression Profiling Reveals Enhanced Defense Responses in an Invasive Weed Compared to Its Native Congener During Pathogenesis. International Journal of Molecular Sciences, 20(19), 4916. https://doi.org/10.3390/ijms20194916






