The Ratio of Oxidized Lipoprotein(a) to Native Lipoprotein(a) and the Endothelial Function in Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
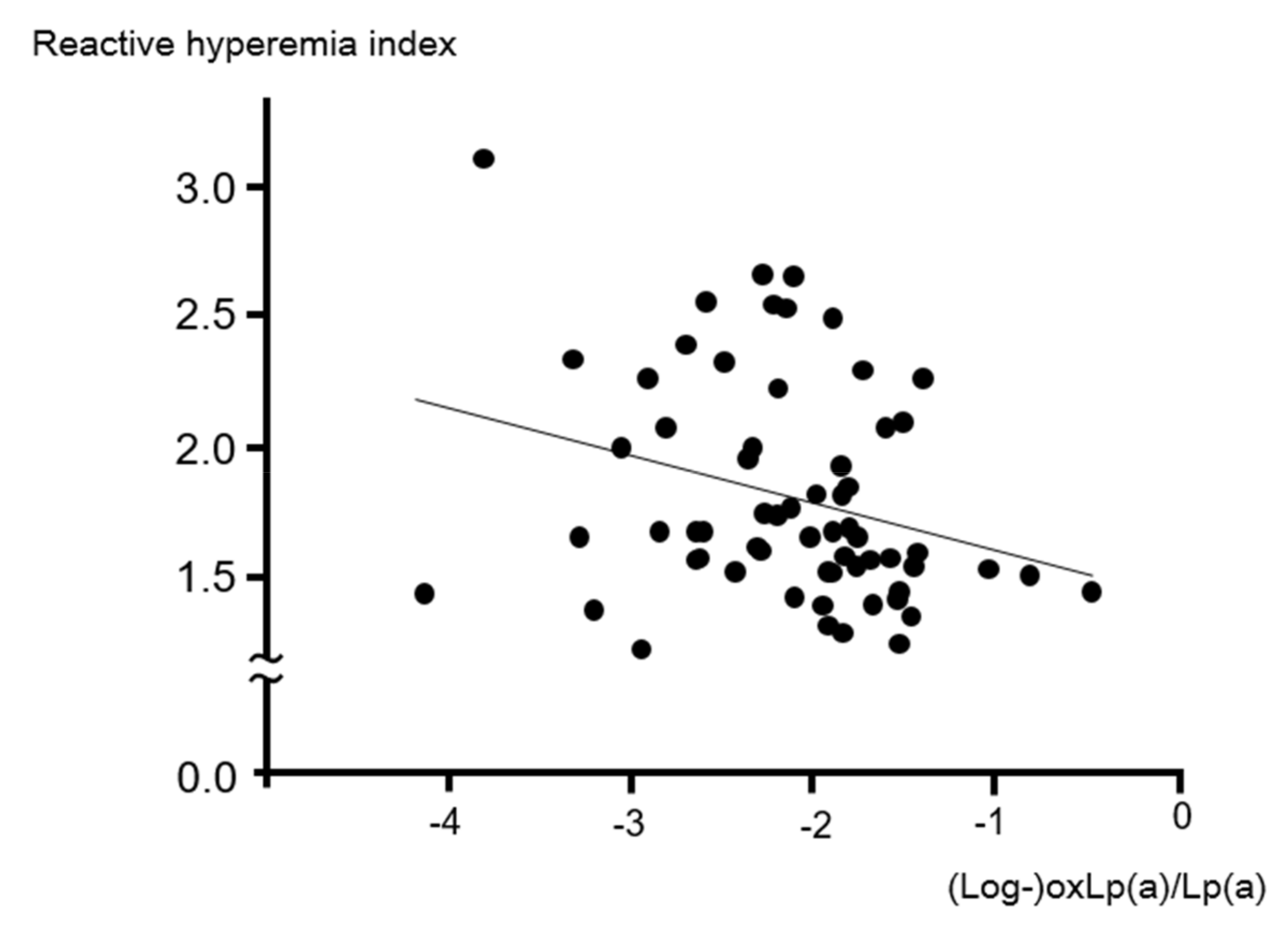
2. Results
3. Discussion
4. Methods
Author Contributions
Funding
Conflicts of Interest
References
- Saito, I. Epidemiological evidence of type 2 diabetes mellitus, metabolic syndrome, and cardiovascular disease in Japan. Circ. J. 2012, 76, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, G.; Landmesser, U. Endothelial dysfunction as an early sign of atherosclerosis. Herz 2007, 32, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Axtell, A.L.; Gomari, F.A.; Cooke, J.P. Assessing endothelial vasodilator function with the Endo-PAT 2000. J. Vis. Exp. 2010, 44, e2167. [Google Scholar] [CrossRef] [PubMed]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxid. Med. Cell Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, D.; Borsa, C.; Ionescu, C.; Prada, G.I. Oxidized LDL and NO synthesis - Biomarkers of endothelial dysfunction and ageing. Mech. Ageing Dev. 2015, 151, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, J.G.; Dallinga-Thie, G.M.; Kroon, J. The role of (modified) lipoproteins in vascular function: A duet between monocytes and the endothelium. Curr. Med. Chem. 2019, 26, 1594–1609. [Google Scholar] [CrossRef] [PubMed]
- Kotani, K.; Serban, M.C.; Penson, P.; Lippi, G.; Banach, M. Evidence-based assessment of lipoprotein(a) as a risk biomarker for cardiovascular diseases - Some answers and still many questions. Crit. Rev. Clin. Lab. Sci. 2016, 53, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Forbes, C.A.; Quek, R.G.; Deshpande, S.; Worthy, G.; Wolff, R.; Stirk, L.; Kleijnen, J.; Gandra, S.R.; Djedjos, S.; Wong, N.D. The relationship between Lp(a) and CVD outcomes: A systematic review. Lipids Health Dis. 2016, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, T.; Kobayashi, T.; Okubo, M.; Nakanishi, K.; Sugimoto, T.; Ohashi, Y.; Murase, T. Prospective study of lipoprotein(a) as a risk factor for atherosclerotic cardiovascular disease in patients with diabetes. Diabetes Care 1995, 18, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Okubo, M.; Amemiya-Kudo, M.; Ebara, T.; Mori, Y. Impact of elevated serum lipoprotein(a) concentrations on the risk of coronary heart disease in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 791–795. [Google Scholar] [CrossRef]
- Kotani, K.; Yamada, S.; Uurtuya, S.; Yamada, T.; Taniguchi, N.; Sakurabayashi, I. The association between blood glucose and oxidized lipoprotein(a) in healthy young women. Lipids Health Dis. 2010, 9, 103. [Google Scholar] [PubMed]
- Kotani, K.; Yamada, S.; Yamada, T.; Taniguchi, N.; Sakurabayashi, I. The relationship between oxidized lipoprotein(a) and carotid atherosclerosis in asymptomatic subjects: A comparison with native lipoprotein(a). Lipids Health Dis. 2010, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Kotani, K.; Yamada, S.; Yamada, T.; Kario, K.; Taniguchi, N. Oxidized lipoprotein(a) and cardio-ankle vascular index (CAVI) in hypertensive subjects. Heart Vessels 2013, 28, 461–466. [Google Scholar] [CrossRef]
- Folli, F.; Corradi, D.; Fanti, P.; Davalli, A.; Paez, A.; Giaccari, A.; Perego, C.; Muscogiuri, G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 2011, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Neele, D.M.; de Wit, E.C.; Princen, H.M. Insulin suppresses apolipoprotein(a) synthesis by primary cultures of cynomolgus monkey hepatocytes. Diabetologia 1999, 42, 41–44. [Google Scholar] [CrossRef]
- Ding, L.; Song, A.; Dai, M.; Xu, M.; Sun, W.; Xu, B.; Sun, J.; Wang, T.; Xu, Y.; Lu, J.; et al. Serum lipoprotein (a) concentrations are inversely associated with T2D, prediabetes, and insulin resistance in a middle-aged and elderly Chinese population. J. Lipid Res. 2015, 56, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Kreisberg, R.A. Diabetic dyslipidemia. Am. J. Cardiol. 1998, 82, U67–U73 & U85–U86. [Google Scholar] [CrossRef]
- Regensteiner, J.G.; Golden, S.; Huebschmann, A.G.; Barrett-Connor, E.; Chang, A.Y.; Chyun, D.; Fox, C.S.; Kim, C.; Mehta, N.; Reckelhoff, J.F.; et al. Sex differences in the cardiovascular consequences of diabetes mellitus: A scientific statement from the American Heart Association. Circulation 2015, 132, 2424–2447. [Google Scholar]
- Liu, L.; Miura, K.; Kadota, A.; Fujiyoshi, A.; Gracely, E.J.; Xue, F.; Liu, Z.; Takashima, N.; Miyagawa, N.; Ohkubo, T.; et al. The impact of sex on risk of cardiovascular disease and all-cause mortality in adults with or without diabetes mellitus: A comparison between the U.S. and Japan. J. Diabetes Complicat. 2019, 33, 417–423. [Google Scholar] [CrossRef]
- Ogita, M.; Miyauchi, K.; Dohi, T.; Wada, H.; Tuboi, S.; Miyazaki, T.; Nishino, A.; Yokoyama, T.; Kojima, T.; Yokoyama, K.; et al. Gender-based outcomes among patients with diabetes mellitus after percutaneous coronary intervention in the drug-eluting stent era. Int. Heart J. 2011, 52, 348–352. [Google Scholar] [CrossRef][Green Version]
- Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus; Seino, Y.; Nanjo, K.; Tajima, N.; Kadowaki, T.; Kashiwagi, A.; Araki, E.; Ito, C.; Inagaki, N.; Iwamoto, Y.; et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 2010, 1, 212–228. [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean ± SD/Median (lQR) |
|---|---|
| Age (years) | 59 ± 12 |
| Gender (men/women) | 41/22 |
| Smoker (n) | 17 (27%) |
| Body mass index (kg/m2) | 26.3 ± 2.7 |
| Mean blood pressure (mmHg) | 93.7 ± 11.5 |
| Total cholesterol (mmol/L) | 5.35 ± 1.09 |
| HDL cholesterol (mmol/L) | 1.39 ± 0.43 |
| Triglyceride (mmol/L) | 1.45 (1.05–2.15) |
| Glucose (mmol/L) | 7.89 ± 2.73 |
| Hemoglobin A1c (%) | 7.8 ± 2.1 |
| Lp(a) (mmol/L) | 0.46 (0.24–0.86) |
| OxLp(a) (nmol/L) | 0.11 (0.04–0.28) |
| OxLp(a)/Lp(a) (ratio) | 0.28 (0.07–0.54) |
| Reactive hyperemia index | 1.8 ± 0.4 |
| Variable | r (p) | β (p) |
|---|---|---|
| Age (years) | 0.01 (0.99) | NE |
| Gender (men) | −0.29 (0.02) | −0.25 (0.04) |
| Smoker | 0.03 (0.81) | NE |
| Body mass index (kg/m2) | −0.04 (0.73) | NE |
| Mean blood pressure (mmHg) | −0.10 (0.46) | NE |
| Total cholesterol (mmol/L) | 0.05 (0.69) | NE |
| HDL cholesterol (mmol/L) | 0.09 (0.50) | NE |
| Triglyceride (mmol/L) | 0.03 (0.83) | NE |
| Glucose (mmol/L) | −0.21 (0.10) | NE |
| Hemoglobin A1c (%) | −0.16 (0.20) | NE |
| Lp(a) (mmol/L) | 0.13 (0.30) | NE |
| OxLp(a) (nmol/L) | −0.19 (0.14) | NE |
| OxLp(a)/Lp(a) (ratio) | −0.29 (0.02) | −0.26 (0.04) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotani, K.; Yamada, S.; Takahashi, H.; Iwazu, Y.; Yamada, T. The Ratio of Oxidized Lipoprotein(a) to Native Lipoprotein(a) and the Endothelial Function in Patients with Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2019, 20, 4909. https://doi.org/10.3390/ijms20194909
Kotani K, Yamada S, Takahashi H, Iwazu Y, Yamada T. The Ratio of Oxidized Lipoprotein(a) to Native Lipoprotein(a) and the Endothelial Function in Patients with Type 2 Diabetes Mellitus. International Journal of Molecular Sciences. 2019; 20(19):4909. https://doi.org/10.3390/ijms20194909
Chicago/Turabian StyleKotani, Kazuhiko, Shingo Yamada, Hirokazu Takahashi, Yoshitaka Iwazu, and Toshiyuki Yamada. 2019. "The Ratio of Oxidized Lipoprotein(a) to Native Lipoprotein(a) and the Endothelial Function in Patients with Type 2 Diabetes Mellitus" International Journal of Molecular Sciences 20, no. 19: 4909. https://doi.org/10.3390/ijms20194909
APA StyleKotani, K., Yamada, S., Takahashi, H., Iwazu, Y., & Yamada, T. (2019). The Ratio of Oxidized Lipoprotein(a) to Native Lipoprotein(a) and the Endothelial Function in Patients with Type 2 Diabetes Mellitus. International Journal of Molecular Sciences, 20(19), 4909. https://doi.org/10.3390/ijms20194909







