Membrane Transporters in Human Parotid Gland-Targeted Proteomics Approach
Abstract
1. Introduction
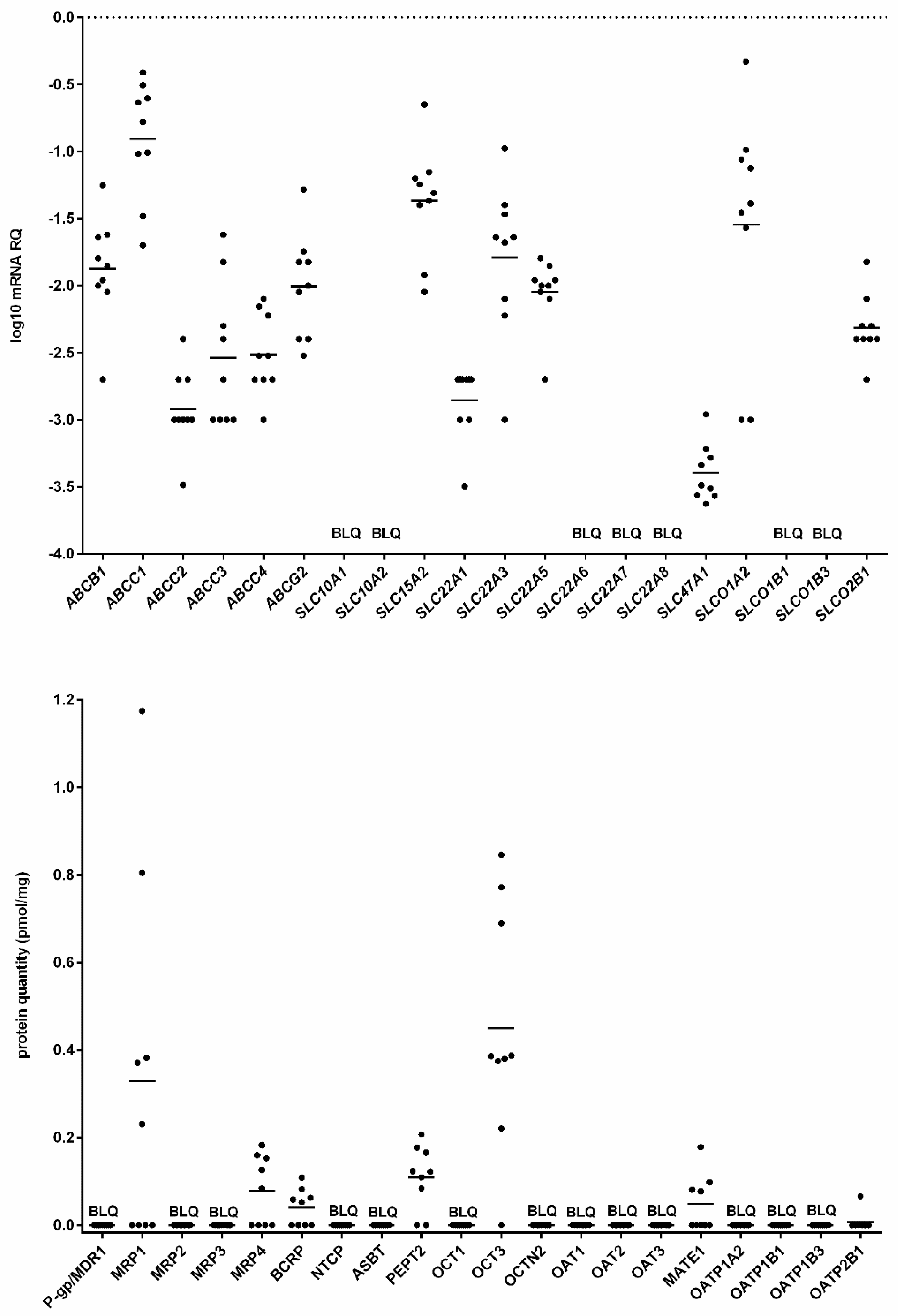
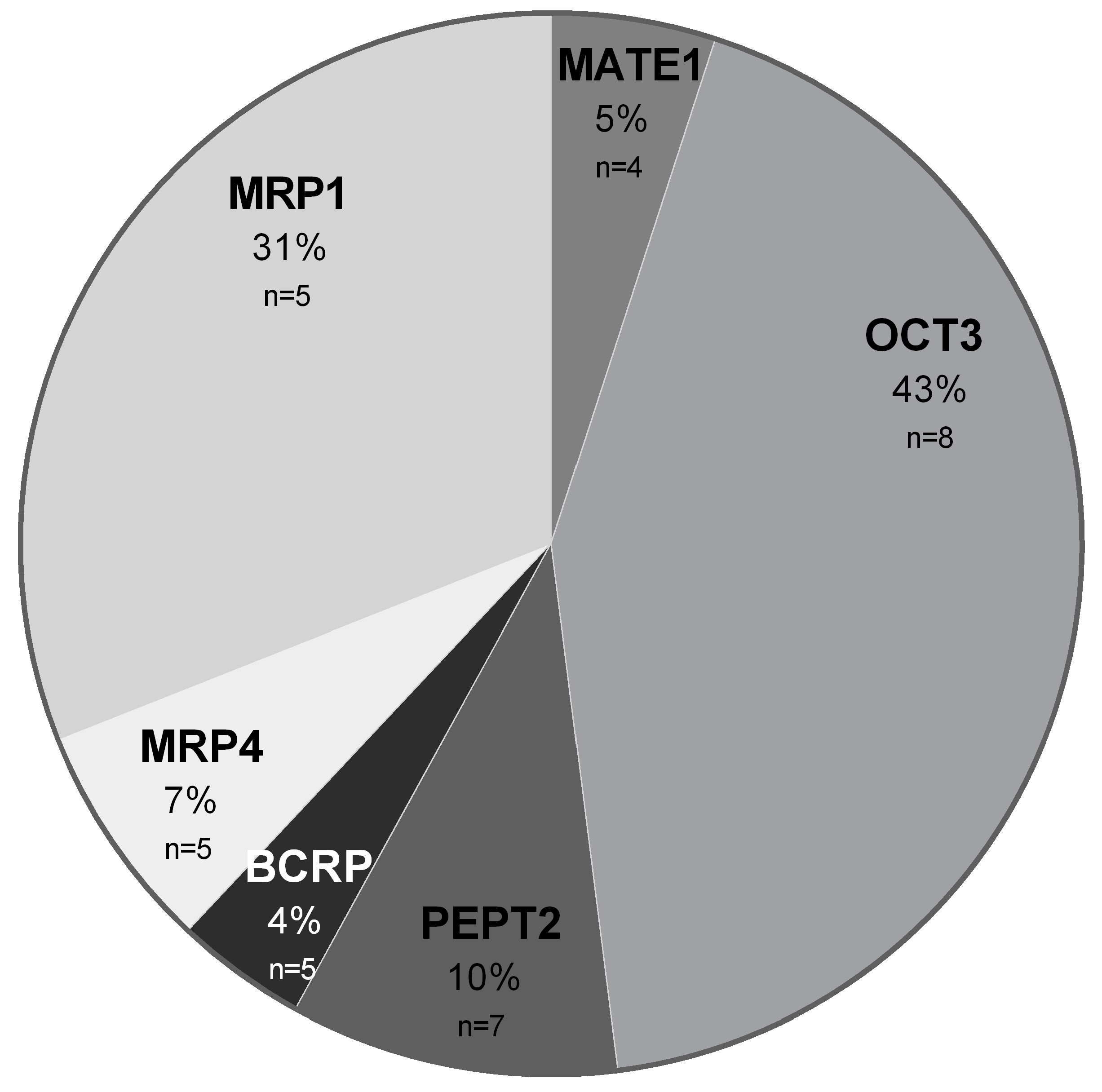
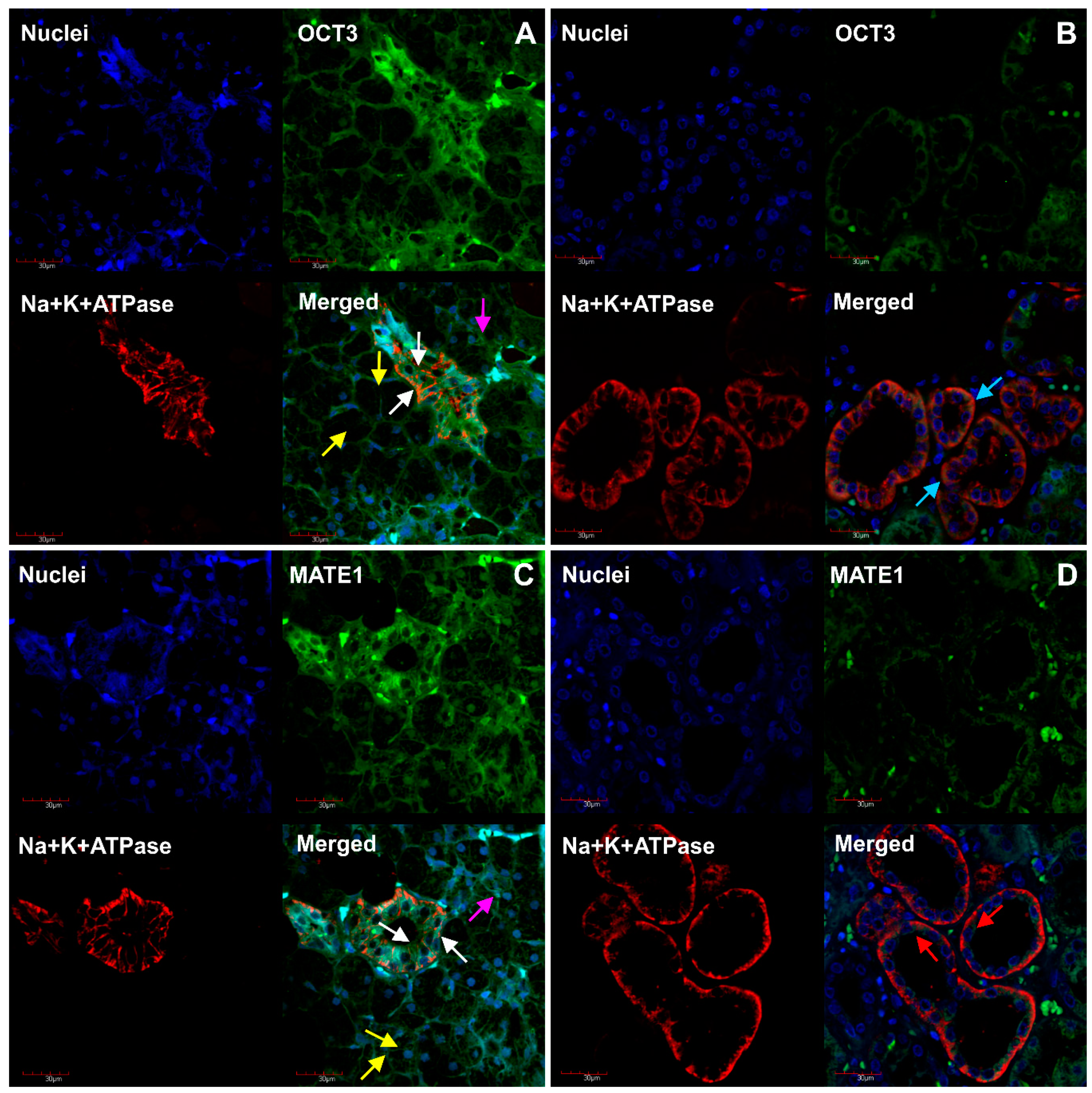
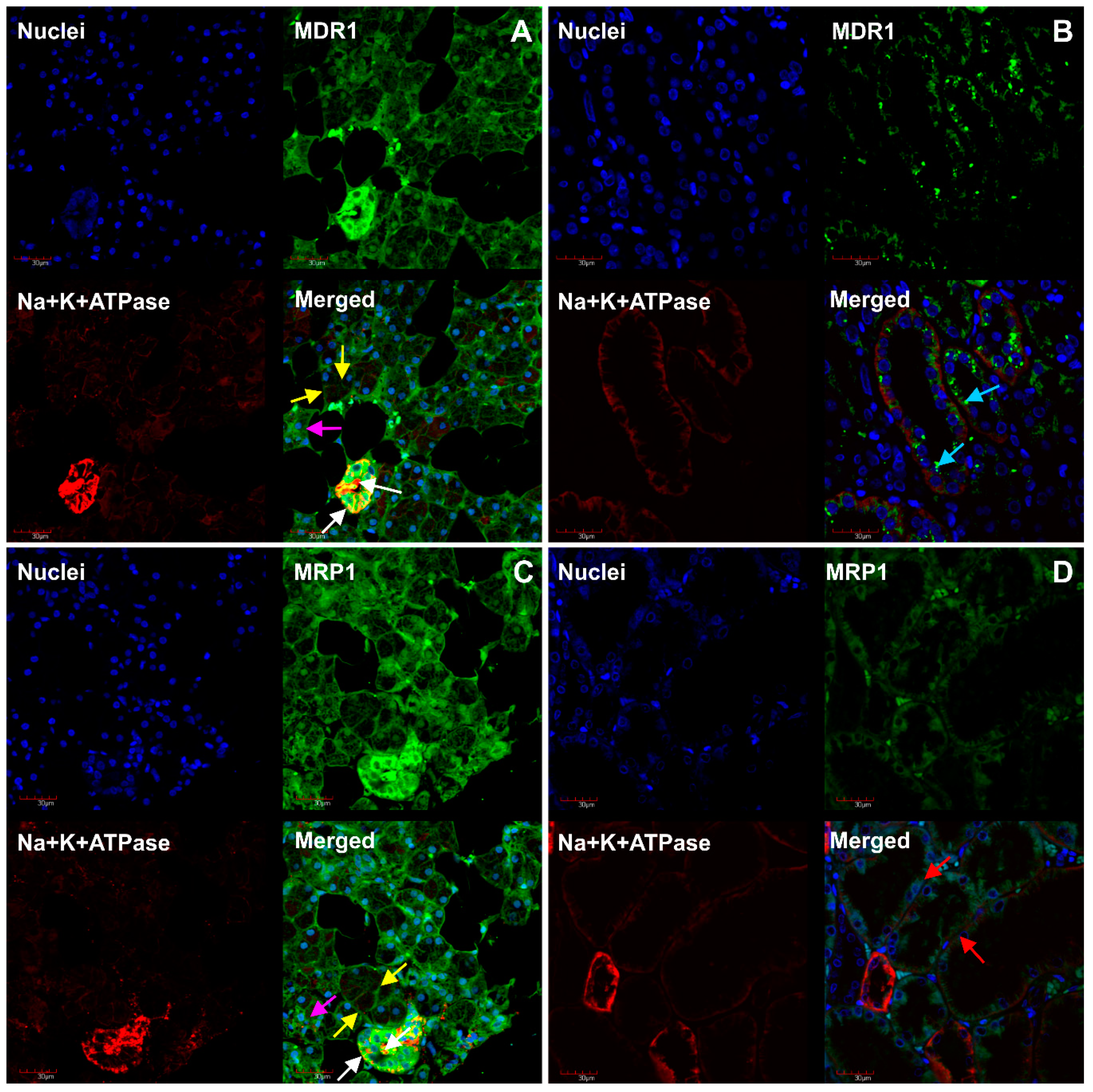
2. Results
3. Discussion
4. Materials and Methods
4.1. Parotid Gland Specimens
4.2. mRNA Isolation and Quantitative Real Time PCR
4.3. Protein Quantification Using LC–MS/MS
4.4. Immunofluorescent Analysis of MDR1, MRP1, OCT3, MATE1 and Na+/K+-ATPase
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genom. 2009, 3, 281–290. [Google Scholar] [CrossRef]
- Zamek-Gliszczynski, M.J.; Taub, M.E.; Chothe, P.P.; Chu, X.; Giacomini, K.M.; Kim, R.B.; Ray, A.S.; Stocker, S.L.; Unadkat, J.D.; Wittwer, M.B.; et al. Transporters in drug development: 2018 ITC recommendations for transporters of emerging clinical importance. Clin. Pharm. Ther. 2018, 104, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Catalán, M.A.; Nakamoto, T.; Melvin, J.E. The salivary gland fluid secretion mechanism. J. Med. Investig. 2009, 56, 192–196. [Google Scholar] [CrossRef]
- Roussa, E. Channels and transporters in salivary glands. Cell Tissue Res. 2011, 343, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Naito, S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab. Pharm. 2005, 20, 452–477. [Google Scholar] [CrossRef]
- Sun, Q.F.; Sun, Q.H.; Du, J.; Wang, S. Differential gene expression profiles of normal human parotid and submandibular glands. Oral Dis. 2008, 14, 500–509. [Google Scholar] [CrossRef]
- Drozdzik, M.; Mysliwiec, K.; Lewinska-Chelstowska, M.; Banach, J.; Drozdzik, A.; Grabarek, J. P-glycoprotein drug transporter MDR1 gene polymorphism in renal transplant patients with and without gingival overgrowth. J. Clin. Periodontol. 2004, 31, 758–763. [Google Scholar] [CrossRef]
- Uematsu, T.; Yamaoka, M.; Matsuura, T.; Doto, R.; Hotomi, H.; Yamada, A.; Hasumi-Nakayama, Y.; Kayamoto, D. P-glycoprotein expression in human major and minor salivary glands. Arch. Oral Biol. 2001, 46, 521–527. [Google Scholar] [CrossRef]
- Uematsu, T.; Yamaoka, M.; Doto, R.; Tanaka, H.; Matsuura, T.; Furusawa, K. Expression of ATP-binding cassette transporter in human salivary ducts. Arch. Oral Biol. 2003, 48, 87–90. [Google Scholar] [CrossRef]
- Ikarashi, R.; Shibasaki, K.; Yamaguchi, A. Immunohistochemical studies of organic anion transporters and urate transporter 1 expression in human salivary gland. Acta Odontol. Scand. 2013, 71, 312–316. [Google Scholar] [CrossRef]
- Lee, N.; Duan, H.; Hebert, M.F.; Liang, C.J.; Rice, K.M.; Wang, J. Taste of a pill: Organic cation transporter-3 (OCT3) mediates metformin accumulation and secretion in salivary glands. J. Biol. Chem. 2014, 289, 27055–27064. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C.; Wong, D.T.W. Role of saliva and salivary diagnostics in the advancement of oral health. J Dent. Res. 2019, 98, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, H.; Bailey, D.G.; Dresser, G.K.; Gregor, J.C.; Schwarz, U.I.; McGrath, J.S.; Jolicoeur, E.; Lee, W.; Leake, B.F.; Tirona, R.G.; et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin. Pharmacol. Ther. 2007, 81, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Drozdzik, M.; Gröer, C.; Penski, J.; Lapczuk, J.; Ostrowski, M.; Lai, Y.; Prasad, B.; Unadkat, J.D.; Siegmund, W.; Oswald, S. Protein abundance of clinically relevant multidrug transporters along the entire length of the human intestine. Mol. Pharm. 2014, 11, 3547–3555. [Google Scholar] [CrossRef] [PubMed]
- Bruckmueller, H.; Martin, P.; Kähler, M.; Haenisch, S.; Ostrowski, M.; Drozdzik, M.; Siegmund, W.; Cascorbi, I.; Oswald, S. Clinically relevant multidrug transporters are regulated by microRNAs along the human intestine. Mol. Pharm. 2017, 14, 2245–2253. [Google Scholar] [CrossRef]
- Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M.L.; Remião, F. Cellular Models and In Vitro Assays for the Screening of modulators of P-gp, MRP1 and BCRP. Molecules 2017, 22, 600. [Google Scholar] [CrossRef]
- Oliff, A.; Bleyer, W.A.; Poplack, D.G. Methotrexate-induced oral mucositis and salivary methotrexate concentrations. Cancer Chemother. Pharmacol. 1979, 2, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Celio, L.A.; DiGregorio, G.J.; Ruch, E.; Pace, J.; Piraino, A.J. Doxorubicin and 5-fluorouracil plasma concentrations and detectability in parotid saliva. Eur. J. Clin. Pharmacol. 1983, 24, 261–266. [Google Scholar] [CrossRef]
- Ritschel, W.A.; Bykadi, G.; Norman, E.J.; Cluxton, R.J.; Denton, D. Salivary elimination of cyclophosphamide in man. J. Clin. Pharmacol. 1981, 21, 461–465. [Google Scholar] [CrossRef]
- Piercy, E.A.; Bawdon, R.E.; Mackowiak, P.A. Penetration of ciprofloxacin into saliva and nasal secretions and effect of the drug on the oropharyngeal flora of ill subjects. Antimicrob. Agents Chemother. 1989, 33, 1645–1646. [Google Scholar] [CrossRef]
- MacPhee, D.J. Methodological considerations for improving Western blot analysis. J. Pharmcol. Toxicol. Methods 2010, 61, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kamiie, J.; Ohtsuki, S.; Iwase, R.; Ohmine, K.; Katsukura, Y.; Yanai, K.; Sekine, Y.; Uchida, Y.; Ito, S.; Terasaki, T. Quantitative atlas of membrane transporter proteins: Development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm. Res. 2008, 25, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, S.; Schaefer, O.; Kawakami, H.; Inoue, T.; Liehner, S.; Saito, A.; Ishiguro, N.; Kishimoto, W.; Ludwig Schwellinger, E.; Ebner, T.; et al. Simultaneous absolute protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases as a novel approach for the characterization of individual human liver: Comparison with mRNA levels and activities. Drug Metab. Dispos. 2012, 40, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Oswald, S.; Gröer, C.; Drozdzik, M.; Siegmund, W. Mass spectrometry-based targeted proteomics as a tool to elucidate the expression and function of intestinal drug transporters. AAPS J. 2013, 15, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.K.; Prasad, B. Critical Issues and Optimized Practices in Quantification of Protein Abundance Level to Determine Interindividual Variability in DMET Proteins by LC-MS/MS Proteomics. Clin. Pharmacol. Ther. 2018, 103, 619–630. [Google Scholar] [CrossRef]
- Gröer, C.; Brück, S.; Lai, Y.; Paulick, A.; Busemann, A.; Heidecke, C.D.; Siegmund, W.; Oswald, S. LC-MS/MS-based quantification of clinically relevant intestinal uptake and efflux transporter proteins. J. Pharm. Biomed. Anal. 2013, 85, 253–261. [Google Scholar] [CrossRef]
- Sakamoto, A.; Matsumaru, T.; Ishiguro, N.; Schaefer, O.; Ohtsuki, S.; Inoue, T.; Kawakami, H.; Terasaki, T. Reliability and robustness of simultaneous absolute quantification of drug transporters, cytochrome P450 enzymes, and Udp-glucuronosyltransferases in human liver tissue by multiplexed MRM/selected reaction monitoring mode tandem mass spectrometry with nano-liquid chromatography. J. Pharm. Sci. 2011, 100, 4037–4043. [Google Scholar]
- Gröer, C.; Busch, D.; Patrzyk, M.; Beyer, K.; Busemann, A.; Heidecke, C.D.; Drozdzik, M.; Siegmund, W.; Oswald, S. Absolute protein quantification of clinically relevant cytochrome P450 enzymes and UDP-glucuronosyltransferases by mass spectrometry-based targeted proteomics. J. Pharm. Biomed. Anal. 2014, 100, 393–401. [Google Scholar] [CrossRef]
- Kamal, M.A.; Keep, R.F.; Smith, D.E. Role and relevance of PEPT2 in drug disposition, dynamics, and toxicity. Drug Metab. Pharmacokinet. 2008, 23, 236–242. [Google Scholar] [CrossRef]
- Luckner, P.; Brandsch, M. Interaction of 31 beta-lactam antibiotics with the H+/peptide symporter PEPT2: Analysis of affinity constants and comparison with PEPT1. Eur. J. Pharm. Biopharm. 2005, 59, 17–24. [Google Scholar] [CrossRef]
- Idkaidek, N.; Arafat, T. Saliva versus plasma pharmacokinetics: Theory and application of a salivary excretion classification system. Mol. Pharm. 2012, 9, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Troeltzsch, M.; Pache, C.; Probst, F.A.; Troeltzsch, M.; Ehrenfeld, M.; Otto, S. Antibiotic concentrations in saliva: A systematic review of the literature, with clinical implications for the treatment of sialadenitis. J. Oral Maxillofac. Surg. 2014, 72, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kurzawski, M.; Szelag-Pieniazek, S.; Lapczuk, J.; Wrzesinski, M.; Sienko, J.; Oswald, S.; Drozdzik, M. The reference liver—ABC and SLC drug transporters in healthy donor and metastatic livers. Pharmacol. Rep. 2019, 71, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Nies, A.T.; Koepsell, H.; Winter, S.; Burk, O.; Klein, K.; Kerb, R.; Zanger, U.M.; Keppler, D.; Schwab, M.; Schaeffeler, E. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology 2009, 50, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Riches, Z.; Abanda, N.; Collier, A.C. BCRP protein levels do not differ regionally in adult human livers, but decline in the elderly. Chem. Biol. Interact. 2015, 242, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Deo, A.K.; Prasad, B.; Balogh, L.; Lai, Y.; Unadkat, J.D. Interindividual variability in hepatic expression of the multidrug resistance-associated protein 2 (MRP2/ABCC2): Quantification by liquid chromatography/tandem mass spectrometry. Drug Metab. Dispos. 2012, 40, 852–855. [Google Scholar] [CrossRef]
- Urquhart, B.L.; Ware, J.A.; Tirona, R.G.; Ho, R.H.; Leake, B.F.; Schwarz, U.I.; Zaher, H.; Palandra, J.; Gregor, J.C.; Dresser, G.K.; et al. Breast cancer resistance protein (ABCG2) and drug disposition: Intestinal expression, polymorphisms and sulfasalazine as an in vivo probe. Pharmacogenet. Genom. 2008, 18, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Aslanian, A.; Yates, J.R. Mass spectrometry for proteomics. Curr. Opin. Chem. Biol. 2008, 12, 483–490. [Google Scholar] [CrossRef]
- Bartnicka, L.; Kurzawski, M.; Drozdzik, A.; Plonska-Gosciniak, E.; Gornik, W.; Drozdzik, M. Effect of ABCB1 (MDR1) 3435C >T and 2677G >A,T polymorphisms and P-glycoprotein inhibitors on salivary digoxin secretion in congestive heart failure patients. Pharmacol. Rep. 2007, 59, 323–329. [Google Scholar]
- Schaiquevich, P.; Viviana, N.; Omar, T.; Modesto, R. Evaluation of acetaminophen P-glycoprotein-mediated salivary secretion by rat submandibular glands. Arch. Oral Biol. 2004, 49, 895–901. [Google Scholar] [CrossRef]
- Wagner, D.J.; Hu, T.; Wang, J. Polyspecific organic cation transporters and their impact on drug intracellular levels and pharmacodynamics. Pharmacol. Res. 2016, 111, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Mineshita, S.; Uchiyama, Y.; Shudo, I.; Shimamura, K.; Togashi, H.; Saito, H. Monitoring of procainamide and N-acetylprocainamide concentration in saliva after oral administration of procainamide. Am. J. Ther. 1996, 3, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.J.; Shireman, L.M.; Ahn, S.; Shen, D.D.; Wang, J. Disposition of methamphetamine and major metabolites in mice: Role of organic cation transporter 3 in tissue-selective accumulation of para-hydroxymethamphetamine. Drug Metab. Dispos. 2018, 46, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Dobson, N.R.; Liu, X.; Rhein, L.M.; Darnall, R.A.; Corwin, M.J.; McEntire, B.L.; Ward, R.M.; James, L.P.; Sherwin, C.M.; Heeren, T.C.; et al. Salivary caffeine concentrations are comparable to plasma concentrations in preterm infants receiving extended caffeine therapy. Br. J. Clin. Pharmacol. 2016, 82, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Stegaev, V.; Nies, A.T.; Porola, P.; Mieliauskaite, D.; Sánchez-Jiménez, F.; Urdiales, J.L.; Sillat, T.; Schwelberger, H.G.; Chazot, P.L.; Katebe, M.; et al. Histamine transport and metabolism are deranged in salivary glands in Sjogren’s syndrome. Rheumatology 2013, 52, 1599–1608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mullangi, R.; Agrawal, S.; Srinivas, N.R. Measurement of xenobiotics in saliva: Is saliva an attractive alternative matrix? Case studies and analytical perspectives. Biomed. Chromatogr. 2009, 23, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Luo, J.; Huang, W.; Tang, J.; Zhou, H.; Zhang, W. The Pharmacological and Physiological Role of Multidrug-Resistant Protein 4. J. Pharmacol. Exp. Ther. 2015, 354, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Kiang, T.K.; Ensom, M.H. A Qualitative Review on the Pharmacokinetics of Antibiotics in Saliva: Implications on Clinical Pharmacokinetic Monitoring in Humans. Clin. Pharmacokinet. 2016, 55, 313–358. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, L.; Sinclair, M.; Reid, B.; Burnett, K.; Callan, B. A descriptive systematic review of salivary therapeutic drug monitoring in neonates and infants. Br. J. Clin. Pharmacol. 2018, 84, 1089–1108. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Scialis, R.J.; Lehman-McKeeman, L. Xenobiotic Transporters in the Kidney: Function and Role in Toxicity. Semin. Nephrol. 2019, 39, 159–175. [Google Scholar] [CrossRef] [PubMed]
| mRNA vs. Protein | Salivary Gland |
|---|---|
| ABCC1 | 0.305 |
| ABCC4 | 0.392 |
| ABCG2 | −0.783 |
| PEPT2 | 0.000 |
| OCT3 | −0.133 |
| MATE1 | 0.511 |
| Na+/K+ATPase | 0.017 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapczuk-Romanska, J.; Busch, D.; Gieruszczak, E.; Drozdzik, A.; Piotrowska, K.; Kowalczyk, R.; Oswald, S.; Drozdzik, M. Membrane Transporters in Human Parotid Gland-Targeted Proteomics Approach. Int. J. Mol. Sci. 2019, 20, 4825. https://doi.org/10.3390/ijms20194825
Lapczuk-Romanska J, Busch D, Gieruszczak E, Drozdzik A, Piotrowska K, Kowalczyk R, Oswald S, Drozdzik M. Membrane Transporters in Human Parotid Gland-Targeted Proteomics Approach. International Journal of Molecular Sciences. 2019; 20(19):4825. https://doi.org/10.3390/ijms20194825
Chicago/Turabian StyleLapczuk-Romanska, Joanna, Diana Busch, Ewa Gieruszczak, Agnieszka Drozdzik, Katarzyna Piotrowska, Robert Kowalczyk, Stefan Oswald, and Marek Drozdzik. 2019. "Membrane Transporters in Human Parotid Gland-Targeted Proteomics Approach" International Journal of Molecular Sciences 20, no. 19: 4825. https://doi.org/10.3390/ijms20194825
APA StyleLapczuk-Romanska, J., Busch, D., Gieruszczak, E., Drozdzik, A., Piotrowska, K., Kowalczyk, R., Oswald, S., & Drozdzik, M. (2019). Membrane Transporters in Human Parotid Gland-Targeted Proteomics Approach. International Journal of Molecular Sciences, 20(19), 4825. https://doi.org/10.3390/ijms20194825






