Type XVIII Collagen Modulates Keratohyalin Granule Formation and Keratinization in Oral Mucosa
Abstract
1. Introduction
2. Results
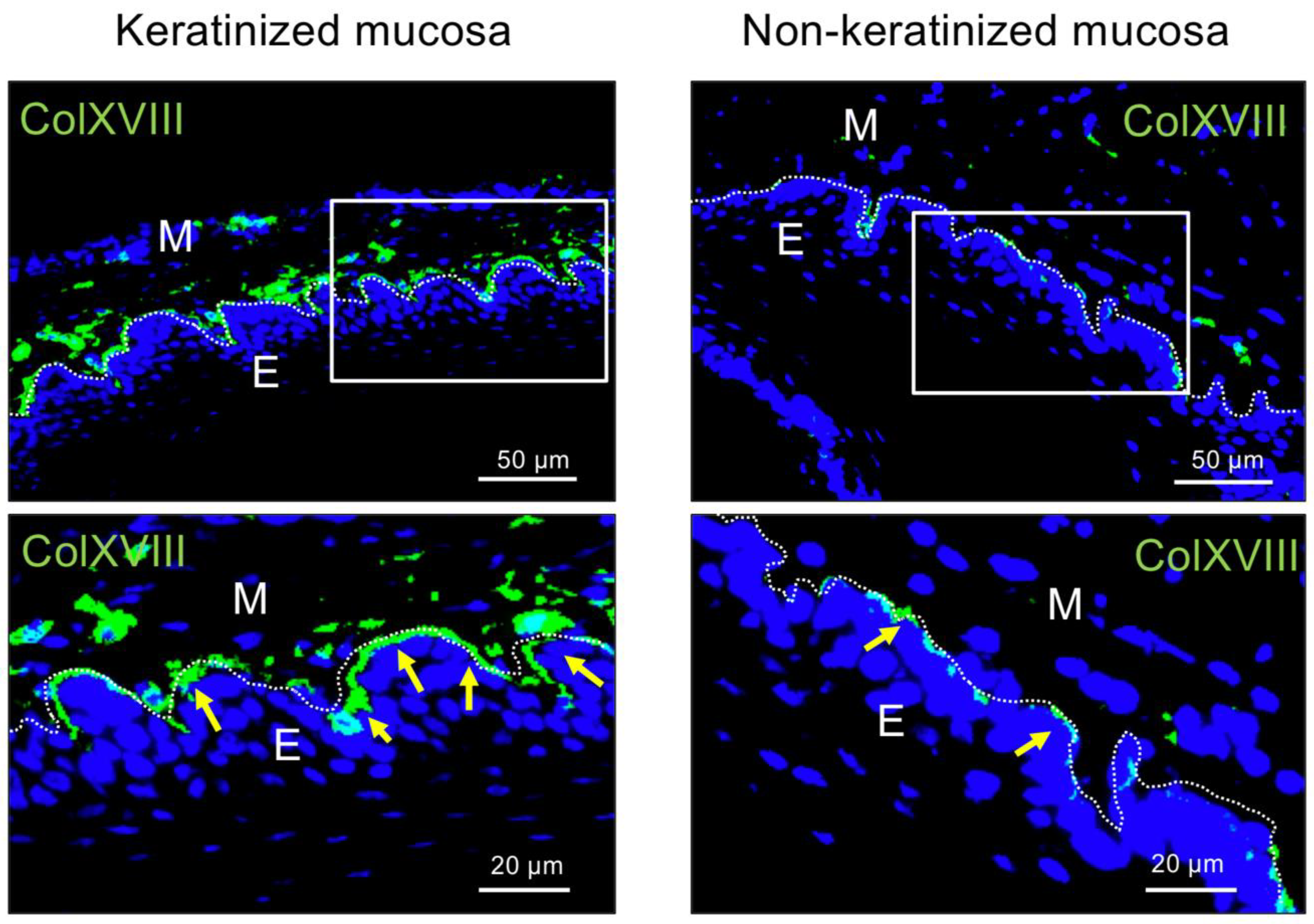
2.1. Immunohistochemical Analysis of Type XVIII Collagen in Bms of Oral Mucosa
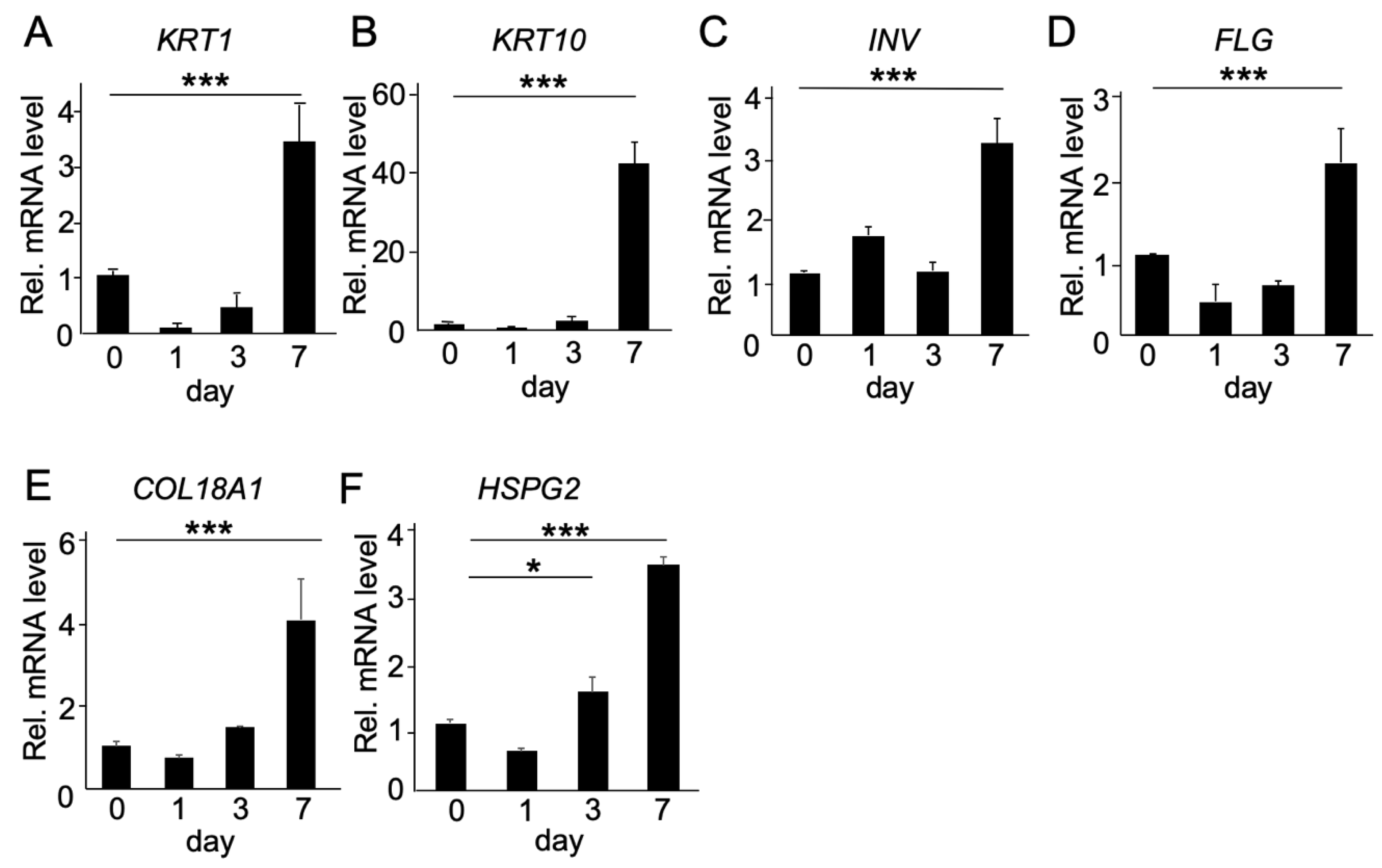
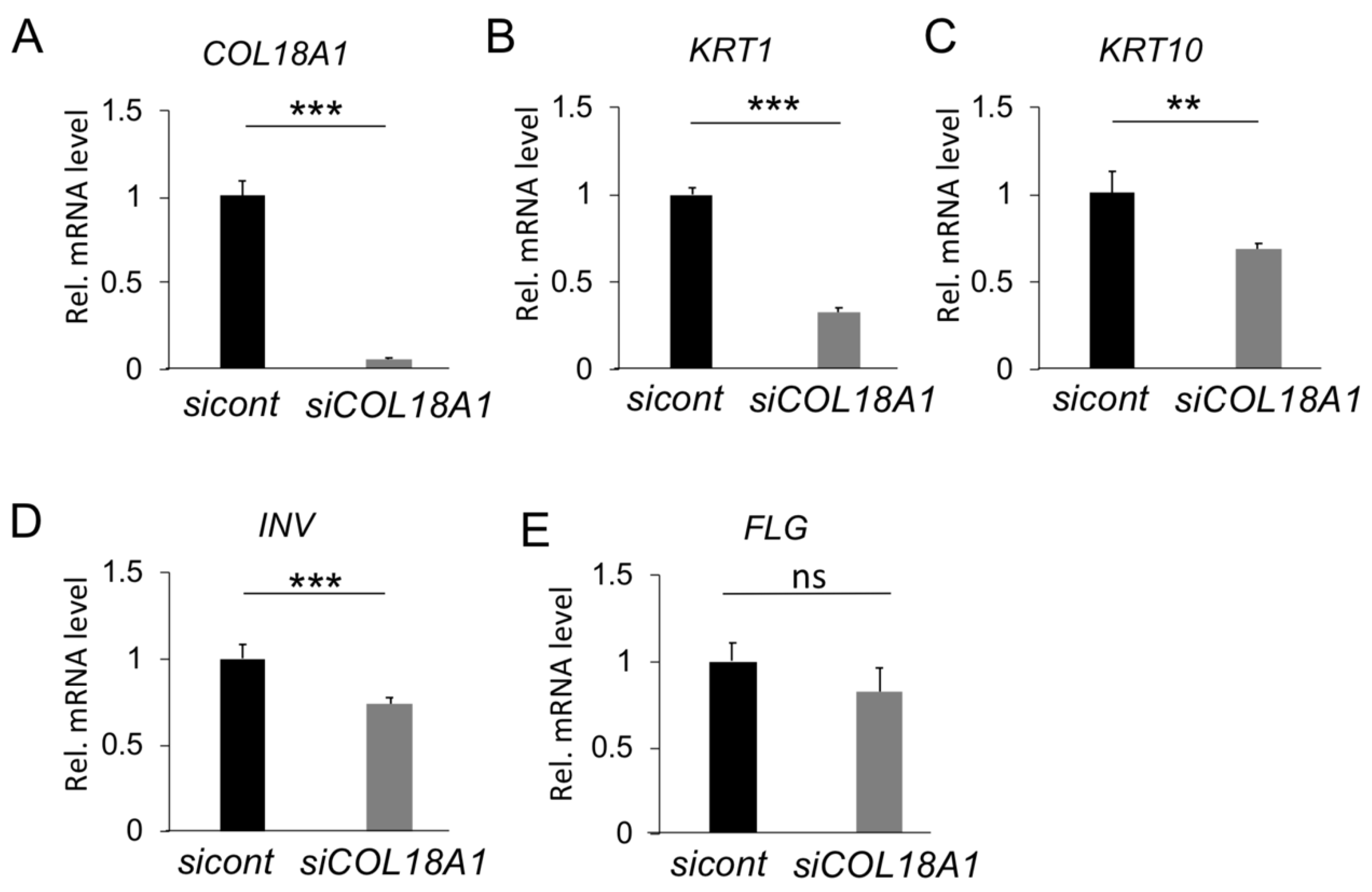
2.2. Functional Analysis of COL18A1 Gene In Vitro
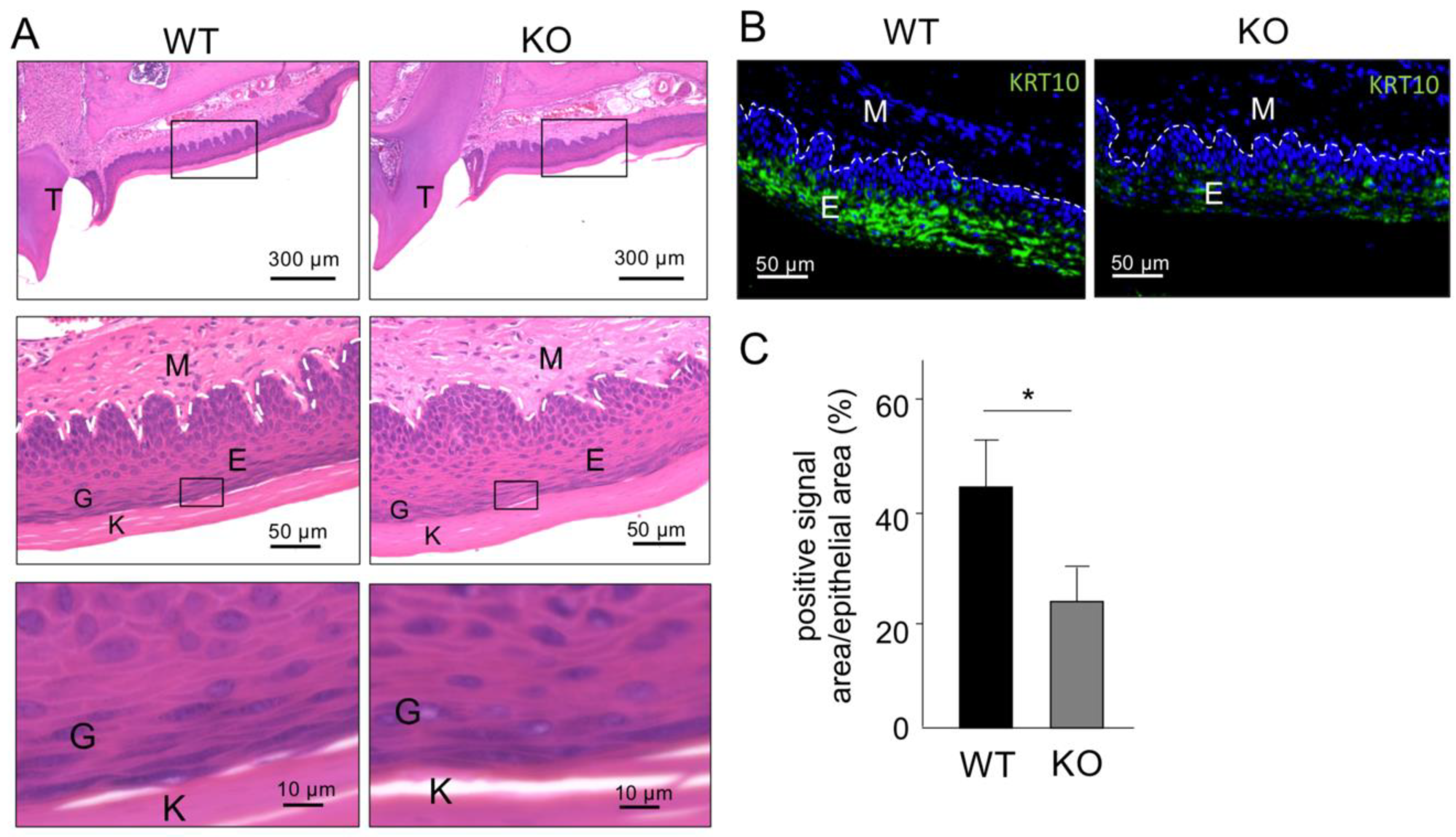
2.3. Histological Analysis of Keratinized Oral Mucosa between Wide-Type and Col18-KO Mice
2.4. Ultrastructural Analysis of Keratinized Oral Mucosa between Wide-Type and Col18-KO Mice
3. Discussion
4. Materials and Methods
4.1. Cells and Culture Methods
4.2. Real-Time RT PCR Analysis
4.3. Animals
4.4. Hematoxylin and EOSIN Staining
4.5. Immunohistochemical Staining
4.6. Transmission Electronic Microscope
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Squier, C.A.; Kremer, M.J. Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 2001, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Cruchley, A.T.; Bergmeier, L.A. Structure and Functions of the Oral Mucosa. In Oral Mucosa in Health and Disease; Bergmeier, L., Ed.; Springer Int Publishing: Cham, Switzerland, 2018; pp. 1–18. [Google Scholar]
- Squier, C.A.; Brogden, K.A. Human Oral Mucosa: Development, Structure, and Function; Wiley-Blackwell: Chichester, UK, 2011; pp. 9–10. [Google Scholar]
- Adams, D. Keratinization of the oral epithelium. Ann. R. Coll. Surg. Engl. 1976, 58, 351–358. [Google Scholar] [PubMed]
- Brito, C.; Tenenbaum, H.C.; Wong, B.K.; Schmitt, C.; Nogueira-Filho, G. Is keratinized mucosa indispensable to maintain peri-implant health? A systematic review of the literature. J. Biomed. Mater. Res. B. Appl. Biomater. 2014, 102, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, R.G.; Rana, A.; Sarkar, A. Gingival Biotype Assessment in a Healthy Periodontium: Transgingival Probing Method. J. Clin. Diagn. Res. 2015, 9, ZC66–ZC69. [Google Scholar] [PubMed]
- Moraschini, V.; Luz, D.; Velloso, G.; Barboza, E.D.P. Quality assessment of systematic reviews of the significance of keratinized mucosa on implant health. Int. J. Oral Maxillofac. Surg. 2017, 46, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.W.; Lee, S.Y.; Lin, Y.C.; Lai, Y.L. Significance of the width of keratinized mucosa on peri-implant health. J. Chin. Med. Assoc. 2015, 78, 389–394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calenic, B.; Greabu, M.; Caruntu, C.; Tanase, C.; Battino, M. Oral keratinocyte stem/progenitor cells: Specific markers, molecular signaling pathways and potential uses. Periodontol 2000 2015, 69, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Ono, M.; Hara, E.S.; Ueda, J.; Nguyen, H.T.T.; Nguyen, H.T.; Yonezawa, T.; Maeba, T.; Kimura-Ono, A.; Takarada, T.; et al. Type IV collagen alpha6 chain is a regulator of keratin 10 in keratinization of oral mucosal epithelium. Sci. Rep. 2018, 8, 2612. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, M. Basement membrane proteins: Structure, assembly, and cellular interactions. Crit. Rev. Biochem. Mol. Biol. 1992, 27, 93–127. [Google Scholar] [PubMed]
- Yurchenco, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 57, 1–11. [Google Scholar] [CrossRef]
- Halfter, W.; Oertle, P.; Monnier, C.A.; Camenzind, L.; Reyes-Lua, M.; Hu, H.; Candiello, J.; Labilloy, A.; Balasubramani, M.; Henrich, P.B.; et al. New concepts in basement membrane biology. FEBS J. 2015, 282, 4466–4479. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, U.; Couchman, J.; Kimata, K.; Esko, J.D. Proteoglycans and Sulfated Glycosaminoglycans. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017. [Google Scholar]
- Groffen, A.J.; Veerkamp, J.H.; Monnens, L.A.; van den Heuvel, L.P. Recent insights into the structure and functions of heparan sulfate proteoglycans in the human glomerular basement membrane. Nephrol. Dial. Transplant. 1999, 14, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, C.; Mayer, U.; Aumailley, M.; Timpl, R. Basement-membrane heparan sulfate proteoglycan binds to laminin by its heparan sulfate chains and to nidogen by sites in the protein core. Eur. J. Biochem. 1992, 208, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Costell, M.; Gustafsson, E.; Aszódi, A.; Mörgelin, M.; Bloch, W.; Hunziker, E.; Addicks, K.; Timpl, R.; Fässler, R. Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 1999, 147, 1109–1122. [Google Scholar] [CrossRef]
- Martinez, J.R.; Grindel, B.J.; Hubka, K.M.; Dodge, G.R.; Farach-Carson, M.C. Perlecan/HSPG2: Signaling role of domain IV in chondrocyte clustering with implications for Schwartz-Jampel Syndrome. J. Cell Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Halfter, W.; Dong, S.; Schurer, B.; Cole, G.J. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J. Biol. Chem. 1998, 273, 25404–25412. [Google Scholar] [CrossRef]
- Mahajan, V.B.; Olney, A.H.; Garrett, P.; Chary, A.; Dragan, E.; Lerner, G.; Murray, J.; Bassuk, A.G. Collagen XVIII mutation in Knobloch syndrome with acute lymphoblastic leukemia. Am. J. Med. Genet. A 2010, 152A, 2875–2879. [Google Scholar] [CrossRef] [PubMed]
- Utriainen, A.; Sormunen, R.; Kettunen, M.; Carvalhaes, L.S.; Sajanti, E.; Eklund, L.; Kauppinen, R.; Kitten, G.T.; Pihlajaniemi, T. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum. Mol. Genet. 2004, 13, 2089–2099. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef]
- Seppinen, L.; Pihlajaniemi, T. The multiple functions of collagen XVIII in development and disease. Matrix Biol. 2011, 30, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Seppinen, L.; Sormunen, R.; Soini, Y.; Elamaa, H.; Heljasvaara, R.; Pihlajaniemi, T. Lack of collagen XVIII accelerates cutaneous wound healing, while overexpression of its endostatin domain leads to delayed healing. Matrix Biol. 2008, 27, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Loe, H. The relationship between the width of keratinized gingiva and gingival health. J. Periodontol. 1972, 43, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Bouri, A., Jr.; Bissada, N.; Al-Zahrani, M.S.; Faddoul, F.; Nouneh, I. Width of keratinized gingiva and the health status of the supporting tissues around dental implants. Int. J. Oral Maxillofac. Implant. 2008, 23, 323–326. [Google Scholar]
- Schwarzbauer, J. Basement membranes: Putting up the barriers. Curr. Biol. 1999, 9, R242–R244. [Google Scholar] [CrossRef]
- Morrissey, M.A.; Sherwood, D.R. An active role for basement membrane assembly and modification in tissue sculpting. J. Cell Sci. 2015, 128, 1661–1668. [Google Scholar] [CrossRef]
- Dos Santos, M.; Michopoulou, A.; Andre-Frei, V.; Boulesteix, S.; Guicher, C.; Dayan, G.; Whitelock, J.; Damour, O.; Rousselle, P. Perlecan expression influences the keratin 15-positive cell population fate in the epidermis of aging skin. Aging (Albany NY) 2016, 8, 751–768. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.C.; Sonthalia, S. Histology, Keratohyalin Granules. In StatPearls [Internet]; StatPearls Pulishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Fukai, N.; Eklund, L.; Marneros, A.G.; Oh, S.P.; Keene, D.R.; Tamarkin, L.; Niemela, M.; Ilves, M.; Li, E.; Pihlajaniemi, T.; et al. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 2002, 21, 1535–1544. [Google Scholar] [CrossRef]
- Elamaa, H.; Snellman, A.; Rehn, M.; Autio-Harmainen, H.; Pihlajaniemi, T. Characterization of the human type XVIII collagen gene and proteolytic processing and tissue location of the variant containing a frizzled motif. Matrix Biol. 2003, 22, 427–442. [Google Scholar] [CrossRef]
- Muragaki, Y.; Timmons, S.; Griffith, C.M.; Oh, S.P.; Fadel, B.; Quertermous, T.; Olsen, B.R. Mouse Col18a1 is expressed in a tissue specific manner as three alternative variants and is localized in basement membrane zones. Proc. Natl. Acad. Sci. USA 1995, 92, 8763–8767. [Google Scholar] [CrossRef] [PubMed]
- Saarela, J.; Rehn, M.; Oikarinen, A.; Autio-Harmainen, H.; Pihlajaniemi, T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am. J. Pathol. 1998, 153, 611–626. [Google Scholar] [CrossRef]
- Heljasvaara, R.; Aikio, M.; Ruotsalainen, H.; Pihlajaniemi, T. Collagen XVIII in tissue homeostasis and dysregulation—Lessons learned from model organisms and human patients. Matrix Biol. 2017, 57, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Maeba, T.; Yonezawa, T.; Ono, M.; Heljasvaara, R.; Pihlajaniemi, T.; Inagawa, K.; Oohashi, T. Collagen XVIII deposition in the basement membrane zone beneath the newly forming epidermis during wound healing in mice. Acta Med. Okayama 2019, 73, 135–146. [Google Scholar] [PubMed]
- Kinnunen, A.I.; Sormunen, R.; Elamaa, H.; Seppinen, L.; Miller, R.T.; Ninomiya, Y.; Janmey, P.A.; Pihlajaniemi, T. Lack of collagen XVIII long isoforms affects kidney podocytes, whereas the short form is needed in the proximal tubular basement membrane. J. Biol. Chem. 2011, 286, 7755–7764. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.R. Life without perlecan has its problems. J. Cell Biol. 1999, 147, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Whitelock, J.M.; Melrose, J.; Iozzo, R.V. Diverse cell signaling events modulated by perlecan. Biochemistry 2008, 47, 11174–11183. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, G.; Eichstetter, I.; Iozzo, R.V. A role for the perlecan protein core in the activation of the keratinocyte growth factor receptor. Biochem. J. 2001, 359, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ettner, N.; Göhring, W.; Sasaki, T.; Mann, K.; Timpl, R. The N-terminal globular domain of the laminin alpha1 chain binds to alpha1beta1 and alpha2beta1 integrins and to the heparan sulfate-containing domains of perlecan. FEBS Lett. 1998, 430, 217–221. [Google Scholar] [CrossRef]
- Brown, J.C.; Sasaki, T.; Göhring, W.; Yamada, Y.; Timpl, R. The C-terminal domain V of perlecan promotes beta1 integrin-mediated cell adhesion, binds heparin, nidogen and fibulin-2 and can be modified by glycosaminoglycans. Eur. J. Biochem. 1997, 250, 39–46. [Google Scholar] [CrossRef]
- Bengtsson, E.; Mörgelin, M.; Sasaki, T.; Timpl, R.; Heinegård, D.; Aspberg, A. The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J. Biol. Chem. 2002, 277, 15061–15068. [Google Scholar] [CrossRef] [PubMed]
- Arikawa-Hirasawa, E.; Watanabe, H.; Takami, H.; Hassell, J.R.; Yamada, Y. Perlecan is essential for cartilage and cephalic development. Nat. Genet. 1999, 23, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Morita, H.; Sormunen, R.; Airenne, S.; Kreivi, M.; Wang, L.; Fukai, N.; Olsen, B.R.; Tryggvason, K.; Soininen, R. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003, 22, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Gatseva, A.; Sin, Y.Y.; Brezzo, G.; Agtmael, T.V. Basement membrane collagens and disease mechanisms. Essays Biochem. 2019, 63, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, R.; Yamada, K.M. Basement Membranes in Development and Disease. Curr. Top. Dev. Biol. 2018, 130, 143–191. [Google Scholar] [PubMed]
- Sher, I.; Zisman-Rozen, S.; Eliahu, L.; Whitelock, J.M.; Maas-Szabowski, N.; Yamada, Y.; Breitkreutz, D.; Fusenig, N.E.; Arikawa-Hirasawa, E.; Iozzo, R.V.; et al. Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation. J. Biol. Chem. 2006, 281, 5178–5187. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Fukai, N.; Mann, K.; Göhring, W.; Olsen, B.R.; Timpl, R. Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 1998, 17, 4249–4256. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Féraud, O.; Lortat-Jacob, H.; Rencurosi, A.; Fukai, N.; Dkhissi, F.; Vittet, D.; Imberty, A.; Olsen, B.R.; van der Rest, M. Characterization of endostatin binding to heparin and heparan sulfate by surface plasmon resonance and molecular modeling: Role of divalent cations. J. Biol. Chem. 2004, 279, 2927–2936. [Google Scholar] [CrossRef] [PubMed]
- Miosge, N.; Simniok, T.; Sprysch, P.; Herken, R. The collagen type XVIII endostatin domain is co-localized with perlecan in basement membranes in vivo. J. Histochem. & Cytochem. 2003, 51, 285–296. [Google Scholar]
- Tomono, Y.; Naito, I.; Ando, K.; Yonezawa, T.; Sado, Y.; Hirakawa, S.; Arata, J.; Okigaki, T.; Ninomiya, Y. Epitope-defined monoclonal antibodies against multiplexin collagens demonstrate that type XV and XVIII collagens are expressed in specialized basement membranes. Cell Struct. Funct. 2002, 27, 9–20. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Encoded Protein | Type | GeneBank Registration Number | Primer Set |
|---|---|---|---|---|
| S29 | Ribosome protein S29 | Human | BC032813 | 5′-TCTCGCTCTTGTCGTGTCTGTTC-3′(S) |
| 5′-ACACTGGCGGCACATATTGAGG-3′(AS) | ||||
| COL18A1 | Type XVIII collagen | Human | BC063833 | 5′-TCCAGAGAATGCCGCTTG-3′(S) |
| 5′-GGAACTTGTCAGGGTCCG-3′(AS) | ||||
| KRT1 | Keratin 1 | Human | BC063697 | 5′-CTTACTCTACCTTGCTCCTACT-3′(S) |
| 5′-AAATCTCCCACCACCTCC-3′(AS) | ||||
| KRT10 | Keratin 10 | Human | NM_000421 | 5′-GCATCACCATGTCTGTTC-3′(S) |
| 5′-GCTAGAAATTCTTAGGGATGAC-3′(AS) | ||||
| INV | Involucrin | Human | BC046391 | 5′-CCTCAGATCGTCTCATACAAG-3′(S) |
| 5′-ACAGAGTCAAGTTCACAGATG -3′(AS) | ||||
| FLG | Filaggrin | Human | NM_002016 | 5′-AGACTCTAGTACCGCTAAGG-3′(S) |
| 5′-CGTGACTGTATTCCTGAGTG-3′(AS) | ||||
| HSPG2 | Perlecan | Human | M85289 | 5′-GCCTTCACTTCCAGATGG -3′(S) |
| 5′-CCACCCCAACTCTTACCA-3′(AS) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.T.T.; Ono, M.; Hara, E.S.; Komori, T.; Edamatsu, M.; Yonezawa, T.; Kimura-Ono, A.; Maekawa, K.; Kuboki, T.; Oohashi, T. Type XVIII Collagen Modulates Keratohyalin Granule Formation and Keratinization in Oral Mucosa. Int. J. Mol. Sci. 2019, 20, 4739. https://doi.org/10.3390/ijms20194739
Nguyen HTT, Ono M, Hara ES, Komori T, Edamatsu M, Yonezawa T, Kimura-Ono A, Maekawa K, Kuboki T, Oohashi T. Type XVIII Collagen Modulates Keratohyalin Granule Formation and Keratinization in Oral Mucosa. International Journal of Molecular Sciences. 2019; 20(19):4739. https://doi.org/10.3390/ijms20194739
Chicago/Turabian StyleNguyen, Ha Thi Thu, Mitsuaki Ono, Emilio Satoshi Hara, Taishi Komori, Midori Edamatsu, Tomoko Yonezawa, Aya Kimura-Ono, Kenji Maekawa, Takuo Kuboki, and Toshitaka Oohashi. 2019. "Type XVIII Collagen Modulates Keratohyalin Granule Formation and Keratinization in Oral Mucosa" International Journal of Molecular Sciences 20, no. 19: 4739. https://doi.org/10.3390/ijms20194739
APA StyleNguyen, H. T. T., Ono, M., Hara, E. S., Komori, T., Edamatsu, M., Yonezawa, T., Kimura-Ono, A., Maekawa, K., Kuboki, T., & Oohashi, T. (2019). Type XVIII Collagen Modulates Keratohyalin Granule Formation and Keratinization in Oral Mucosa. International Journal of Molecular Sciences, 20(19), 4739. https://doi.org/10.3390/ijms20194739






