AFM-based Analysis of Wharton’s Jelly Mesenchymal Stem Cells
Abstract
1. Introduction
2. Results
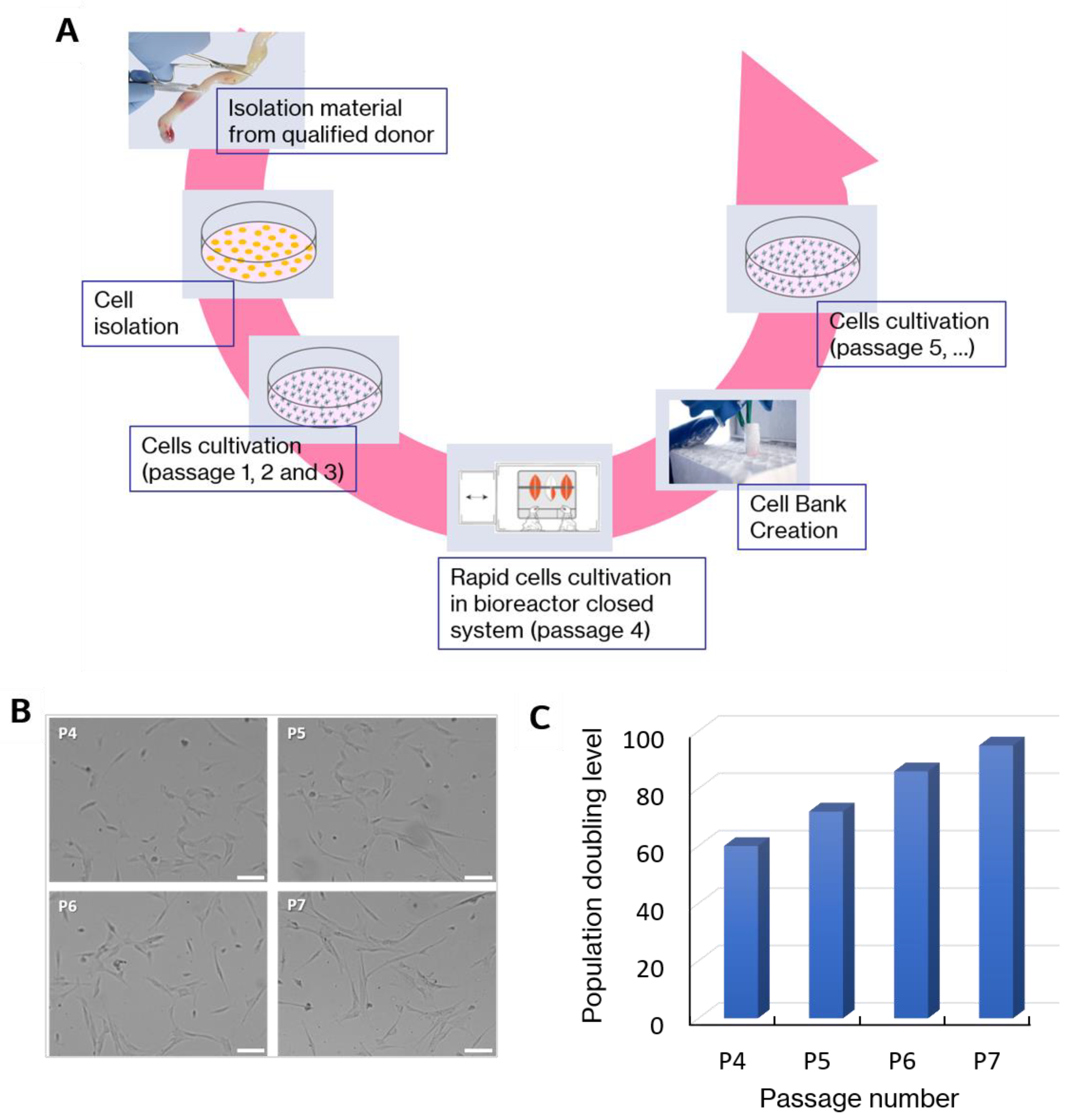
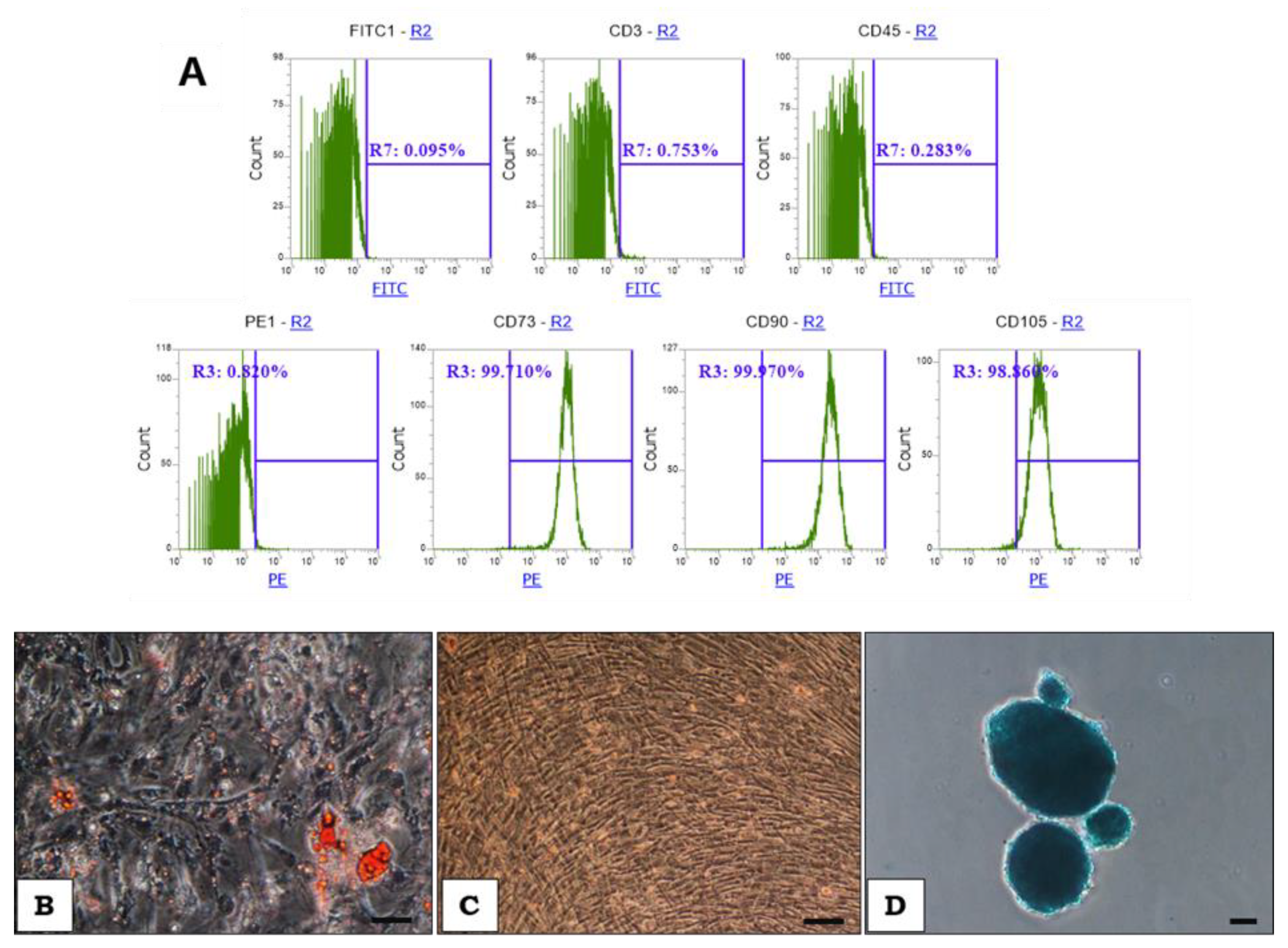
2.1. WJ-MSCs Manufacturing and Cell Characterization
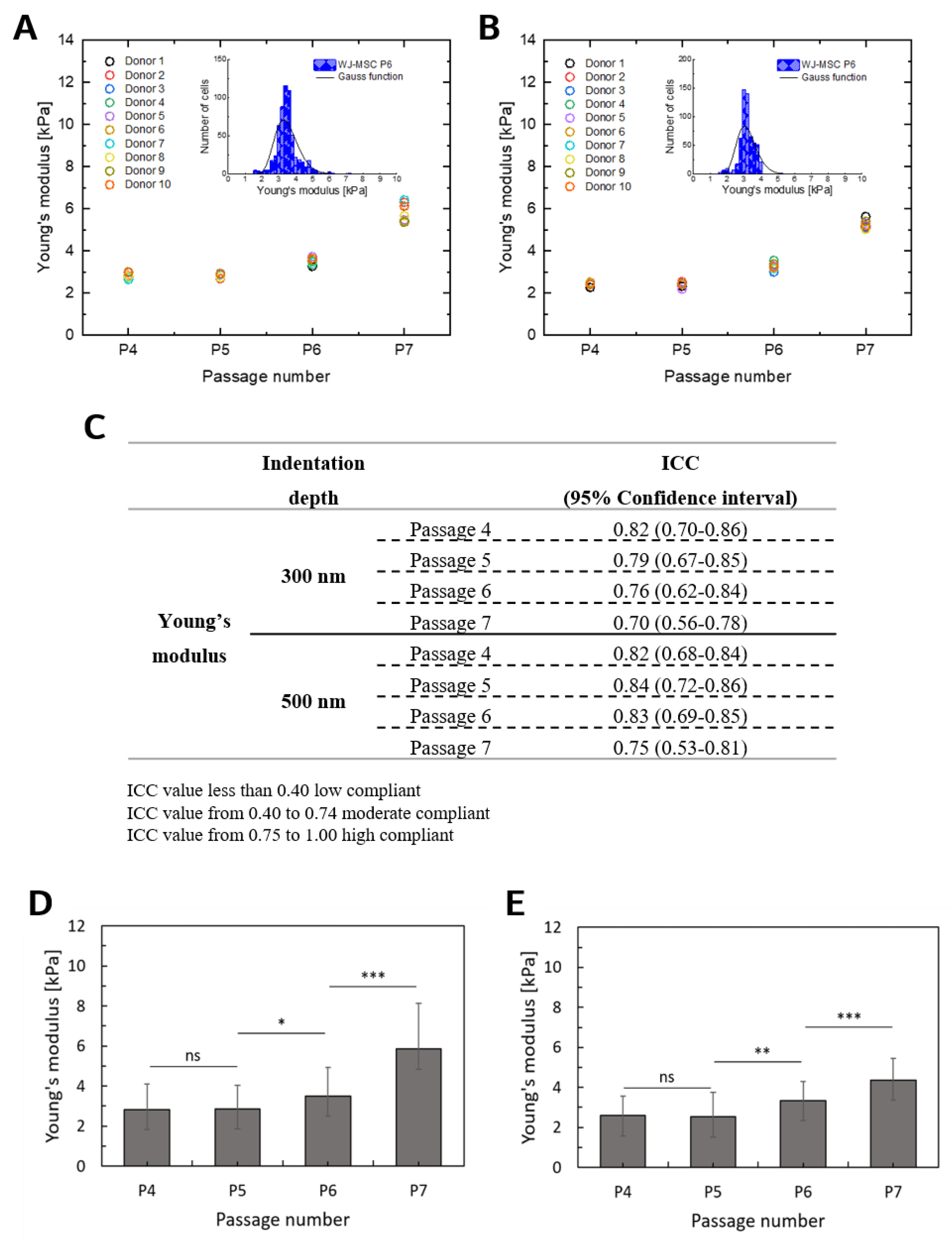
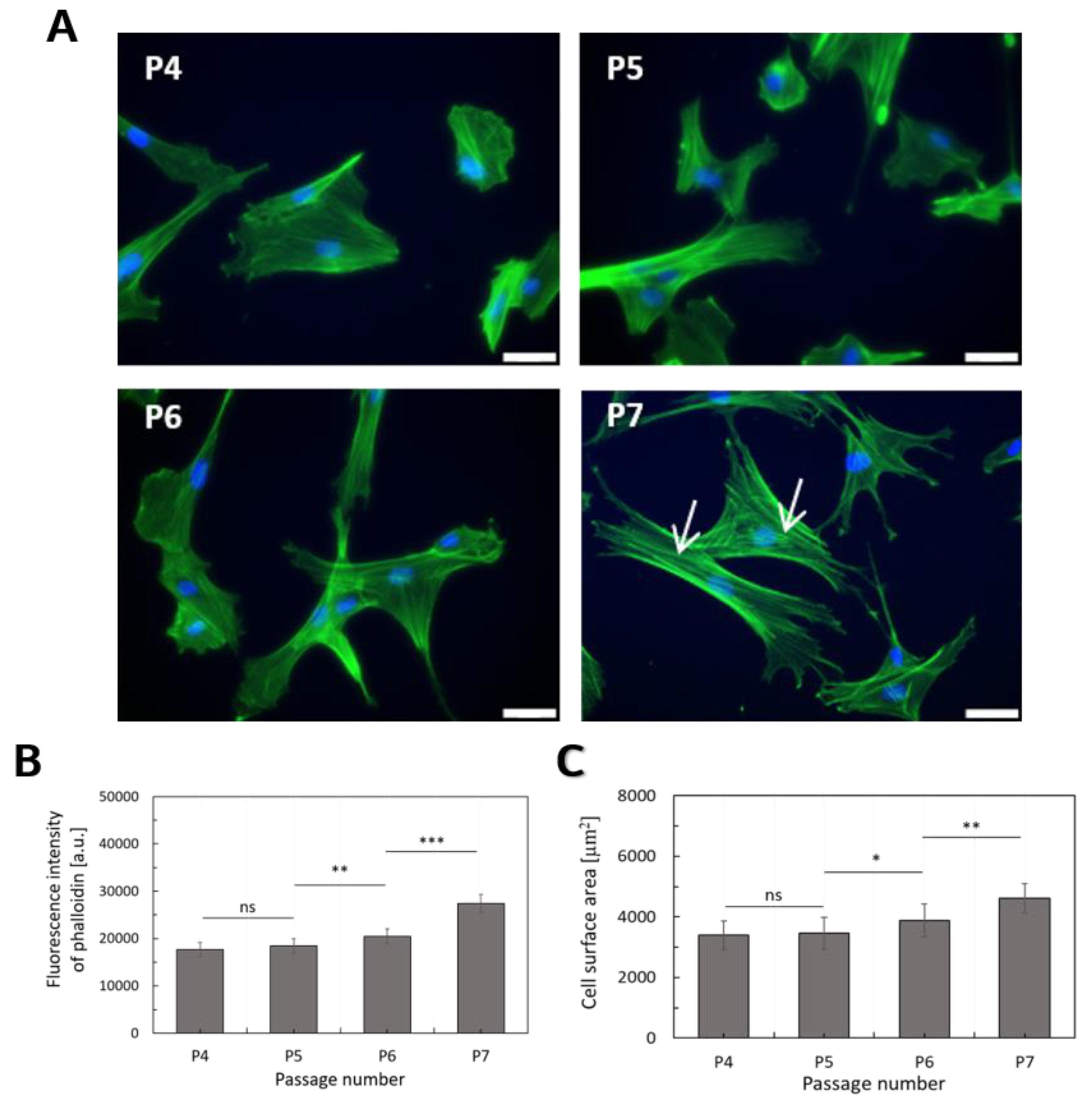
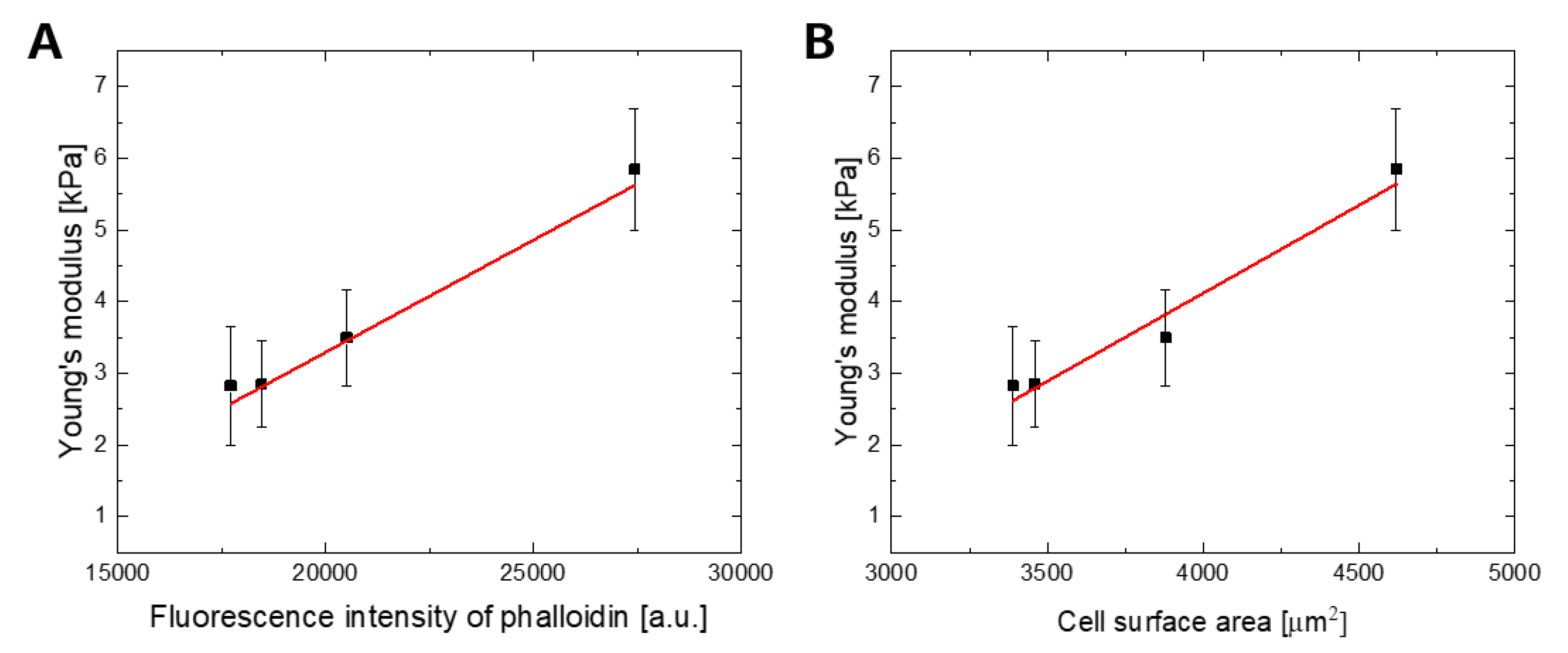
2.2. Deformability of WJ-MSCs Changes during in vitro Cultivation
2.3. The F-actin Cytoskeleton is the Main Determinant of WJ-MSCs Deformability
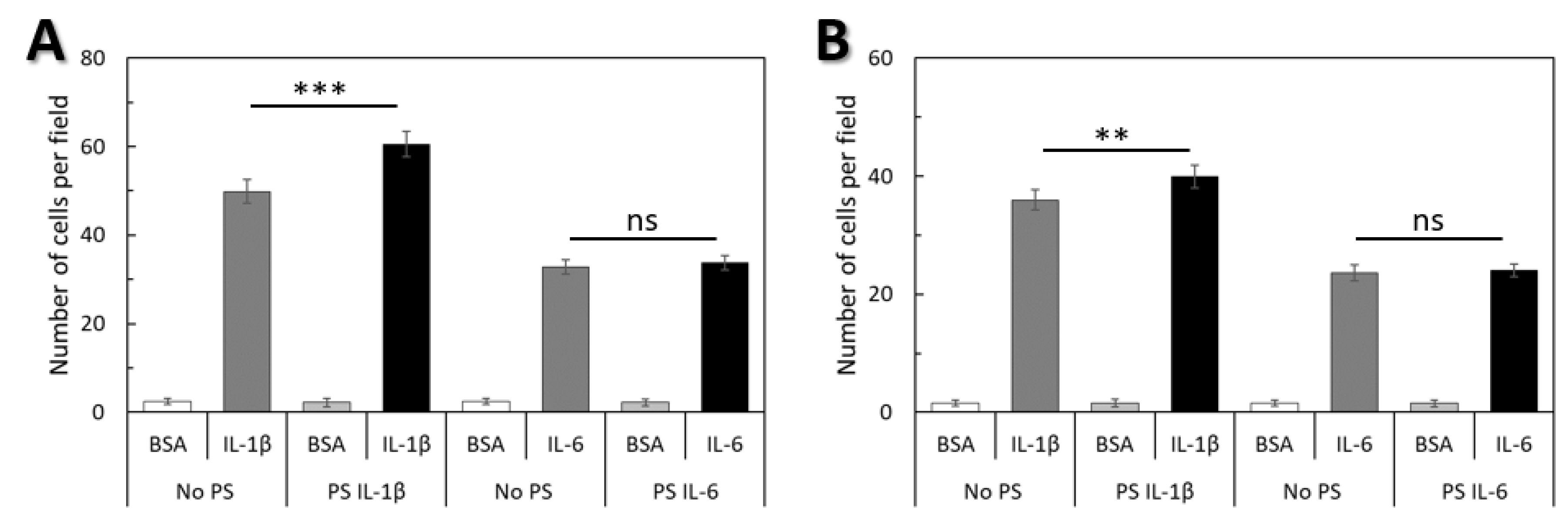
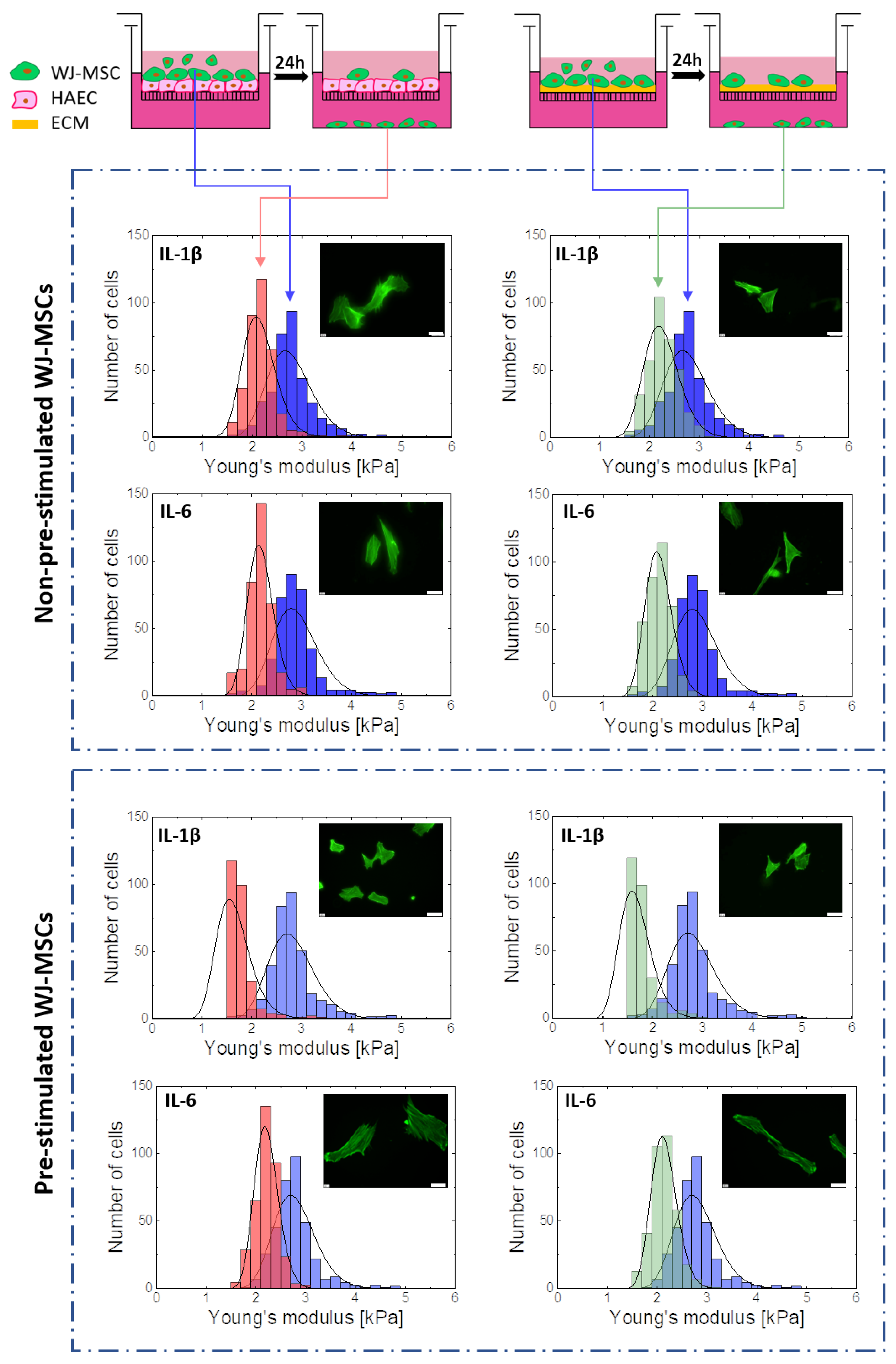
2.4. Cellular Deformability is a Key Factor of WJ-MSCs Transmigration
3. Discussion
4. Materials and Methods
4.1. Human WJ-MSCs Isolation and Cultivation
4.2. Evaluation of Surface Markers Expression
4.3. Adipogenic, Osteogenic and Chondrogenic Differentiation
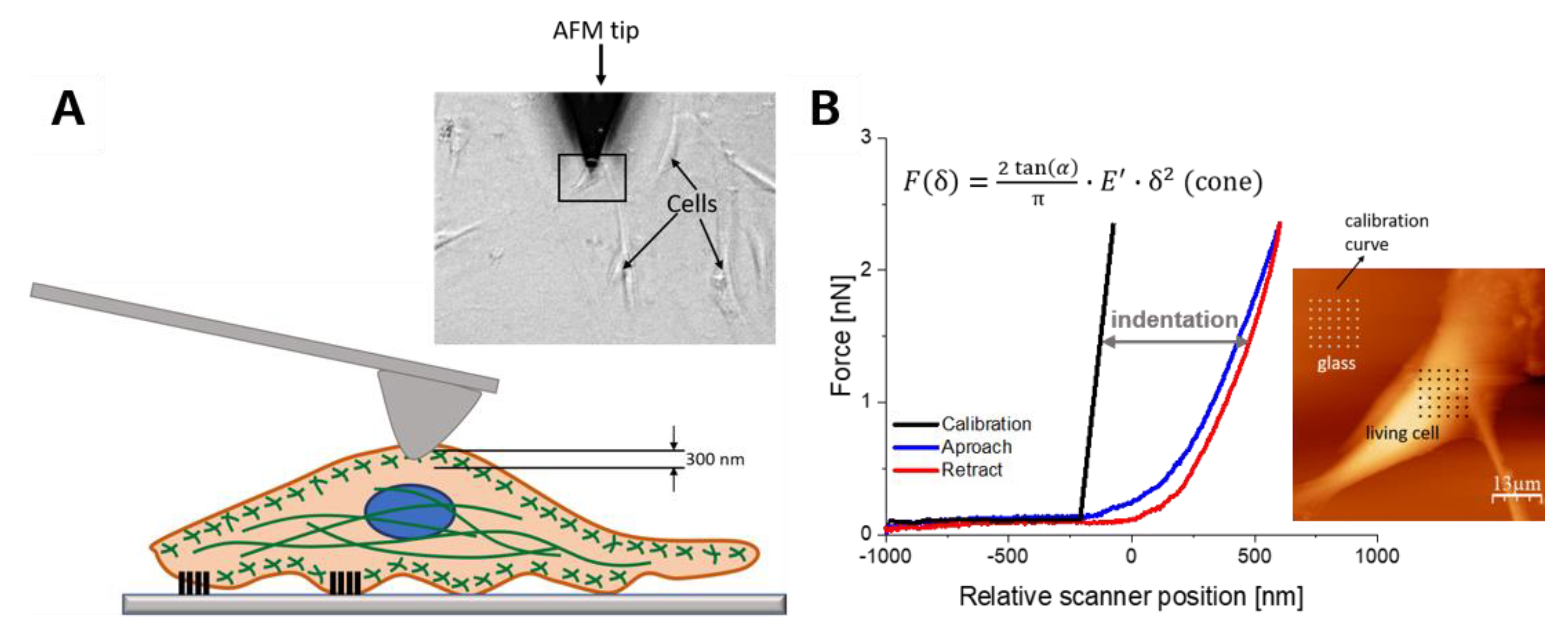
4.4. Sample Preparation for AFM Measurements
4.5. AFM Nanoindentation Measurements
4.6. Fluorescence Staining
4.7. Image Recording
4.8. Cell Spreading Area Determination
4.9. F-actin Content Determination
4.10. Transmigration Assays
4.11. Statistical Significance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| GMP | Good Manufacturing Practice |
| GLP | Good Laboratory Practice |
| MSCs | Mesenchymal Stem Cells |
| WJ-MSCs | Wharton’s Jelly Mesenchymal Stem Cells |
References
- Da Silva Meirelles, L. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- da Silva Meirelles, L.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of Bone, Marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and clinical applications of mesenchymal stem cells state-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells 2014, 6, 195–202. [Google Scholar] [CrossRef]
- Batsali, A.K.; Batsali, A.; Kastrinaki, M.-C.A.; Papadaki, H.; Pontikoglou, C. Mesenchymal Stem Cells Derived from Wharton's Jelly of the Umbilical Cord: Biological Properties and Emerging Clinical Applications. Curr. Stem Cell Res. Ther. 2013, 8, 144–155. [Google Scholar] [CrossRef]
- Moretti, P.; Hatlapatka, T.; Marten, D.; Lavrentieva, A.; Majore, I.; Hass, R.; Kasper, C. Mesenchymal Stromal Cells Derived from Human Umbilical Cord Tissues: Primitive Cells with Potential for Clinical and Tissue Engineering Applications. Adv. Biochem. Eng. Biotechnol. 2010, 123, 29–54. [Google Scholar] [CrossRef]
- Majka, M.; Sułkowski, M.; Badyra, B.; Musiałek, P. Concise review: Mesenchymal stem cells in cardiovascular regeneration: Emerging research directions and clinical applications. Stem Cells Translational Med. 2017. [Google Scholar] [CrossRef]
- Dmitrieva, R.I.; Minullina, R.; Bilibina, A.A.; Tarasova, O.V.; Anisimov, S.V.; Zaritskey, A.Y. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: Differences and similarities. Cell Cycle 2012. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.K.; Ogando, C.R.; Wang, S.C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; McAndrews, K.M.; Dawson, M.R. Biomechanical analysis predicts decreased human mesenchymal stem cell function before molecular differences. Exp. Cell Res. 2013. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Chen, Y.J.; Yew, T.L.; Chen, L.L.; Wang, J.Y.; Chiu, C.H.; Hung, S.C. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-P21 by HIF-TWIST. Blood 2011. [Google Scholar] [CrossRef] [PubMed]
- Boregowda, S.V.; Krishnappa, V.; Chambers, J.W.; Lograsso, P.V.; Lai, W.T.; Ortiz, L.A.; Phinney, D.G. Atmospheric oxygen inhibits growth and differentiation of marrow-derived mouse mesenchymal stem cells via a P53-dependent mechanism: Implications for long-term culture expansion. Stem Cells 2012. [Google Scholar] [CrossRef]
- Shibata, K.R.; Aoyama, T.; Shima, Y.; Fukiage, K.; Otsuka, S.; Furu, M.; Kohno, Y.; Ito, K.; Fujibayashi, S.; Neo, M.; et al. Expression of the P16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells 2007. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.A. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 2004. [Google Scholar] [CrossRef]
- Maloney, J.M.; Nikova, D.; Lautenschläger, F.; Clarke, E.; Langer, R.; Guck, J.; Van Vliet, K.J. Mesenchymal Stem cell mechanics from the attached to the suspended state. Biophys. J. 2010. [Google Scholar] [CrossRef]
- LeBlon, C.E.; Casey, M.E.; Fodor, C.R.; Zhang, T.; Zhang, X.; Jedlicka, S.S. Correlation between in vitro expansion-related cell stiffening and differentiation potential of human mesenchymal stem cells. Differentiation 2015. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature. 2010. [Google Scholar] [CrossRef] [PubMed]
- Ambriz, X.; de Lanerolle, P.; Ambrosio, J.R. The mechanobiology of the actin cytoskeleton in stem cells during differentiation and interaction with biomaterials. Stem Cells Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Dulińska-Molak, I.; Pasikowska, M.; Pogoda, K.; Lewandowska, M.; Eris, I.; Lekka, M. Age-related changes in the mechanical properties of human fibroblasts and its prospective reversal after anti-wrinkle tripeptide treatment. Int. J. Pept. Res. Ther. 2014. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nature Rev. Mol. Cell Biol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.D.; Gerlach, B.D. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zemła, J.; Danilkiewicz, J.; Orzechowska, B.; Pabijan, J.; Seweryn, S.; Lekka, M. Atomic force microscopy as a tool for assessing the cellular elasticity and adhesiveness to identify cancer cells and tissues. Semin. Cell Dev. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rianna, C.; Radmacher, M. Cell mechanics as a marker for diseases: Biomedical applications of AFM. In AIP Conference Proceedings, Penang, Malaysia, 26 September 2016; AIP Publishing: College Park, MD, USA. [CrossRef]
- Lieber, S.C.; Vatner, S.F.; Aubry, N.; Diaz, G.; Pain, J.; Kim, S.-J. Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am. J. Physiol. Circ. Physiol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Han, I.; Kwon, B.S.; Park, H.K.; Kim, K.S. Differentiation potential of mesenchymal stem cells is related to their intrinsic mechanical properties. Int. Neurourol. J. 2017. [Google Scholar] [CrossRef]
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. Concise review: MSC adhesion cascade—insights into homing and transendothelial migration. Stem Cells 2017. [Google Scholar] [CrossRef]
- Rüster, B.; Göttig, S.; Ludwig, R.J.; Bistrian, R.; Müller, S.; Seifried, E.; Gille, J.; Henschler, R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006. [Google Scholar] [CrossRef]
- Berry, M.F.; Engler, A.J.; Joseph, W.Y.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Lee, S.H. Regulation and function of stem cells in the cardiovascular system mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Hear. Circ. Physiol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.M.; Chamberlain, G.; Ashton, B.A.; Middleton, J. Recent advances into the understanding of mesenchymal stem cell trafficking. Brit. J. Haematol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Sohni, A.; Verfaillie, C.M. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, Y. The role of chemokines in mesenchymal stromal cell homing to sites of inflammation, including infarcted myocardium. In The Biology and Therapeutic Application of Mesenchymal Cells; Atkinson, K., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Ip, J.E.; Wu, Y.; Huang, J.; Zhang, L.; Pratt, R.E.; Dzau, V.J. Mesenchymal stem cells use integrin 1 NOT CXC chemokine receptor 4 for myocardial migration and engraftment. Mol. Biol. Cell 2007. [Google Scholar] [CrossRef] [PubMed]
- Steingen, C.; Brenig, F.; Baumgartner, L.; Schmidt, J.; Schmidt, A.; Bloch, W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J. Mol. Cell. Cardiol. 2008. [Google Scholar] [CrossRef]
- Kia, N.A.; Bahrami, A.R.; Ebrahimi, M.; Matin, M.M.; Neshati, Z.; Almohaddesin, M.R.; Aghdami, N.; Bidkhori, H.R. comparative analysis of chemokine receptor’s expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J. Mol. Neurosci. 2011. [Google Scholar] [CrossRef]
- Honczarenko, M.; Le, Y.; Swierkowski, M.; Ghiran, I.; Glodek, A.M.; Silberstein, L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 2005. [Google Scholar] [CrossRef]
- Ringe, J.; Strassburg, S.; Neumann, K.; Endres, M.; Notter, M.; Burmester, G.R.; Kaps, C.; Sittinger, M. Towards in situ tissue repair: Human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but Not CCL2. J. Cell Biochem. 2007. [Google Scholar] [CrossRef]
- Olson, M.F.; Sahai, E. The actin cytoskeleton in cancer cell motility. Clin. Exp. Metastasis 2009. [Google Scholar] [CrossRef]
- Iwanicki, M.P.; Davidowitz, R.A.; Ng, M.R.; Besser, A.; Muranen, T.; Merritt, M.; Danuser, G.; Ince, T.; Brugge, J.S. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov. 2011. [Google Scholar] [CrossRef]
- Rudzka, D.A.; Spennati, G.; McGarry, D.J.; Chim, Y.-H.; Neilson, M.; Ptak, A.; Munro, J.; Kalna, G.; Hedley, A.; Moralli, D.; et al. Migration through physical constraints is enabled by MAPK-induced cell softening via actin cytoskeleton re-organization. J. Cell Sci. 2019, 132, jcs224071. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, K.; Jaczewska, J.; Wiltowska-Zuber, J.; Klymenko, O.; Zuber, K.; Fornal, M.; Lekka, M. Depth-sensing analysis of cytoskeleton organization based on AFM data. Eur. Biophys. J. 2012. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Kopen, G.; Righter, W.; Webster, S.; Tremain, N.; Prockop, D.J. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J. Cell. Biochem. 1999. [Google Scholar] [CrossRef]
- Beane, O.S.; Fonseca, V.C.; Cooper, L.L.; Koren, G.; Darling, E.M. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Titushkin, I.; Cho, M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys. J. 2007. [Google Scholar] [CrossRef] [PubMed]
- Yourek, G.; Hussain, M.A.; Mao, J.J. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO J. 2007. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Padula, D.; Popov, C.; Mutschler, W.; Clausen-Schaumann, H.; Schieker, M. Researching into the cellular shape, volume and elasticity of mesenchymal stem cells, osteoblasts and osteosarcoma cells by atomic force microscopy: stem cells. J. Cell. Mol. Med. 2008. [Google Scholar] [CrossRef]
- Schillers, H.; Rianna, C.; Schäpe, J.; Luque, T.; Doschke, H.; Wälte, M.; Uriarte, J.J.; Campillo, N.; Michanetzis, G.P.A.; Bobrowska, J.; et al. Standardized Nanomechanical atomic force microscopy procedure (SNAP) for measuring soft and biological samples. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Rico, F.; Roca-Cusachs, P.; Gavara, N.; Farré, R.; Rotger, M.; Navajas, D. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys. Rev. E 2005. [Google Scholar] [CrossRef]
- Banfi, A.; Muraglia, A.; Dozin, B.; Mastrogiacomo, M.; Cancedda, R.; Quarto, R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp. Hematol. 2000. [Google Scholar] [CrossRef]
- Bershadsky, A.D.; Balaban, N.Q.; Geiger, B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003. [Google Scholar] [CrossRef] [PubMed]
- Wexler, S.A.; Donaldson, C.; Denning-Kendall, P.; Rice, C.; Bradley, B.; Hows, J.M. adult bone marrow is a rich source of human mesenchymal “stem” cells but umbilical cord and mobilized adult blood are not. Br. J. Haematol. 2003. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. Antecedents of cell aging research. Exp. Gerontol. 1989. [Google Scholar] [CrossRef]
- Chen, M.S.; Lin, C.Y.; Chiu, Y.H.; Chen, C.P.; Tsai, P.J.; Wang, H.S. IL-1β-Induced matrix metalloprotease-1 promotes mesenchymal stem cell migration via PAR1 and G-protein-coupled signaling pathway. Stem Cells Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.C.; Chiu, Y.H.; Chen, C.P.; Wang, H.S. Interleukin-1β induces CXCR3-mediated chemotaxis to promote umbilical cord mesenchymal stem cell transendothelial migration. Stem Cell Res. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pankajakshan, D.; Agrawal, D.K. Mesenchymal stem cell paracrine factors in vascular repair and regeneration. J. Biomed. Technol. Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.J.; Palma, J.; Gupta, D.; Torres, D.; Gaughan, J.P.; Houser, S.; Macha, M. Reverse remodeling is associated with changes in extracellular matrix proteases and tissue inhibitors after mesenchymal stem cell (MSC) treatment of pressure overload hypertrophy. J. Tissue Eng. Regen. Med. 2009. [Google Scholar] [CrossRef]
- Qi, J.; Fox, A.M.; Alexopoulos, L.G.; Chi, L.; Bynum, D.; Guilak, F.; Banes, A.J. IL-1β decreases the elastic modulus of human tenocytes. J. Appl. Physiol. 2006. [Google Scholar] [CrossRef]
- Sader, J.E.; Larson, I.; Mulvaney, P.; White, L.R. Method for the calibration of atomic force microscope cantilevers. Rev. Sci. Instrum. 1995. [Google Scholar] [CrossRef]
- Lekka, M.; Laidler, P.; Ignacak, J.J.; Labd, M.; Lekki, J.; Struszczyk, H.; Stachura, Z.; Hrynkiewicz, A.Z. The effect of chitosan on stiffness and glycolytic activity of human bladder cells. Biochim. Biophys. Acta Mol. Cell Res. 2001. [Google Scholar] [CrossRef]
- Prauzner-Bechcicki, S.; Raczkowska, J.; Madej, E.; Pabijan, J.; Lukes, J.; Sepitka, J.; Rysz, J.; Awsiuk, K.; Bernasik, A.; Budkowski, A.; et al. PDMS Substrate stiffness affects the morphology and growth profiles of cancerous prostate and melanoma cells. J. Mech. Behav. Biomed. Mater. 2015. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydlak, R.; Majka, M.; Lekka, M.; Kot, M.; Laidler, P. AFM-based Analysis of Wharton’s Jelly Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 4351. https://doi.org/10.3390/ijms20184351
Szydlak R, Majka M, Lekka M, Kot M, Laidler P. AFM-based Analysis of Wharton’s Jelly Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2019; 20(18):4351. https://doi.org/10.3390/ijms20184351
Chicago/Turabian StyleSzydlak, Renata, Marcin Majka, Małgorzata Lekka, Marta Kot, and Piotr Laidler. 2019. "AFM-based Analysis of Wharton’s Jelly Mesenchymal Stem Cells" International Journal of Molecular Sciences 20, no. 18: 4351. https://doi.org/10.3390/ijms20184351
APA StyleSzydlak, R., Majka, M., Lekka, M., Kot, M., & Laidler, P. (2019). AFM-based Analysis of Wharton’s Jelly Mesenchymal Stem Cells. International Journal of Molecular Sciences, 20(18), 4351. https://doi.org/10.3390/ijms20184351





