A Molecular Dynamics Study of Vasoactive Intestinal Peptide Receptor 1 and the Basis of Its Therapeutic Antagonism
Abstract
1. Introduction
2. Results
2.1. Little Ligand Selectivity of VPAC1 and Other Class B GPCRs
2.2. Insights Into the Mechaninism of Action of VPAC1 Antagonists
2.2.1. Experimental Data for the Biological Activity of VPAC1 Antagonists
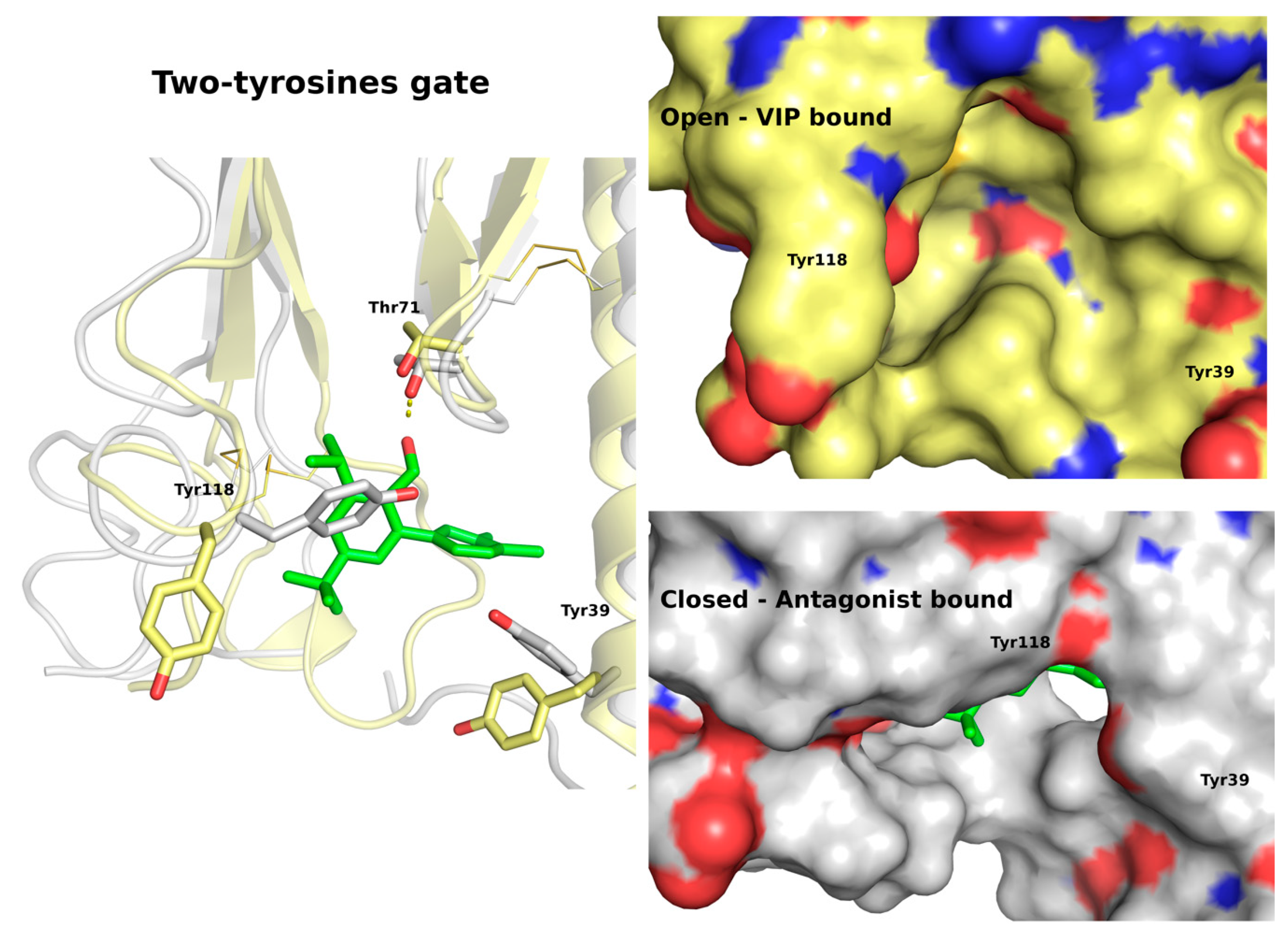
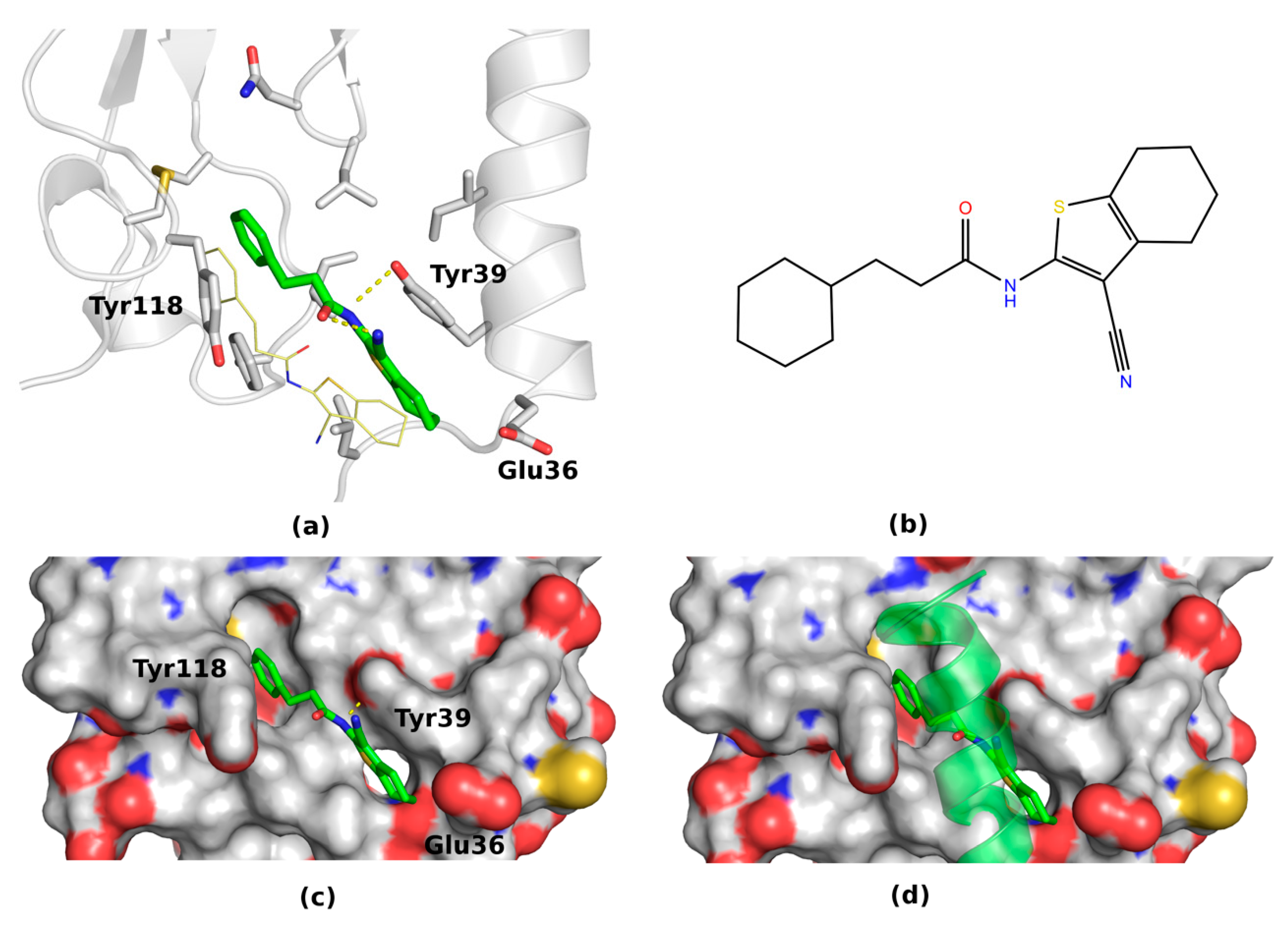
2.2.2. Binding Modes of VPAC1 Small-Molecule Antagonists Proposed by Molecular Docking
2.2.3. Binding Modes of VPAC1 Antagonists Refined by Molecular Dynamics Simulations
3. Discussion and Conclusions
4. Materials and Methods
4.1. Homology Modeling and Molecular Docking
4.1.1. An Inactive Transmembrane Conformation of VPAC1 for Testing Allosteric Modulators
4.1.2. An ECD Domain of VPAC1 for Testing Antagonists
4.1.3. Active and Partly Active Two-Domains Models of VPAC1 with VIP
4.2. Molecular Dynamics Simulations of VPAC1 and Its Complexes With Antagonists
4.2.1. MD Simulations of the ECD Domain Complexes
4.2.2. MD Simulations of the TMD Domain Complexes
4.2.3. MD Simulations of an Active Conformation of VPAC1.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| VIP | Vasoactive intestinal peptide |
| VPAC1 | Vasoactive intestinal peptide receptor 1 |
| VPAC2 | Vasoactive intestinal peptide receptor 2 |
| PACAP | Pituitary adenylate cyclase-activating peptide |
| PAC1R | Pituitary adenylate cyclase-activating peptide type I receptor |
| GCGR | Glucagon receptor |
| GLP-1R | Glucagon-like peptide-1 receptor |
| CGRP | Calcitonin gene-related peptide receptor |
| RAMP | Receptor-activity modifying protein |
| GPCR | G protein-coupled receptor |
| ECD | Ectodomain (extracellular domain) of class B GPCRs |
| TMD | Transmembrane domain of GPCRs |
| EC2 | Extracellular loop 2 of GPCR |
| NMR | Nucleic Magnetic Resonance |
| MD | Molecular Dynamics |
| RMSD | Root-mean-square deviation of atomic positions |
| NVT | An ensemble of a constant number of particles, volume and temperature |
| NPT | An ensemble of a constant number of particles, pressure and temperature |
References
- Hollenstein, K.; de Graaf, C.; Bortolato, A.; Wang, M.W.; Marshall, F.H.; Stevens, R.C. Insights into the structure of class B GPCRs. Trends Pharmacol. Sci. 2014, 35, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.L.; Dong, M.; Miller, L.J. Differential determinants for coupling of distinct G proteins with the class B secretin receptor. Am. J. Physiol. Cell Physiol. 2012, 302, C1202–C1212. [Google Scholar] [CrossRef] [PubMed]
- Harmar, A.J.; Arimura, A.; Gozes, I.; Journot, L.; Laburthe, M.; Pisegna, J.R.; Rawlings, S.R.; Robberecht, P.; Said, S.I.; Sreedharan, S.P.; et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998, 50, 265–270. [Google Scholar] [PubMed]
- Mayo, K.E.; Miller, L.J.; Bataille, D.; Dalle, S.; Goke, B.; Thorens, B.; Drucker, D.J. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol. Rev. 2003, 55, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Tache, Y.; Larauche, M.; Yuan, P.Q.; Million, M. Brain and Gut CRF Signaling: Biological Actions and Role in the Gastrointestinal Tract. Curr. Mol. Pharmacol. 2018, 11, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Kim, K.B.; Yoon, S.M.; Han, J.H.; Chae, H.B.; Park, S.M.; Youn, S.J. Corticotropin-releasing factor stimulates colonic motility via muscarinic receptors in the rat. World J. Gastroenterol. 2017, 23, 3825–3831. [Google Scholar] [CrossRef]
- Siu, F.Y.; He, M.; de Graaf, C.; Han, G.W.; Yang, D.; Zhang, Z.; Zhou, C.; Xu, Q.; Wacker, D.; Joseph, J.S.; et al. Structure of the human glucagon class B G-protein-coupled receptor. Nature 2013, 499, 444–449. [Google Scholar] [CrossRef]
- Song, G.; Yang, D.; Wang, Y.; de Graaf, C.; Zhou, Q.; Jiang, S.; Liu, K.; Cai, X.; Dai, A.; Lin, G.; et al. Human GLP-1 receptor transmembrane domain structure in complex with allosteric modulators. Nature 2017, 546, 312–315. [Google Scholar] [CrossRef]
- Hollenstein, K.; Kean, J.; Bortolato, A.; Cheng, R.K.; Dore, A.S.; Jazayeri, A.; Cooke, R.M.; Weir, M.; Marshall, F.H. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature 2013, 499, 438–443. [Google Scholar] [CrossRef]
- Liang, Y.L.; Khoshouei, M.; Radjainia, M.; Zhang, Y.; Glukhova, A.; Tarrasch, J.; Thal, D.M.; Furness, S.G.B.; Christopoulos, G.; Coudrat, T.; et al. Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature 2017, 546, 118–123. [Google Scholar] [CrossRef]
- Ehrenmann, J.; Schoppe, J.; Klenk, C.; Rappas, M.; Kummer, L.; Dore, A.S.; Pluckthun, A. High-resolution crystal structure of parathyroid hormone 1 receptor in complex with a peptide agonist. Nat. Struct. Mol. Biol. 2018, 25, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.L.; Khoshouei, M.; Deganutti, G.; Glukhova, A.; Koole, C.; Peat, T.S.; Radjainia, M.; Plitzko, J.M.; Baumeister, W.; Miller, L.J.; et al. Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nature 2018, 561, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Chow, B.K.; Kasamatsu, J.; Kasahara, M.; Lee, L.T. Agnathan VIP, PACAP and their receptors: Ancestral origins of today’s highly diversified forms. PLoS ONE 2012, 7, e44691. [Google Scholar] [CrossRef] [PubMed]
- Langer, I. Mechanisms involved in VPAC receptors activation and regulation: Lessons from pharmacological and mutagenesis studies. Front. Endocrinol. (Lausanne) 2012, 3, 129. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.; Arsenescu, R.; Arsenescu, V. Neuro-Immuno-Gastroenterology, 1st ed.; Springer International Publishing: Basel, Switzerland, 2016; p. 337. [Google Scholar]
- Moody, T.W.; Ramos-Alvarez, I.; Jensen, R.T. Neuropeptide G Protein-Coupled Receptors as Oncotargets. Front. Endocrinol. (Lausanne) 2018, 9, 345. [Google Scholar] [CrossRef]
- Pinter, E.; Pozsgai, G.; Hajna, Z.; Helyes, Z.; Szolcsanyi, J. Neuropeptide receptors as potential drug targets in the treatment of inflammatory conditions. Br. J. Clin. Pharmacol. 2014, 77, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Chorny, A.; Delgado, M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat. Rev. Immunol. 2007, 7, 52–63. [Google Scholar] [CrossRef]
- Gray, S.L.; Cline, D.L. Chapter 21-PACAP: Regulator of the Stress Response. In Stress: Physiology, Biochemistry, and Pathology, 1st ed.; Fink, G., Ed.; Academic Press: Cambridge, Massachusetts, MA, USA, 2019; Volume 3, p. 420. [Google Scholar]
- Reglodi, D.; Illes, A.; Opper, B.; Schafer, E.; Tamas, A.; Horvath, G. Presence and Effects of Pituitary Adenylate Cyclase Activating Polypeptide Under Physiological and Pathological Conditions in the Stomach. Front. Endocrinol. (Lausanne) 2018, 9, 90. [Google Scholar] [CrossRef]
- Rudecki, A.P.; Gray, S.L. PACAP in the Defense of Energy Homeostasis. Trends Endocrinol. Metab. 2016, 27, 620–632. [Google Scholar] [CrossRef]
- Reglodi, D.; Atlasz, T.; Szabo, E.; Jungling, A.; Tamas, A.; Juhasz, T.; Fulop, B.D.; Bardosi, A. PACAP deficiency as a model of aging. Geroscience 2018, 40, 437–452. [Google Scholar] [CrossRef]
- Couvineau, A.; Ceraudo, E.; Tan, Y.V.; Nicole, P.; Laburthe, M. The VPAC1 receptor: Structure and function of a class B GPCR prototype. Front. Endocrinol. (Lausanne) 2012, 3, 139. [Google Scholar] [CrossRef] [PubMed]
- Solano, R.M.; Langer, I.; Perret, J.; Vertongen, P.; Juarranz, M.G.; Robberecht, P.; Waelbroeck, M. Two basic residues of the h-VPAC1 receptor second transmembrane helix are essential for ligand binding and signal transduction. J. Biol. Chem. 2001, 276, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, S.M.; Tams, J.W.; Fahrenkrug, J. Functional roles of conserved transmembrane prolines in the human VPAC(1) receptor. FEBS Lett. 2001, 503, 126–130. [Google Scholar] [CrossRef]
- Chugunov, A.O.; Simms, J.; Poyner, D.R.; Dehouck, Y.; Rooman, M.; Gilis, D.; Langer, I. Evidence that interaction between conserved residues in transmembrane helices 2, 3, and 7 are crucial for human VPAC1 receptor activation. Mol. Pharmacol. 2010, 78, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Ceraudo, E.; Hierso, R.; Tan, Y.V.; Murail, S.; Rouyer-Fessard, C.; Nicole, P.; Robert, J.C.; Jamin, N.; Neumann, J.M.; Robberecht, P.; et al. Spatial proximity between the VPAC1 receptor and the amino terminus of agonist and antagonist peptides reveals distinct sites of interaction. Faseb J. 2012, 26, 2060–2071. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, L.S.; Srivastava, N.; Kayser, L.E.; Nirschl, D.S.; Kumaragurubaran, K.; Roy, A.; Gupta, A.; Karmakar, S.; Karatt, T.; Mathur, A.; et al. Identification and optimization of small molecule antagonists of vasoactive intestinal peptide receptor-1 (VIPR1). Bioorg. Med. Chem. Lett. 2012, 22, 2287–2290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lutz, E.M.; Ronaldson, E.; Shaw, P.; Johnson, M.S.; Holland, P.J.; Mitchell, R. Characterization of novel splice variants of the PAC1 receptor in human neuroblastoma cells: Consequences for signaling by VIP and PACAP. Mol. Cell. Neurosci. 2006, 31, 193–209. [Google Scholar] [CrossRef]
- Harmar, A.J.; Fahrenkrug, J.; Gozes, I.; Laburthe, M.; May, V.; Pisegna, J.R.; Vaudry, D.; Vaudry, H.; Waschek, J.A.; Said, S.I. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br. J. Pharmacol. 2012, 166, 4–17. [Google Scholar] [CrossRef]
- Sakamoto, K.; Koyama, R.; Kamada, Y.; Miwa, M.; Tani, A. Discovery of artificial VIPR2-antagonist peptides possessing receptor- and ligand-selectivity. Biochem. Biophys. Res. Commun. 2018, 503, 1973–1979. [Google Scholar] [CrossRef]
- Booe, J.M.; Walker, C.S.; Barwell, J.; Kuteyi, G.; Simms, J.; Jamaluddin, M.A.; Warner, M.L.; Bill, R.M.; Harris, P.W.; Brimble, M.A.; et al. Structural Basis for Receptor Activity-Modifying Protein-Dependent Selective Peptide Recognition by a G Protein-Coupled Receptor. Mol. Cell. 2015, 58, 1040–1052. [Google Scholar] [CrossRef]
- Gingell, J.J.; Simms, J.; Barwell, J.; Poyner, D.R.; Watkins, H.A.; Pioszak, A.A.; Sexton, P.M.; Hay, D.L. Erratum: An allosteric role for receptor activity-modifying proteins in defining GPCR pharmacology. Cell. Discov. 2016, 2, 16020. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.L.; Garelja, M.L.; Poyner, D.R.; Walker, C.S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 2018, 175, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, A.; Christopoulos, G.; Morfis, M.; Udawela, M.; Laburthe, M.; Couvineau, A.; Kuwasako, K.; Tilakaratne, N.; Sexton, P.M. Novel receptor partners and function of receptor activity-modifying proteins. J. Biol. Chem. 2003, 278, 3293–3297. [Google Scholar] [CrossRef] [PubMed]
- Wootten, D.; Lindmark, H.; Kadmiel, M.; Willcockson, H.; Caron, K.M.; Barwell, J.; Drmota, T.; Poyner, D.R. Receptor activity modifying proteins (RAMPs) interact with the VPAC2 receptor and CRF1 receptors and modulate their function. Br. J. Pharmacol. 2013, 168, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Tamas, A. Pituitary Adenylate Cyclase Activating Polypeptide — PACAP, 1st ed.; Springer International Publishing: Basel, Switzerland, 2016; Volume 11. [Google Scholar]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Herrick-Davis, K.; Milligan, G.; Di Giovanni, G. G-protein-coupled Receptor Dimers, 1st ed.; Humana Press: Totowa, NJ, USA, 2017; Volume 33. [Google Scholar]
- Iemolo, A.; Seiglie, M.; Blasio, A.; Cottone, P.; Sabino, V. Pituitary adenylate cyclase-activating polypeptide (PACAP) in the central nucleus of the amygdala induces anxiety via melanocortin receptors. Psychopharmacology (Berl.) 2016, 233, 3269–3277. [Google Scholar] [CrossRef] [PubMed]
- Dedic, N.; Chen, A.; Deussing, J.M. The CRF Family of Neuropeptides and their Receptors - Mediators of the Central Stress Response. Curr. Mol. Pharmacol. 2018, 11, 4–31. [Google Scholar] [CrossRef] [PubMed]
- Ronan, P.J.; Summers, C.H. Molecular signaling and translational significance of the corticotropin releasing factor system. Prog. Mol. Biol. Transl. Sci. 2011, 98, 235–292. [Google Scholar]
- Gourlet, P.; Vandermeers, A.; Van Rampelbergh, J.; De Neef, P.; Cnudde, J.; Waelbroeck, M.; Robberecht, P. Analogues of VIP, helodermin, and PACAP discriminate between rat and human VIP1 and VIP2 receptors. Ann. N. Y. Acad. Sci. 1998, 865, 247–252. [Google Scholar] [CrossRef]
- Rekasi, Z.; Varga, J.L.; Schally, A.V.; Halmos, G.; Groot, K.; Czompoly, T. Antagonistic actions of analogs related to growth hormone-releasing hormone (GHRH) on receptors for GHRH and vasoactive intestinal peptide on rat pituitary and pineal cells in vitro. Proc. Natl. Acad. Sci. USA 2000, 97, 1218–1223. [Google Scholar] [CrossRef]
- Moretti, C.; Mencacci, C.; Frajese, G.V.; Cerilli, M.; Frajese, G. Growth hormone-releasing hormone and pituitary adenylate cyclase-activating polypeptide in the reproductive system. Trends Endocrinol. Metab. 2002, 13, 428–435. [Google Scholar] [CrossRef]
- Hauger, R.L.; Risbrough, V.; Brauns, O.; Dautzenberg, F.M. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: New molecular targets. CNS Neurol. Disord. Drug Targets 2006, 5, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Song, D.; Davis-Taber, R.A.; Barrett, L.W.; Scott, V.E.; Richardson, P.L.; Pereda-Lopez, A.; Uchic, M.E.; Solomon, L.R.; Lake, M.R.; et al. Solution structure and mutational analysis of pituitary adenylate cyclase-activating polypeptide binding to the extracellular domain of PAC1-RS. Proc. Natl. Acad. Sci. USA 2007, 104, 7875–7880. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pioszak, A.; Zhang, C.; Swaminathan, K.; Xu, H.E. Crystal structure of the PAC1R extracellular domain unifies a consensus fold for hormone recognition by class B G-protein coupled receptors. PLoS ONE 2011, 6, e19682. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E. Chapter 14: Unraveling the Origin of Substituents Effects in π-Stacking Interactions. In Noncovalent Forces, 1st ed.; Scheiner, S., Ed.; Springer International Publishing: Basel, Switzerland, 2015; Volume 19, p. VIII 532. [Google Scholar]
- Chu, A.; Caldwell, J.S.; Chen, Y.A. Identification and characterization of a small molecule antagonist of human VPAC(2) receptor. Mol. Pharmacol. 2010, 77, 95–101. [Google Scholar] [CrossRef]
- Zhang, H.; Qiao, A.; Yang, D.; Yang, L.; Dai, A.; de Graaf, C.; Reedtz-Runge, S.; Dharmarajan, V.; Zhang, H.; Han, G.W.; et al. Structure of the full-length glucagon class B G-protein-coupled receptor. Nature 2017, 546, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Gourlet, P.; De Neef, P.; Cnudde, J.; Waelbroeck, M.; Robberecht, P. In vitro properties of a high affinity selective antagonist of the VIP1 receptor. Peptides 1997, 18, 1555–1560. [Google Scholar] [CrossRef]
- Moody, T.W.; Jensen, R.T.; Fridkin, M.; Gozes, I. (N-stearyl, norleucine17)VIPhybrid is a broad spectrum vasoactive intestinal peptide receptor antagonist. J. Mol. Neurosci. 2002, 18, 29–35. [Google Scholar] [CrossRef]
- Doan, N.D.; Bourgault, S.; Dejda, A.; Letourneau, M.; Detheux, M.; Vaudry, D.; Vaudry, H.; Chatenet, D.; Fournier, A. Design and in vitro characterization of PAC1/VPAC1-selective agonists with potent neuroprotective effects. Biochem. Pharmacol. 2011, 81, 552–561. [Google Scholar] [CrossRef]
- Zhao, S.J.; Wang, D.H.; Li, Y.W.; Han, L.; Xiao, X.; Ma, M.; Wan, D.C.; Hong, A.; Ma, Y. A novel selective VPAC2 agonist peptide-conjugated chitosan modified selenium nanoparticles with enhanced anti-type 2 diabetes synergy effects. Int. J. Nanomed. 2017, 12, 2143–2160. [Google Scholar] [CrossRef]
- Oparil, S.; Schmieder, R.E. New approaches in the treatment of hypertension. Circ. Res. 2015, 116, 1074–1095. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Liu, H.; Li, H. In silico classification and prediction of VIP derivatives as VPAC1/ VPAC2 receptor agonists/antagonists. Comb. Chem High. Throughput Screen 2015, 18, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.; Gomella, L.G.; Thakur, M.L.; Trabulsi, E.J. VPAC1-targeted PET/CT scan: Improved molecular imaging for the diagnosis of prostate cancer using a novel cell surface antigen. World J. Urol. 2018, 36, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Alvarez, I.; Mantey, S.A.; Nakamura, T.; Nuche-Berenguer, B.; Moreno, P.; Moody, T.W.; Maderdrut, J.L.; Coy, D.H.; Jensen, R.T. A structure-function study of PACAP using conformationally restricted analogs: Identification of PAC1 receptor-selective PACAP agonists. Peptides 2015, 66, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Yang, Y.; Cui, Z.; Zheng, L.; Zeng, Z.; Zhang, H. Novel peptide VIP-TAT with higher affinity for PAC1 inhibited scopolamine induced amnesia. Peptides 2014, 60, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sethi, V.; Rubinstein, I.; Kuzmis, A.; Kastrissios, H.; Artwohl, J.; Onyuksel, H. Novel, biocompatible, and disease modifying VIP nanomedicine for rheumatoid arthritis. Mol. Pharm. 2013, 10, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Mathioudakis, A.; Chatzimavridou-Grigoriadou, V.; Evangelopoulou, E.; Mathioudakis, G. Vasoactive intestinal Peptide inhaled agonists: Potential role in respiratory therapeutics. Hippokratia 2013, 17, 12–16. [Google Scholar] [PubMed]
- Yu, R.; Zeng, Z.; Guo, X.; Zhang, H.; Liu, X.; Ding, Y.; Chen, J. The TAT peptide endows PACAP with an enhanced ability to traverse bio-barriers. Neurosci. Lett. 2012, 527, 1–5. [Google Scholar] [CrossRef]
- Paulis, L.; Rajkovicova, R.; Simko, F. New developments in the pharmacological treatment of hypertension: Dead-end or a glimmer at the horizon? Curr. Hypertens. Rep. 2015, 17, 557. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef]
- Ter Haar, E.; Koth, C.M.; Abdul-Manan, N.; Swenson, L.; Coll, J.T.; Lippke, J.A.; Lepre, C.A.; Garcia-Guzman, M.; Moore, J.M. Crystal structure of the ectodomain complex of the CGRP receptor, a class-B GPCR, reveals the site of drug antagonism. Structure 2010, 18, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Coleman, R.G.; Smyth, K.T.; Cao, Q.; Soulard, P.; Caffrey, D.R.; Salzberg, A.C.; Huang, E.S. Structure-based maximal affinity model predicts small-molecule druggability. Nat. Biotechnol. 2007, 25, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2018–1: Schrödinger, L. New York, NY, USA, 2018. Available online: https://www.schrodinger.com/news/announcing-schr%C3%B6dinger-software-release-2018-1 (accessed on 15 February 2018).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Longenecker, K.L.; Stamper, G.F.; Hajduk, P.J.; Fry, E.H.; Jakob, C.G.; Harlan, J.E.; Edalji, R.; Bartley, D.M.; Walter, K.A.; Solomon, L.R.; et al. Structure of MurF from Streptococcus pneumoniae co-crystallized with a small molecule inhibitor exhibits interdomain closure. Protein Sci. 2005, 14, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Nittinger, E.; Gibbons, P.; Eigenbrot, C.; Davies, D.R.; Maurer, B.; Yu, C.L.; Kiefer, J.R.; Kuglstatter, A.; Murray, J.; Ortwine, D.F.; et al. Water molecules in protein-ligand interfaces. Evaluation of software tools and SAR comparison. J. Comput. Aided Mol. Des. 2019, 33, 307–330. [Google Scholar] [CrossRef] [PubMed]
- Jaladanki, C.K.; Taxak, N.; Varikoti, R.A.; Bharatam, P.V. Toxicity Originating from Thiophene Containing Drugs: Exploring the Mechanism using Quantum Chemical Methods. Chem. Res. Toxicol. 2015, 28, 2364–2376. [Google Scholar] [CrossRef]
- Liao, C.; Zhao, X.; Brewer, M.; May, V.; Li, J. Conformational Transitions of the Pituitary Adenylate Cyclase-Activating Polypeptide Receptor, a Human Class B GPCR. Sci. Rep. 2017, 7, 5427. [Google Scholar] [CrossRef]
- Takasaki, I.; Watanabe, A.; Yokai, M.; Watanabe, Y.; Hayakawa, D.; Nagashima, R.; Fukuchi, M.; Okada, T.; Toyooka, N.; Miyata, A.; et al. In Silico Screening Identified Novel Small-molecule Antagonists of PAC1 Receptor. J. Pharmacol. Exp. Ther. 2018, 365, 1–8. [Google Scholar] [CrossRef]
- Sarkar, A.; Sen, S. 3D structure prediction of VAPC1 and identification of dual natural inhibitors for VPAC1 and EGFR. J. Bioenerg. Biomembr. 2019, 51, 89–102. [Google Scholar] [CrossRef]
- Latek, D.; Pasznik, P.; Carlomagno, T.; Filipek, S. Towards improved quality of GPCR models by usage of multiple templates and profile-profile comparison. PLoS ONE 2013, 8, e56742. [Google Scholar] [CrossRef]
- Latek, D.; Rutkowska, E.; Niewieczerzal, S.; Cielecka-Piontek, J. Drug-induced diabetes type 2: In silico study involving class B GPCRs. PLoS ONE 2019, 14, e0208892. [Google Scholar] [CrossRef] [PubMed]
- Latek, D.; Bajda, M.; Filipek, S. A Hybrid Approach to Structure and Function Modeling of G Protein-Coupled Receptors. J. Chem. Inf. Model. 2016, 56, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Latek, D. Rosetta Broker for membrane protein structure prediction: Concentrative nucleoside transporter 3 and corticotropin-releasing factor receptor 1 test cases. BMC Struct. Biol. 2017, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Miszta, P.; Pasznik, P.; Jakowiecki, J.; Sztyler, A.; Latek, D.; Filipek, S. GPCRM: A homology modeling web service with triple membrane-fitted quality assessment of GPCR models. Nucleic Acids Res. 2018. [Google Scholar] [CrossRef]
- Pasznik, P.; Rutkowska, E.; Niewieczerzal, S.; Cielecka-Piontek, J.; Latek, D. Potential off-target effects of beta-blockers on gut hormone receptors: In silico study including GUT-DOCK-A web service for small-molecule docking. PLoS ONE 2019, 14, e0210705. [Google Scholar] [CrossRef]
- Kufareva, I.; Katritch, V.; Stevens, R.C.; Abagyan, R. Advances in GPCR modeling evaluated by the GPCR Dock 2013 assessment: Meeting new challenges. Structure 2014, 22, 1120–1139. [Google Scholar] [CrossRef]
- Kufareva, I.; Rueda, M.; Katritch, V.; Stevens, R.C.; Abagyan, R. Status of GPCR modeling and docking as reflected by community-wide GPCR Dock 2010 assessment. Structure 2011, 19, 1108–1126. [Google Scholar] [CrossRef]
- Pandy-Szekeres, G.; Munk, C.; Tsonkov, T.M.; Mordalski, S.; Harpsoe, K.; Hauser, A.S.; Bojarski, A.J.; Gloriam, D.E. GPCRdb in 2018: Adding GPCR structure models and ligands. Nucleic Acids Res. 2018, 46, D440–D446. [Google Scholar] [CrossRef]
- Worth, C.L.; Kreuchwig, F.; Tiemann, J.K.S.; Kreuchwig, A.; Ritschel, M.; Kleinau, G.; Hildebrand, P.W.; Krause, G. GPCR-SSFE 2.0-a fragment-based molecular modeling web tool for Class A G-protein coupled receptors. Nucleic Acids Res. 2017, 45, W408–W415. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Davila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Lomize, M.A.; Pogozheva, I.D.; Joo, H.; Mosberg, H.I.; Lomize, A.L. OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucleic Acids Res. 2012, 40, D370–D376. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, A.; Gil, C.; Dalton, J.A.R.; Giraldo, J. Structural insights into positive and negative allosteric regulation of a G protein-coupled receptor through protein-lipid interactions. Sci. Rep. 2018, 8, 4456. [Google Scholar] [CrossRef] [PubMed]
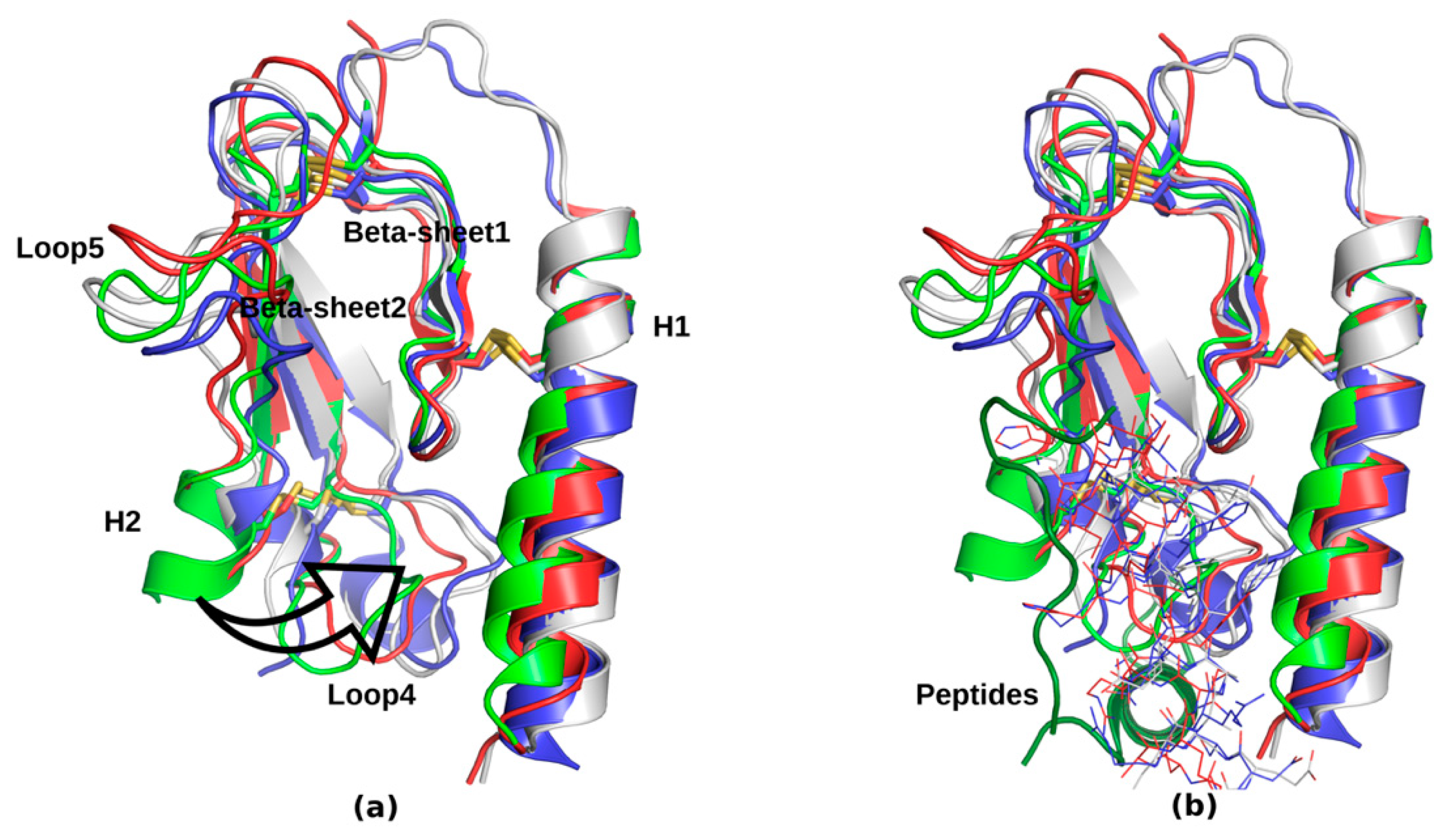
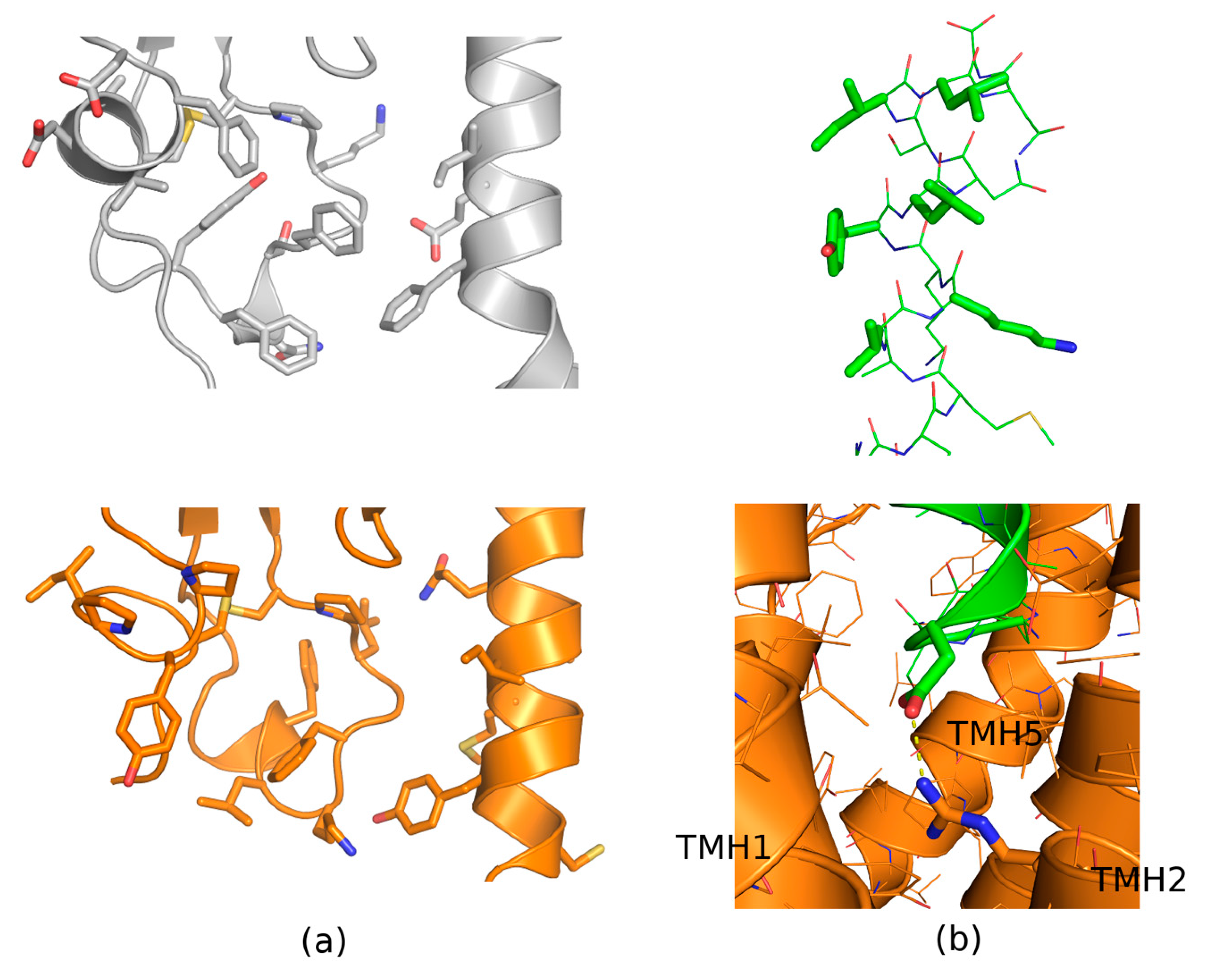
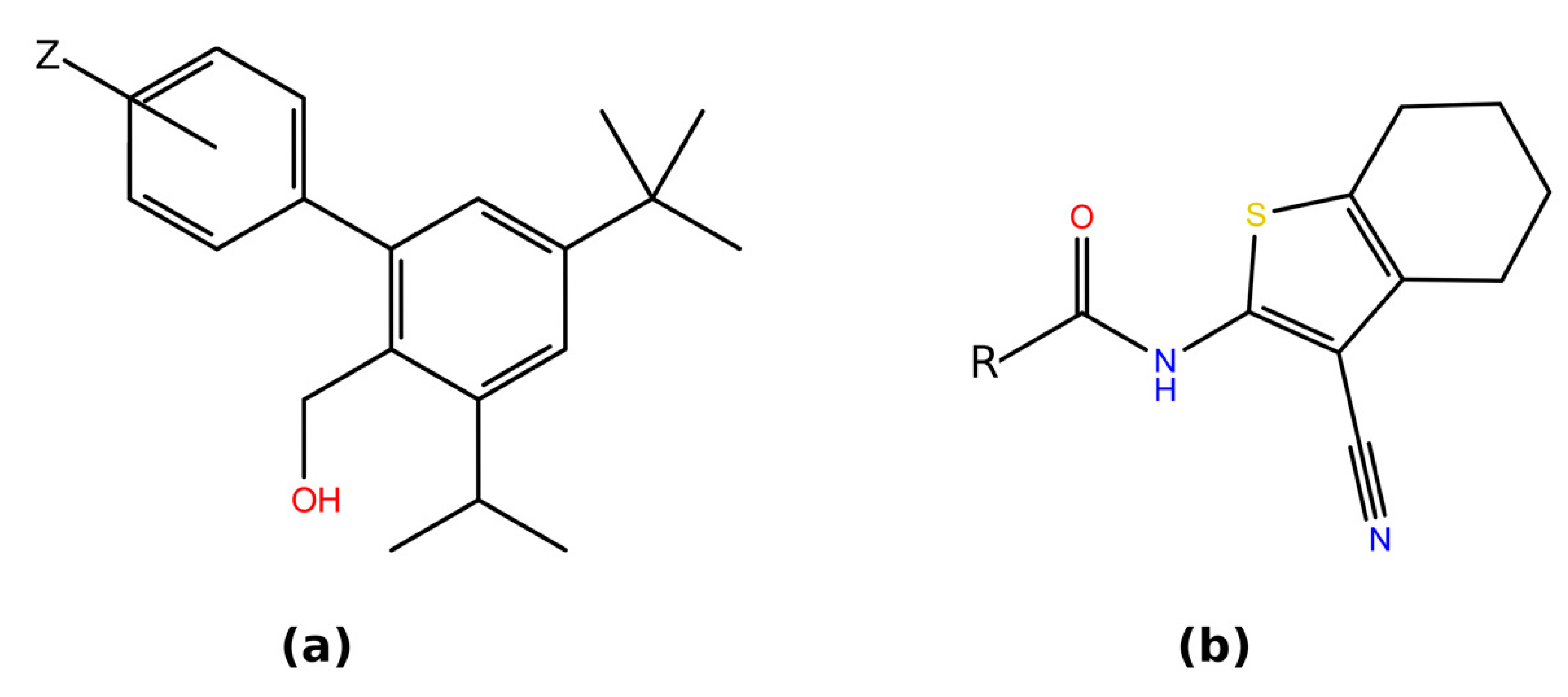

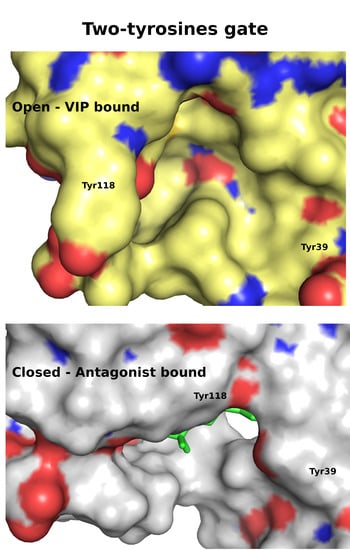
| Peptide Hormone 1 | Primary GPCR Target | Other GPCR Targets |
|---|---|---|
| Secretin | SCTR | VPAC1, VPAC2 [39] |
| Amylin [34] | AMY1, AMY2, AMY3 | CTR |
| Adrenomedullin | AM1, AM2 | CGRP receptor |
| Adrenomedullin 2 | AM2 | AM1 |
| GHRH (GRF) | GHRHR | VPAC1, VPAC2 2 |
| PACAP 3 | PAC1, VPAC1, VPAC2 | melanocortin receptors (class A) [40] |
| VIP | VPAC1, VPAC2 | PAC1 |
| CRF [41,42] 4 | CRFR1 5 | CRFR2 |
| Urocortin1 [41] | CRFR1, CRFR2 | |
| Urocortin2 [41] | CRFR2 | CRFR1 |
| Urocortin3 [41] | CRFR2 | CRFR1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latek, D.; Langer, I.; Krzysko, K.A.; Charzewski, L. A Molecular Dynamics Study of Vasoactive Intestinal Peptide Receptor 1 and the Basis of Its Therapeutic Antagonism. Int. J. Mol. Sci. 2019, 20, 4348. https://doi.org/10.3390/ijms20184348
Latek D, Langer I, Krzysko KA, Charzewski L. A Molecular Dynamics Study of Vasoactive Intestinal Peptide Receptor 1 and the Basis of Its Therapeutic Antagonism. International Journal of Molecular Sciences. 2019; 20(18):4348. https://doi.org/10.3390/ijms20184348
Chicago/Turabian StyleLatek, Dorota, Ingrid Langer, Krystiana A. Krzysko, and Lukasz Charzewski. 2019. "A Molecular Dynamics Study of Vasoactive Intestinal Peptide Receptor 1 and the Basis of Its Therapeutic Antagonism" International Journal of Molecular Sciences 20, no. 18: 4348. https://doi.org/10.3390/ijms20184348
APA StyleLatek, D., Langer, I., Krzysko, K. A., & Charzewski, L. (2019). A Molecular Dynamics Study of Vasoactive Intestinal Peptide Receptor 1 and the Basis of Its Therapeutic Antagonism. International Journal of Molecular Sciences, 20(18), 4348. https://doi.org/10.3390/ijms20184348