Protection of Male Rat Offspring against Hypertension Programmed by Prenatal Dexamethasone Administration and Postnatal High-Fat Diet with the Nrf2 Activator Dimethyl Fumarate during Pregnancy
Abstract
1. Introduction
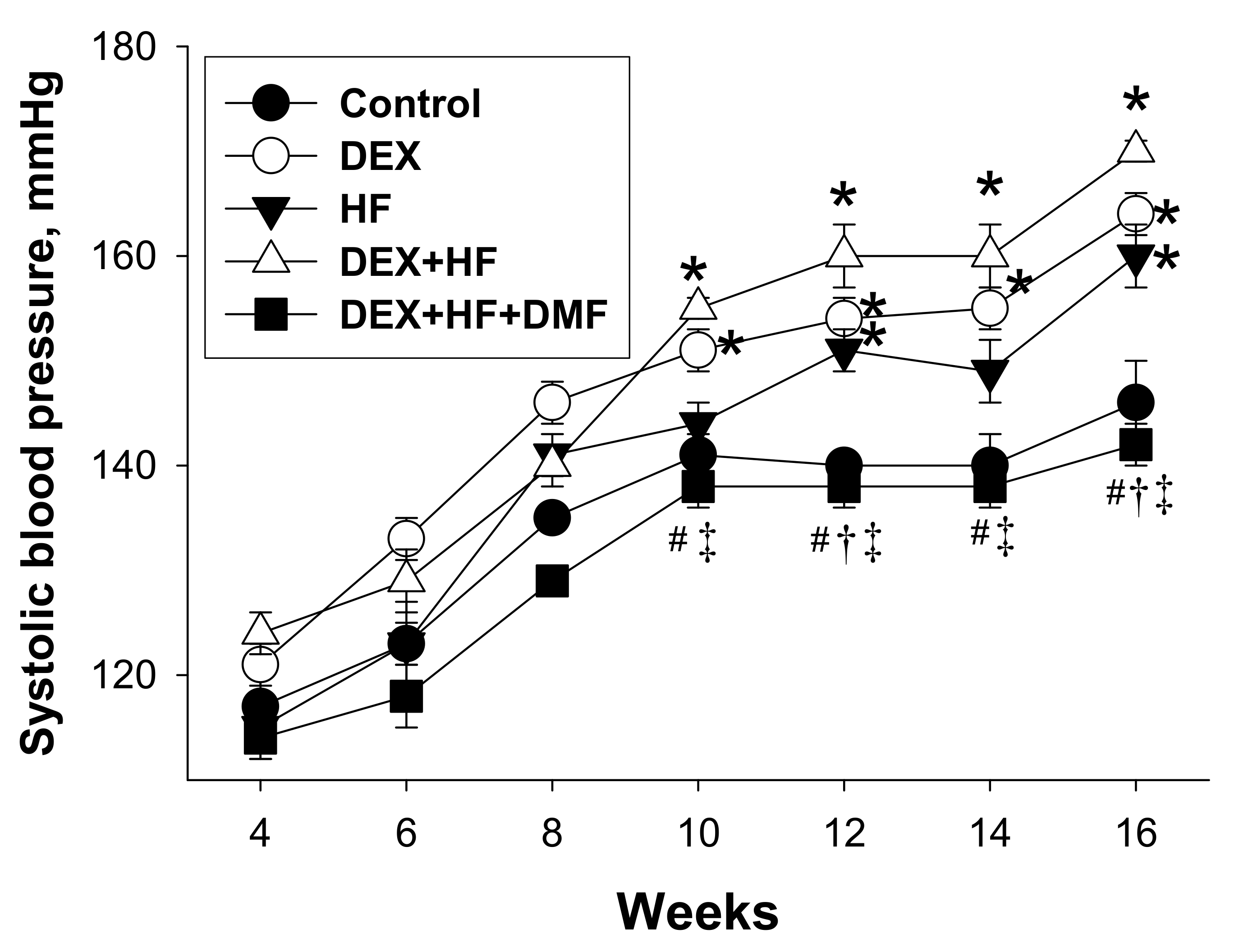
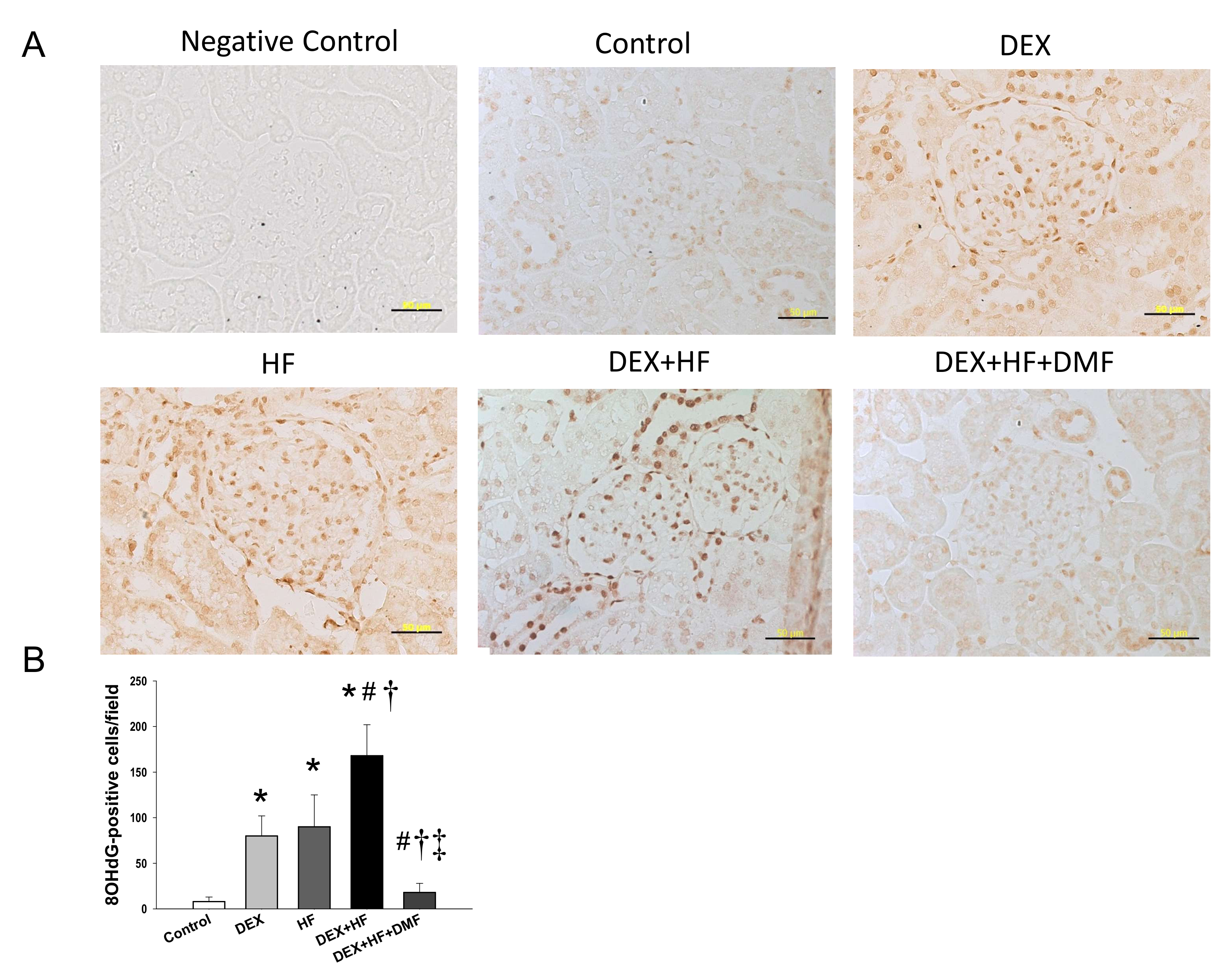
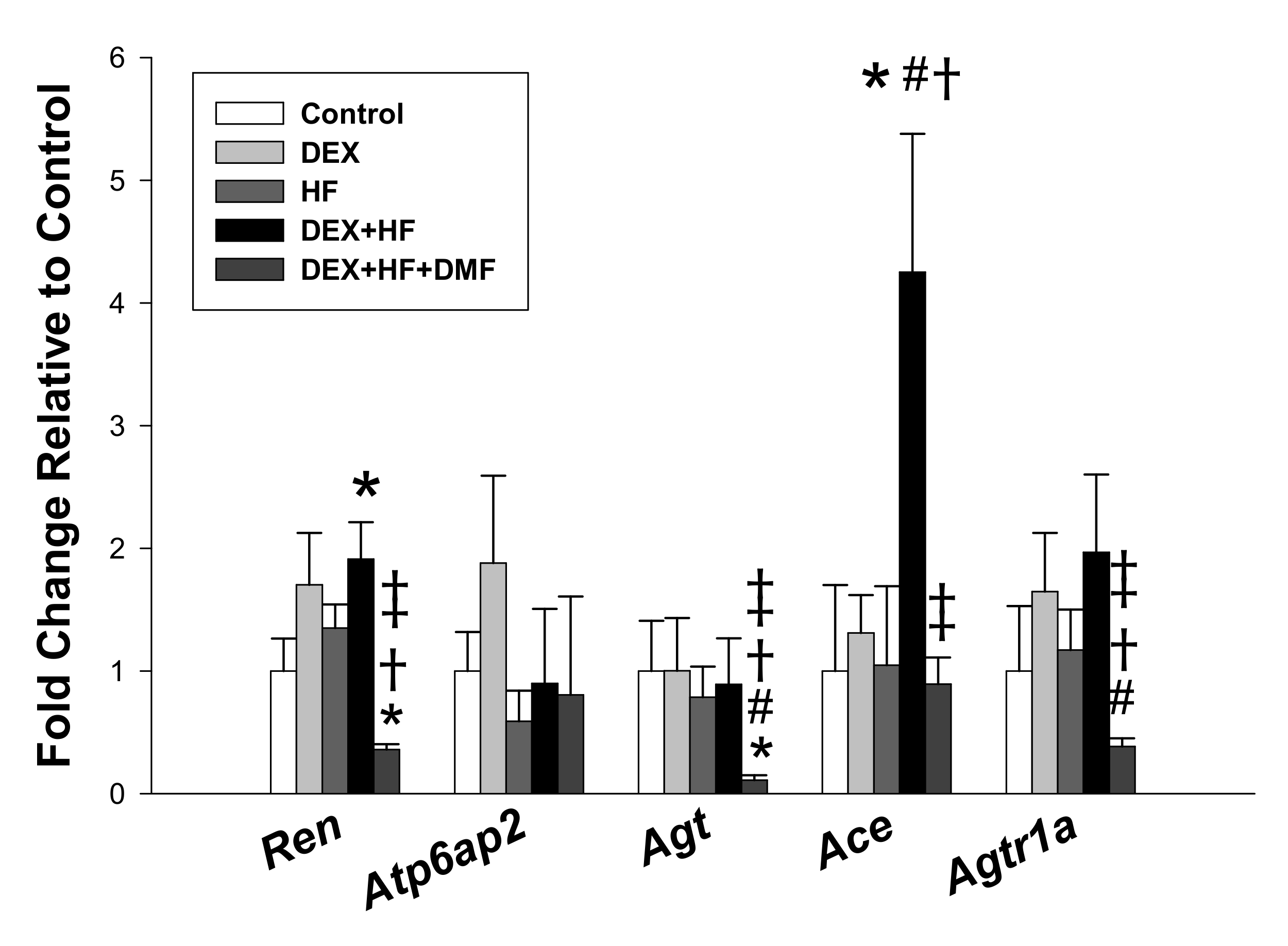
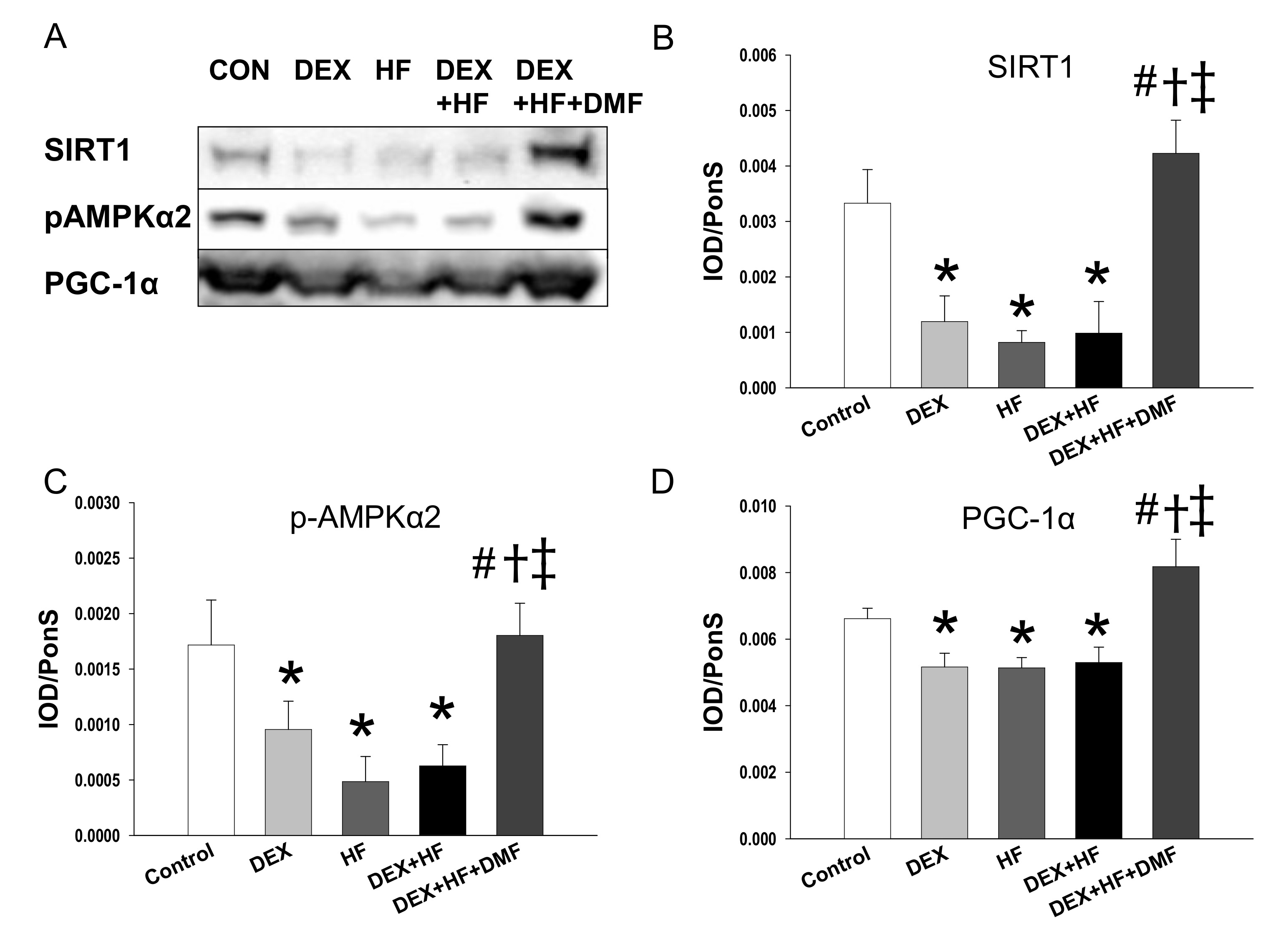
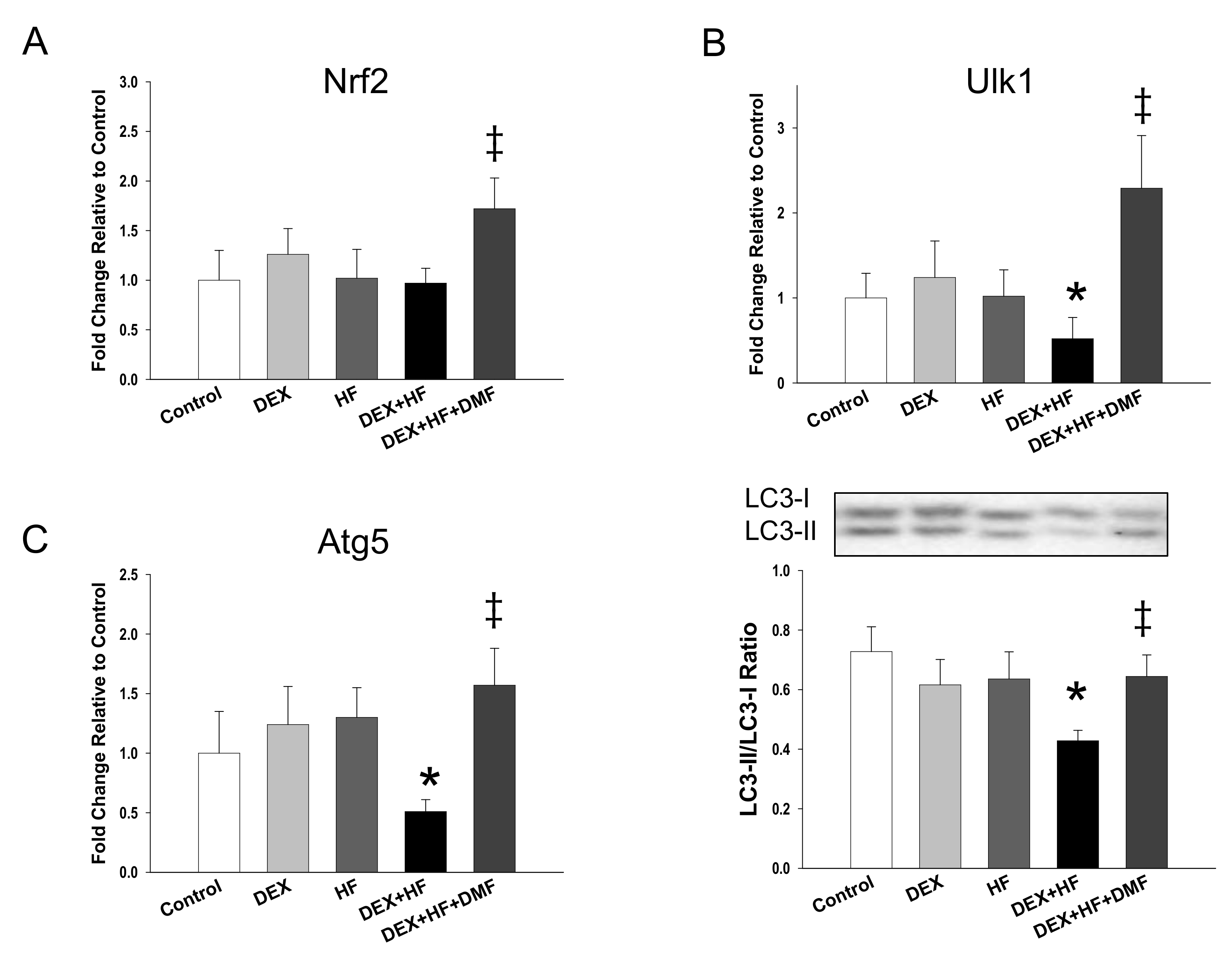
2. Results
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.3. High-Performance Liquid Chromatography (HPLC)
4.4. Western Blotting
4.5. Immunohistochemical Staining
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barker, D.J.; Bagby, S.P.; Hanson, M.A. Mechanisms of disease: In utero programming in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2006, 2, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.P.; Al-Hasan, Y. Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R913–R935. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.G.; Echeverri, I.; De Plata, C.A.; Castillo, A. Impact of oxidative stress during pregnancy on fetal epigenetic patterns and early origin of vascular diseases. Nutr. Rev. 2015, 73, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Interplay between oxidative stress and nutrient sensing signaling in the developmental origins of cardiovascular disease. Int. J. Mol. Sci. 2017, 18, 841. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Dalziel, S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2006, 3, CD004454. [Google Scholar]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef]
- Nascimento, L.; Freitas, C.M.; Silva-Filho, R.; Leite, A.C.; Silva, A.B.; Da Silva, A.I.; Ferreira, D.S.; Pedroza, A.A.; Maia, M.B.; Fernandes, M.P.; et al. The effect of maternal low-protein diet on the heart of adult offspring: Role of mitochondria and oxidative stress. Appl. Physiol. Nutr. Metab. 2014, 39, 880–887. [Google Scholar] [CrossRef]
- Tai, I.H.; Sheen, J.M.; Lin, Y.J.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Huang, L.T.; Tain, Y.L. Maternal N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and prevents programmed hypertension in male offspring exposed to prenatal dexamethasone and postnatal high-fat diet. Nitric Oxide 2016, 53, 6–12. [Google Scholar] [CrossRef]
- Tain, Y.L.; Sheen, J.M.; Yu, H.R.; Chen, C.C.; Tiao, M.M.; Hsu, C.N.; Lin, Y.J.; Kuo, K.C.; Huang, L.T. Maternal melatonin therapy rescues prenatal dexamethasone and postnatal high-fat diet induced programmed hypertension in male rat offspring. Front. Physiol. 2015, 6, 377. [Google Scholar] [CrossRef]
- Kett, M.M.; Denton, K.M. Renal programming: Cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R791–R803. [Google Scholar] [CrossRef] [PubMed]
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and fetal programming part 2: Mechanisms. Nat. Rev. Endocrinol. 2014, 10, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Huang, L.T.; Chang, H.Y.; Tain, Y.L. Prenatal dexamethasone induced programmed hypertension and renal programming. Life Sci. 2015, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Chan, J.Y.; Lee, C.T. Transcriptome analysis in rat kidneys: Importance of genes involved in programmed hypertension. Int. J. Mol. Sci. 2015, 16, 4744–4758. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 2005, 7, 385–394. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Javkhedkar, A.A.; Quiroz, Y.; Rodriguez-Iturbe, B.; Vaziri, N.D.; Lokhandwala, M.F.; Banday, A.A. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R840–R846. [Google Scholar] [CrossRef]
- Lin, Y.J.; Lin, I.C.; Yu, H.R.; Sheen, J.M.; Huang, L.T.; Tain, Y.L. Early Postweaning Treatment with Dimethyl Fumarate Prevents Prenatal Dexamethasone- and Postnatal High-Fat Diet-Induced Programmed Hypertension in Male Rat Offspring. Oxid. Med. Cell Longev. 2018, 2018, 5343462. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 62, e1800066. [Google Scholar] [CrossRef]
- Tintoré, M.; Sastre-Garriga, J. Multiple sclerosis: Dimethyl fumarate is coming of age. Nat. Rev. Neurol. 2016, 12, 436–437. [Google Scholar] [CrossRef]
- Atwan, A.; Ingram, J.R.; Abbott, R.; Kelson, M.J.; Pickles, T.; Bauer, A.; Piguet, V. Oral fumaric acid esters for psoriasis. Cochrane Database Syst. Rev. 2015, 8, CD010497. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.P.; Portaccio, E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: Impact of disease-modifying drugs. CNS Drugs 2015, 29, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Takasu, C.; Vaziri, N.D.; Li, S.; Robles, L.; Vo, K.; Takasu, M.; Pham, C.; Liu, S.; Farzaneh, S.H.; Foster, C.E., 3rd.; et al. Treatment with dimethyl fumarate attenuates calcineurin inhibitor-induced nephrotoxicity. Transplantation 2015, 99, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Targeting on asymmetric dimethylarginine related nitric oxide-reactive oxygen species imbalance to reprogram the development of hypertension. Int. J. Mol. Sci. 2016, 17, 2020. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Scalera, F.; Ignarro, L.J. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Powell, T.L. Role of placental nutrient sensing in developmental programming. Clin. Obstet. Gynecol. 2013, 56, 591–601. [Google Scholar] [CrossRef]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef]
- Zhang, J. Teaching the basics of autophagy and mitophagy to redox biologists—Mechanisms and experimental approaches. Redox Biol. 2015, 4, 242–259. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lin, Y.J.; Sheen, J.M.; Lin, I.C.; Yu, H.R.; Huang, L.T.; Hsu, C.N. Resveratrol prevents the combined maternal plus postweaning high-fat-diets-induced hypertension in male offspring. J. Nutr. Biochem. 2017, 48, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.E.; Lin, Y.J.; Lin, I.C.; Yu, H.R.; Sheen, J.M.; Tsai, C.C.; Huang, L.T.; Tain, Y.L. Resveratrol prevents combined prenatal NG-Nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient-sensing signals, oxidative stress and gut microbiota. J. Nutr. Biochem. 2019, 70, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Norlander, A.E.; Madhur, M.S.; Harrison, D.G. The immunology of hypertension. J. Exp. Med. 2018, 215, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G.; Guzik, T.J.; Lob, H.E.; Madhur, M.S.; Marvar, P.J.; Thabet, S.R.; Vinh, A.; Weyand, C.M. Inflammation, immunity, and hypertension. Hypertension 2011, 57, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Kanazawa, T.; Daigaku, Y.; Fujimura, S.; Miotto, G.; Kadowaki, M. Cytosolic LC3 ratio as a sensitive index of macroautophagy in isolated rat hepatocytes and H4-II-E cells. Autophagy 2007, 3, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Van Goor, H.; Havinga, R.; Baller, J.F.; Bloks, V.W.; Van der Leij, F.R.; Sauer, P.J.; Kuipers, F.; Navis, G.; De Borst, M.H. Neonatal dexamethasone administration causes progressive renal damage due to induction of an early inflammatory response. Am. J. Physiol. Renal Physiol. 2008, 294, F768–F776. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, K.; Li, X.; Coker, M.; Flesch, M.; Barger, P.M.; Sivasubramanian, N.; Mann, D.L. Cross-regulation between the renin-angiotensin system and inflammatory mediators in cardiac hypertrophy and failure. Cardiovasc. Res. 2004, 63, 433–442. [Google Scholar] [CrossRef]
- Satou, R.; Penrose, H.; Navar, L.G. Inflammation as a Regulator of the Renin-Angiotensin System and Blood Pressure. Curr. Hypertens. Rep. 2018, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Lipton, S. Recent advances in understanding NRF2 as a druggable target: Development of pro-electrophilic and non-covalent NRF2 activators to overcome systemic side effects of electrophilic drugs like dimethyl fumarate. F1000Research 2017, 6, 2138. [Google Scholar] [CrossRef]
- McSweeney, S.R.; Warabi, E.; Siow, R.C. Nrf2 as an Endothelial Mechanosensitive Transcription Factor: Going With the Flow. Hypertension 2016, 67, 20–29. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Giampieri, F.; Pistollato, F.; Sureda, A.; De Oliveira, M.R.; Pittalà, V.; Fallarino, F.; Nabavi, S.F.; Atanasov, A.G.; Nabavi, S.M. Nrf2 as regulator of innate immunity: A molecular Swiss army knife! Biotechnol. Adv. 2018, 36, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Wakabayashi, J.; Yates, M.S.; Wakabayashi, N.; Dolan, P.M.; Aja, S.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur. J. Pharmacol. 2009, 620, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
| Groups | Control | DEX | HF | DEX+HF | DEX+HF+DMF |
|---|---|---|---|---|---|
| n = 11 | n = 12 | n = 13 | n = 12 | n = 13 | |
| Mortality | 0% | 0% | 0% | 0% | 0% |
| Body weight (BW) (g) | 542 ± 15 | 499 ± 16 | 531 ± 13 | 499 ± 12 | 507 ± 12 |
| Left kidney weight (g) | 1.74 ± 0.06 | 1.73 ± 0.06 | 1.69 ± 0.06 | 1.53 ± 0.04 | 1.72 ± 0.05 |
| Left kidney weight/100g BW | 0.32 ± 0.01 | 0.35 ± 0.01 | 0.32 ± 0.01 | 0.31 ± 0.01 | 0.34 ± 0.01 |
| Systolic blood pressure (mmHg) | 140 ± 3 | 164 ± 2 * | 151 ± 2 * | 170 ± 1 *#† | 142 ± 2 #†‡ |
| Diastolic blood pressure (mmHg) | 69 ± 2 | 79 ± 2 * | 88 ± 2* | 90 ± 4 *# | 72 ± 3 #†‡ |
| Mean arterial pressure (mmHg) | 92 ± 2 | 107 ± 2 * | 109 ± 1 * | 117 ± 3 *#† | 95 ± 23 #†‡ |
| Groups | Control | DEX | HF | DEX+HF | DEX+HF+DMF |
|---|---|---|---|---|---|
| l-Citrulline (μM) | 55.8 ± 5.4 | 56.4 ± 4.6 | 66.1 ± 5.9 | 63.4 ± 4.5 | 36 ± 2.1 #†‡ |
| l-Arginine (μM) | 305.1 ± 27.2 | 348.5 ± 19.8 | 284.5 ± 18.1 | 292.9 ± 31 | 252.7 ± 19 |
| ADMA (μM) | 1.94 ± 0.34 | 1.92 ± 0.2 | 2.47 ± 0.22 | 2.27 ± 0.24 | 1.19 ± 0.24†‡ |
| SDMA (μM) | 1.81 ± 0.25 | 1.74 ± 0.15 | 2.05 ± 0.11 | 1.88 ± 0.2 | 1.95 ± 0.17 |
| l-Arginine-to-ADMA ratio (μM/μM) | 200 ± 40 | 209 ± 44 | 120 ± 9 | 144 ± 26 | 289 ± 65 †‡ |
| Gene | Forward | Reverse |
|---|---|---|
| Nrf2 | 5 cccattgagggctgtgatct 3 | 5 tcagtgaaatgccggagtca 3 |
| Ren | 5 aacattaccagggcaactttcact 3 | 5 acccccttcatggtgatctg 3 |
| Atp6ap2 | 5 gaggcagtgaccctcaacat 3 | 5 ccctcctcacacaacaaggt 3 |
| Agt | 5 gcccaggtcgcgatgat 3 | 5 tgtacaagatgctgagtgaggcaa 3 |
| Ace | 5 caccggcaaggtctgctt 3 | 5 cttggcatagtttcgtgaggaa 3 |
| Agtr1a | 5 gctgggcaacgagtttgtct 3 | 5 cagtccttcagctggatcttca 3 |
| Ulk1 | 5 gagtacccgcaccagaatgt 3 | 5 gctgtgtagggtttccgtgt 3 |
| Atg5 | 5 ttggcctactgttcgatcttctt 3 | 5 ggacagtgcagaaggtcctttt 3 |
| Rn18s | 5 gccgcggtaattccagctcca 3 | 5 cccgcccgctcccaagatc 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-N.; Lin, Y.-J.; Yu, H.-R.; Lin, I.-C.; Sheen, J.-M.; Huang, L.-T.; Tain, Y.-L. Protection of Male Rat Offspring against Hypertension Programmed by Prenatal Dexamethasone Administration and Postnatal High-Fat Diet with the Nrf2 Activator Dimethyl Fumarate during Pregnancy. Int. J. Mol. Sci. 2019, 20, 3957. https://doi.org/10.3390/ijms20163957
Hsu C-N, Lin Y-J, Yu H-R, Lin I-C, Sheen J-M, Huang L-T, Tain Y-L. Protection of Male Rat Offspring against Hypertension Programmed by Prenatal Dexamethasone Administration and Postnatal High-Fat Diet with the Nrf2 Activator Dimethyl Fumarate during Pregnancy. International Journal of Molecular Sciences. 2019; 20(16):3957. https://doi.org/10.3390/ijms20163957
Chicago/Turabian StyleHsu, Chien-Ning, Yu-Ju Lin, Hong-Ren Yu, I-Chun Lin, Jiunn-Ming Sheen, Li-Tung Huang, and You-Lin Tain. 2019. "Protection of Male Rat Offspring against Hypertension Programmed by Prenatal Dexamethasone Administration and Postnatal High-Fat Diet with the Nrf2 Activator Dimethyl Fumarate during Pregnancy" International Journal of Molecular Sciences 20, no. 16: 3957. https://doi.org/10.3390/ijms20163957
APA StyleHsu, C.-N., Lin, Y.-J., Yu, H.-R., Lin, I.-C., Sheen, J.-M., Huang, L.-T., & Tain, Y.-L. (2019). Protection of Male Rat Offspring against Hypertension Programmed by Prenatal Dexamethasone Administration and Postnatal High-Fat Diet with the Nrf2 Activator Dimethyl Fumarate during Pregnancy. International Journal of Molecular Sciences, 20(16), 3957. https://doi.org/10.3390/ijms20163957








