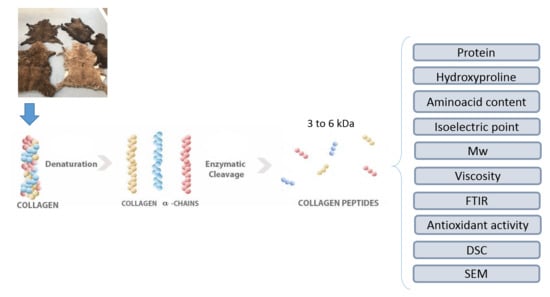
Hydrolysed Collagen from Sheepskins as a Source of Functional Peptides with Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Protein Content
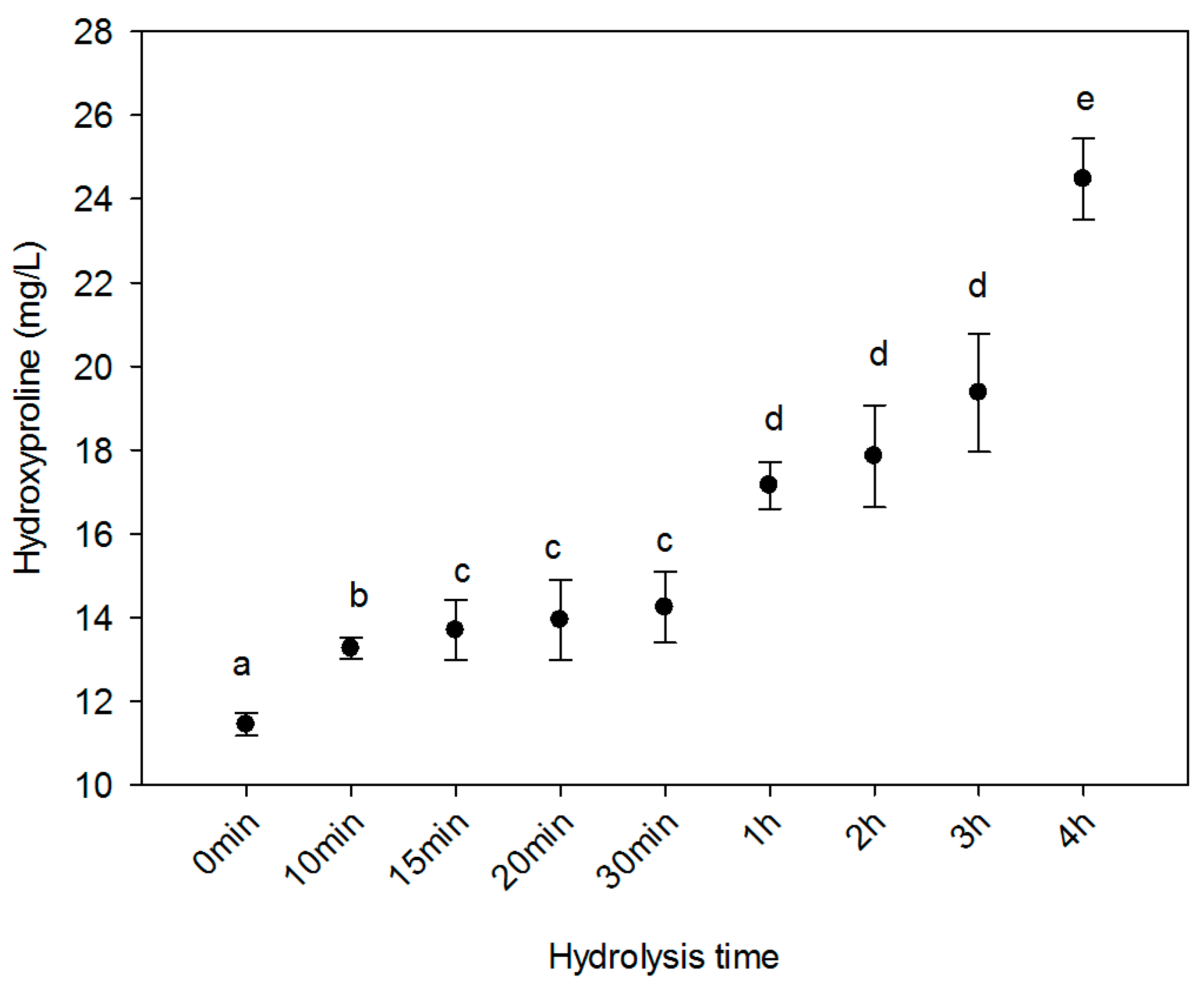
2.2. Hydroxyproline Content
2.3. Amino Acid Content in Hydrolysed Collagen as a Function of Hydrolysis Time
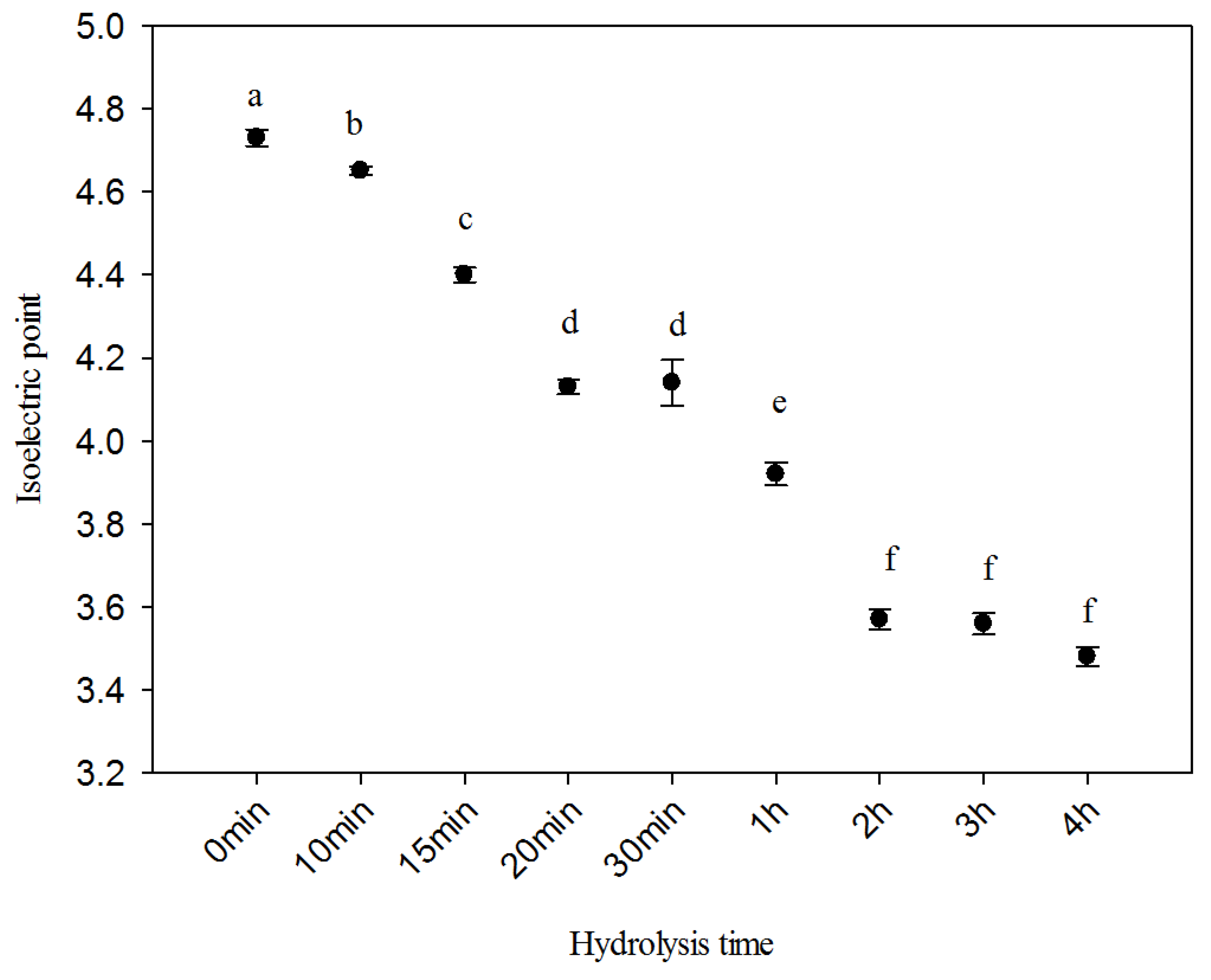
2.4. Isoelectric Point
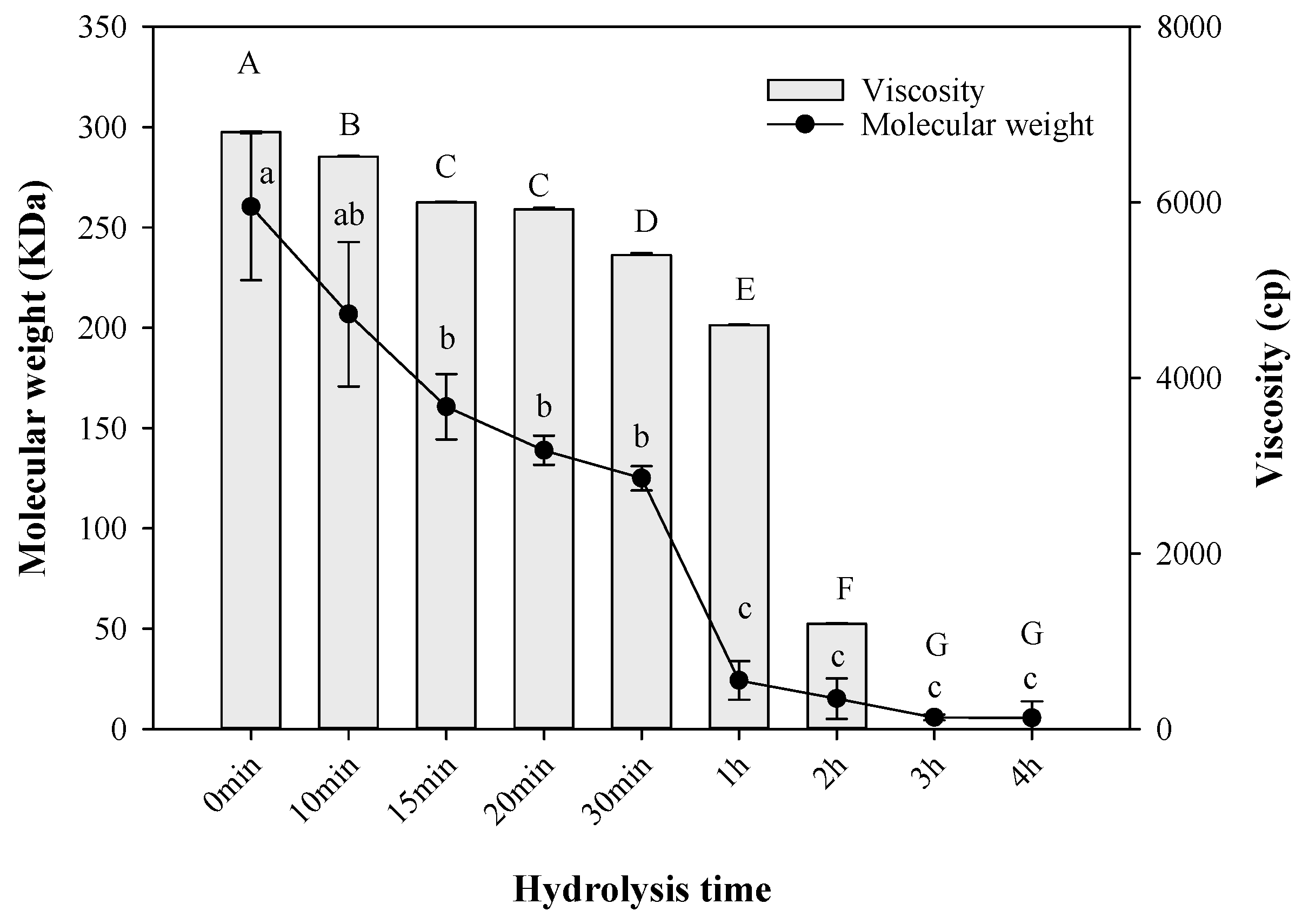
2.5. Molecular Weight and Viscosity
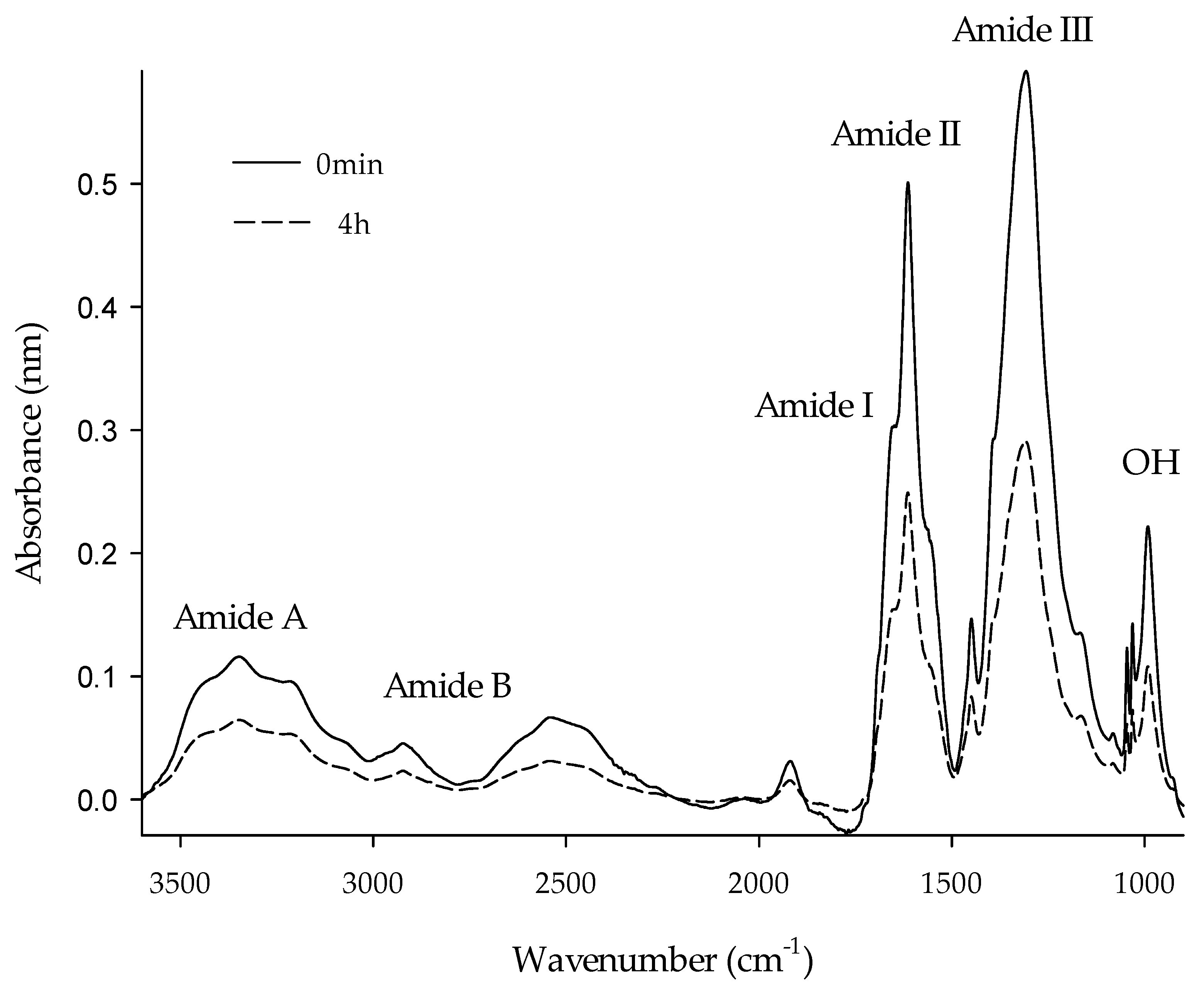
2.6. Fourier Transform-Infrared Spectroscopy (FTIR)
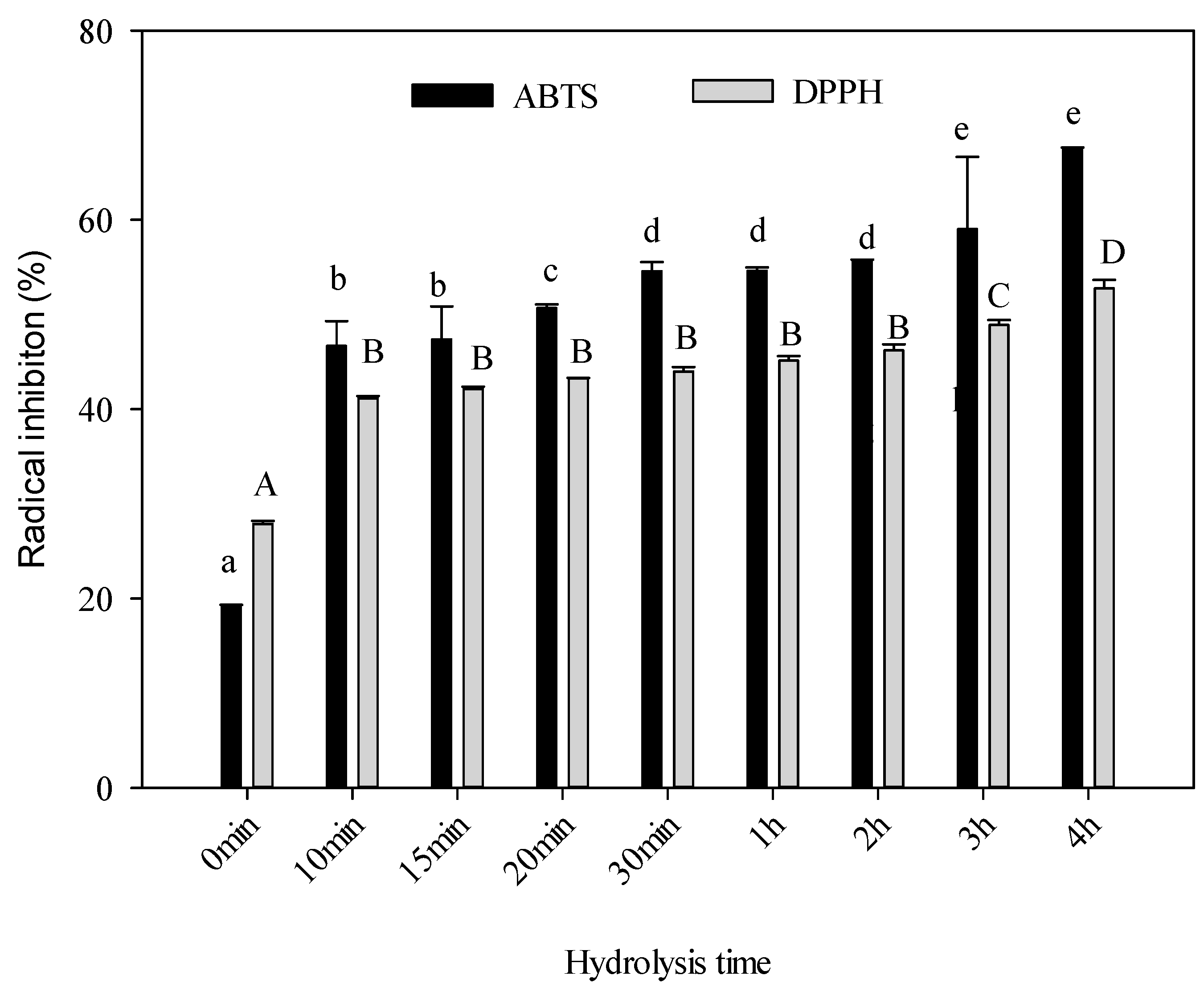
2.7. Antioxidant Activity
2.8. Thermal Properties
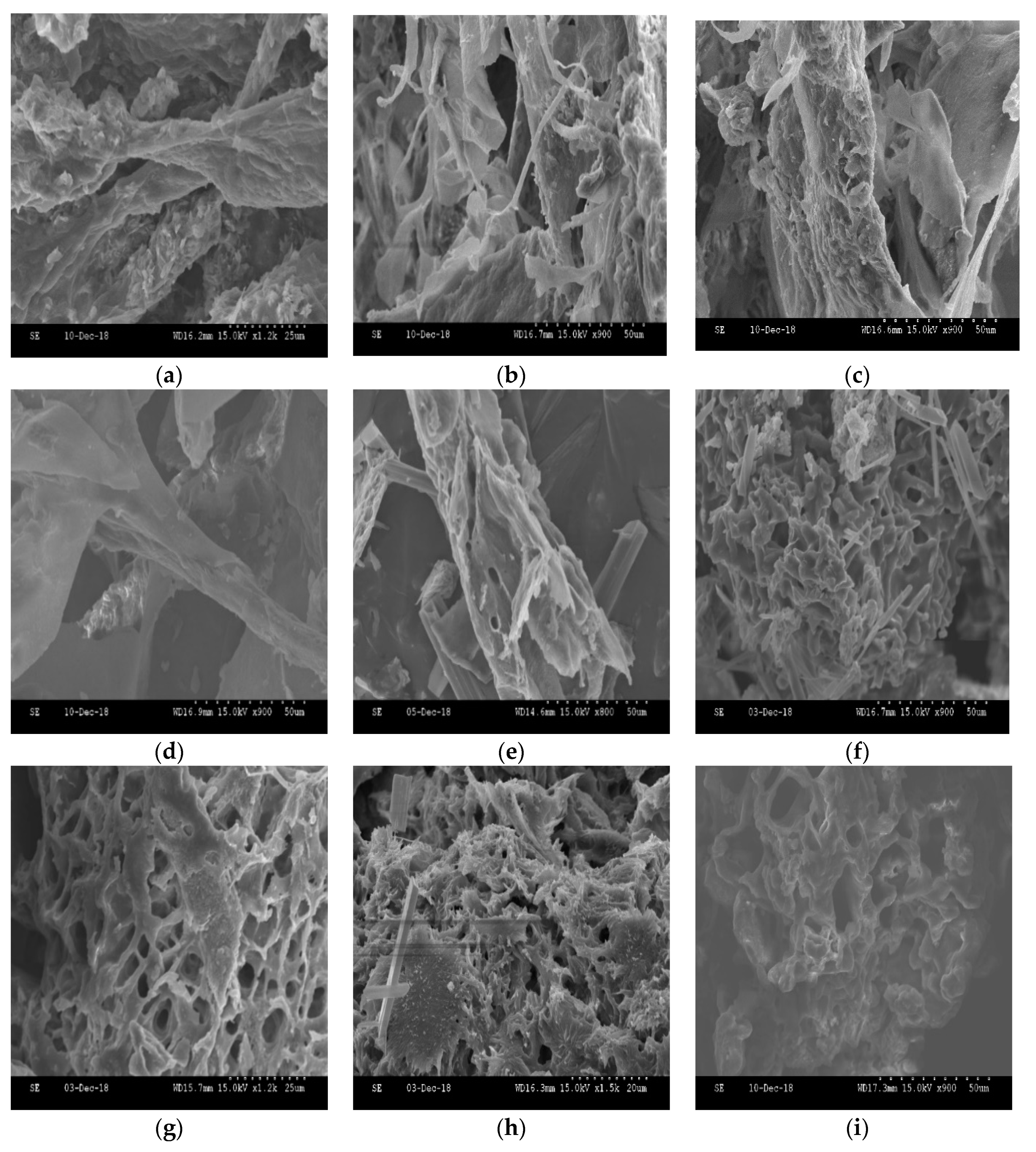
2.9. SEM Images
2.10. Relationship between Mw, Viscosity, Antioxidant Activity and Thermal Properties
3. Materials and Methods
3.1. Conditioning of The Skin before Collagen Extraction
3.2. Collagen Extraction from Ovine Skin
3.3. Hydrolysis of Collagen
3.4. Protein Determination
3.5. Isoelectric Point
3.6. Hydroxyproline Quantification
- Y = Hydroxyproline concentration from the standard curve
- W = sample weight
- V = volume in mL to adjust the 100 mL
3.7. Viscosity Analysis
3.8. Molecular Weight
3.9. Fourier Transform-Infrared (FTIR) Spectroscopy
3.10. Differential Scanning Calorimetry (DSC)
3.11. Amino Acid Determination
3.12. Antioxidant Activity
3.13. Scanning Electron Microscopy (SEM)
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HC | Hydrolysed collagen |
| SDS-PAGE | Sodium dodecyl sulphate polyacrylamide gel Electrophoresis |
| Mw | Molecular weight |
| FTIR | Fourier transform-infrared spectroscopy |
| DSC | Differential scanning calorimetry |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis, 3-ethylbenzothiazoline-6-sulphonic acid |
| ANOVA | Analysis of variance |
| SEM | Scanning electron microscopy |
References
- Gao, L.-L.; Wang, Z.-Y.; Li, Z.; Zhang, C.-X.; Zhang, D.-Q. The characterization of acid and pepsin soluble collagen from ovine bones (Ujumuqin sheep). J. Integr. Agric. 2018, 17, 704–711. [Google Scholar] [CrossRef]
- Liu, D.; Liang, L.; Regenstein, J.M.; Zhou, P. Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem. 2012, 133, 1441–1448. [Google Scholar] [CrossRef]
- Rodriguez, M.I.A.; Barroso, L.G.R. Sanchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Min, S.G.; Jo, Y.J. Functionality of porcine skin hydrolysates produced by hydrothermal processing for liposomal delivery system. J. Food Biochem. 2018, 42, e12464. [Google Scholar] [CrossRef]
- O’Sullivan, S.M.; Lafarga, T.; Hayes, M.; O’Brien, N.M. Bioactivity of bovine lung hydrolysates prepared using papain, pepsin, and Alcalase. J. Food Biochem. 2017, 41, e12406. [Google Scholar] [CrossRef]
- Benjakul, S.; Karnjanapratum, S.; Visessanguan, W. Production and Characterization of Odorless Antioxidative Hydrolyzed Collagen from Seabass (Lates calcarifer) Skin without Descaling. Waste Biomass Valori. 2018, 9, 549–559. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Tuo, F.Y.; Song, L.; Liu, Y.X.; Dong, X.P.; Li, D.M.; Zhou, D.Y.; Shahidi, F. Action of trypsin on structural changes of collagen fibres from sea cucumber (Stichopus japonicus). Food Chem. 2018, 256, 113–118. [Google Scholar] [CrossRef]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef]
- Martínez-Ortiz, M.A.; Hernández-Fuentes, A.D.; Pimentel-González, D.J.; Campos-Montiel, R.G.; Vargas-Torres, A.; Aguirre-Álvarez, G. Extraction and characterization of collagen from rabbit skin: Partial characterization. Cyta J. Food 2015, 13, 253–258. [Google Scholar] [CrossRef]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An Overview of the Beneficial Effects of Hydrolysed Collagen as a Nutraceutical on Skin Properties: Scientific Background and Clinical Studies. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef]
- Bilek, S.E.; Bayram, S.K. Fruit juice drink production containing hydrolyzed collagen. J. Funct. Foods 2015, 14, 562–569. [Google Scholar] [CrossRef]
- Denis, A.; Brambati, N.; Dessauvages, B.; Guedj, S.; Ridoux, C.; Meffre, N.; Autier, C. Molecular weight determination of hydrolyzed collagens. Food Hydrocoll. 2008, 22, 989–994. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.-l.; Zhang, Q.; Li, P. Composite electrospun nanomembranes of fish scale collagen peptides/chito-oligosaccharides: antibacterial properties and potential for wound dressing. Int J Nanomed. 2011, 6, 667–676. [Google Scholar]
- Moskowitz, R.W. Role of collagen hydrolysate in bone and joint disease. Semin. Arthritis Rheum. 2000, 30, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Zague, V. A new view concerning the effects of collagen hydrolysate intake on skin properties. Arch. Dermatol. Res. 2008, 300, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, G.; Shi, B. Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes. J. Soc. Leather Technol. Chem. 2006, 90, 23. [Google Scholar]
- Lorenzo, J.M.; Munekata, P.E.S.; Gomez, B.; Barba, F.J.; Mora, L.; Perez-Santaescolastica, C.; Toldra, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Jin, H.X.; Xu, H.P.; Li, Y.; Zhang, Q.W.; Xie, H. Preparation and Evaluation of Peptides with Potential Antioxidant Activity by Microwave Assisted Enzymatic Hydrolysis of Collagen from Sea Cucumber Acaudina Molpadioides Obtained from Zhejiang Province in China. Mar. Drugs 2019, 17, 169. [Google Scholar] [CrossRef]
- Zhao, W.H.; Luo, Q.B.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Preparation, identification, and activity evaluation of ten antioxidant peptides from protein hydrolysate of swim bladders of miiuy croaker (Miichthys miiuy). J. Funct. Foods 2018, 47, 503–511. [Google Scholar] [CrossRef]
- Hong, G.P.; Min, S.G.; Jo, Y.J. Anti-Oxidative and Anti-Aging Activities of Porcine By-Product Collagen Hydrolysates Produced by Commercial Proteases: Effect of Hydrolysis and Ultrafiltration. Molecules 2019, 24, 1104. [Google Scholar] [CrossRef]
- Vidal, A.R.; Ferreira, T.E.; Mello, R.D.; Schmidt, M.M.; Kubota, E.H.; Demiate, I.M.; Zielinski, A.A.F.; Dornelles, R.C.P. Effects of enzymatic hydrolysis (Flavourzyme (R)) assisted by ultrasound in the structural and functional properties of hydrolyzates from different bovine collagens. Food Sci. Technol. 2018, 38, 103–108. [Google Scholar] [CrossRef]
- Liu, C.Y.; Ma, X.M.; Che, S.; Wang, C.W.; Li, B.F. The Effect of Hydrolysis with Neutrase on Molecular Weight, Functional Properties, and Antioxidant Activities of Alaska Pollock Protein Isolate. J. Ocean Univ. China 2018, 17, 1423–1431. [Google Scholar] [CrossRef]
- Blanco, M.; Vazquez, J.A.; Perez-Martin, R.I.; Sotelo, C.G. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Comparative assessment of physico-chemical characteristics and fibril formation capacity of thermostable carp scales collagen. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 70, 32–40. [Google Scholar] [CrossRef]
- Lee, H.-J.; Chae, S.-J.; Saravana, P.S.; Chun, B.-S. Physical and functional properties of tunicate (Styela clava) hydrolysate obtained from pressurized hydrothermal process. Fish. Aquat. Sci. 2017, 20, 14. [Google Scholar] [CrossRef]
- Felician, F.F.; Xia, C.L.; Qi, W.Y.; Xu, H.M. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef]
- Sae-leaw, T.; Benjakul, S. Antioxidant activities of hydrolysed collagen from salmon scale ossein prepared with the aid of ultrasound. Int. J. Food Sci. Technol. 2018, 53, 2786–2795. [Google Scholar] [CrossRef]
- Paul, R.; Adzet, J.M.; Brouta-Agnésa, M.; Balsells, S.; Esteve, H. Hydrolyzed collagen: A novel additive in cotton and leather dyeing. Dye. Pigment. 2012, 94, 475–480. [Google Scholar] [CrossRef]
- Offengenden, M.; Chakrabarti, S.; Wu, J. Chicken collagen hydrolysates differentially mediate anti-inflammatory activity and type I collagen synthesis on human dermal fibroblasts. Food Sci. Hum. Wellness 2018, 7, 138–147. [Google Scholar] [CrossRef]
- Bateman, J.F.; Lamande, S.R.; Ramshaw, J.A. Collagen superfamily. Extracell. Matrix 1996, 2, 22–67. [Google Scholar]
- Atma, Y.; Hanifah, N.L.; Endang, P.; Hermawan, S.; Moh, T.; Dita, F.; Apon, Z.M. The hydroxyproline content of fish bone gelatin from Indonesian pangasius catfish by enzymatic hydrolysis for producing the bioactive peptide. Biofarmasi. J. Nat. Prod. Biochem. 2018, 6, 64–68. [Google Scholar]
- Ao, J.; Li, B. Amino acid composition and antioxidant activities of hydrolysates and peptide fractions from porcine collagen. Food Sci. Technol. Int. 2012, 18, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Li-Chan, E.C.Y.; Hunag, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Peptides Derived from Atlantic Salmon Skin Gelatin as Dipeptidyl-peptidase IV Inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef]
- Benjakul, S.; Oungbho, K.; Visessanguan, W.; Thiansilakul, Y.; Roytrakul, S. Characteristics of gelatin from the skins of bigeye snapper, Priacanthus tayenus and Priacanthus macracanthus. Food Chem. 2009, 116, 445–451. [Google Scholar] [CrossRef]
- Gómez-Lizárraga, K.; Piña-Barba, C.; Rodríguez-Fuentes, N.; Romero, M. Obtención y caracterización de colágena tipo I a partir de tendón bovino. Superf. Y Vacío 2011, 24, 137–140. [Google Scholar]
- Soladoye, O.P.; Saldo, J.; Peiro, L.; Rovira, A.; Mor-Mur, M. Antioxidant and Angiotensin 1 Converting Enzyme Inhibitory Functions from Chicken Collagen Hydrolysates. J. Nutr. Food Sci. 2015, 5, 1. [Google Scholar] [CrossRef]
- Friess, W. Collagen–biomaterial for drug delivery. Eur. J. Pharm. Biopharm. 1998, 45, 113–136. [Google Scholar] [CrossRef]
- Paschalis, E.; Verdelis, K.; Doty, S.; Boskey, A.; Mendelsohn, R.; Yamauchi, M. Spectroscopic characterization of collagen cross-links in bone. J. Bone Miner. Res. 2001, 16, 1821–1828. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Wu, H.C.; Chen, H.M.; Shiau, C.Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Aguirre-Alvarez, G.; Foster, T.; Hill, S.E. Impact of the origin of gelatins on their intrinsic properties. Cyta-J. Food 2012, 10, 306–312. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.T.; Byun, H.G.; Nam, K.S.; Joo, D.S.; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J. Agric. Food Chem. 2001, 49, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Caessens, P.W.; Daamen, W.F.; Gruppen, H.; Visser, S.; Voragen, A.G. β-Lactoglobulin hydrolysis. 2. Peptide identification, SH/SS exchange, and functional properties of hydrolysate fractions formed by the action of plasmin. J. Agric. Food Chem. 1999, 47, 2980–2990. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, W.T.; Li, G.Y. Isolation and characterisation of collagens from the skin of largefin longbarbel catfish (Mystus macropterus). Food Chem. 2009, 115, 826–831. [Google Scholar] [CrossRef]
- Chi, C.; Hu, F.; Li, Z.; Wang, B.; Luo, H. Influence of Different Hydrolysis Processes by Trypsin on the Physicochemical, Antioxidant, and Functional Properties of Collagen Hydrolysates from Sphyrna lewini, Dasyatis akjei, and Raja porosa. J. Aquat. Food Prod. Technol. 2016, 25, 616–632. [Google Scholar] [CrossRef]
- Khiari, Z.; Ndagijimana, M.; Betti, M. Low molecular weight bioactive peptides derived from the enzymatic hydrolysis of collagen after isoelectric solubilization/precipitation process of turkey by-products. Poult. Sci. 2014, 93, 2347–2362. [Google Scholar] [CrossRef]
- Yan, L.J.; Jin, T.C.; Chen, Y.L.; Zhan, C.L.; Zhang, L.J.; Weng, L.; Liu, G.M.; Cao, M.J. Characterization of a recombinant matrix metalloproteinase-2 from sea cucumber (Stichopus japonicas) and its application to prepare bioactive collagen hydrolysate. Process Biochem. 2018, 72, 63–70. [Google Scholar] [CrossRef]
- Ogawa, M.; Portier, R.J.; Moody, M.W.; Bell, J.; Schexnayder, M.A.; Losso, J.N. Biochemical properties of bone and scale collagens isolated from the subtropical fish black drum (Pogonia cromis) and sheepshead seabream (Archosargus probatocephalus). Food Chem. 2004, 88, 495–501. [Google Scholar] [CrossRef]
- Pan, B.S.; Chen, H.E.; Sung, W.C. Molecular and thermal characteristics of acid-soluble collagen from orbicular batfish: effects of deep-sea water culturing. Int. J. Food Prop. 2018, 21, 1080–1090. [Google Scholar] [CrossRef]
- Barth, A.; Zscherp, C. What vibrations tell us about proteins. Q. Rev. Biophys. 2002, 35, 369–430. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Shanab, S.M.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo-Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Foh, M.B.K.; Amadou, I.; Foh, B.M.; Kamara, M.T.; Xia, W. Functionality and antioxidant properties of Tilapia (Oreochromis niloticus) as influenced by the degree of hydrolysis. Int. J. Mol. Sci. 2010, 11, 1851–1869. [Google Scholar] [CrossRef]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino Acid Composition and Antioxidant Properties of Pea Seed (Pisum sativum L.) Enzymatic Protein Hydrolysate Fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef]
- Nam, K.A.; You, S.G.; Kim, S.M. Molecular and physical characteristics of squid (Todarodes pacificus) skin collagens and biological properties of their enzymatic hydrolysates. J. Food Sci. 2008, 73, C249–C255. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Khantaphant, S.; Benjakul, S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2008, 151, 410–419. [Google Scholar] [CrossRef]
- Busnel, J.P.; Morris, E.R.; Rossmurphy, S.B. Interpretation of the renaturation kinetics of gelatin solutions. Int. J. Biol. Macromol. 1989, 11, 119–125. [Google Scholar] [CrossRef]
- Harrington, W.F.; Rao, N.V. Collagen structure in solution. I. Kinetics of helix regeneration in single-chain gelatins. Biochemistry 1970, 9, 3714–3724. [Google Scholar] [CrossRef]
- Djabourov, M.; Nishinari, K.; Ross-Murphy, S.B.; Djabourov, M.; Nishinari, K.; RossMurphy, S.B. Helical Structures from Neutral Biopolymers; Cambridge Univ Press: Cambridge, UK, 2013; pp. 182–221. [Google Scholar]
- Chi, C.F.; Cao, Z.H.; Wang, B.; Hu, F.Y.; Li, Z.R.; Zhang, B. Antioxidant and functional properties of collagen hydrolysates from Spanish mackerel skin as influenced by average molecular weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef]
- Aguirre-Alvarez, G.; Pimentel-Gonzalez, D.J.; Campos-Montiel, R.G.; Foster, T.; Hill, S.E. The effect of drying temperature on mechanical properties of pig skin gelatin films. Cyta-J. Food 2011, 9, 243–249. [Google Scholar] [CrossRef]
- Chuaychan, S.; Benjakul, S.; Kishimura, H. Characteristics of acid- and pepsin-soluble collagens from scale of seabass (Lates calcarifer). Lwt Food Sci. Technol. 2015, 63, 71–76. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- AOAC Authors. Official methods of analysis Amino Acids. Analysis Acid hydrolysis Hydroxyproline—Item 77. In Food Analysis Methods, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2006; Reference data: Method 990.26 (39.1.27); NFNAP; NITR; HYP. [Google Scholar]
- Yu, D.; Chi, C.-F.; Wang, B.; Ding, G.-F.; Li, Z.-R. Characterization of acid-and pepsin-soluble collagens from spines and skulls of skipjack tuna (Katsuwonus pelamis). Chin. J. Nat. Med. 2014, 12, 712–720. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Fanelli, S.; Zimmermann, A.; Totoli, E.G.; Salgado, H.R.N. FTIR Spectrophotometry as a Green Tool for Quantitative Analysis of Drugs: Practical Application to Amoxicillin. J. Chem. 2018. [Google Scholar] [CrossRef]
- Cohen, S.A. Amino acids analysis using precolumn derivatization with 6-Aminoquinolyl-N-Hidroxysuccinimidyl Carbamate. In Amino Acid Analysis Protocols. Methods in Molecular Biology; Cooper, C., Packer, N., Williams, K., Eds.; Humana Press: Totowa, NJ, USA, 2001; Volume 159, pp. 39–47. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying and improve ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berse, C. Use of a free radical method to evaluate antioxidant activity. Lwt Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]

| Amino Acid | 0 min | 10 min | 20 min | 30 min | 1 h |
|---|---|---|---|---|---|
| Aspartic acid | 4.04 ± 0.03 a | 10.86 ± 0.05 d | 5.06 ± 0.04 f | 28.03 ± 0.036 g | 32.85 ± 0.16 a |
| Glutamic acid | 7.99 ± 0.06 a | 7.71 ± 0.04 a | 16.19 ± 0.05 e | 38.21 ± 0.048 f | 16.58 ± 0.03 a |
| Serine | 19.33 ± 0.16 a | 25.99 ± 0.03 c | 17.45 ± 0.03 a | 13.72 ± 0.03 a | 17.87 ± 0.05 a |
| Glycine | 4.86 ± 0.01 a | 4.31 ± 0.04 a | 16.71 ± 0.04 a | 1.92 ± 0.00 a | 3.10 ± 0.00 a |
| Lysine | 6.60 ± 0.04 a | 5.72 ± 0.02 a | 3.32 ± 0.06 a | 2.05 ± 0.00 a | 1.92 ± 0.00 a |
| Histidine | 3.38 ± 0.03 a | 3.25 ± 0.05 a | 2.41 ± 0.01 a | 3.95 ± 0.02 a | 1.59 ± 0.00 a |
| Threonine | 2.72 ± 0.06 a | 4.50 ± 0.02 a | 2.90 ± 0.01 a | 3.18 ± 0.01 a | 3.12 ± 0.00 a |
| Arginine | 8.91 ± 0.01 a | 3.46 ± 0.05 a | 18.62 ± 0.03 b | 2.68 ± 0.02 a | 11.02 ± 0.02 a |
| Alanine | 1.39 ± 0.04 a | 0.40 ± 0.04 a | 1.26 ± 0.00 a | 0.14 ± 0.00 a | 1.39 ± 0.00 a |
| Proline | 2.68 ± 0.04 a | 1.23 ± 0.03 a | 0.86 ± 0.00 a | 0.13 ± 0.00 a | 0.49 ± 0.00 a |
| Cysteine | 1.53 ± 0.02 a | 1.84 ± 0.03 a | 1.00 ± 0.00 a | 0.13 ± 0.00 a | 0.36 ± 0.00 a |
| Tyrosine | 12.36 ± 0.18 a | 3.02 ± 0.02 d | 7.24 ± 0.05 f | 0.78 ± 0.00 g | 2.9 ± 0.01 a |
| Valine | 3.17 ± 0.05 a | 2.73 ± 0.01 a | 1.67 ± 0.00 a | 1.53 ± 0.00 a | 1.26 ± 0.01 a |
| Methionine | 7.80 ± 0.04 a | 7.48 ± 0.04 a | 0.75 ± 0.00 c | 1.36 ± 0.00 d | 1.65 ± 0.00 a |
| Isoleucine | 6.13 ± 0.03 a | 6.49 ± 0.05 a | 0.52 ± 0.00 b | 0.27 ± 0.00 c | 1.21 ± 0.00 a |
| Leucine | 3.41 ± 0.01 a | 6.35 ± 0.03 b | 1.00 ± 0.00 d | 0.24 ± 0.00 e | 1.30 ± 0.00 a |
| Phenylalanine | 2.66 ± 0.07 a | 3.31 ± 0.04 a | 1.66 ± 0.00 a | 0.87 ± 0.00 a | 0.69 ± 0.00 a |
| Hydrolysis Time | Tm (°C) | ΔH (J/g) |
|---|---|---|
| 0 min | 153.38 ± 0.40 a | 20.06 ± 0.10 a |
| 15 min | 147.97 ± 0.80 a | 17.21 ± 0.47ab |
| 20 min | 147.72 ± 0.45 a | 17.48 ± 0.18 ab |
| 30 min | 147.59 ± 0.78 a | 16.91 ± 0.32 ab |
| 1 h | 137.91 ± 0.35 b | 15.61 ± 0.21 ab |
| 2 h | 137.77 ± 0.13 b | 11.46 ± 0.49 ab |
| 3 h | 137.24 ± 0.37 b | 9.09 ± 0.20 ab |
| 4 h | 136.91 ± 0.55 b | 8.93 ± 0.11 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-López, A.; Fuentes-Jiménez, L.; Hernández-Fuentes, A.D.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Hydrolysed Collagen from Sheepskins as a Source of Functional Peptides with Antioxidant Activity. Int. J. Mol. Sci. 2019, 20, 3931. https://doi.org/10.3390/ijms20163931
León-López A, Fuentes-Jiménez L, Hernández-Fuentes AD, Campos-Montiel RG, Aguirre-Álvarez G. Hydrolysed Collagen from Sheepskins as a Source of Functional Peptides with Antioxidant Activity. International Journal of Molecular Sciences. 2019; 20(16):3931. https://doi.org/10.3390/ijms20163931
Chicago/Turabian StyleLeón-López, Arely, Lucía Fuentes-Jiménez, Alma Delia Hernández-Fuentes, Rafael G. Campos-Montiel, and Gabriel Aguirre-Álvarez. 2019. "Hydrolysed Collagen from Sheepskins as a Source of Functional Peptides with Antioxidant Activity" International Journal of Molecular Sciences 20, no. 16: 3931. https://doi.org/10.3390/ijms20163931
APA StyleLeón-López, A., Fuentes-Jiménez, L., Hernández-Fuentes, A. D., Campos-Montiel, R. G., & Aguirre-Álvarez, G. (2019). Hydrolysed Collagen from Sheepskins as a Source of Functional Peptides with Antioxidant Activity. International Journal of Molecular Sciences, 20(16), 3931. https://doi.org/10.3390/ijms20163931