Activity Coefficients for Liquid Organic Reactions: Towards a Better Understanding of True Kinetics with the Synthesis of Jasmin Aldehyde as Showcase
Abstract
:1. Introduction
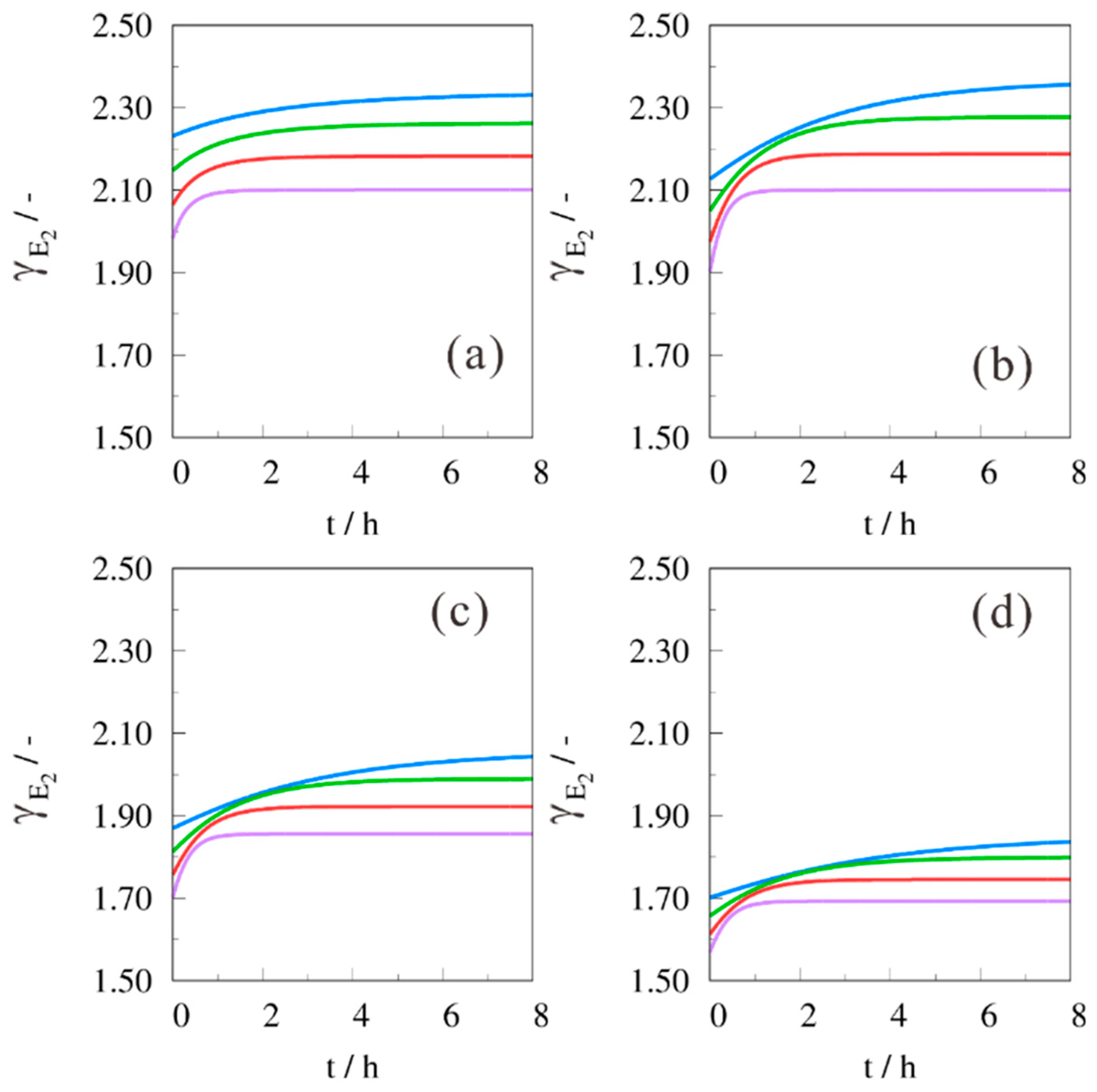
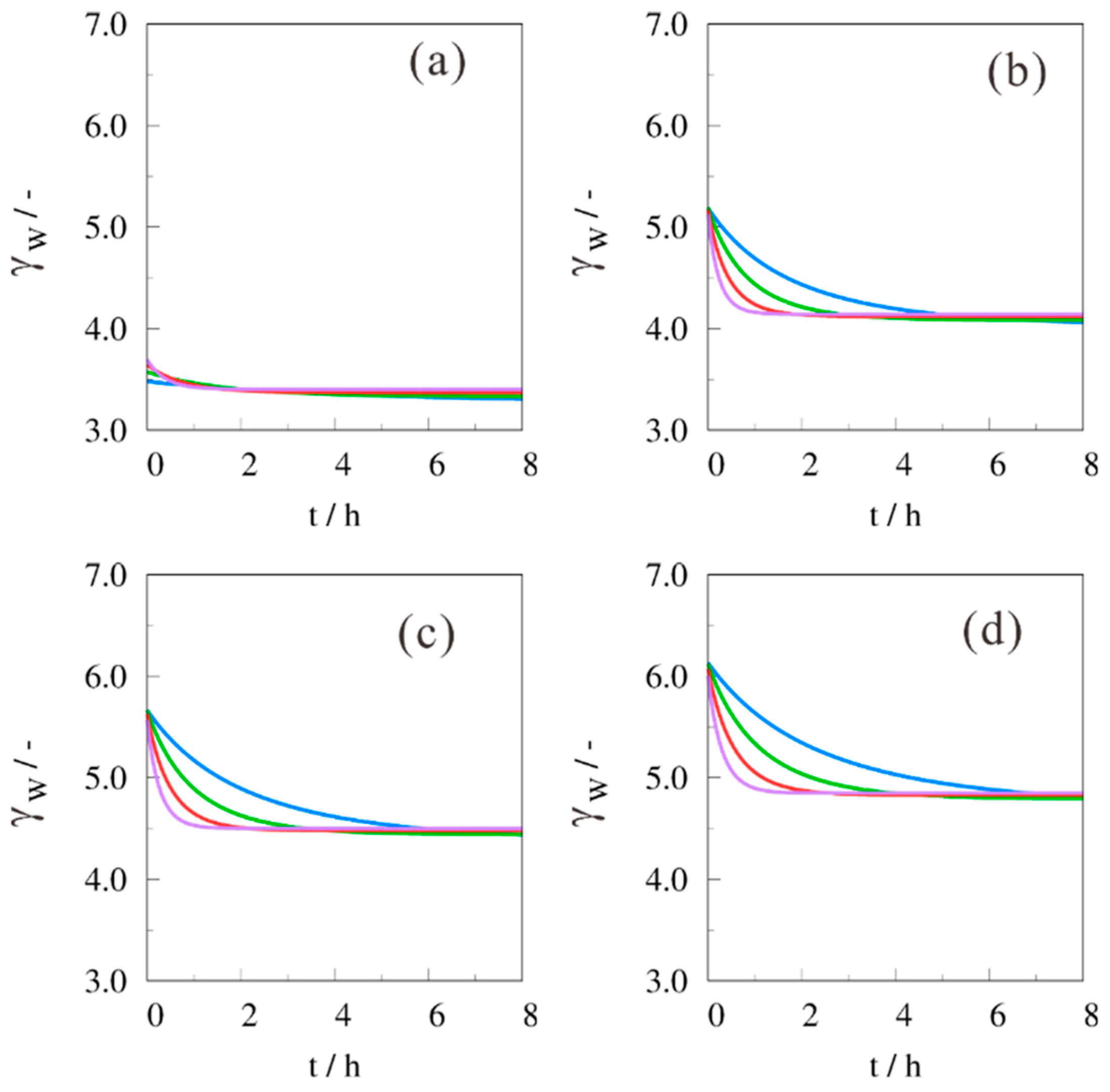
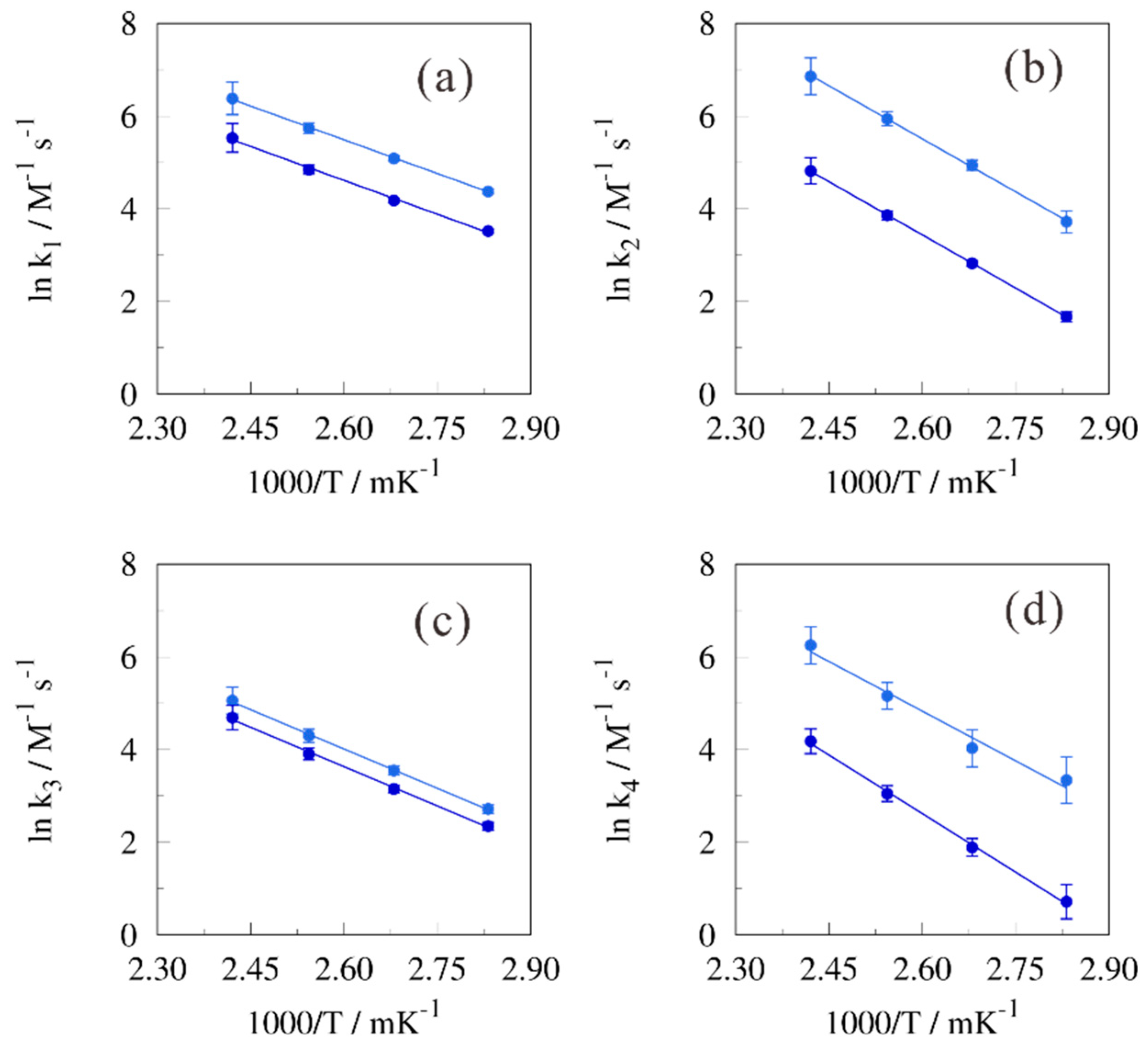
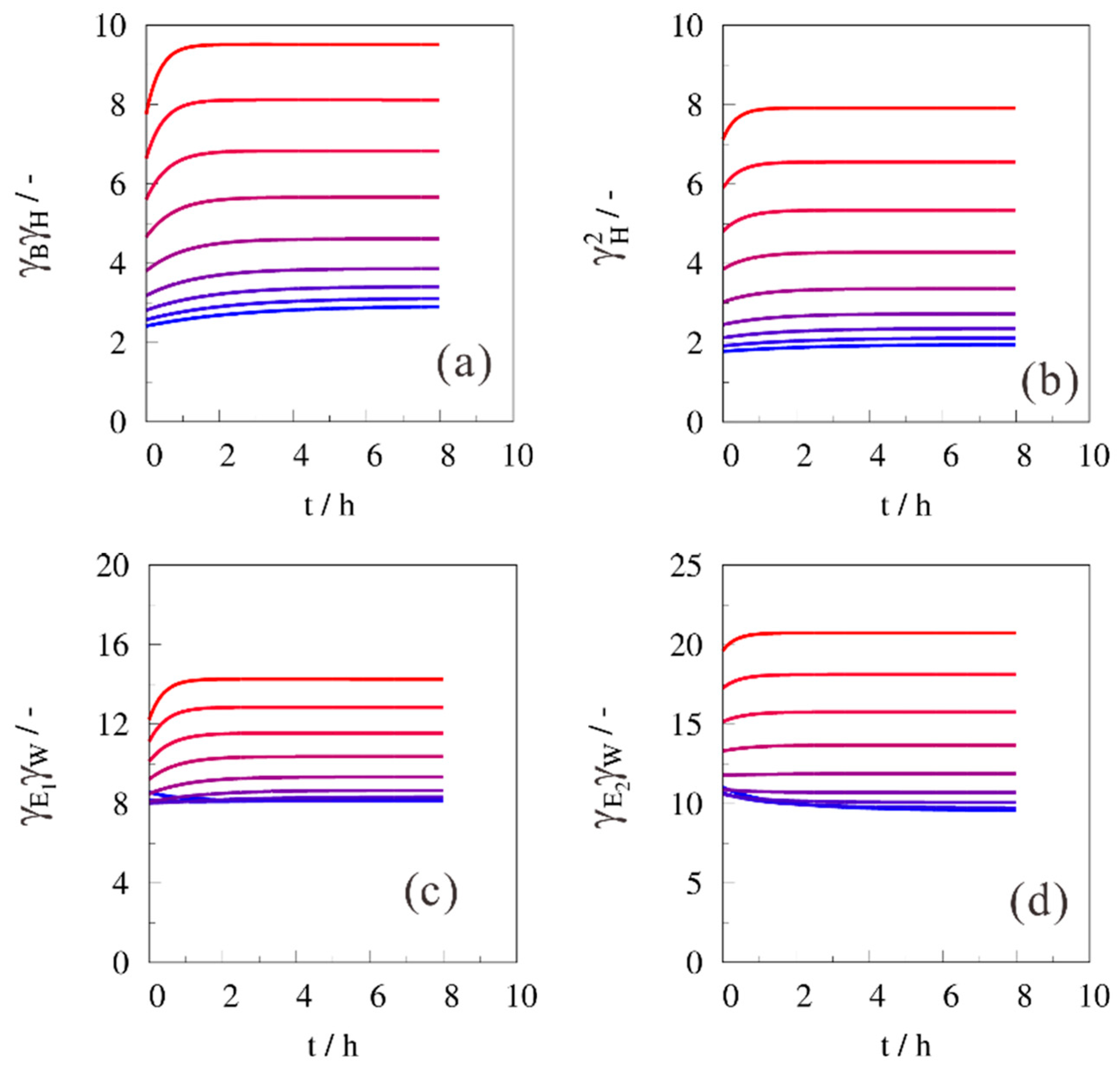
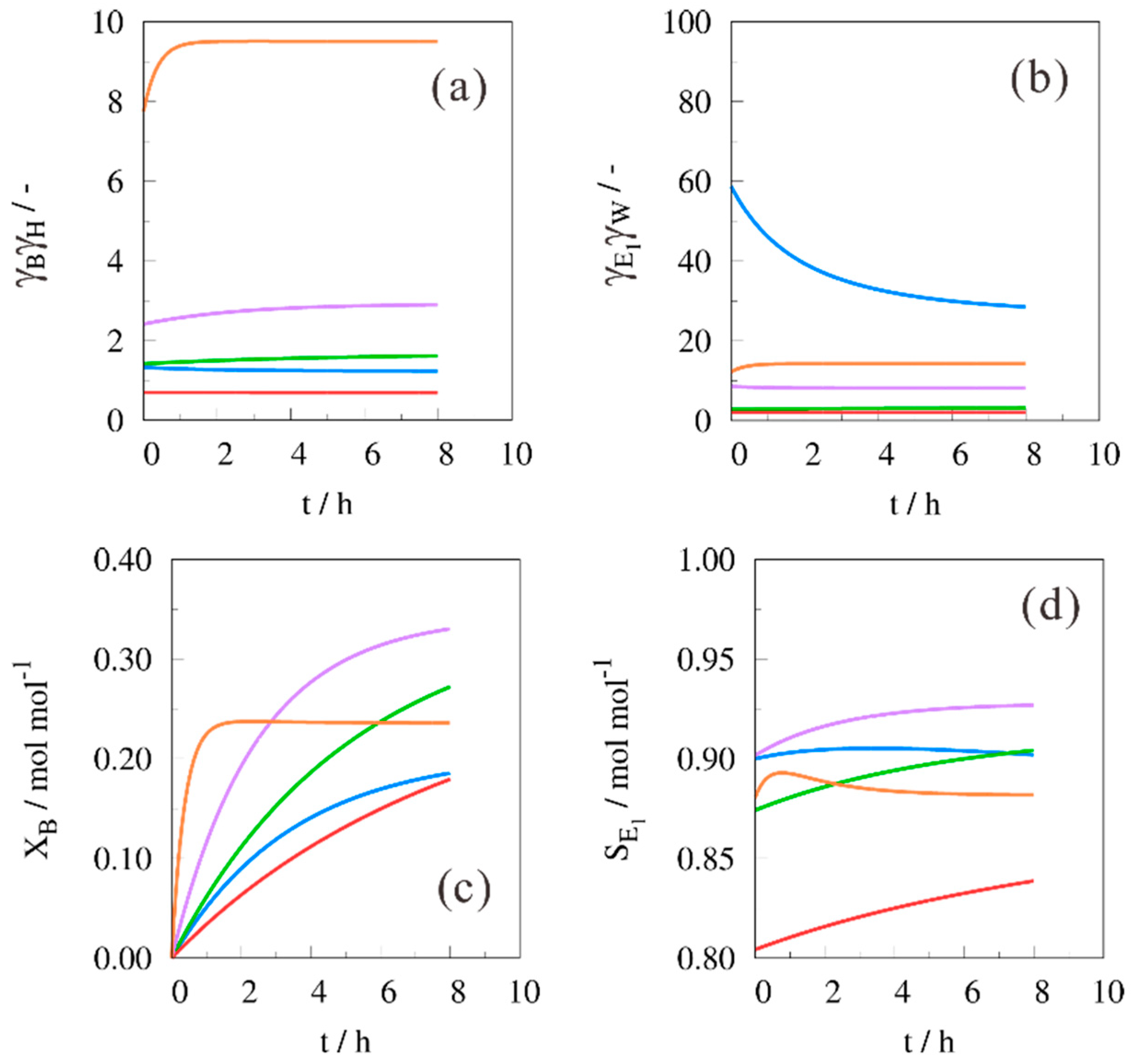
2. Results and Discussion
3. Methods
3.1. Reaction Mechanism
3.2. In-Silico Data and Parameter Estimation Procedure
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| B | benzaldehyde |
| CE | continuity equation |
| DMF | dimethylformamide |
| E1, E2 | end product 1 and 2 (jasmin aldehyde and (Z)-2-pentylnon-2-enal) |
| GC-MS | gas chromatography mass spectrometry |
| H | heptanal |
| HPLC | high pressure liquid chromatography |
| NC | number of components |
| ODR | orthogonal distance regression |
| RSSQ | residual sum of squares |
| S | selectivity (mol·mol−1) |
| t | reaction time (dep.) |
| T | temperature (°C) |
| UNIFAC | UNIQUAC (universal quasichemical) functional-group activity coefficients |
| W | water |
| X | conversion (mol·mol−1) |
| * | ‘A*B’ signifies the product of the activity coefficients for compound A and B |
Appendix A
| Entry | Conditions | Catalyst | X (%) | S (%) | Ref. |
|---|---|---|---|---|---|
| 1 | B (15.8 mmol), H (7.9 mmol), t = 1 h, T = 125 °C | L-proline (40 mol%) + benzoic acid | 99 | 96 | [14] |
| 2 | p-chlorobenzaldehyde (0.5 mmol), isobutyraldehyde (dep.), Cs2O3 as base (10 mol%), solvent THF or xylene (5 mL) | N-heterocyclic carbene catalyst (10 mol%) | (1) | (1) | [15] |
| 3 | aliphatic and aromatic aldehydes (1) | Ti(OR)4 | (1) | (1) | [16] |
| 4 | B (7–79 mmol), H (2–31.6 mmol), tmax = 6 h, T = 100–180 °C | modified chitosan | (2) | (2) | [12] |
| 5 | n-heptanal (2.28 g) dropwise (during 30 min) in benzaldehyde (2.12 g), t = 3 h | K2CO3 (2.76 g) and benzyltriethylammonium chloride as phase transfer catalyst (2.5 g) in CH2Cl2 | (3) | 80 | [17] |
| 6 | B (336 mmol), H (33.6 mmol), t = 2 h, T = 150 °C | Al-MCM-41 supported MgO (0.2 g) | 9.4–96.7 | 40.7–56.2 | [44] |
| 7 | B and H(4), t = 15 min, T = 120 °C | pyrrolidine (30 mol%) | (3) | 93 | [45] |
| 8 | (B/H)0 = 2/1, T = 120 °C, solvent = DMF | MgO | 94 | 68 | [46] |
| 9 | benzaldehyde with C3–C8 linear aldehydes | K2CO3/Al2O3, KOH/Al2O3 | (1) | (1) | [47] |
| 10 | (B/H)0 = 2/1, T = 80–140 °C, t < 7 h | Zn modified mixed Mg/Al Oxides | (1) | (1) | [48] |
| 11 | (B/H)0 = 2/1, T = 100 °C, no solvent | industrially prepared Mg–Al mixed oxides | <70 | <66 | [49] |
Appendix B
References
- Hart, D.J.; Hadad, C.M.; Craine, L.E.; Hart, H. Organic Chemistry: A Short Course; Brooks Cole, Cengage Learning: Belmont, CA, USA, 2012; ISBN1 0618590730. ISBN2 9780618590735. [Google Scholar]
- Wade, L.G. Organic Chemistry; Pearson Education: New York, NY, USA, 2013; ISBN1 0321768418. ISBN2 9780321768414. [Google Scholar]
- Dossin, T.F.; Reyniers, M.-F.; Marin, G.B. Kinetics of heterogeneously MgO-catalyzed transesterification. Appl. Catal. B Environ. 1980, 61, 35–45. [Google Scholar] [CrossRef]
- Dossin, T.F.; Reyniers, M.-F.; Marin, G.B.; Berger, R.J. Simulation of heterogeneously MgO-catalyzed transesterification for fine-chemical and biodiesel industrial production. Appl. Catal. B Environ. 2006, 67, 136–148. [Google Scholar] [CrossRef]
- Ronnback, R.; Salmi, T.; Vuori, A.; Haario, H.; Lehtonen, J.; Sundqvist, A.; Tirronen, E. Development of a kinetic model for the esterification of acetic acid with methanol in the presence of a homogeneous acid catalyst. Chem. Eng. Sci. 1997, 52, 3369–3381. [Google Scholar] [CrossRef]
- Keurentjes, J.T.F.; Janssen, G.H.R.; Gorissen, J.J. The esterification of tartaric acid with ethanol—Kinetics and shifting the equilibrium by means of evaporization. Chem. Eng. Sci. 1994, 49, 4681–4689. [Google Scholar] [CrossRef]
- Galli, F.; Corbetta, M.; Pirola, C.; Manenti, F. Robust kinetic modelling of heterogeneously catalyzed free fatty acids esterification in (monophasic liquid)/solid packed-bed reactor: Rival model discrimination. In PRES 2014, 17th Conference on Process Integration, Modelling and Optimisation for Energy Saving and Pollution Reduction, Prague, Czech Republic, 23–27 August 2014; Pts 1–3; Varbanov, P.S., Klemes, J.J., Liew, P.Y., Yong, J.Y., Stehlik, P., Eds.; Aidic Servizi Srl: Milan, Italy, 2014; pp. 979–981. [Google Scholar]
- Sharma, M.; Wanchoo, R.K.; Toor, A.P. Adsorption and kinetic parameters for synthesis of methyl nonanoate over heterogeneous catalysts. Ind. Eng. Chem. Res. 2012, 51, 14367–14375. [Google Scholar] [CrossRef]
- Sharma, M.; Wanchoo, R.K.; Toor, A.P. Amberlyst 15 catalyzed esterification of nonanoic acid with 1-propanol: Kinetics, modeling, and comparison of its reaction kinetics with lower alcohols. Ind. Eng. Chem. Res. 2014, 53, 2167–2174. [Google Scholar] [CrossRef]
- Hajek, J.; Vandichel, M.; van de Voorde, B.; Bueken, B.; de Vos, D.; Waroquier, M.; van Speybroeck, V. Mechanistic studies of aldol condensations in UiO-66 and UiO-66-NH2 metal organic frameworks. J. Catal. 2015, 331, 1–12. [Google Scholar] [CrossRef]
- Sharma, S.K.; Parikh, P.A.; Jasra, R.V. Eco-friendly synthesis of jasminaldehyde by condensation of 1-heptanal with benzaldehyde using hydrotalcite as a solid base catalyst. J. Mol. Catal. A-Chem. 2008, 286, 55–62. [Google Scholar] [CrossRef]
- Sudheesh, N.; Sharma, S.K.; Khokhar, M.D.; Shukla, R.S. Kinetic investigations on the modified chitosan catalyzed solvent-free synthesis of jasminaldehyde. J. Mol. Catal. A-Chem. 2011, 339, 86–91. [Google Scholar] [CrossRef]
- Heynderickx, P.M. Closing the balance by the CLOBAL procedure: Towards more accurate concentration, conversion and selectivity values. Chem. Eng. J. 2019, 361, 805–811. [Google Scholar] [CrossRef]
- Ganga, V.S.R.; Abdi, S.H.R.; Kureshi, R.I.; Khan, N.H.; Bajaj, H.C. Bifunctional organocatalysts for the synthesis of jasminaldehyde and their derivatives. Indian J. Chem. 2016, 55A, 950–955. [Google Scholar]
- Jin, M.Y.; Kim, S.M.; Mao, H.; Ryu, D.H.; Song, C.E.; Yang, J.W. Chemoselective and repetitive intermolecular cross-acyloin condensation reactions between a variety of aromatic and aliphatic aldehydes using a robust N-heterocyclic carbene catalyst. Org. Biomol. Chem. 2014, 12, 1547–1550. [Google Scholar] [CrossRef]
- Mahrwald, R.; Schick, H. Synthesis of a,b-unsaturated carbonyl compounds by titanium tetraalkoxide-induced aldol condensation under neutral conditions. Synthesis 1990, 12, 592–595. [Google Scholar] [CrossRef]
- Sarkar, A.; Dey, P.K.; Datta, K. An improved synthesis of alpha-normal-amylcinnamaldehyde based on liquid-phase transfer. Ind. J. Chem. B 1986, 25, 656. [Google Scholar]
- Chiappe, C.; Pieraccini, D. Ionic liquids: Solvent properties and organic reactivity. J. Phys. Org. Chem. 2015, 18, 275–297. [Google Scholar] [CrossRef]
- Geerlings, P.; de Proft, F. Chemical reactivity as described by quantum chemical methods. Int. J. Mol. Sci. 2003, 3, 276–309. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Solvent effects on the rates and mechanisms of reaction of phenols with free radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef]
- Pirrung, M.C. Acceleration of organic reactions through aqueous solvent effects. Chem. Eur. J. 2006, 12, 1312–1317. [Google Scholar] [CrossRef]
- Kandel, K.; Althaus, S.M.; Peeraphatdit, C.; Kobyashi, T.; Trewyn, B.G.; Pruski, M.; Slowing, I.I. Solvent-induced reversal of activities between two closely related heterogeneous catalysts in the aldol reaction. ACS Catal. 2013, 3, 265–271. [Google Scholar] [CrossRef]
- Iglesias, M.; Gonzalez-Olmos, R.; Cota, I.; Medina, F. Brønsted ionic liquids: Study of physico-chemical properties and catalytic activity in aldol condensations. Chem. Eng. J. 2010, 162, 802–808. [Google Scholar] [CrossRef]
- Huang, Y. Concerning the solvent effect in the aldol condensation. Mon. Chem. 2000, 131, 521–523. [Google Scholar] [CrossRef]
- Froment, G.F.; Hosten, L.H. Catalytic Kinetics: Modelling. In Catalysis: Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin, Germany, 1981; pp. 97–170. [Google Scholar]
- Heynderickx, P.M.; Thybaut, J.W.; Poelman, H.; Poelman, D.; Marin, G.B. Kinetic modeling of the total oxidation of propane over CuO-CeO2/g-Al2O3. Appl. Catal. B Environ. 2010, 95, 26–38. [Google Scholar] [CrossRef]
- Cueto, J.; Faba, L.; Díaz, E.; Ordóñez, S. Enhancement of furfural-cyclopentanone aldol condensation using binary water-ethanol mixtures as solvent. J. Chem. Technol. Biotechnol. 2018, 93, 1563–1571. [Google Scholar] [CrossRef]
- Heynderickx, P.M.; Thybaut, J.W.; Poelman, H.; Poelman, D.; Marin, G.B. Kinetic modeling of the total oxidation of propane over anatase and vanadia sputter deposited catalysts. Appl. Catal. B Environ. 2010, 90, 295–306. [Google Scholar] [CrossRef]
- Himmelblau, D.M. Process Analysis by Statistical Methods; Wiley: New York, NY, USA, 1970. [Google Scholar]
- Ramos, R.; Tišler, Z.; Kikhtyanin, O.; Kubička, D. Solvent effects in hydrodeoxygenation of furfural-acetone aldol condensation products over Pt/TiO2 catalyst. Appl. Catal. A Gen. 2017, 530, 174–183. [Google Scholar] [CrossRef]
- Noyse, D.S.; Pryor, W.A. Carbonyl reactions. I. Kinetics and mechanism of the acid-catalyzed aldol condensation of benzaldehyde and acetophenone. J. Am. Chem. Soc. 1955, 77, 1397–1401. [Google Scholar] [CrossRef]
- Comisar, C.M.; Savage, P.E. Kinetics of crossed aldol condensations in high-temperature water. Green Chem. 2004, 6, 227–231. [Google Scholar] [CrossRef]
- Doering, F.J.; Schaefer, G.F. Reaction kinetics of the aldol condensation of mixed C7 aldehydes. J. Mol. Catal. 1987, 41, 313–328. [Google Scholar] [CrossRef]
- Casale, M.T.; Richman, A.R.; Elrod, M.J.; Garland, R.M.; Beaver, M.R.; Tolbert, M.A. Kinetics of acid-catalyzed aldol condensation reactions of aliphatic aldehydes. Atmos. Environ. 2007, 41, 6212–6224. [Google Scholar] [CrossRef]
- Fredeslund, A.; Jones, R.L.; Prausnitz, J.M. Group-contribution estimation of activity coefficients in nonideal liquid mixtures. AIChE J. 1975, 21, 1086–1099. [Google Scholar] [CrossRef]
- Hansen, H.K.; Rasmussen, P.; Fredeslund, A.; Schiller, M.; Gmehling, J. Vapor-liquid equilibria by UNIFAC group contribution. 5. Revision and extension. Ind. Eng. Chem. Res. 1991, 30, 2352–2355. [Google Scholar] [CrossRef]
- Wittig, R.; Lohmann, J.; Gmehling, J. Vapor−liquid equilibria by UNIFAC group contribution. 6. Revision and extension. Ind. Eng. Chem. Res. 2003, 42, 183–188. [Google Scholar] [CrossRef]
- Peng, C.; Chan, M.N.; Chan, C.K. The hygroscopic properties of dicarboxylic and multifunctional acids: Measurements and UNIFAC predictions. Environ. Sci. Technol. 2001, 35, 4495–4501. [Google Scholar] [CrossRef]
- Kaufman, M. Principles of Thermodynamics; Marcel Dekker, Inc.: New York, NY, USA, 2002; ISBN 0-8247-0692-7. [Google Scholar]
- Heynderickx, P.M. Acquisition of nonlinear kinetics from linear relations: Application on homogeneous transesterification reactions. Chem. Eng. J. 2018, 342, 41–51. [Google Scholar] [CrossRef]
- Heynderickx, P.M.; Roelant, R. Superposition of artificial experimental error onto calculated time series: Construction of in-silico data sets. Data Brief 2018, 19, 601–610. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook, 7th ed.; Mcgraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Boggs, P.T.; Byrd, R.H.; Schnabel, R.B. A stable and efficient algorithm for nonlinear orthogonal distance regression. SIAM J. Sci. Stat. Comput. 1987, 8, 1052–1078. [Google Scholar] [CrossRef]
- Yu, J.I.; Shiau, S.Y.; Ko, A.N. Al-MCM-41 supported magnesium oxide as catalysts for synthesis of alpha-pentylcinnamaldehyde. Catal. Lett. 2001, 77, 165–169. [Google Scholar] [CrossRef]
- Limnios, D.; Kokotos, C.G. Microwave-assisted organocatalytic cross-aldol condensation of aldehydes. RSC Adv. 2013, 3, 4496–4499. [Google Scholar] [CrossRef]
- Vrbková, E.; Vyskočilova, E.; Krupka, J.; Červený, L. Aldol condensation of benzaldehyde with heptanal using solid-supported caesium and potassium catalysts. Prog. React. Kinet. 2016, 41, 289–300. [Google Scholar] [CrossRef]
- Vrbková, E.; Vyskočilova, E.; Červený, L. Potassium modified alumina as a catalyst for the aldol condensation of benzaldehyde with linear C3-C8 aldehydes. React. Kinet. Mech. Catal. 2017, 121, 307–316. [Google Scholar] [CrossRef]
- Tiśler, Z.; Vrbková, E.; Kocík, J.; Kadlec, D.; Vyskočilova, E.; Červený, L. Aldol condensation of benzaldehyde and heptanal over zinc modified mixed Mg/Al oxides. Catal. Lett. 2018, 148, 2042–2057. [Google Scholar] [CrossRef]
- Vrbková, E.; Tiśler, Z.; Vyskočilova, E.; Kadlec, D.; Červený, L. Aldol condensation of benzaldehyde and heptanal: A comparative study of laboratory and industrially prepared Mg-Al mixed oxides. J. Chem. Technol. Biotechnol. 2018, 93, 166–173. [Google Scholar] [CrossRef]
- Xiong, C.; Liang, N.; An, H.; Zhao, X.; Wang, Y. n-Butyraldehyde self-condensation catalyzed by Ce-modified g-Al2O3. ACS Adv. 2013, 5, 103523–103533. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Shcherban, N.; Lozachmeur, C.; Eränen, K.; Aho, A.; Smeds, A.; Kumar, N.; Peltonen, J.; Peurla, M.; Russo, V.; et al. Aldol condensation of cyclopentanone with valeraldehyde over metal oxides. Catal. Lett. 2019, 149, 1383–1395. [Google Scholar] [CrossRef]
- Aldehydes and Ketones II: Reactions at the α-Carbon. Available online: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/aldket2.htm (accessed on 5 May 2019).
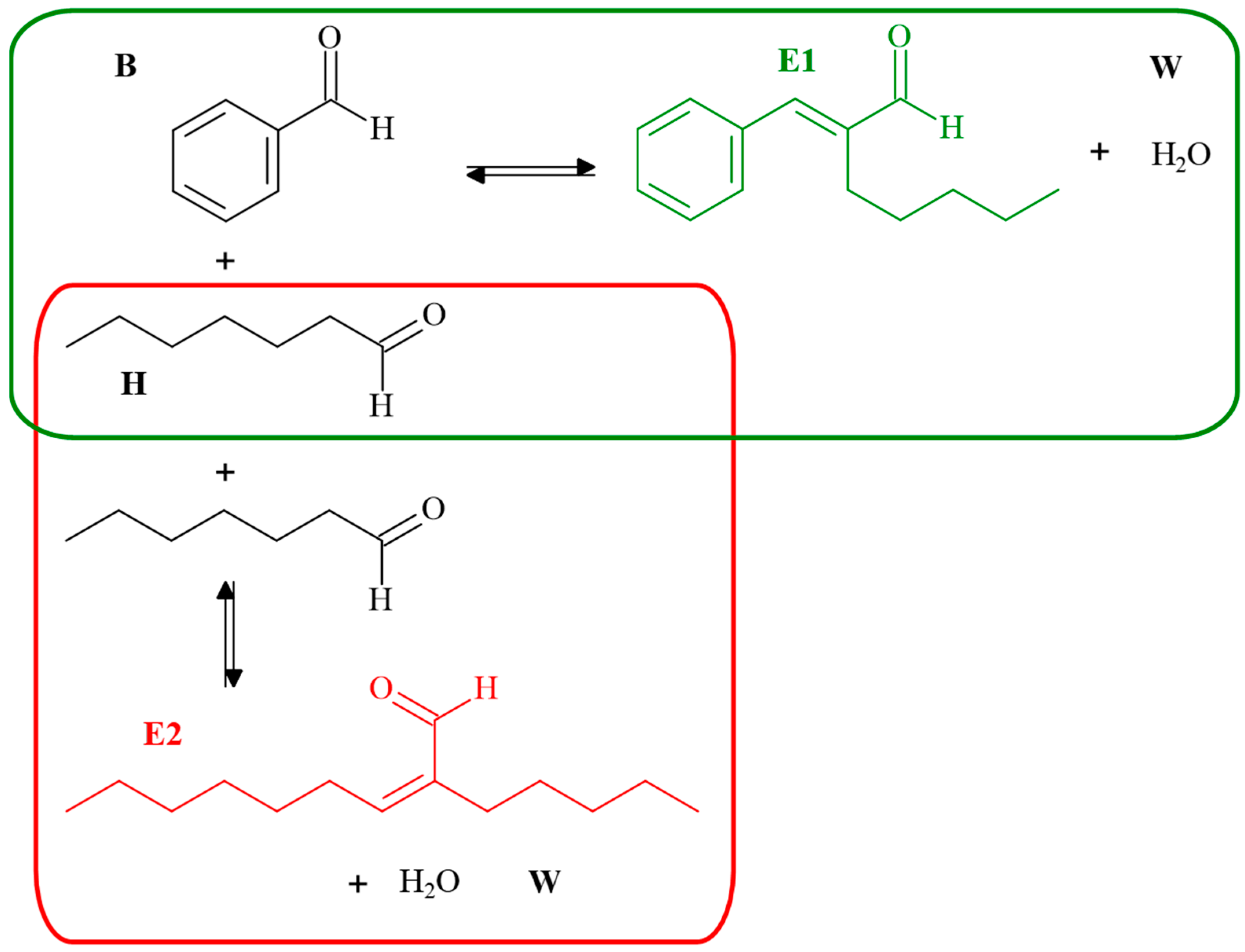
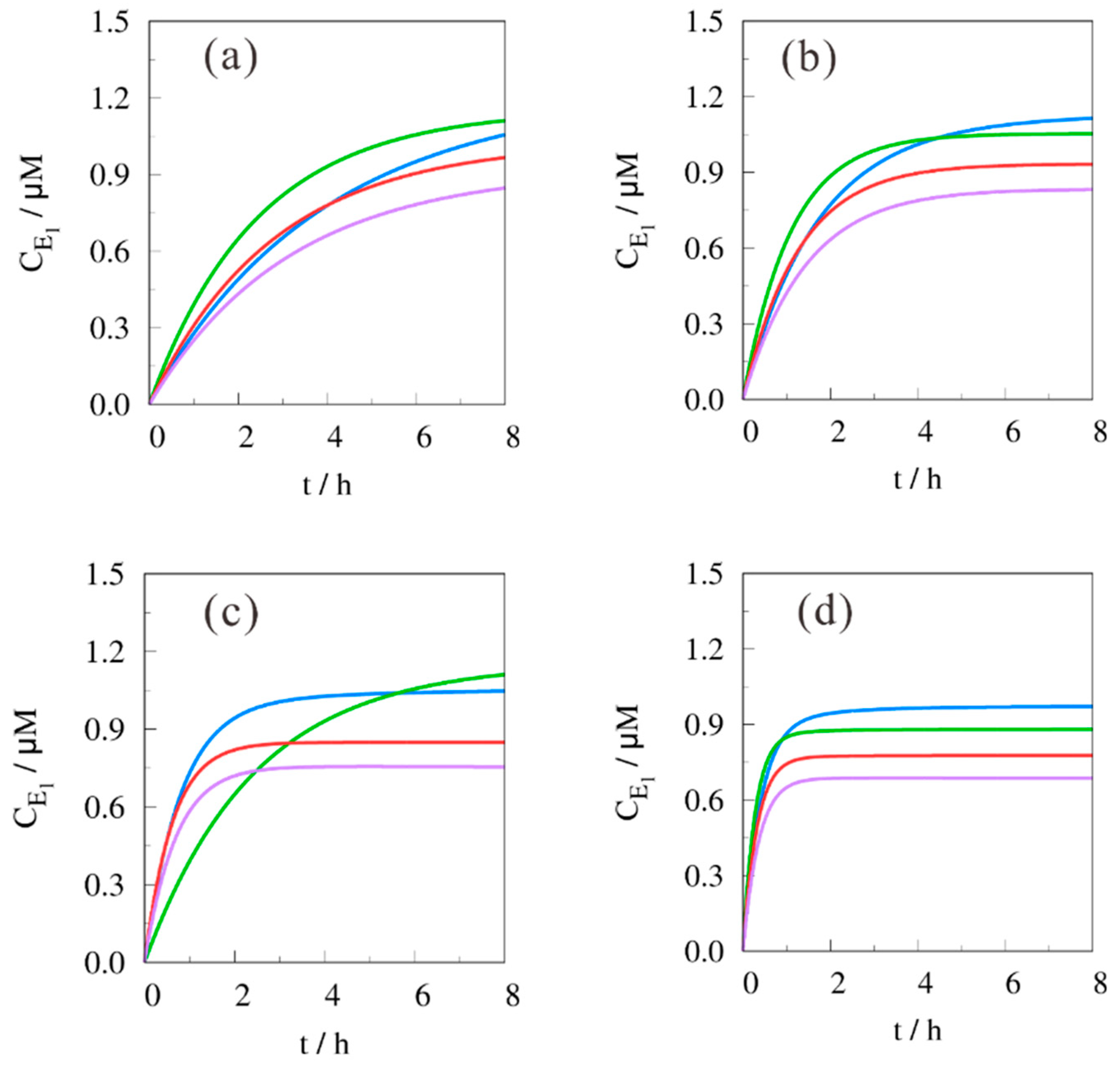

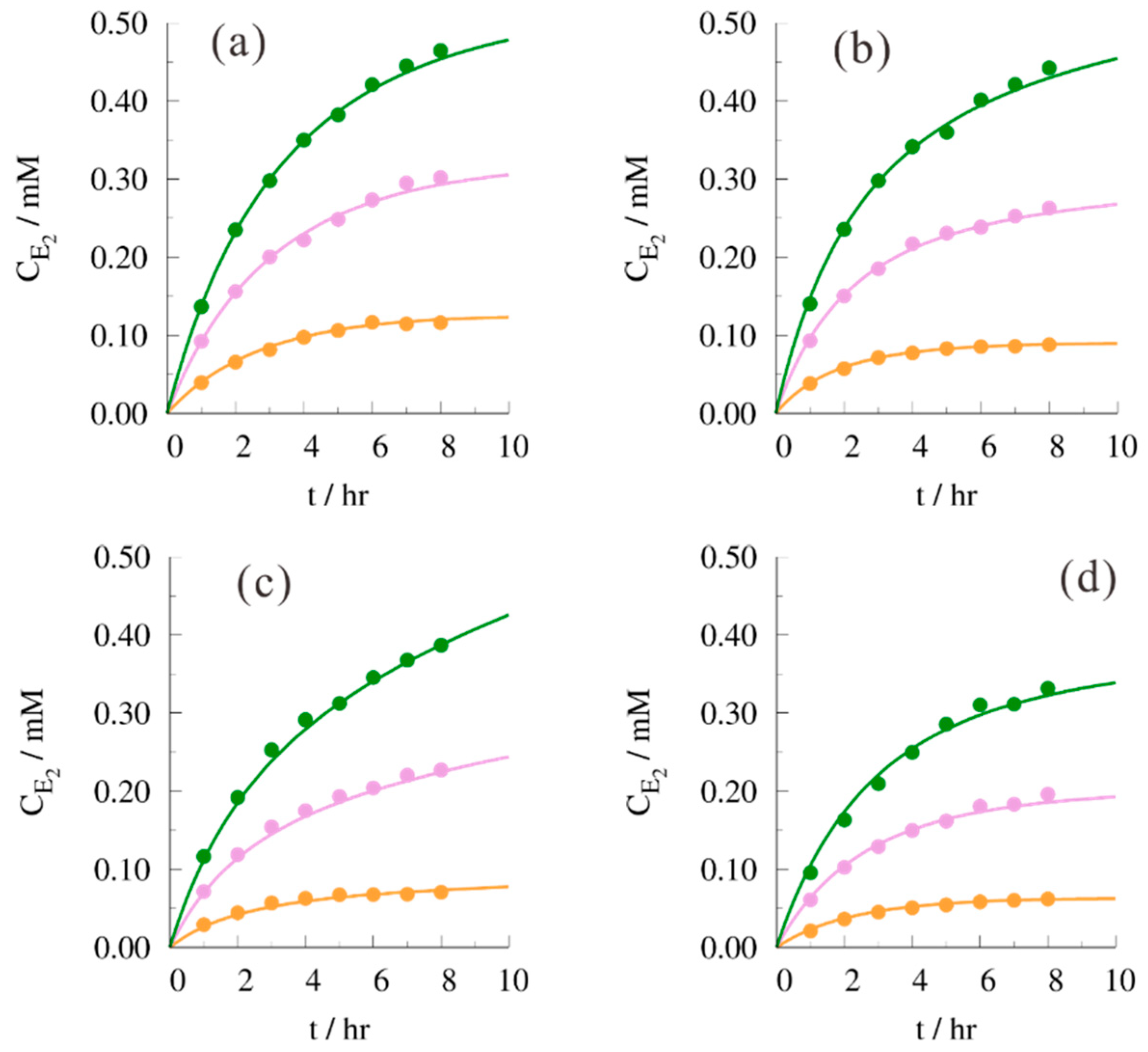
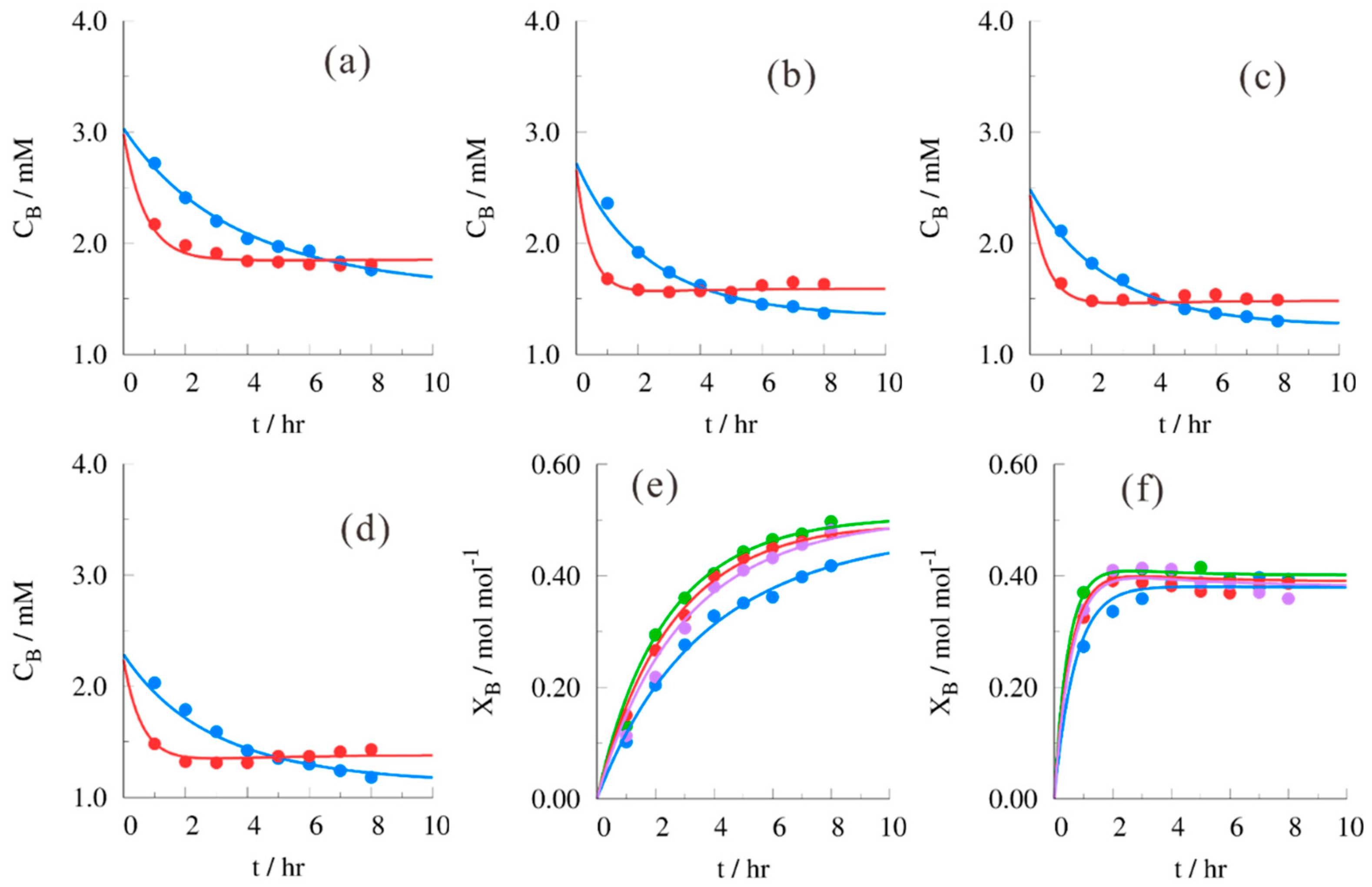
| (B/H)0 | T (In °C) | B*H | H*H | E1*W | E2*W |
|---|---|---|---|---|---|
| 2 | 80 | 2.752 ± 0.048 | 1.900 ± 0.022 | 8.251 ± 0.107 | 9.947 ± 0.049 |
| 100 | 2.740 ± 0.045 | 1.903 ± 0.022 | 7.996 ± 0.104 | 9.537 ± 0.048 | |
| 120 | 2.680 ± 0.042 | 1.885 ± 0.021 | 7.726 ± 0.098 | 9.182 ± 0.048 | |
| 140 | 2.603 ± 0.039 | 1.856 ± 0.021 | 7.450 ± 0.093 | 8.836 ± 0.047 | |
| 1 | 80 | 2.722 ± 0.069 | 1.763 ± 0.036 | 8.101 ± 0.152 | 9.155 ± 0.050 |
| 100 | 2.720 ± 0.064 | 1.774 ± 0.035 | 7.850 ± 0.148 | 8.788 ± 0.048 | |
| 120 | 2.649 ± 0.058 | 1.756 ± 0.033 | 7.574 ± 0.140 | 8.479 ± 0.048 | |
| 140 | 2.556 ± 0.054 | 1.725 ± 0.032 | 7.295 ± 0.132 | 8.185 ± 0.048 | |
| 0.5 | 80 | 2.676 ± 0.080 | 1.669 ± 0.045 | 8.097 ± 0.180 | 8.811 ± 0.051 |
| 100 | 2.683 ± 0.074 | 1.686 ± 0.043 | 7.838 ± 0.177 | 8.451 ± 0.050 | |
| 120 | 2.610 ± 0.067 | 1.669 ± 0.040 | 7.553 ± 0.168 | 8.155 ± 0.049 | |
| 140 | 2.515 ± 0.062 | 1.646 ± 0.041 | 7.267 ± 0.155 | 7.910 ± 0.049 |
| Parameter | Unit | Without Correction | With Correction |
|---|---|---|---|
| k1,∞ | M−1·s−1 | (1.36 ± 0.01) × 104 (1.70) | (7.62 ± 0.12) × 103 (1.05) |
| E1 | kJ·mol−1 | 40.8 ± 0.6 | 40.0 ± 0.9 |
| k2,∞ | s−1 | (4.36 ± 0.16) × 107 (31.4) | (4.71 ± 0.10) × 106 (3.39) |
| E2 | kJ·mol−1 | 66.4 ± 2.4 | 64.2 ± 1.4 |
| k3,∞ | M−1·s−1 | (2.89 ± 0.02) × 104 (1.25) | (2.84 ± 0.03) × 104 (1.27) |
| E3 | kJ·mol−1 | 46.8 ± 0.5 | 47.3 ± 0.6 |
| k4,∞ | s−1 | (4.44 ± 0.26) × 106 (3.13) | (4.91 ± 0.23) × 106 (2.85) |
| E4 | kJ·mol−1 | 60.1 ± 4.4 | 65.9 ± 3.5 |
| RSSQ | μM2 | 0.470 | 0.342 |
| Parameter | Unit | Without Correction | With Correction |
|---|---|---|---|
| k1,∞ | M−1·s−1 | (1.69 ± 0.01) × 104 (2.11) | (1.01 ± 0.01) × 104 (1.26) |
| E1 | kJ·mol−1 | 39.6 ± 0.3 | 41.0 ± 0.5 |
| k2,∞ | s−1 | (1.32 ± 0.02) × 107 (9.54) | (3.82 ± 0.04) × 106 (2.75) |
| E2 | kJ·mol−1 | 61.2 ± 0.8 | 63.8 ± 0.7 |
| k3,∞ | M−1·s−1 | (8.31 ± 0.11) × 104 (2.30) | (5.35 ± 0.09) × 104 (1.48) |
| E3 | kJ·mol−1 | 49.1 ± 0.8 | 49.4 ± 1.1 |
| k4,∞ | s−1 | (1.32 ± 0.04) × 108 (9.52) | (4.06 ± 0.12) × 107 (2.92) |
| E4 | kJ·mol−1 | 70.8 ± 2.8 | 73.9 ± 2.5 |
| RSSQ | μM2 | 0.298 | 0.287 |
| Parameter | Unit | Without Correction | With Correction |
|---|---|---|---|
| k1,∞ | M−1·s−1 | (1.87 ± 0.04) × 104 (2.34) | (1.14 ± 0.03) × 104 (1.42) |
| E1 | kJ·mol−1 | 40.0 ± 1.1 | 41.4 ± 1.5 |
| k2,∞ | S−1 | (1.31 ± 0.01) × 107 (9.44) | (3.48 ± 0.04) × 106 (2.51) |
| E2 | kJ·mol−1 | 61.1 ± 0.6 | 63.5 ± 0.8 |
| k3,∞ | M−1·s−1 | (8.72 ± 0.14) × 104 (2.41) | (5.94 ± 0.07) × 104 (1.65) |
| E3 | kJ·mol-1 | 49.6 ± 1.0 | 49.8 ± 0.7 |
| k4,∞ | s−1 | (7.27 ± 0.19) × 107 (5.24) | (1.39 ± 0.05) × 107 (1.00) |
| E4 | kJ·mol−1 | 69.0 ± 2.1 | 70.5 ± 2.7 |
| RSSQ | μM2 | 0.276 | 0.265 |
| Parameter | Unit | Without Correction | With Correction |
|---|---|---|---|
| k1,∞ | M−1·s−1 | (1.98 ± 0.03) × 104 (2.48) | (8.98 ± 0.27) × 103 (1.12) |
| E1 | kJ·mol−1 | 40.3 ± 0.7 | 40.5 ± 1.6 |
| k2,∞ | s−1 | (2.72 ± 0.03) × 107 (19.6) | (3.79 ± 0.02) × 106 (2.73) |
| E2 | kJ·mol−1 | 63.3 ± 0.7 | 63.6 ± 0.3 |
| k3,∞ | M−1 s−1 | (3.69 ± 0.07) × 104 (1.02) | (2.56 ± 0.06) × 104 (1.41) |
| E3 | kJ·mol−1 | 47.0 ± 1.0 | 49.8 ± 0.7 |
| k4,∞ | s−1 | (4.06 ± 0.03) × 106 (3.43) | (1.24 ± 0.03) × 107 (1.12) |
| E4 | kJ·mol−1 | 59.4 ± 6.2 | 70.0 ± 2.0 |
| RSSQ | μM2 | 0.201 | 0.197 |
| Kinetic Coefficient | Unit | Reported Range | Ballpark Value |
|---|---|---|---|
| k1,∞ | M−1·s−1 | 8.2 × 103 [31] | 8.0 × 103 |
| E1 | kJ·mol−1 | 20–40 [32], 48.5 [31], 39.7–49.9 [33] | 40 |
| k2,∞ | s−1 | 1.4 × 106 | |
| E2 | kJ·mol−1 | 60 | |
| k3,∞ | M−1·s−1 | 1.35 × 103–1.0 × 106 [34] | 3.6 × 104 |
| E3 | kJ·mol−1 | 42.7–53.8 [34] | 48 |
| k4,∞ | s−1 | 1.4 × 107 | |
| E4 | kJ·mol−1 | 70 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heynderickx, P.M. Activity Coefficients for Liquid Organic Reactions: Towards a Better Understanding of True Kinetics with the Synthesis of Jasmin Aldehyde as Showcase. Int. J. Mol. Sci. 2019, 20, 3819. https://doi.org/10.3390/ijms20153819
Heynderickx PM. Activity Coefficients for Liquid Organic Reactions: Towards a Better Understanding of True Kinetics with the Synthesis of Jasmin Aldehyde as Showcase. International Journal of Molecular Sciences. 2019; 20(15):3819. https://doi.org/10.3390/ijms20153819
Chicago/Turabian StyleHeynderickx, Philippe M. 2019. "Activity Coefficients for Liquid Organic Reactions: Towards a Better Understanding of True Kinetics with the Synthesis of Jasmin Aldehyde as Showcase" International Journal of Molecular Sciences 20, no. 15: 3819. https://doi.org/10.3390/ijms20153819
APA StyleHeynderickx, P. M. (2019). Activity Coefficients for Liquid Organic Reactions: Towards a Better Understanding of True Kinetics with the Synthesis of Jasmin Aldehyde as Showcase. International Journal of Molecular Sciences, 20(15), 3819. https://doi.org/10.3390/ijms20153819





