Effect of Water Chemistry on Antimony Removal by Chemical Coagulation: Implications of ζ-Potential and Size of Precipitates
Abstract
:1. Introduction
2. Results and Discussion
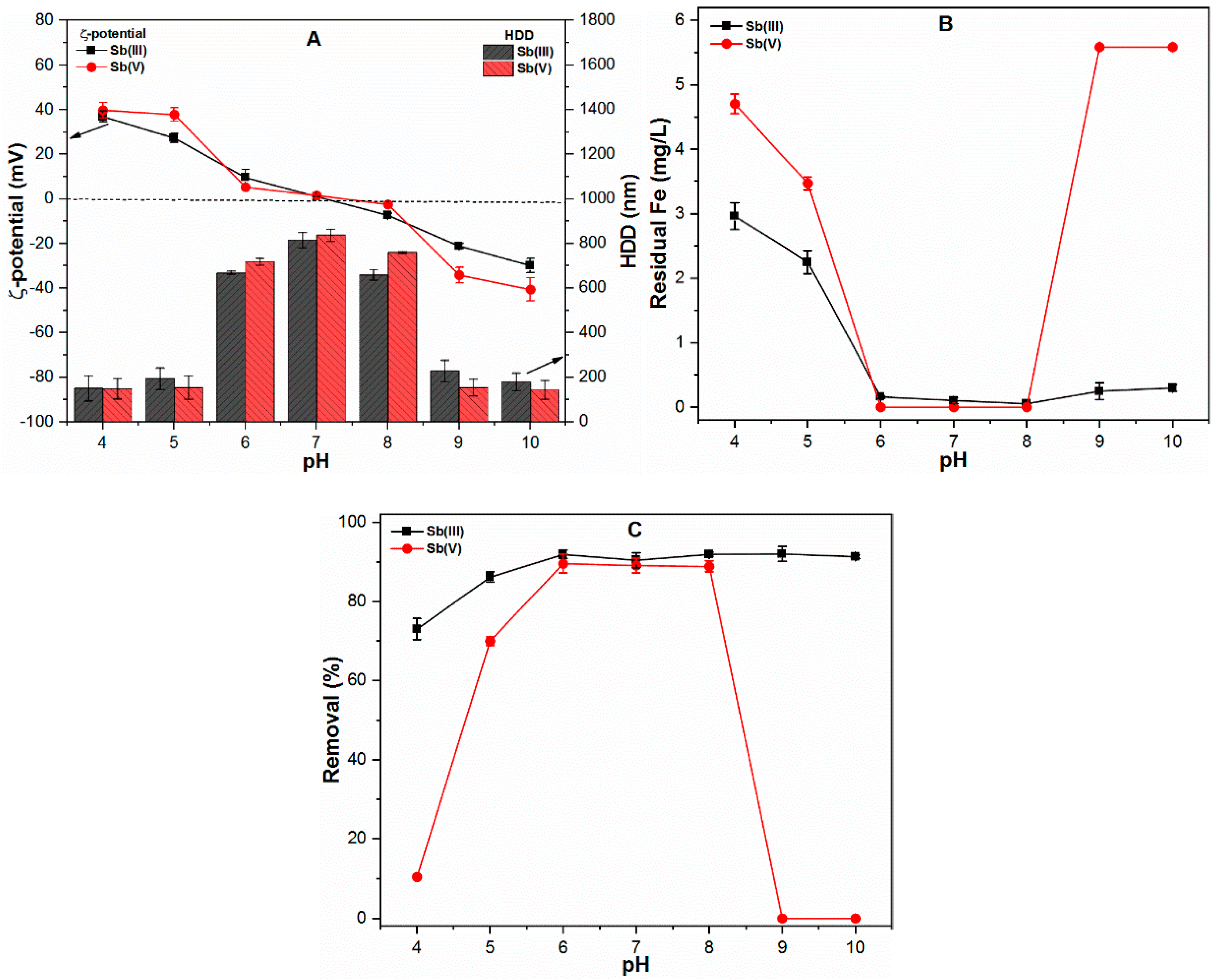
2.1. Effect of pH on Physico-chemical Properties of Precipitates and Sb Removal
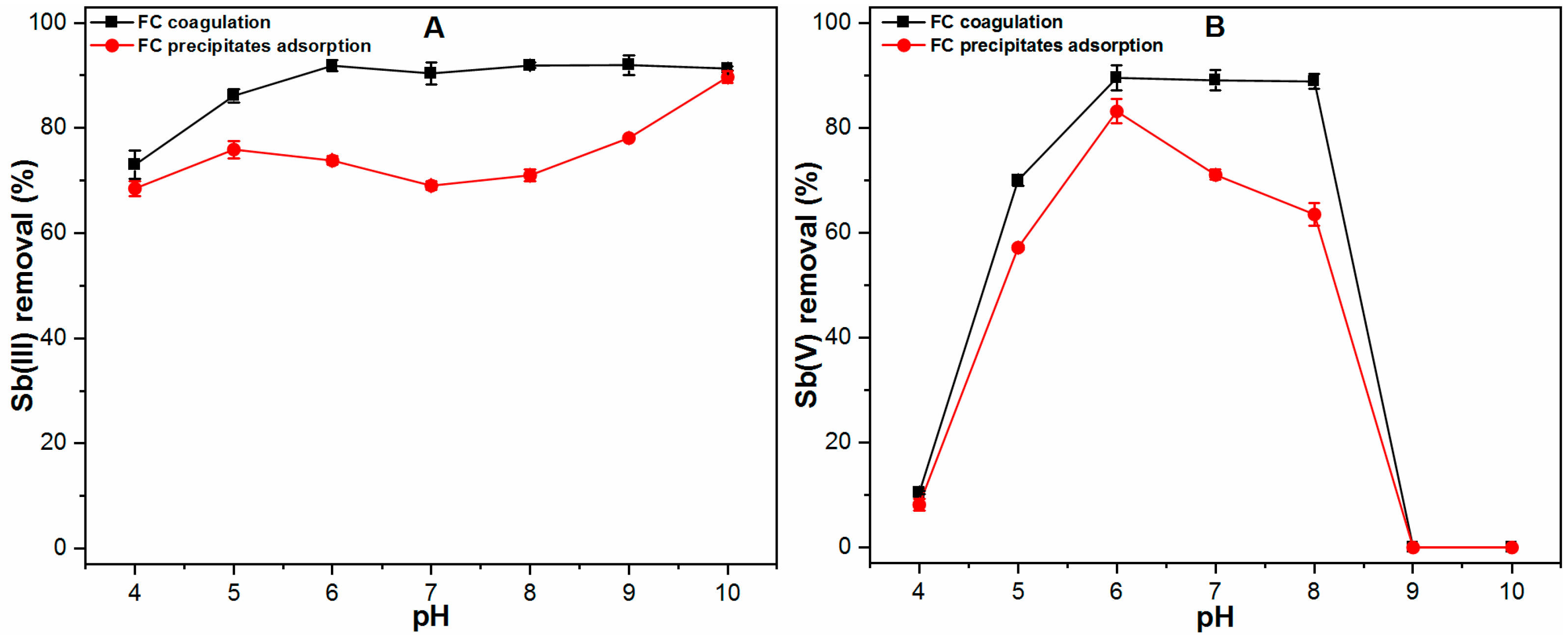
2.2. Dominant Mechanism Involved in Sb Removal
2.3. Effect of Metal Cations on Physico-chemical Properties of Precipitates and Sb Removal
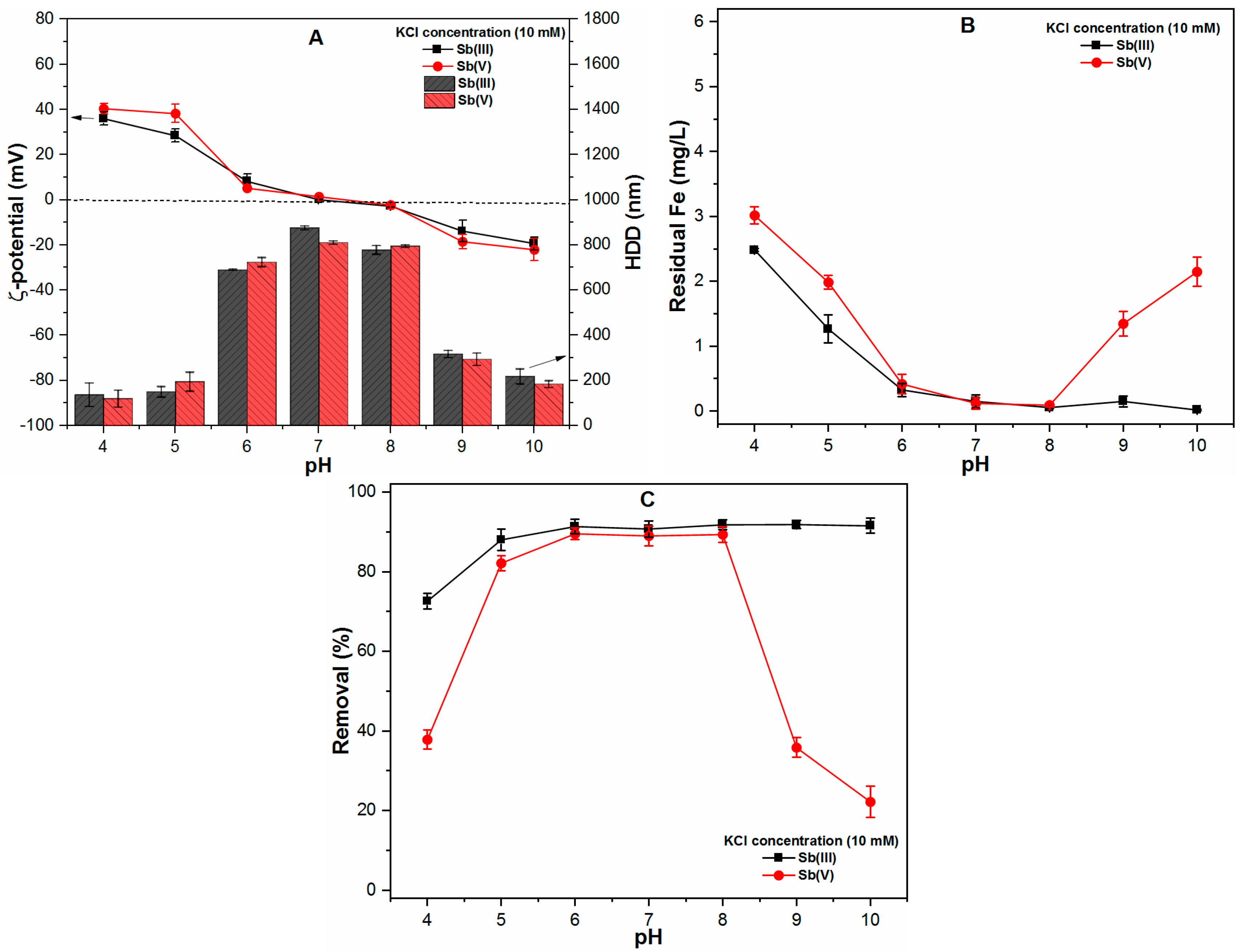
2.3.1. Monovalent Cation
2.3.2. Divalent Cation
2.4. Effect of Organic Matter on Physico-chemical Properties of Precipitates and Sb Removal
3. Materials and Methods
3.1. Chemicals and Solutions Preparation
3.2. Chemical Coagulation Experiments
3.2.1. Experimental Procedures
3.2.2. Experimental Conditions
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HDD | Hydrodynamic diameter |
| HA | Humic acid |
| DI | Deionized |
| RSD | Relative standard deviation |
| TOC | Total organic carbon |
| USEPA | United States Environmental Protection Agency |
| EU | European Union |
| WHO | World Health Organization |
| pzc | Point of zero charge |
| EDL | Electrical double layer |
References
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the environment: A review focused on natural waters I. Occurence. Earth Sci. Rev. 2002, 57, 125–176. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manage. 2015, 151, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, M.; Xi, J.; Lu, X. Antimony distribution and mobility in rivers around the world’s largest antimony mine of Xikuangshan, Hunan Province, China. Microchem. J. 2011, 97, 4–11. [Google Scholar] [CrossRef]
- Ritchie, V.J.; Ilgen, A.G.; Mueller, S.H.; Trainor, T.P.; Goldfarb, R.J. Mobility and chemical fate of antimony and arsenic in historic mining environments of the Kantishna Hills district, Denali National Park and Preserve, Alaska. Chem. Geol. 2013, 335, 172–188. [Google Scholar] [CrossRef]
- Hiller, E.; Lalinská, B.; Chovan, M.; Jurkovič, Ľ.; Klimko, T.; Jankulár, M.; Hovorič, R.; Šottník, P.; Fľaková, R.; Ženišová, Z. Arsenic and antimony contamination of waters, stream sediments and soils in the vicinity of abandoned antimony mines in the Western Carpathians, Slovakia. Appl. Geochem. 2012, 27, 598–614. [Google Scholar] [CrossRef]
- Gebel, T. Aresnic and antimony: Comparative approach on mechanistic toxicology. Chem. Biol. Interact. 1997, 107, 131–144. [Google Scholar] [CrossRef]
- Herath, I.; Vithanage, M.; Bundschuh, J. Antimony as a global dilemma: Geochemistry, mobility, fate and transport Title. Environ. Pollut. 2017, 223, 545–559. [Google Scholar] [CrossRef]
- MOE Korea. Available online: http://www.me.go.kr/home/web/index.do?menuId=68 (accessed on 30 April 2019).
- Jo, M.; Kim, T.; Choi, S.; Jung, J.; Song, H.; Lee, H.; Park, G.; Lim, S.; Sung, Y.; Oh, J. Investigation of Antimony in Natural Water and Leaching from Polyethylene Terephthalate (PET) Bottled Water. In Proceedings of the 3rd World Congress on New Technologies (NewTech’17), Rome, Italy, 6–8 June 2017; pp. 6–8. [Google Scholar]
- Guo, X.; Wu, Z.; He, M. Removal of antimony (V) and antimony (III) from drinking water by coagulation-flocculation-sedimentation (CFS). Water Res. 2009, 43, 4327–4335. [Google Scholar] [CrossRef]
- Inam, M.A.; Khan, R.; Park, D.R.; Khan, S.; Uddin, A.; Yeom, I.T. Complexation of Antimony with Natural Organic Matter: Performance Evaluation during Coagulation-Flocculation Process. Int. J. Environ. Res. Public Health 2019, 16, 1092. [Google Scholar] [CrossRef]
- Inam, M.A.; Khan, R.; Park, D.R.; Lee, Y.W.; Yeom, I.T. Removal of Sb (III) and Sb (V) by ferric chloride coagulation: Implications of Fe solubility. Water 2018, 10, 418. [Google Scholar] [CrossRef]
- Inam, M.A.; Khan, R.; Park, D.R.; Ali, B.A.; Uddin, A.; Yeom, I.T. Influence of pH and Contaminant Redox Form on the Competitive Removal of Arsenic and Antimony from Aqueous Media by Coagulation. Minerals 2018, 8, 574. [Google Scholar] [CrossRef]
- Wu, Z.; He, M.; Guo, X.; Zhou, R. Removal of antimony (III) and antimony (V) from drinking water by ferric chloride coagulation: Competing ion effect and the mechanism analysis. Sep. Purif. Technol. 2010, 76, 184–190. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kamei, T.; Magara, Y. Comparing polyaluminum chloride and ferric chloride for antimony removal. Water Res. 2003, 37, 4171–4179. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, J.; Liu, S.; Li, W.; van Leeuwen, J.; Mulcahy, D. Removal of As (III) and As (V) by ferric salts coagulation–Implications of particle size and zeta potential of precipitates. Sep. Purif. Technol. 2014, 135, 64–71. [Google Scholar] [CrossRef]
- Khan, R.; Inam, M.; Park, D.; Zam Zam, S.; Shin, S.; Khan, S.; Akram, M.; Yeom, I. Influence of Organic Ligands on the Colloidal Stability and Removal of ZnO Nanoparticles from Synthetic Waters by Coagulation. Processes 2018, 6, 170. [Google Scholar] [CrossRef]
- Guo, W.; Fu, Z.; Wang, H.; Liu, S.; Wu, F.; Giesy, J.P. Removal of antimonate (Sb (V)) and antimonite (Sb (III)) from aqueous solutions by coagulation-flocculation-sedimentation (CFS): Dependence on influencing factors and insights into removal mechanisms. Sci. Total Environ. 2018, 644, 1277–1285. [Google Scholar] [CrossRef]
- Duan, J.; Wang, J.; Graham, N.; Wilson, F. Coagulation of humic acid by aluminium sulphate in saline water conditions. Desalination 2002, 150, 1–14. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Xia, S.; Yang, Y.; Wu, R.; Li, G. Calcium-Enhanced Ferric Hydroxide Co-Precipitation of Arsenic in the Presence of Silicate. Water Environ. Res. 2007, 79, 2260–2264. [Google Scholar]
- Wilkie, J.A.; Hering, J.G. Adsorption of arsenic onto hydrous ferric oxide: Effects of adsorbate/adsorbent ratios and co-occurring solutes. Colloids Surf. A Physicochem. Eng. Asp. 1996, 107, 97–110. [Google Scholar] [CrossRef]
- Qiao, J.; Jiang, Z.; Sun, B.; Sun, Y.; Wang, Q.; Guan, X. Arsenate and arsenite removal by FeCl3: Effects of pH, As/Fe ratio, initial As concentration and co-existing solutes. Sep. Purif. Technol. 2012, 92, 106–114. [Google Scholar] [CrossRef]
- Gu, B.; Schmitt, J.; Chen, Z.; Liang, L.; McCarthy, J.F. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, J.; Sigg, L. Antimony (III) binding to humic substances: Influence of pH and type of humic acid. Environ. Sci. Technol. 2004, 38, 4535–4541. [Google Scholar] [CrossRef] [PubMed]
- Redman, A.D.; Macalady, D.L.; Ahmann, D. Natural organic matter affects arsenic speciation and sorption onto hematite. Environ. Sci. Technol. 2002, 36, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Dries, J.; Bastiaens, L.; Springael, D.; Kuypers, S.; Agathos, S.N.; Diels, L. Effect of humic acids on heavy metal removal by zero-valent iron in batch and continuous flow column systems. Water Res. 2005, 39, 3531–3540. [Google Scholar] [CrossRef] [PubMed]
- Inam, M.A.; Khan, R.; Akram, M.; Khan, S.; Park, D.R.; Yeom, I.T. Interaction of Arsenic Species with Organic Ligands : Competitive Removal from Water by Coagulation-Flocculation-Sedimentation (C/F/S). Molecules 2019, 24, 1619. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inam, M.A.; Khan, R.; Akram, M.; Khan, S.; Yeom, I.T. Effect of Water Chemistry on Antimony Removal by Chemical Coagulation: Implications of ζ-Potential and Size of Precipitates. Int. J. Mol. Sci. 2019, 20, 2945. https://doi.org/10.3390/ijms20122945
Inam MA, Khan R, Akram M, Khan S, Yeom IT. Effect of Water Chemistry on Antimony Removal by Chemical Coagulation: Implications of ζ-Potential and Size of Precipitates. International Journal of Molecular Sciences. 2019; 20(12):2945. https://doi.org/10.3390/ijms20122945
Chicago/Turabian StyleInam, Muhammad Ali, Rizwan Khan, Muhammad Akram, Sarfaraz Khan, and Ick Tae Yeom. 2019. "Effect of Water Chemistry on Antimony Removal by Chemical Coagulation: Implications of ζ-Potential and Size of Precipitates" International Journal of Molecular Sciences 20, no. 12: 2945. https://doi.org/10.3390/ijms20122945
APA StyleInam, M. A., Khan, R., Akram, M., Khan, S., & Yeom, I. T. (2019). Effect of Water Chemistry on Antimony Removal by Chemical Coagulation: Implications of ζ-Potential and Size of Precipitates. International Journal of Molecular Sciences, 20(12), 2945. https://doi.org/10.3390/ijms20122945






