Protein Phosphatase Ppz1 Is Not Regulated by a Hal3-Like Protein in Plant Pathogen Ustilago maydis
Abstract
1. Introduction
2. Results
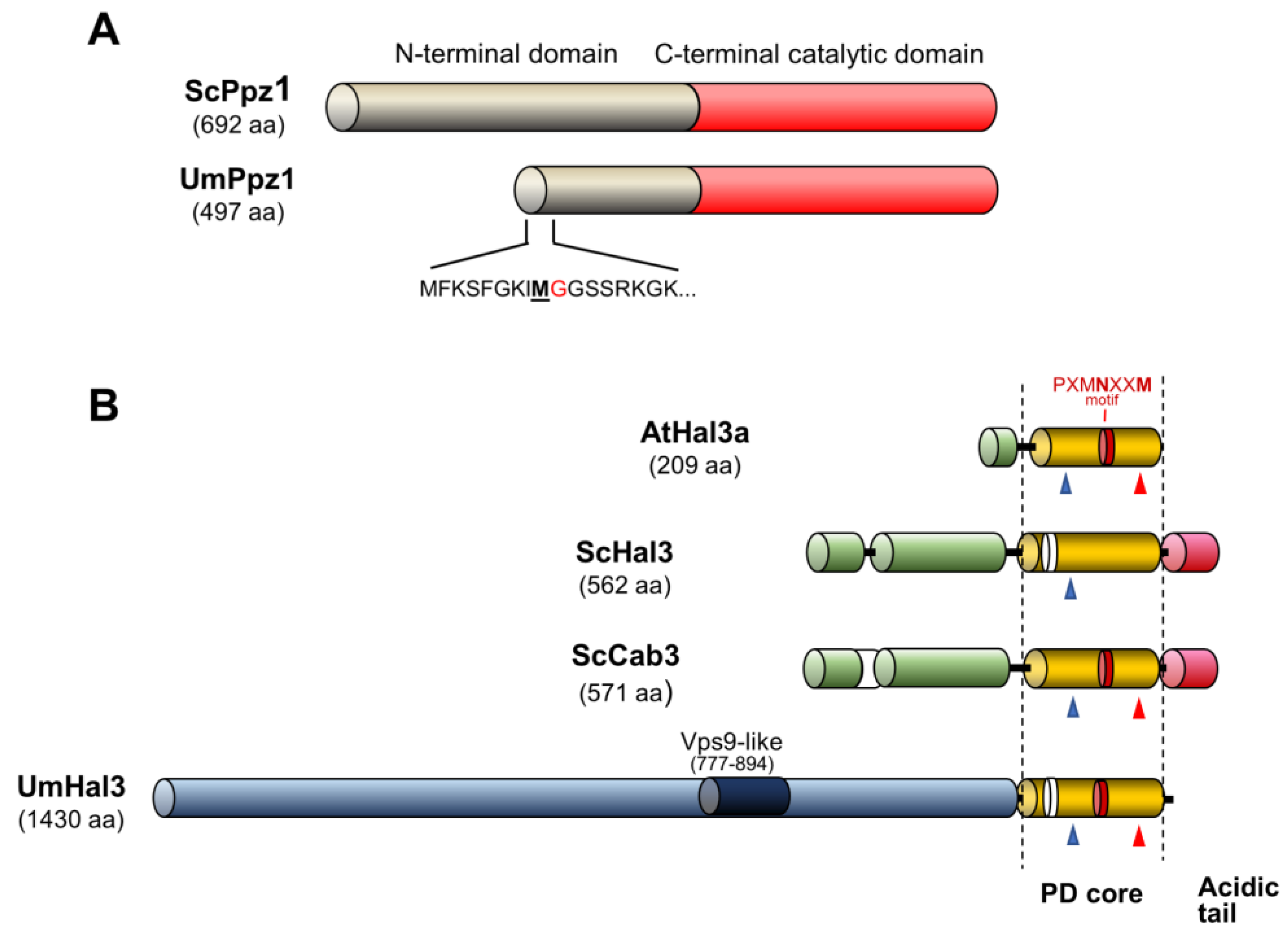
2.1. Identification of Ustilago maydis Ppz1 and Hal3-Like Proteins
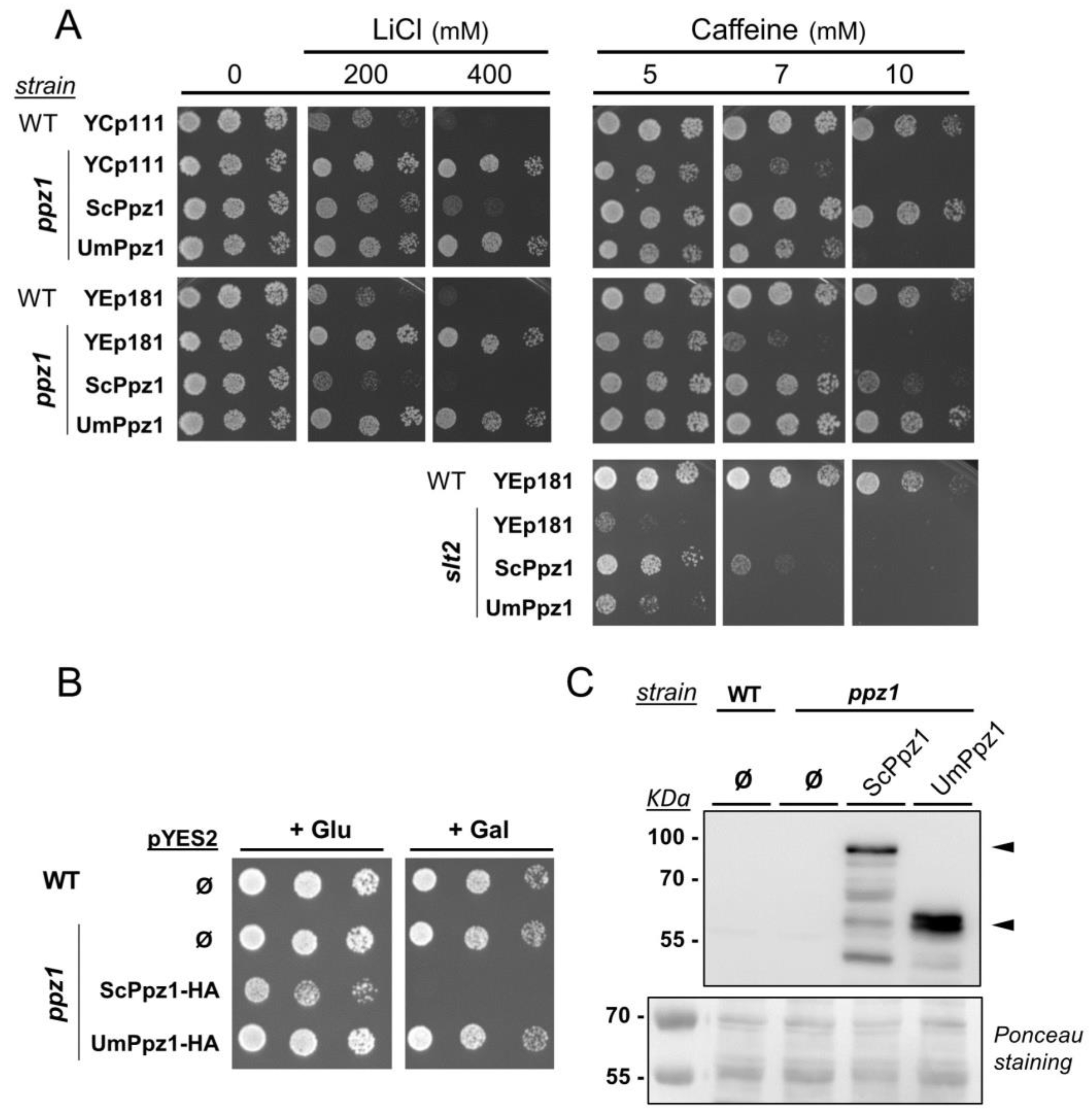
2.2. Expression and Functional Characterization of UmPpz1 in S. cerevisiae
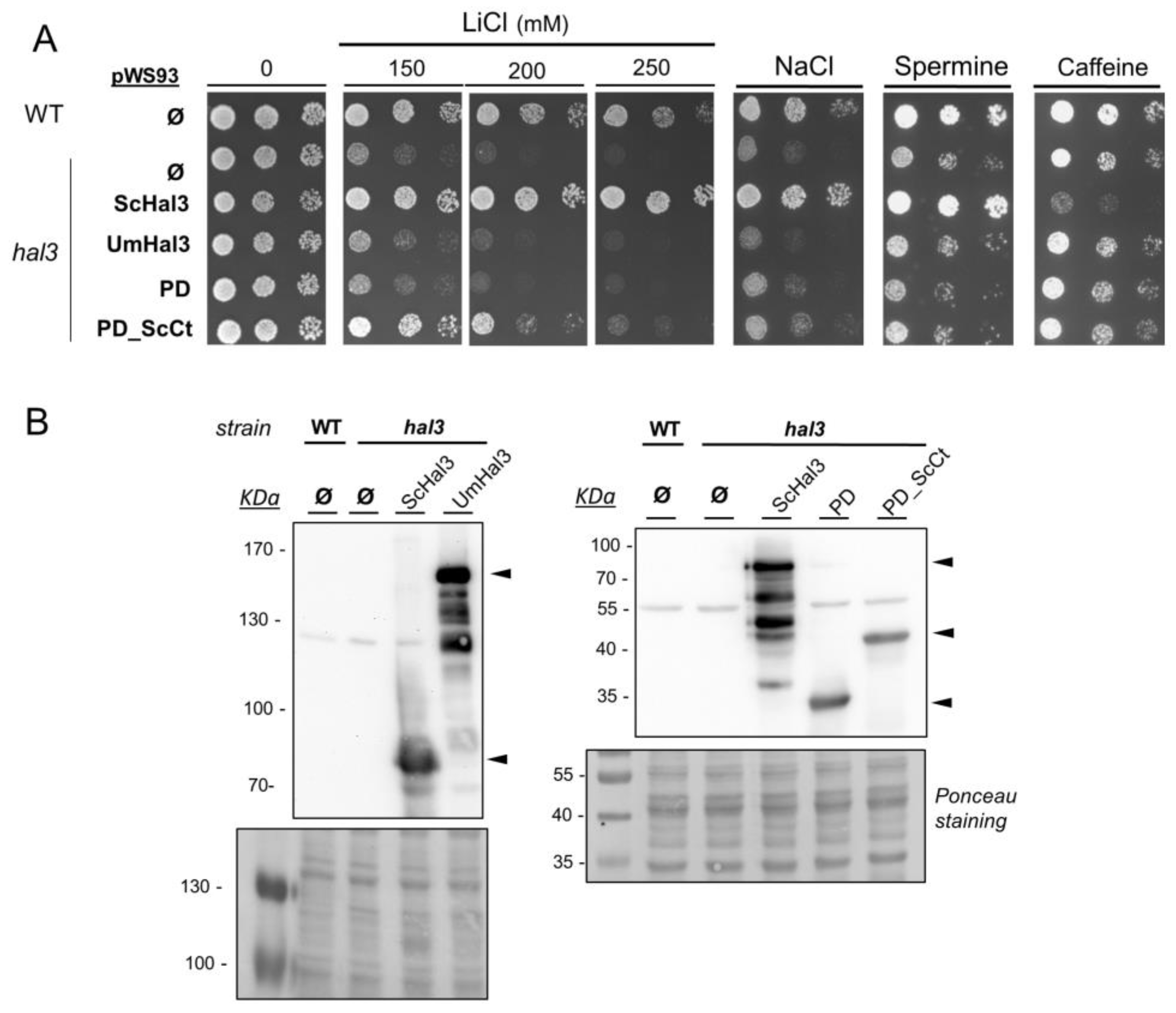
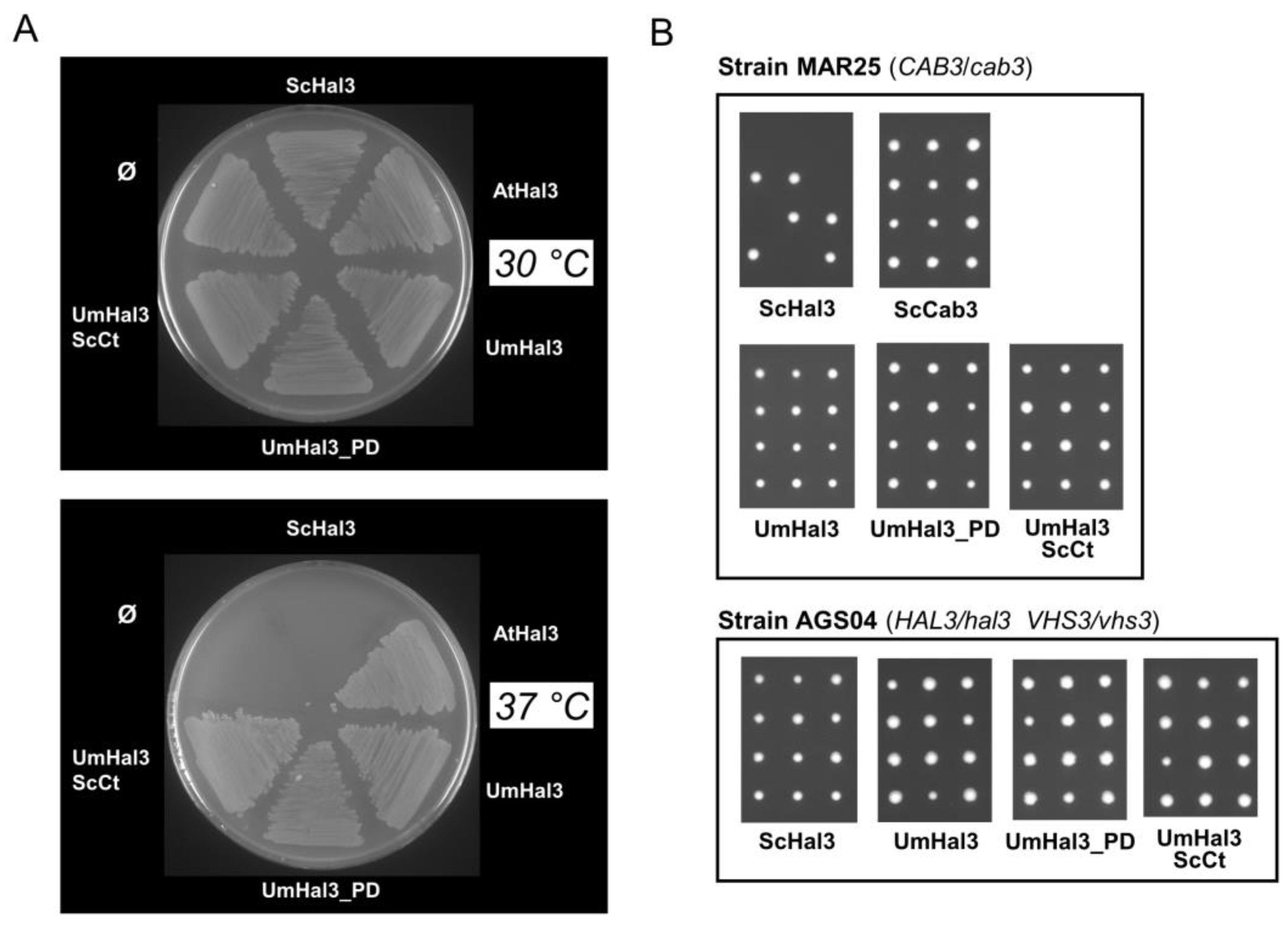
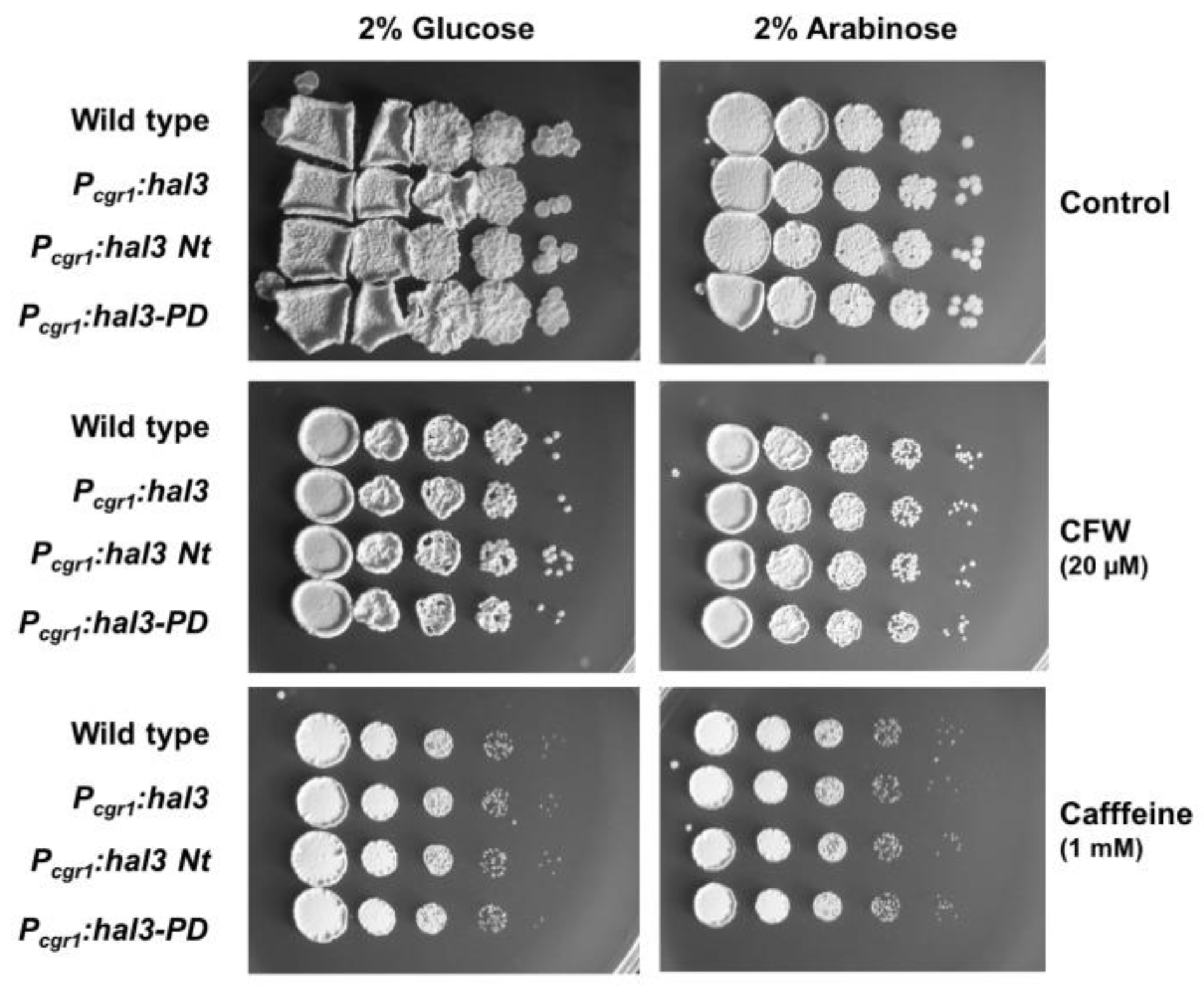
2.3. Functional Characterization of UmHal3 in S. cerevisiae
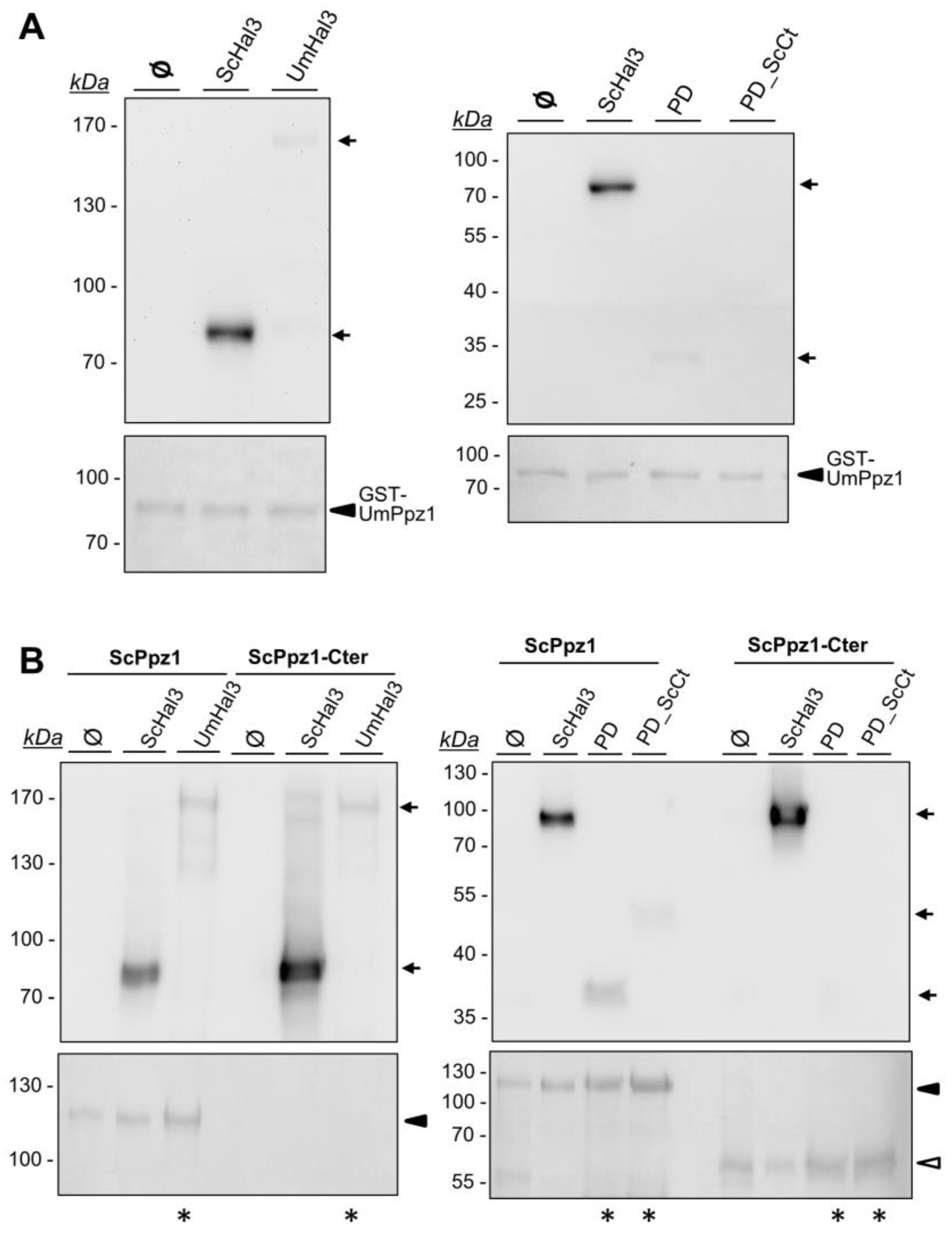
2.4. Characterization of Recombinant UmPpz1 and UmHal3 Proteins
2.5. UmHal3 Is a Functional PPCDC Enzyme
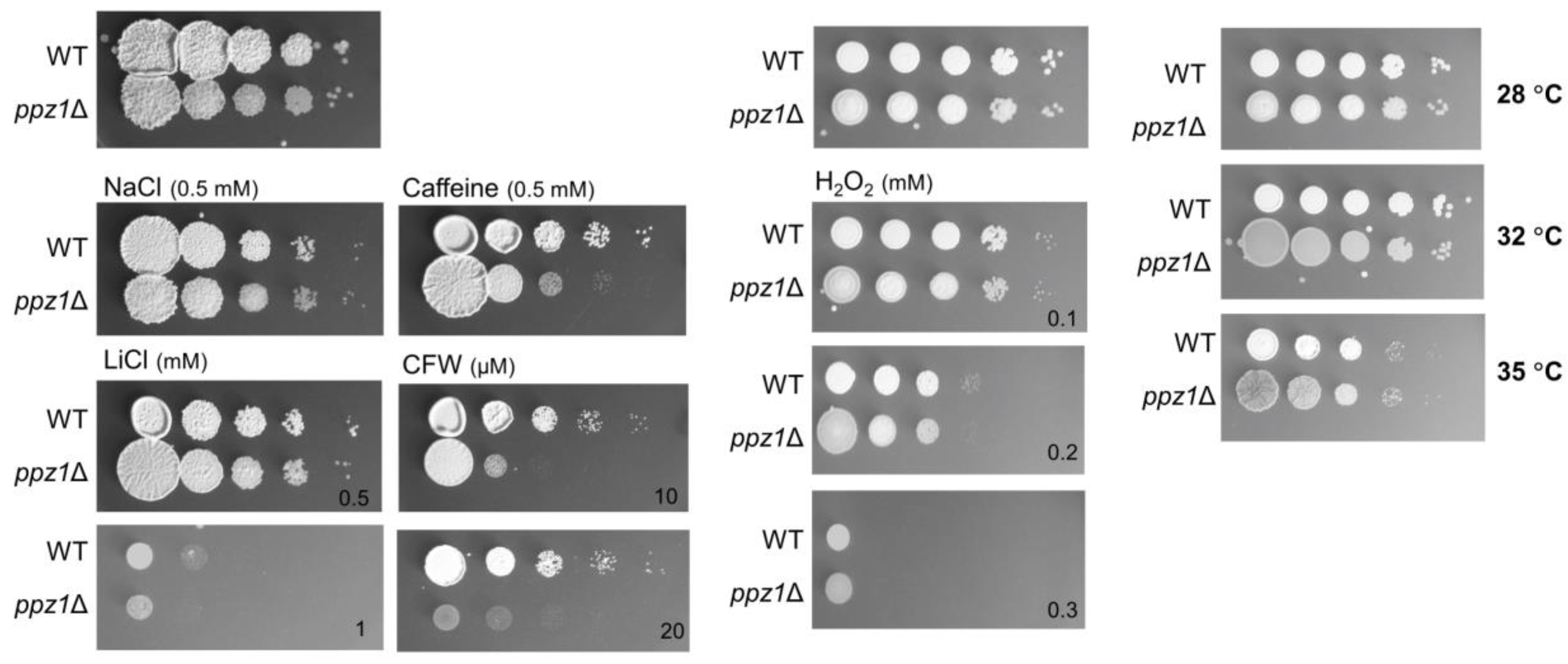
2.6. Phenotypic Characterization of UmPpz1 and UmHal3 Deletion Mutants
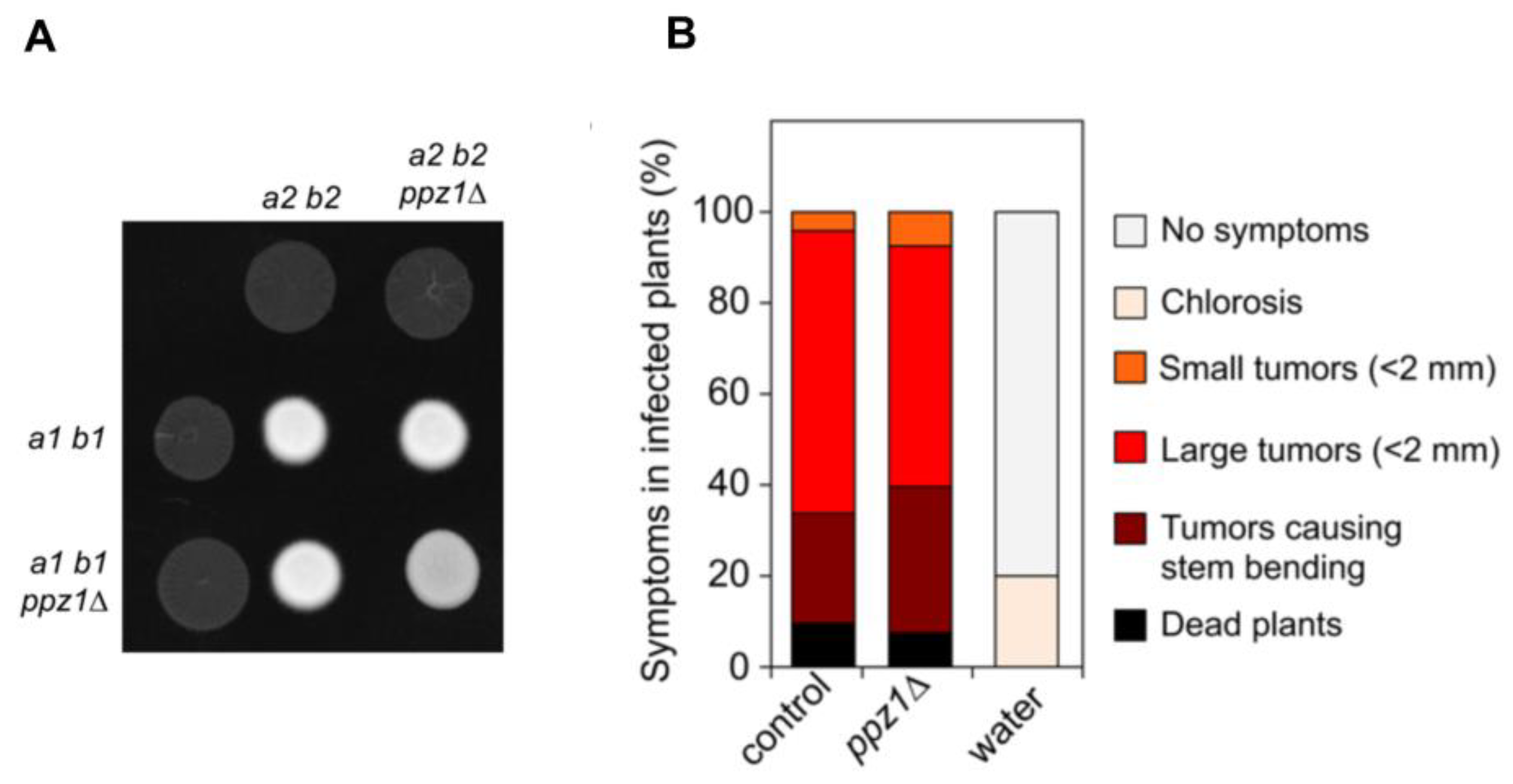
2.7. Ppz1 Is Dispensable for Virulence in U. maydis
3. Discussion
4. Materials and Methods
4.1. Yeast Strains and Growth Conditions
4.2. Generation of Fungal Deletion Mutant Strains
4.3. Recombinant DNA Techniques and Plasmid Construction
4.4. Phenotypic Analysis
4.5. Expression and Purification of Recombinant Proteins
4.6. Yeasts Protein Extraction and Immunoblot Analysis
4.7. In Vitro Interaction Experiments
4.8. Other Techniques
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ariño, J. Novel protein phosphatases in yeast. Eur. J. Biochem. 2002, 269, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Ariño, J.; Velázquez, D.; Casamayor, A. Ser/Thr protein phosphatases in fungi: Structure, regulation and function. Microb. Cell 2019, 6, 217–256. [Google Scholar] [CrossRef] [PubMed]
- Posas, F.; Casamayor, A.; Morral, N.; Ariño, J. Molecular cloning and analysis of a yeast protein phosphatase with an unusual amino-terminal region. J. Biol. Chem. 1992, 267, 11734–11740. [Google Scholar] [PubMed]
- Lee, K.S.; Hines, L.K.; Levin, D.E. A pair of functionally redundant yeast genes (PPZ1 and PPZ2) encoding type 1-related protein phosphatases function within the PKC1-mediated pathway. Mol. Cell Biol. 1993, 13, 5843–5853. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Posas, F.; Camps, M.; Ariño, J. The PPZ protein phosphatases are important determinants of salt tolerance in yeast cells. J. Biol. Chem. 1995, 270, 13036–13041. [Google Scholar] [CrossRef]
- Ruiz, A.; Yenush, L.; Arino, J. Regulation of ENA1 Na(+)-ATPase gene expression by the Ppz1 protein phosphatase is mediated by the calcineurin pathway. Eukaryot. Cell 2003, 2937–2948. [Google Scholar]
- Yenush, L.; Merchan, S.; Holmes, J.; Serrano, R. pH-Responsive, Posttranslational Regulation of the Trk1 Potassium Transporter by the Type 1-Related Ppz1 Phosphatase. Mol. Celluar Biol. 2005, 25, 8683–8692. [Google Scholar] [CrossRef]
- Yenush, L.; Mulet, J.M.; Ariño, J.; Serrano, R. The Ppz protein phosphatases are key regulators of K+ and pH homeostasis: Implications for salt tolerance, cell wall integrity and cell cycle progression. EMBO J. 2002, 21, 920–929. [Google Scholar] [CrossRef]
- De Nadal, E.; Clotet, J.; Posas, F.; Serrano, R.; Gomez, N.; Ariño, J. The yeast halotolerance determinant Hal3p is an inhibitory subunit of the Ppz1p Ser/Thr protein phosphatase. Proc. Natl. Acad. Sci. USA 1998, 95, 7357–7362. [Google Scholar] [CrossRef]
- Merchan, S.; Bernal, D.; Serrano, R.; Yenush, L. Response of the Saccharomyces cerevisiae Mpk1 mitogen-activated protein kinase pathway to increases in internal turgor pressure caused by loss of Ppz protein phosphatases. Eukaryot. Cell 2004, 3, 100–107. [Google Scholar] [CrossRef]
- Clotet, J.; Garí, E.; Aldea, M.; Ariño, J. The yeast Ser/Thr phosphatases Sit4 and Ppz1 play opposite roles in regulation of the cell cycle. Mol. Cell Biol. 1999, 19, 2408–2415. [Google Scholar] [CrossRef] [PubMed]
- Makanae, K.; Kintaka, R.; Makino, T.; Kitano, H.; Moriya, H. Identification of dosage-sensitive genes in Saccharomyces cerevisiae using the genetic tug-of-war method. Genome Res. 2013, 23, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.; Kron, S.J.; Rios, G.; Fink, G.R.; Serrano, R. Regulation of cation transport in Saccharomyces cerevisiae by the salt tolerance gene HAL3. Mol. Cell Biol. 1995, 15, 5470–5481. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.; Simón, E.; Casals, N.; Clotet, J.; Ariño, J. Identification of multicopy suppressors of cell cycle arrest at the G1 - S transition in Saccharomyces cerevisiae. Yeast 2003, 20, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Muñoz, I.; Serrano, R.; Gonzalez, A.; Simon, E.; Arino, J. Functional characterization of the Saccharomyces cerevisiae VHS3 gene: A regulatory subunit of the Ppz1 protein phosphatase with novel, phosphatase-unrelated functions. J. Biol. Chem. 2004, 279, 34421–34430. [Google Scholar] [CrossRef]
- Abrie, J.A.; Molero, C.; Ariño, J.; Strauss, E. Complex stability and dynamic subunit interchange modulates the disparate activities of the yeast moonlighting proteins Hal3 and Vhs3. Sci. Rep. 2015, 5, 15774. [Google Scholar] [CrossRef] [PubMed]
- Balcells, L.; Gomez, N.; Casamayor, A.; Clotet, J.; Arino, J. Regulation of salt tolerance in fission yeast by a protein-phosphatase-Z-like Ser/Thr protein phosphatase. Eur. J. Biochem. 1997, 250, 476–483. [Google Scholar] [CrossRef]
- Vissi, E.; Clotet, J.; de Nadal, E.; Barcelo, A.; Bako, E.; Gergely, P.; Dombradi, V.; Arino, J. Functional analysis of the Neurospora crassa PZL-1 protein phosphatase by expression in budding and fission yeast. Yeast 2001, 18, 115–124. [Google Scholar] [CrossRef]
- Minhas, A.; Sharma, A.; Kaur, H.; Fnu, Y.; Ganesan, K.; Mondal, A.K. A conserved Ser/Arg rich motif in PPZ orthologs from fungi is important for its role in cation tolerance. J. Biol. Chem. 2012, 287, 7301–7312. [Google Scholar] [CrossRef]
- Ádám, C.; Erdei, É.; Casado, C.; Kovács, L.; González, A.; Majoros, L.; Petrényi, K.; Bagossi, P.; Farkas, I.; Molnar, M.; et al. Protein phosphatase CaPpz1 is involved in cation homeostasis, cell wall integrity and virulence of Candida albicans. Microbiology 2012, 158, 1258–1267. [Google Scholar] [CrossRef]
- Kovacs, L.; Farkas, I.; Majoros, L.; Miskei, M.; Pocsi, I.; Dombradi, V. The polymorphism of protein phosphatase Z1 gene in Candida albicans. J. Basic Microbiol. 2010, 50, S74–S82. [Google Scholar] [CrossRef]
- Leiter, É.; González, A.; Erdei, É.; Casado, C.; Kovács, L.; Ádám, C.; Oláh, J.; Miskei, M.; Molnar, M.; Farkas, I.; et al. Protein phosphatase Z modulates oxidative stress response in fungi. Fungal Genet. Biol. 2012, 49, 708–716. [Google Scholar] [CrossRef]
- Manfiolli, A.O.; de Castro, P.A.; Dos Reis, T.F.; Dolan, S.; Doyle, S.; Jones, G.; Riaño Pachón, D.M.; Ulaş, M.; Noble, L.M.; Mattern, D.J.; et al. Aspergillus fumigatus protein phosphatase PpzA is involved in iron assimilation, secondary metabolite production, and virulence. Cell. Microbiol. 2017, e12770. [Google Scholar] [CrossRef]
- Zhang, C.; García-Rodas, R.; Molero, C.; de Oliveira, H.C.; Tabernero, L.; Reverter, D.; Zaragoza, O.; Ariño, J. Characterization of the atypical Ppz/Hal3 phosphatase system from the pathogenic fungus Cryptococcus neoformans. Mol. Microbiol. 2019, 111, 898–917. [Google Scholar] [CrossRef]
- Clotet, J.; Posas, F.; De Nadal, E.; Arino, J. The NH2-terminal extension of protein phosphatase PPZ1 has an essential functional role. J. Biol. Chem. 1996, 271, 26349–26355. [Google Scholar] [CrossRef]
- Ruiz, A.; Gonzalez, A.; Munoz, I.; Serrano, R.; Abrie, J.A.; Strauss, E.; Arino, J. Moonlighting proteins Hal3 and Vhs3 form a heteromeric PPCDC with Ykl088w in yeast CoA biosynthesis. Nat. Chem. Biol. 2009, 5, 920–928. [Google Scholar] [CrossRef]
- Petrényi, K.; Molero, C.; Kónya, Z.; Erdődi, F.; Ariño, J.; Dombrádi, V. Analysis of Two Putative Candida albicans Phosphopantothenoylcysteine Decarboxylase / Protein Phosphatase Z Regulatory Subunits Reveals an Unexpected Distribution of Functional Roles. PLoS ONE 2016, 11, e0160965. [Google Scholar] [CrossRef]
- Molero, C.; Petrényi, K.; González, A.; Carmona, M.; Gelis, S.; Abrie, J.A.; Strauss, E.; Ramos, J.; Dombradi, V.; Hidalgo, E.; et al. The Schizosaccharomyces pombe fusion gene hal3 encodes three distinct activities. Mol. Microbiol. 2013, 90, 367–382. [Google Scholar]
- Vollmeister, E.; Schipper, K.; Baumann, S.; Haag, C.; Pohlmann, T.; Stock, J.; Feldbrügge, M. Fungal development of the plant pathogen Ustilago maydis. Fems Microbiol. Rev. 2012, 36, 59–77. [Google Scholar] [CrossRef]
- Steinberg, G.; Perez-Martin, J. Ustilago maydis, a new fungal model system for cell biology. Trends Cell Biol. 2008, 18, 61–67. [Google Scholar] [CrossRef]
- Bologna, G.; Yvon, C.; Duvaud, S.; Veuthey, A.-L. N-Terminal myristoylation predictions by ensembles of neural networks. Proteomics 2004, 4, 1626–1632. [Google Scholar] [CrossRef]
- Spitzer, E.D.; Weiss, B. dfp Gene of Escherichia coli K-12, a locus affecting DNA synthesis, codes for a flavoprotein. J. Bacteriol. 1985, 164, 994–1003. [Google Scholar]
- Spitzer, E.D.; Jimenez-Billini, H.E.; Weiss, B. beta-Alanine auxotrophy associated with dfp, a locus affecting DNA synthesis in Escherichia coli. J. Bacteriol. 1988, 170, 872–876. [Google Scholar] [CrossRef]
- Carbó, N.; Pérez-Martín, J. Activation of the Cell Wall Integrity Pathway Promotes Escape from G2 in the Fungus Ustilago maydis. PLoS Genet. 2010, 6, 1001009. [Google Scholar] [CrossRef]
- Banuett, F.; Herskowitz, I. Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc. Natl. Acad. Sci. USA 1989, 86, 5878–5882. [Google Scholar] [CrossRef]
- Banuett, F.; Herskowitz, I. Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development 1996, 122, 2965–2976. [Google Scholar]
- Szabó, K.; Kónya, Z.; Erdődi, F.; Farkas, I.; Dombrádi, V. Dissection of the regulatory role for the N-terminal domain in Candida albicans protein phosphatase Z1. PLoS ONE 2019, 14, e0211426. [Google Scholar] [CrossRef]
- Lee, S.; Ho, H.-C.; Tumolo, J.M.; Hsu, P.-C.; MacGurn, J.A. Methionine triggers Ppz-mediated dephosphorylation of Art1 to promote cargo-specific endocytosis. J. Cell Biol. 2019, 218, 977–992. [Google Scholar] [CrossRef]
- Benito, B.; Garciadeblás, B.; Pérez-Martín, J.; Rodríguez-Navarro, A. Growth at High pH and Sodium and Potassium Tolerance in Media above the Cytoplasmic pH Depend on ENA ATPases in Ustilago maydis. Eukaryot. Cell 2009, 8, 821–829. [Google Scholar] [CrossRef]
- Harrison, J.C.; Bardes, E.S.; Ohya, Y.; Lew, D.J. A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat. Cell Biol. 2001, 3, 417–420. [Google Scholar] [CrossRef]
- Tatjer, L.; González, A.; Serra-Cardona, A.; Barceló, A.; Casamayor, A.; Ariño, J. The Saccharomyces cerevisiae Ptc1 protein phosphatase attenuates G2-M cell cycle blockage caused by activation of the cell wall integrity pathway. Mol. Microbiol. 2016, 101, 671–687. [Google Scholar] [CrossRef]
- Munoz, I.; Ruiz, A.; Marquina, M.; Barcelo, A.; Albert, A.; Arino, J. Functional characterization of the yeast Ppz1 phosphatase inhibitory subunit Hal3: A mutagenesis study. J. Biol. Chem. 2004, 279, 42619–42627. [Google Scholar] [CrossRef]
- Molero, C.; Casado, C.; Ariño, J. The inhibitory mechanism of Hal3 on the yeast Ppz1 phosphatase: A mutagenesis analysis. Sci. Rep. 2017, 7, 8819. [Google Scholar] [CrossRef]
- Holt, L.J.; Tuch, B.B.; Villen, J.; Johnson, A.D.; Gygi, S.P.; Morgan, D.O. Global Analysis of Cdk1 Substrate Phosphorylation Sites Provides Insights into Evolution. Science 2009, 325, 1682–1686. [Google Scholar] [CrossRef]
- Swaney, D.L.; Beltrao, P.; Starita, L.; Guo, A.; Rush, J.; Fields, S.; Krogan, N.J.; Villén, J. Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat. Methods 2013, 10, 676–682. [Google Scholar] [CrossRef]
- Selvan, L.D.N.; Renuse, S.; Kaviyil, J.E.; Sharma, J.; Pinto, S.M.; Yelamanchi, S.D.; Puttamallesh, V.N.; Ravikumar, R.; Pandey, A.; Prasad, T.S.K.; et al. Phosphoproteome of Cryptococcus neoformans. J. Proteom. 2014, 97, 287–295. [Google Scholar] [CrossRef]
- Ingrell, C.R.; Miller, M.L.; Jensen, O.N.; Blom, N. NetPhosYeast: Prediction of protein phosphorylation sites in yeast. Bioinformatics 2007, 23, 895–897. [Google Scholar] [CrossRef]
- Hama, H.; Tall, G.G.; Horazdovsky, B.F. Vps9p is a guanine nucleotide exchange factor involved in vesicle-mediated vacuolar protein transport. J. Biol. Chem. 1999, 274, 15284–15291. [Google Scholar] [CrossRef]
- Moolman, W.J.A.; de Villiers, M.; Strauss, E. Recent advances in targeting coenzyme A biosynthesis and utilization for antimicrobial drug development. Biochem. Soc. Trans. 2014, 42, 1080–1086. [Google Scholar] [CrossRef]
- Grütter, C.; Simard, J.R.; Mayer-Wrangowski, S.C.; Schreier, P.H.; Pérez-Martín, J.; Richters, A.; Getlik, M.; Gutbrod, O.; Braun, C.A.; Beck, M.E.; et al. Targeting GSK3 from Ustilago maydis: Type-II Kinase Inhibitors as Potential Antifungals. ACS Chem. Biol. 2012, 7, 1257–1267. [Google Scholar] [CrossRef]
- Holliday, R. Ustilago maydis. In Handbook of Genetics; King, R.C., Ed.; Plenum Press: New York, NY, USA, 1974; pp. 575–595. [Google Scholar]
- Adams, A.; Gottschling, D.E.; Kaiser, C.A.; Stearns, T. Methods in Yeast Genetics; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1997. [Google Scholar]
- Terfrüchte, M.; Joehnk, B.; Fajardo-Somera, R.; Braus, G.H.; Riquelme, M.; Schipper, K.; Feldbrügge, M. Establishing a versatile Golden Gate cloning system for genetic engineering in fungi. Fungal Genet. Biol. 2014, 62, 1–10. [Google Scholar] [CrossRef]
- Tsukuda, T.; Carleton, S.; Fotheringham, S.; Holloman, W.K. Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol. Cell. Biol. 1988, 8, 3703–3709. [Google Scholar] [CrossRef]
- Gillissen, B.; Bergemann, J.; Sandmann, C.; Schroeer, B.; Bölker, M.; Kahmann, R. A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell 1992, 68, 647–657. [Google Scholar] [CrossRef]
- Brachmann, A.; Weinzierl, G.; Kämper, J.; Kahmann, R. Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol. Microbiol. 2001, 42, 1047–1063. [Google Scholar] [CrossRef]
- Song, W.; Carlson, M. Srb/mediator proteins interact functionally and physically with transcriptional repressor Sfl1. EMBO J. 1998, 17, 5757–5765. [Google Scholar] [CrossRef]
- Abrie, J.A.A.; González, A.; Strauss, E.; Ariño, J. Functional mapping of the disparate activities of the yeast moonlighting protein Hal3. Biochem. J. 2012, 442, 357–368. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]

| Organism | Strain | Genotype | Source/Reference |
|---|---|---|---|
| U. maydis | |||
| FB1 | a1 b1 | [35] | |
| FB2 | a2 b2 | [35] | |
| FBD11 | a1a2 b1b2 | [35] | |
| UMP341 | a1a2 b1b2 hal3Δ::HygR/hal3 | This work | |
| UMP342 | a1a2 b1b2 ppz1Δ::HygR/ppz1 | This work | |
| UMP351 | a1 b1 ppz1Δ::HygR | This work | |
| UMP352 | a2 b2 ppz1Δ::HygR | This work | |
| S. cerevisiae | |||
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Euroscarf | |
| ppz1 | BY4741 ppz1::kanMx4 | Euroscarf | |
| hal3 | BY4741 hal3::kanMx4 | Euroscarf | |
| slt2 | BY4741 slt2::kanMx4 | Euroscarf | |
| IM21 | JA100 (MATa, ura3-52 leu2-3,112 trp1-1 his4 can-1r) ppz1::kanMx4 hal3::LEU2 | [42] | |
| AGS04 | 1788 (a/α, ura3-52 leu2-3,112 trp1-1 his4 can-1r) VHS3/vhs3::kanMx4 HAL3/hal3::LEU2 | [15] | |
| MAR25 | 1788 (a/α, ura3-52 leu2-3,112 trp1-1 his4 can-1r) CAB3/cab3::kanMx4 | [25] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; de la Torre, A.; Pérez-Martín, J.; Ariño, J. Protein Phosphatase Ppz1 Is Not Regulated by a Hal3-Like Protein in Plant Pathogen Ustilago maydis. Int. J. Mol. Sci. 2019, 20, 3817. https://doi.org/10.3390/ijms20153817
Zhang C, de la Torre A, Pérez-Martín J, Ariño J. Protein Phosphatase Ppz1 Is Not Regulated by a Hal3-Like Protein in Plant Pathogen Ustilago maydis. International Journal of Molecular Sciences. 2019; 20(15):3817. https://doi.org/10.3390/ijms20153817
Chicago/Turabian StyleZhang, Chunyi, Antonio de la Torre, José Pérez-Martín, and Joaquín Ariño. 2019. "Protein Phosphatase Ppz1 Is Not Regulated by a Hal3-Like Protein in Plant Pathogen Ustilago maydis" International Journal of Molecular Sciences 20, no. 15: 3817. https://doi.org/10.3390/ijms20153817
APA StyleZhang, C., de la Torre, A., Pérez-Martín, J., & Ariño, J. (2019). Protein Phosphatase Ppz1 Is Not Regulated by a Hal3-Like Protein in Plant Pathogen Ustilago maydis. International Journal of Molecular Sciences, 20(15), 3817. https://doi.org/10.3390/ijms20153817







