Mesenchymal Stem Cells in Homeostasis and Systemic Diseases: Hypothesis, Evidences, and Therapeutic Opportunities
Abstract
1. Introduction
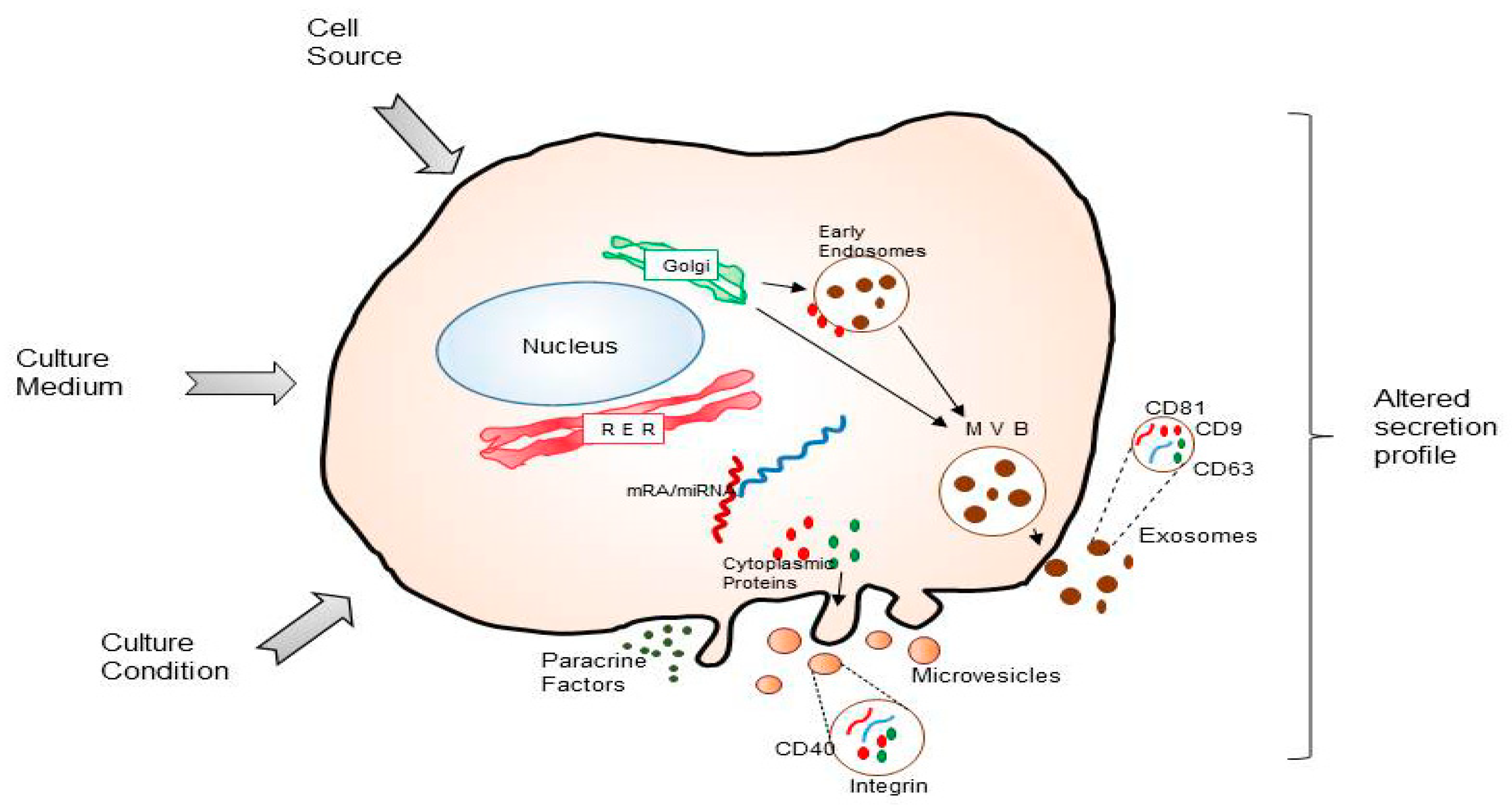
2. MSCs and Its Secretome in Intercellular Communication
3. MSCs Dysfunction in Systemic Diseases and Aging
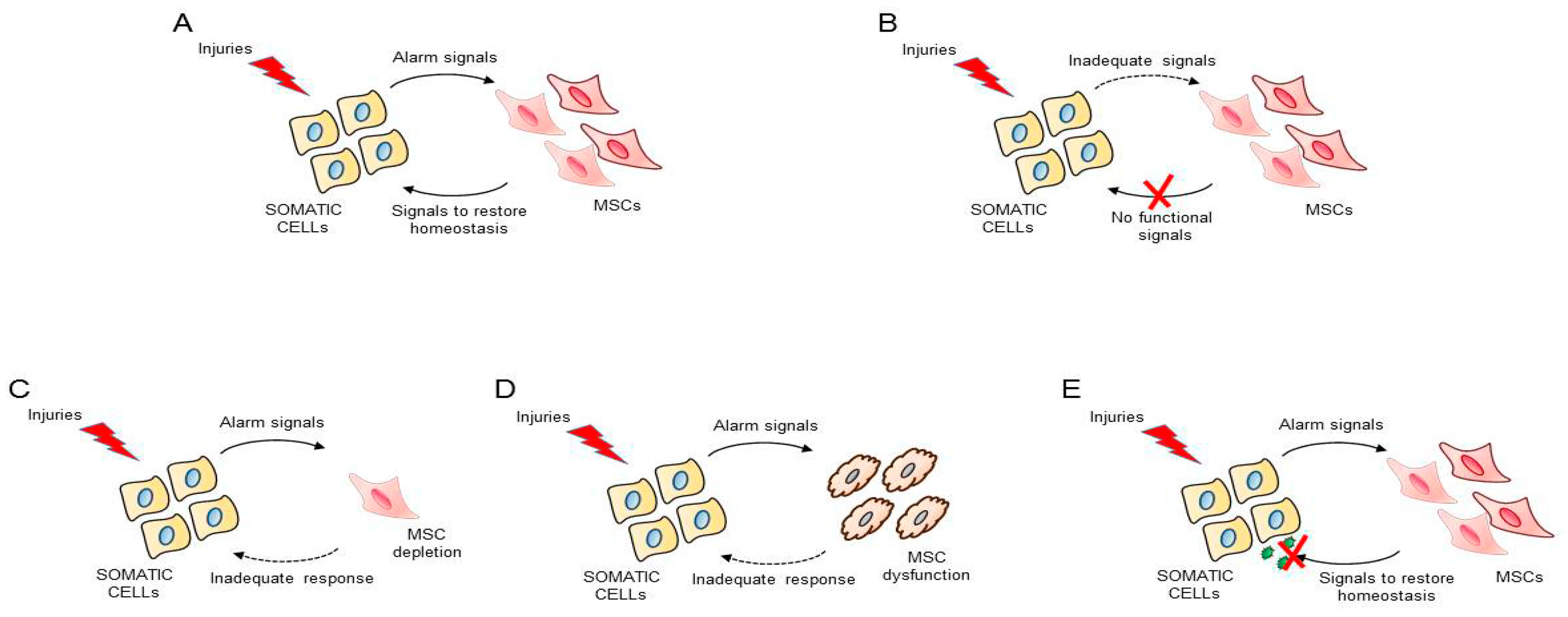
4. Control of Tissue Homeostasis by MSCs: Hypothesis and Therapeutic Opportunities
5. MSC-Derived Secretome Products as Therapeutic Agents
5.1. Origin of MSCs
5.2. Donor Condition
5.3. Bioprocess Development for Secretome-Derived Products
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| α–ΣMA | Alpha-smooth muscle actin |
| BM | Bone marrow |
| CCLs | Chemokine (C-C motif) ligands |
| CCL2 | Chemokine (C-C motif) ligand-2 |
| CCL5 | Chemokine (C-C motif) ligand-5 |
| CD | Cluster of differentiation |
| CM | Conditioned medium |
| CXCLs | Chemokine (C-X-C motif) ligands |
| DM | Diabetes mellitus |
| DNA | Deoxyribonucleic acid |
| EGF | Epidermal growth factor |
| Evs | Extracellular vesicles |
| FcγRIIB | Inhibitory Fcγ receptor Iib |
| FGFs | Fibroblast growth factors |
| FLT-3 ligand | Fms-related tyrosine kinase 3 ligand |
| G-CSF | Granulocyte colony-stimulating factor |
| GMP | Good manufacturing Practice |
| GvHD | Graft-versus-host disease |
| H2O2 | Hydrogen peroxide |
| HGF | Hepatocyte growth factor |
| IDO | Indolamine 2,3-deoxygenase |
| IFN γ | Interferon γ |
| IGF-1 | Insulin-like growth factor-1 |
| IL | Interleukin |
| IP10 | Interferon-gamma-inducible protein-10 |
| LAP | Latency-associated peptide |
| LIF | Leukemia inhibitory factor |
| LPS | Lipopolysaccharide |
| MCP-1 | Monocyte chemotactic protein-1 |
| MMP | Matrix metalloproteinase |
| MSC | Mesenchymal stem cell |
| mTOR | mammalian Target of Rapamycin |
| NT-3 | Neurothrofin-3 |
| OAZ | Olfatory-1/early B-cell factor |
| Pbx1 | Pre-B-cell leukemia homeobox 1 |
| PD-1 | Programmed death 1 |
| PDGF | Platelet-derived growth factor |
| PGE-2 | Prostaglandin E2 |
| RA | Rheumatoid arthritis |
| ROS | Reactive oxygen species |
| RNA | Ribonucleic acid |
| SLE | Systemic lupus erythematosus |
| STAT1 | Signal transducer and activator of transcription 1 |
| STC-1 | Stanniocalcin-1 |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| TGF-b | Transforming growth factor-b |
| TIMP | Tissue inhibitor of metalloproteinases |
| TNFa | Tumor necrosis factor alpha |
| uPAR | Urokinase-type plasminogen activator receptor |
| VE-cadherin | vascular endothelial cadherin |
| VEGF | Vascular endothelial growth factor |
References
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.B.; He, M. Effect of mesenchymal stromal (stem) cell (MSC) transplantation in asthmatic animal models: A systematic review and meta-analysis. Pulm. Pharmacol. Ther. 2019, 54, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Afanasyev, B.V.; Elstner, E.E.; Zander, A.R.A.J. Friedenstein, founder of the mesenchymal stem cell concept. Cell. Ther. Transplant. 2009, 1, 35–38. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Corselli, M.; Chen, C.W.; Crisan, M.; Lazzari, L.; Peault, B. Perivascular ancestors of adult multipotent stem cells. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1104–1109. [Google Scholar] [CrossRef]
- Elahi, K.C.; Klein, G.; Avci-Adali, M.; Sievert, K.D.; MacNeil, S.; Aicher, W.K. Human Mesenchymal Stromal Cells from Different Sources Diverge in Their Expression of Cell Surface Proteins and Display Distinct Differentiation Patterns. Stem Cells Int. 2016, 2016, 5646384. [Google Scholar] [CrossRef]
- Chen, J.Y.; Mou, X.Z.; Du, X.C.; Xiang, C. Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins. Asian Pac. J. Trop. Med. 2015, 8, 739–746. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.C.; Guo, S.C.; Li, M.; Ke, Q.F.; Guo, Y.P.; Zhang, C.Q. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. Stem Cells Transl. Med. 2017, 6, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound HealingCorneal MSC Exosomes Promote Corneal Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Perets, N.; Hertz, S.; London, M.; Offen, D. Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic-like behaviors of BTBR mice. Mol. Autism 2018, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Liver Fibrosis. Stem Cells Dev. 2013, 22, 845–854. [Google Scholar] [CrossRef]
- Mohammed, E.; Khalil, E.; Sabry, D. Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats. Biomolecules 2018, 8, 167. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Author Correction: Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2018, 8, 7066. [Google Scholar] [CrossRef] [PubMed]
- Dalirfardouei, R.; Jamialahmadi, K.; Jafarian, A.H.; Mahdipour, E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J. Tissue Eng. Regen. Med. 2019, 13, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Tan, Y.; Zou, S.; Zhang, H.; Mao, F.; Gong, A.; Qian, H.; Xu, W. hucMSC Exosome-Derived GPX1 Is Required for the Recovery of Hepatic Oxidant Injury. Mol. Ther. 2017, 25, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jin, S.; Zhang, Y. Ischemic preconditioning potentiates the protective effect of mesenchymal stem cells on endotoxin-induced acute lung injury in mice through secretion of exosome. Int. J. Clin. Exp. Med. 2015, 8, 3825–3832. [Google Scholar]
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef]
- Guo, S.-C.; Tao, S.-C.; Yin, W.-J.; Qi, X.; Sheng, J.-G.; Zhang, C.-Q. Exosomes from Human Synovial-Derived Mesenchymal Stem Cells Prevent Glucocorticoid-Induced Osteonecrosis of the Femoral Head in the Rat. Int. J. Biol. Sci. 2016, 12, 1262–1272. [Google Scholar] [CrossRef]
- Monsel, A.; Zhu, Y.-G.; Gennai, S.; Hao, Q.; Hu, S.; Rouby, J.-J.; Rosenzwajg, M.; Matthay, M.A.; Lee, J.W. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am. J. Respir. Crit. Care Med. 2015, 192, 324–336. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Feng, X.-M.; Abbott, J.; Fang, X.-H.; Hao, Q.; Monsel, A.; Qu, J.-M.; Matthay, M.A.; Lee, J.W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef]
- Stone, M.L.; Zhao, Y.; Robert Smith, J.; Weiss, M.L.; Kron, I.L.; Laubach, V.E.; Sharma, A.K. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir. Res. 2017, 18, 212. [Google Scholar] [CrossRef]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Ban, T.; Rhim, T. Therapeutic use of stem cell transplantation for cell replacement or cytoprotective effect of microvesicle released from mesenchymal stem cell. Mol. Cells 2014, 37, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Pouriran, R.; Abdollahifar, M.-A.; Abbaszadeh, H.A.; Ghoreishi, S.K.; Chien, S.; Bayat, M. Stereological and molecular studies on the combined effects of photobiomodulation and human bone marrow mesenchymal stem cell conditioned medium on wound healing in diabetic rats. J. Photochem. Photobiol. B Biol. 2018, 182, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, J.; Su, L.; Cheng, S.; Zhou, J.; Ye, X.; Dong, Y.; Sun, S.; Qi, F.; Liu, Z.; et al. Human adipose tissue-derived stem cells inhibit the activity of keloid fibroblasts and fibrosis in a keloid model by paracrine signaling. Burns 2018, 44, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, M.A.; Sendon-Lago, J.; Eiro, N.; Treviño, M.; Gonzalez, F.; Yebra-Pimentel, E.; Giraldez, M.J.; Macia, M.; Lamelas, M.L.; Saa, J.; et al. Corneal Epithelial Wound Healing and Bactericidal Effect of Conditioned Medium from Human Uterine Cervical Stem CellsEffect of CM-hUCESCs on Wound Healing in Dry Eye. Investig. Ophthalmol. Vis. Sci. 2015, 56, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Sendon-Lago, J.; Seoane, S.; Martinez-Ordoñez, A.; Eiro, N.; Saa, J.; Vizoso, F.J.; Gonzalez, F.; Perez-Fernandez, R.; Bermudez, M.A. Corneal regeneration by conditioned medium of human uterine cervical stem cells is mediated by TIMP-1 and TIMP-2. Exp. Eye Res. 2019, 180, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, M.A.; Sendon-Lago, J.; Seoane, S.; Eiro, N.; Gonzalez, F.; Saa, J.; Vizoso, F.; Perez-Fernandez, R. Anti-inflammatory effect of conditioned medium from human uterine cervical stem cells in uveitis. Exp. Eye Res. 2016, 149, 84–92. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Yang, L.; Zhang, G.; Wang, Y.; Zhang, S. The Effects of Conditioned Medium Derived from Mesenchymal Stem Cells Cocultured with Hepatocytes on Damaged Hepatocytes and Acute Liver Failure in Rats. Stem Cells Int. 2018, 2018, 9156560. [Google Scholar] [CrossRef]
- Giacoppo, S.; Thangavelu, S.R.; Diomede, F.; Bramanti, P.; Conti, P.; Trubiani, O.; Mazzon, E. Anti-inflammatory effects of hypoxia-preconditioned human periodontal ligament cell secretome in an experimental model of multiple sclerosis: A key role of IL-37. FASEB J. 2017, 31, 5592–5608. [Google Scholar] [CrossRef]
- Brini, A.T.; Amodeo, G.; Ferreira, L.M.; Milani, A.; Niada, S.; Moschetti, G.; Franchi, S.; Borsani, E.; Rodella, L.F.; Panerai, A.E.; et al. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci. Rep. 2017, 7, 9904. [Google Scholar] [CrossRef]
- Devaney, J.; Horie, S.; Masterson, C.; Elliman, S.; Barry, F.; O’Brien, T.; Curley, G.F.; O’Toole, D.; Laffey, J.G. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax 2015, 70, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Helal, O.; Gabr, H.; El Hady, A.; Ahmed, S. Histological and immunohistochemical study of the role of stem cells, conditioned medium and microvesicles in treatment of experimentally induced acute kidney injury in rats. J. Med. Histol. 2017, 1, 69–83. [Google Scholar] [CrossRef]
- Timmers, L.; Lim, S.K.; Arslan, F.; Armstrong, J.S.; Hoefer, I.E.; Doevendans, P.A.; Piek, J.J.; El Oakley, R.M.; Choo, A.; Lee, C.N.; et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2008, 1, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front. Immunol. 2017, 8, 339. [Google Scholar] [CrossRef]
- Nery, A.A.; Nascimento, I.C.; Glaser, T.; Bassaneze, V.; Krieger, J.E.; Ulrich, H. Human mesenchymal stem cells: From immunophenotyping by flow cytometry to clinical applications. Cytom. Part A J. Int. Soc. Anal. Cytol. 2013, 83, 48–61. [Google Scholar] [CrossRef]
- Price, M.J.; Chou, C.-C.; Frantzen, M.; Miyamoto, T.; Kar, S.; Lee, S.; Shah, P.K.; Martin, B.J.; Lill, M.; Forrester, J.S.; et al. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int. J. Cardiol. 2006, 111, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Ben Menachem-Zidon, O.; Gropp, M.; Ben Shushan, E.; Reubinoff, B.; Shveiky, D. Systemically transplanted mesenchymal stem cells induce vascular-like structure formation in a rat model of vaginal injury. PLoS ONE 2019, 14, e0218081. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, C. Effects of Hyperlipidemia and Cardiovascular Diseases on Proliferation, Differentiation and Homing of Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2017, 12, 377–387. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. Enhancement of Functionality and Therapeutic Efficacy of Cell-Based Therapy Using Mesenchymal Stem Cells for Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 982. [Google Scholar] [CrossRef]
- Jiang, W.; Ma, A.; Wang, T.; Han, K.; Liu, Y.; Zhang, Y.; Zhao, X.; Dong, A.; Du, Y.; Huang, X.; et al. Intravenous transplantation of mesenchymal stem cells improves cardiac performance after acute myocardial ischemia in female rats. Ransplant Int. 2006, 19, 570–580. [Google Scholar] [CrossRef]
- Yue, W.M.; Liu, W.; Bi, Y.W.; He, X.P.; Sun, W.Y.; Pang, X.Y.; Gu, X.H.; Wang, X.P. Mesenchymal stem cells differentiate into an endothelial phenotype, reduce neointimal formation, and enhance endothelial function in a rat vein grafting model. Stem Cells Dev. 2008, 17, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Eun, L.Y.; Song, H.; Choi, E.; Lee, T.G.; Moon, D.W.; Hwang, D.; Byun, K.H.; Sul, J.H.; Hwang, K.C. Implanted bone marrow-derived mesenchymal stem cells fail to metabolically stabilize or recover electromechanical function in infarcted hearts. Tissue Cell 2011, 43, 238–245. [Google Scholar] [CrossRef] [PubMed]
- McGinley, L.M.; McMahon, J.; Stocca, A.; Duffy, A.; Flynn, A.; O’Toole, D.; O’Brien, T. Mesenchymal stem cell survival in the infarcted heart is enhanced by lentivirus vector-mediated heat shock protein 27 expression. Hum. Gene Ther. 2013, 24, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Lei, M.; Hu, W.; Han, S.; Wang, Q. A brief review: The therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res. Ther. 2017, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Togel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Ren. Physiol. 2005, 289, F31–F42. [Google Scholar] [CrossRef] [PubMed]
- Yeo, R.W.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Rosca, A.M.; Rayia, D.M.; Tutuianu, R. Emerging Role of Stem Cells-Derived Exosomes as Valuable Tools for Cardiovascular Therapy. Curr. Stem Cell Res. Ther. 2017, 12, 134–138. [Google Scholar] [CrossRef]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Linero, I.; Chaparro, O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef] [PubMed]
- Inukai, T.; Katagiri, W.; Yoshimi, R.; Osugi, M.; Kawai, T.; Hibi, H.; Ueda, M. Novel application of stem cell-derived factors for periodontal regeneration. Biochem. Biophys. Res. Commun. 2013, 430, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Crisostomo, P.R.; Herring, C.; Meldrum, K.K.; Meldrum, D.R. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2006, 291, R880–R884. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Ivanova, G.; Caseiro, A.R.; Barbosa, P.; Bártolo, P.J.; Santos, J.D.; Luís, A.L.; Maurício, A.C. MSCs conditioned media and umbilical cord blood plasma metabolomics and composition. PLoS ONE 2014, 9, e113769. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polacek, M.; Bruun, J.-A.; Elvenes, J.; Figenschau, Y.; Martinez, I. The Secretory Profiles of Cultured Human Articular Chondrocytes and Mesenchymal Stem Cells: Implications for Autologous Cell Transplantation Strategies. Cell Transplant. 2011, 20, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Hwang, S.-M. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine 2005, 32, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef]
- Eiro, N.; Sendon-Lago, J.; Seoane, S.; Bermudez, M.A.; Lamelas, M.L.; Garcia-Caballero, T.; Schneider, J.; Perez-Fernandez, R.; Vizoso, F.J. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget 2014, 5, 10692–10708. [Google Scholar] [CrossRef]
- Schneider, J.; Mateo, E.; Marcos-Arias, C.; Eiro, N.; Vizoso, F.; Perez-Fernandez, R.; Eraso, E.; Quindos, G. Antifungal Activity of the Human Uterine Cervical Stem Cells Conditioned Medium (hUCESC-CM) Against Candida albicans and Other Medically Relevant Species of Candida. Front. Microbiol. 2018, 9, 2818. [Google Scholar] [CrossRef]
- Adkins, D.R.; Abidi, M.H.; Brown, R.A.; Khoury, H.; Goodnough, L.T.; Vij, R.; Westervelt, P.; DiPersio, J.F. Resolution of psoriasis after allogeneic bone marrow transplantation for chronic myelogenous leukemia: Late complications of therapy. Bone Marrow Transpl. 2000, 26, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, H.A.; Kritikos, H.D.; Gemetzi, C.; Koutala, H.; Marsh, J.C.; Boumpas, D.T.; Eliopoulos, G.D. Bone marrow progenitor cell reserve and function and stromal cell function are defective in rheumatoid arthritis: Evidence for a tumor necrosis factor alpha-mediated effect. Blood 2002, 99, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Kastrinaki, M.C.; Sidiropoulos, P.; Roche, S.; Ringe, J.; Lehmann, S.; Kritikos, H.; Vlahava, V.M.; Delorme, B.; Eliopoulos, G.D.; Jorgensen, C.; et al. Functional, molecular and proteomic characterisation of bone marrow mesenchymal stem cells in rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Zhang, H.Y.; Feng, X.B.; Hou, Y.Y.; Lu, L.W.; Fan, L.M. Abnormality of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus 2007, 16, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Lau, C.; Lie, A.; Chan, G.; Mok, M. Defective phenotype of mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus 2010, 19, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Li, X.; Chen, H.; Che, N.; Zhou, S.; Lu, Z.; Shi, S.; Sun, L. High level of reactive oxygen species impaired mesenchymal stem cell migration via overpolymerization of F-actin cytoskeleton in systemic lupus erythematosus. Pathol. Biol. 2014, 62, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Meng, D.; Wang, D.; Zhang, J.; Shi, D.; Liu, H.; Xu, H.; Lu, L.; Sun, L. Enhanced apoptosis and senescence of bone-marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Stem Cells Dev. 2012, 21, 2387–2394. [Google Scholar] [CrossRef]
- Geng, L.; Li, X.; Feng, X.; Zhang, J.; Wang, D.; Chen, J.; Liu, R.; Chen, H.; Sun, L. Association of TNF-α with impaired migration capacity of mesenchymal stem cells in patients with systemic lupus erythematosus. J. Immunol. Res. 2014, 2014, 169082. [Google Scholar] [CrossRef]
- Gu, Z.; Jiang, J.; Tan, W.; Xia, Y.; Cao, H.; Meng, Y.; Da, Z.; Liu, H.; Cheng, C. p53/p21 Pathway involved in mediating cellular senescence of bone marrow-derived mesenchymal stem cells from systemic lupus erythematosus patients. Clin. Dev. Immunol. 2013, 2013, 134243. [Google Scholar] [CrossRef]
- Gao, L.; Bird, A.K.; Meednu, N.; Dauenhauer, K.; Liesveld, J.; Anolik, J.; Looney, R.J. Bone Marrow-Derived Mesenchymal Stem Cells from Patients with Systemic Lupus Erythematosus Have a Senescence-Associated Secretory Phenotype Mediated by a Mitochondrial Antiviral Signaling Protein-Interferon-β Feedback Loop. Arthr. Rheumatol. 2017, 69, 1623–1635. [Google Scholar] [CrossRef]
- Gu, Z.; Cao, X.; Jiang, J.; Li, L.; Da, Z.; Liu, H.; Cheng, C. Upregulation of p16INK4A promotes cellular senescence of bone marrow-derived mesenchymal stem cells from systemic lupus erythematosus patients. Cell. Signal. 2012, 24, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Feng, X.; Lu, L.; Konkel, J.E.; Zhang, H.; Chen, Z.; Li, X.; Gao, X.; Lu, L.; Shi, S.; et al. A CD8 T cell/indoleamine 2,3-dioxygenase axis is required for mesenchymal stem cell suppression of human systemic lupus erythematosus. Arthr. Rheumatol. 2014, 66, 2234–2245. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ma, X.; Zhang, H.; Gu, Z.; Hou, Y.; Gilkeson, G.S.; Lu, L.; Zeng, X.; Sun, L. Gene expression profile reveals abnormalities of multiple signaling pathways in mesenchymal stem cell derived from patients with systemic lupus erythematosus. Clin. Dev. Immunol. 2012, 2012, 826182. [Google Scholar] [CrossRef] [PubMed]
- Becker-Merok, A.; Eilertsen, G.Ø.; Nossent, J.C. Levels of Transforming Growth Factor-β Are Low in Systemic Lupus Erythematosus Patients with Active Disease. J. Rheumatol. 2010, 37, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Cárdenes, N.; Álvarez, D.; Sellarés, J.; Peng, Y.; Corey, C.; Wecht, S.; Nouraie, S.M.; Shanker, S.; Sembrat, J.; Bueno, M.; et al. Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res. Ther. 2018, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xu, M.; Shen, M.; Cheng, J. Effects of Type 2 Diabetic Serum on Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. J. Diabetes Res. 2018, 2018, 5765478. [Google Scholar] [CrossRef] [PubMed]
- Moseley, K.F.; Doyle, M.E.; Jan De Beur, S.M. Diabetic serum from older women increases adipogenic differentiation in mesenchymal stem cells. Endocr. Res. 2018, 43, 155–165. [Google Scholar] [CrossRef]
- Cramer, C.; Freisinger, E.; Jones, R.K.; Slakey, D.P.; Dupin, C.L.; Newsome, E.R.; Alt, E.U.; Izadpanah, R. Persistent High Glucose Concentrations Alter the Regenerative Potential of Mesenchymal Stem Cells. Stem Cells Dev. 2010, 19, 1875–1884. [Google Scholar] [CrossRef]
- Barbagallo, I.; Li Volti, G.; Galvano, F.; Tettamanti, G.; Pluchinotta, F.R.; Bergante, S.; Vanella, L. Diabetic human adipose tissue-derived mesenchymal stem cells fail to differentiate in functional adipocytes. Exp. Biol. Med. 2017, 242, 1079–1085. [Google Scholar] [CrossRef]
- Beltramo, E.; Lopatina, T.; Berrone, E.; Mazzeo, A.; Iavello, A.; Camussi, G.; Porta, M. Extracellular vesicles derived from mesenchymal stem cells induce features of diabetic retinopathy In Vitro. Acta Diabetol. 2014, 51, 1055–1064. [Google Scholar] [CrossRef]
- Mazzeo, A.; Beltramo, E.; Iavello, A.; Carpanetto, A.; Porta, M. Molecular mechanisms of extracellular vesicle-induced vessel destabilization in diabetic retinopathy. Acta Diabetol. 2015, 52, 1113–1119. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Cheraghi, O.; Nourazarian, A.; Hassanpour, M.; Kazemi, M.; Ghaderi, S.; Faraji, E.; Rahbarghazi, R.; Avci, C.B.; Bagca, B.G.; et al. Type 2 Diabetes Inhibited Human Mesenchymal Stem Cells Angiogenic Response by Over-Activity of the Autophagic Pathway. J. Cell. Biochem. 2017, 118, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, J.; Mehranjani, M.S.; Rahbarghazi, R.; Shariatzadeh, M.A. Angiogenic and Restorative Abilities of Human Mesenchymal Stem Cells Were Reduced Following Treatment with Serum from Diabetes Mellitus Type 2 Patients. J. Cell. Biochem. 2018, 119, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Fijany, A.; Sayadi, L.R.; Khoshab, N.; Banyard, D.A.; Shaterian, A.; Alexander, M.; Lakey, J.R.T.; Paydar, K.Z.; Evans, G.R.D.; Widgerow, A.D. Mesenchymal stem cell dysfunction in diabetes. Mol. Biol. Rep. 2019, 46, 1459–1475. [Google Scholar] [CrossRef] [PubMed]
- Jumabay, M.; Moon, J.H.; Yeerna, H.; Bostrom, K.I. Effect of Diabetes Mellitus on Adipocyte-Derived Stem Cells in Rat. J. Cell. Physiol. 2015, 230, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Madhira, S.L.; Challa, S.S.; Chalasani, M.; Nappanveethl, G.; Bhonde, R.R.; Ajumeera, R.; Venkatesan, V. Promise(s) of mesenchymal stem cells as an in vitro model system to depict pre-diabetic/diabetic milieu in WNIN/GR-Ob mutant rats. PLoS ONE 2012, 7, e48061. [Google Scholar] [CrossRef]
- Kim, H.; Han, J.W.; Lee, J.Y.; Choi, Y.J.; Sohn, Y.D.; Song, M.; Yoon, Y.S. Diabetic Mesenchymal Stem Cells Are Ineffective for Improving Limb Ischemia Due to Their Impaired Angiogenic Capability. Cell Transpl. 2015, 24, 1571–1584. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, M.; Li, L.; Liu, J.; Chen, B.; Chen, Y.; An, X.; Liu, S.; Luo, R.; Long, D.; et al. Mesenchymal stem cell-conditioned media ameliorate diabetic endothelial dysfunction by improving mitochondrial bioenergetics via the Sirt1/AMPK/PGC-1alpha pathway. Clin. Sci. 2016, 130, 2181–2198. [Google Scholar] [CrossRef]
- Redondo, J.; Sarkar, P.; Kemp, K.; Virgo, P.F.; Pawade, J.; Norton, A.; Emery, D.C.; Guttridge, M.G.; Marks, D.I.; Wilkins, A.; et al. Reduced cellularity of bone marrow in multiple sclerosis with decreased MSC expansion potential and premature ageing in vitro. Mult. Scler. 2018, 24, 919–931. [Google Scholar] [CrossRef]
- Sarkar, P.; Redondo, J.; Kemp, K.; Ginty, M.; Wilkins, A.; Scolding, N.J.; Rice, C.M. Reduced neuroprotective potential of the mesenchymal stromal cell secretome with ex vivo expansion, age and progressive multiple sclerosis. Cytotherapy 2018, 20, 21–28. [Google Scholar] [CrossRef]
- Redondo, J.; Sarkar, P.; Kemp, K.; Heesom, K.J.; Wilkins, A.; Scolding, N.J.; Rice, C.M. Dysregulation of Mesenchymal Stromal Cell Antioxidant Responses in Progressive Multiple Sclerosis. Stem Cells Transl. Med. 2018, 7, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Barilani, M.; Lovejoy, C.; Dossena, M.; Viganò, M.; Seresini, A.; Piga, D.; Gandhi, S.; Pezzoli, G.; Abramov, A.Y.; et al. Mitochondrial dysfunction in Parkinsonian mesenchymal stem cells impairs differentiation. Redox Biol. 2017, 14, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.-W.; Noh, M.-Y.; Kim, H.Y.; Koh, S.-H.; Kim, K.-S.; Kim, S.H. Bone Marrow-Derived Stromal Cells from Amyotrophic Lateral Sclerosis Patients Have Diminished Stem Cell Capacity. Stem Cells Dev. 2010, 19, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Bossolasco, P.; Cova, L.; Calzarossa, C.; Servida, F.; Mencacci, N.E.; Onida, F.; Polli, E.; Lambertenghi Deliliers, G.; Silani, V. Metalloproteinase alterations in the bone marrow of ALS patients. J. Mol. Med. 2010, 88, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.-H.; Baik, W.; Noh, M.Y.; Cho, G.W.; Kim, H.Y.; Kim, K.S.; Kim, S.H. The functional deficiency of bone marrow mesenchymal stromal cells in ALS patients is proportional to disease progression rate. Exp. Neurol. 2012, 233, 472–480. [Google Scholar] [CrossRef]
- Liu, R.F.; Wang, F.; Wang, Q.; Zhao, X.C.; Zhang, K.M. Research Note Mesenchymal stem cells from skin lesions of psoriasis patients promote proliferation and inhibit apoptosis of HaCaT cells. Genet. Mol. Res. GMR 2015, 14, 17758–17767. [Google Scholar] [CrossRef] [PubMed]
- Orciani, M.; Campanati, A.; Salvolini, E.; Lucarini, G.; Di Benedetto, G.; Offidani, A.; Di Primio, R. The mesenchymal stem cell profile in psoriasis. Br. J. Dermatol. 2011, 165, 585–592. [Google Scholar] [CrossRef]
- Campanati, A.; Orciani, M.; Consales, V.; Lazzarini, R.; Ganzetti, G.; Di Benedetto, G.; Di Primio, R.; Offidani, A. Characterization and profiling of immunomodulatory genes in resident mesenchymal stem cells reflect the Th1-Th17/Th2 imbalance of psoriasis. Arch. Dermatol. Res. 2014, 306, 915–920. [Google Scholar] [CrossRef]
- Hou, R.; Yan, H.; Niu, X.; Chang, W.; An, P.; Wang, C.; Yang, Y.; Yan, X.; Li, J.; Liu, R.; et al. Gene expression profile of dermal mesenchymal stem cells from patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1782–1791. [Google Scholar] [CrossRef]
- Hou, R.X.; Liu, R.F.; Zhao, X.C.; Jia, Y.R.; An, P.; Hao, Z.P.; Li, J.Q.; Li, X.H.; Yin, G.H.; Zhang, K.M. Increased miR-155-5p expression in dermal mesenchymal stem cells of psoriatic patients: Comparing the microRNA expression profile by microarray. Genet. Mol. Res. GMR 2016, 15. [Google Scholar] [CrossRef]
- Mattiucci, D.; Maurizi, G.; Leoni, P.; Poloni, A. Aging-and Senescence-associated Changes of Mesenchymal Stromal Cells in Myelodysplastic Syndromes. Cell Transpl. 2018, 27, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Feng, X. Genetic contribution to mesenchymal stem cell dysfunction in systemic lupus erythematosus. Stem Cell Res. Ther. 2018, 9, 149. [Google Scholar] [CrossRef]
- Feng, X.; Che, N.; Liu, Y.; Chen, H.; Wang, D.; Li, X.; Chen, W.; Ma, X.; Hua, B.; Gao, X.; et al. Restored immunosuppressive effect of mesenchymal stem cells on B cells after olfactory 1/early B cell factor-associated zinc-finger protein down-regulation in patients with systemic lupus erythematosus. Arthr. Rheumatol. 2014, 66, 3413–3423. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.R.; Aoyama, T.; Shima, Y.; Fukiage, K.; Otsuka, S.; Furu, M.; Kohno, Y.; Ito, K.; Fujibayashi, S.; Neo, M.; et al. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells 2007, 25, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Scully, T. Diabetes in numbers. Nature 2012, 485, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, Y.S.; Ko, E.S.; Lim, S.M.; Lee, C.W.; Kim, D.I. Glucose-stimulated insulin secretion of various mesenchymal stem cells after insulin-producing cell differentiation. J. Biosci. Bioeng. 2012, 113, 771–777. [Google Scholar] [CrossRef]
- Ho, J.H.; Tseng, T.C.; Ma, W.H.; Ong, W.K.; Chen, Y.F.; Chen, M.H.; Lin, M.W.; Hong, C.Y.; Lee, O.K. Multiple intravenous transplantations of mesenchymal stem cells effectively restore long-term blood glucose homeostasis by hepatic engraftment and β-cell differentiation in streptozocin-induced diabetic mice. Cell Transpl. 2012, 21, 997–1009. [Google Scholar] [CrossRef]
- Van de Vyver, M.; Niesler, C.; Myburgh, K.H.; Ferris, W.F. Delayed wound healing and dysregulation of IL6/STAT3 signalling in MSCs derived from pre-diabetic obese mice. Mol. Cell. Endocrinol. 2016, 426, 1–10. [Google Scholar] [CrossRef]
- Khan, M.; Ali, F.; Mohsin, S.; Akhtar, S.; Mehmood, A.; Choudhery, M.S.; Khan, S.N.; Riazuddin, S. Preconditioning diabetic mesenchymal stem cells with myogenic medium increases their ability to repair diabetic heart. Stem Cell Res. Ther. 2013, 4, 58. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Rennert, R.C.; Sorkin, M.; Januszyk, M.; Duscher, D.; Kosaraju, R.; Chung, M.T.; Lennon, J.; Radiya-Dixit, A.; Raghvendra, S.; Maan, Z.N.; et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res. Ther. 2014, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.L.; Yukata, K.; Farnsworth, C.W.; Chen, D.G.; Awad, H.; Hilton, M.J.; O’Keefe, R.J.; Xing, L.; Mooney, R.A.; Zuscik, M.J. Delayed fracture healing and increased callus adiposity in a C57BL/6J murine model of obesity-associated type 2 diabetes mellitus. PLoS ONE 2014, 9, e99656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Paula, D.R.M.; Capuano, V.; Filho, D.M.; Carneiro, A.; de Oliveira Crema, V.; de Oliveira, L.F.; Rodrigues, A.R.A.; Montano, N.; da Silva, V.J.D. Biological properties of cardiac mesenchymal stem cells in rats with diabetic cardiomyopathy. Life Sci. 2017, 188, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kota, S.K.; Meher, L.K.; Jammula, S.; Kota, S.K.; Krishna, S.V.; Modi, K.D. Aberrant angiogenesis: The gateway to diabetic complications. Indian J. Endocrinol. Metab. 2012, 16, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev. 2018, 14, 337–345. [Google Scholar] [CrossRef]
- Digirolamo, C.M.; Stokes, D.; Colter, D.; Phinney, D.G.; Class, R.; Prockop, D.J. Propagation and senescence of human marrow stromal cells in culture: A simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br. J. Haematol. 1999, 107, 275–281. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Alvarez, D.; Levine, M.; Rojas, M. Regenerative medicine in the treatment of idiopathic pulmonary fibrosis: Current position. Stem Cells Cloning Adv. Appl. 2015, 8, 61–65. [Google Scholar] [CrossRef]
- Wu, L.W.; Wang, Y.L.; Christensen, J.M.; Khalifian, S.; Schneeberger, S.; Raimondi, G.; Cooney, D.S.; Lee, W.P.; Brandacher, G. Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transp. Immunol. 2014, 30, 122–127. [Google Scholar] [CrossRef]
- Kizilay Mancini, O.; Shum-Tim, D.; Stochaj, U.; Correa, J.A.; Colmegna, I. Age, atherosclerosis and type 2 diabetes reduce human mesenchymal stromal cell-mediated T-cell suppression. Stem Cell Res. Ther. 2015, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Juhasz, B.; Tosaki, A. Management of multicellular senescence and oxidative stress. J. Cell. Mol. Med. 2013, 17, 936–957. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Rando, T.A. Emerging models and paradigms for stem cell ageing. Nat. Cell Biol. 2011, 13, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.R.; Kang, K.S. Aging-related genes in mesenchymal stem cells: A mini-review. Gerontology 2013, 59, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Raggi, C.; Berardi, A.C. Mesenchymal stem cells, aging and regenerative medicine. Musclesligaments Tendons J. 2012, 2, 239–242. [Google Scholar]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Golpanian, S.; DiFede, D.L.; Pujol, M.V.; Lowery, M.H.; Levis-Dusseau, S.; Goldstein, B.J.; Schulman, I.H.; Longsomboon, B.; Wolf, A.; Khan, A.; et al. Rationale and design of the allogeneiC human mesenchymal stem cells (hMSC) in patients with aging fRAilTy via intravenoUS delivery (CRATUS) study: A phase I/II, randomized, blinded and placebo controlled trial to evaluate the safety and potential efficacy of allogeneic human mesenchymal stem cell infusion in patients with aging frailty. Oncotarget 2016, 7, 11899–11912. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, G.; Leng, S.X. Frailty syndrome: An overview. Clin. Interv. Aging 2014, 9, 433–441. [Google Scholar] [CrossRef]
- Mansilla, E.; Diaz Aquino, V.; Zambon, D.; Marin, G.H.; Martire, K.; Roque, G.; Ichim, T.; Riordan, N.H.; Patel, A.; Sturla, F.; et al. Could metabolic syndrome, lipodystrophy, and aging be mesenchymal stem cell exhaustion syndromes? Stem Cells Int. 2011, 2011, 943216. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Rubin, M.R.; Schwartz, A.V.; Kanis, J.A. Type 2 diabetes and bone. J. Bone Miner. Res. 2012, 27, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Vasam, G.; Joshi, S.; Thatcher, S.E.; Bartelmez, S.H.; Cassis, L.A.; Jarajapu, Y.P. Reversal of Bone Marrow Mobilopathy and Enhanced Vascular Repair by Angiotensin-(1-7) in Diabetes. Diabetes 2017, 66, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.; Suzuki, K.; Qu, J.; Wang, P.; Zhou, J.; Liu, X.; Ren, R.; Xu, X.; Ocampo, A.; et al. Aging stem cells. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 2015, 348, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Barkho, B.Z.; Ruiz, S.; Diep, D.; Qu, J.; Yang, S.L.; Panopoulos, A.D.; Suzuki, K.; Kurian, L.; Walsh, C.; et al. Recapitulation of premature ageing with iPSCs from Hutchinson-Gilford progeria syndrome. Nature 2011, 472, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, G.; Su, J.; Li, W.; Chen, Q.; Shou, P.; Xu, C.; Chen, X.; Huang, Y.; Zhu, Z.; et al. Mesenchymal stem cells: A new strategy for immunosuppression and tissue repair. Cell Res. 2010, 20, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Benias, P.C.; Wells, R.G.; Sackey-Aboagye, B.; Klavan, H.; Reidy, J.; Buonocore, D.; Miranda, M.; Kornacki, S.; Wayne, M.; Carr-Locke, D.L.; et al. Structure and Distribution of an Unrecognized Interstitium in Human Tissues. Sci. Rep. 2018, 8, 4947. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef] [PubMed]
- Tachida, Y.; Sakurai, H.; Okutsu, J. Proteomic Comparison of the Secreted Factors of Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue and Dental Pulp. J. Proteom. Bioinform. 2015, 8. [Google Scholar] [CrossRef]
- Amable, P.R.; Teixeira, M.V.; Carias, R.B.; Granjeiro, J.M.; Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 2014, 5, 53. [Google Scholar] [CrossRef]
- Orciani, M.; Caffarini, M.; Lazzarini, R.; Delli Carpini, G.; Tsiroglou, D.; Di Primio, R.; Ciavattini, A. Mesenchymal Stem Cells from Cervix and Age: New Insights into CIN Regression Rate. Oxid. Med. Cell. Longev. 2018, 2018, 1545784. [Google Scholar] [CrossRef]
- Mendicino, M.; Bailey, A.M.; Wonnacott, K.; Puri, R.K.; Bauer, S.R. MSC-based product characterization for clinical trials: An FDA perspective. Cell Stem Cell 2014, 14, 141–145. [Google Scholar] [CrossRef]
- Panchalingam, K.M.; Jung, S.; Rosenberg, L.; Behie, L.A. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: A review. Stem Cell Res. Ther. 2015, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, A.C.; Verma, A.; Kehoe, D.E.; Jing, D.; Murrell, J.R.; Der, K.A.; Aysola, M.; Rapiejko, P.J.; Punreddy, S.; Rook, M.S. Bioprocessing of human mesenchymal stem/stromal cells for therapeutic use: Current technologies and challenges. Biochem. Eng. J. 2016, 108, 3–13. [Google Scholar] [CrossRef]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef] [PubMed]
- Phelps, J.; Sanati-Nezhad, A.; Ungrin, M.; Duncan, N.A.; Sen, A. Bioprocessing of Mesenchymal Stem Cells and Their Derivatives: Toward Cell-Free Therapeutics. Stem Cells Int. 2018, 2018, 9415367. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.C.; Pochampally, R.R.; Chen, S.C.; Hsu, S.C.; Prockop, D.J. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 2007, 25, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Block, G.J.; Ohkouchi, S.; Fung, F.; Frenkel, J.; Gregory, C.; Pochampally, R.; DiMattia, G.; Sullivan, D.E.; Prockop, D.J. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells 2009, 27, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Xu, P. Smart Mesoporous Silica Nanoparticles for Protein Delivery. Nanomater. 2019, 9, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. Cmater. Biol. Appl. 2018, 92, 1041–1060. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Garcia Garcia, C.; Kiick, K.L. Methods for producing microstructured hydrogels for targeted applications in biology. Acta Biomater. 2019, 84, 34–48. [Google Scholar] [CrossRef]
- Perez-Luna, V.H.; Gonzalez-Reynoso, O. Encapsulation of Biological Agents in Hydrogels for Therapeutic Applications. Gels 2018, 4, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 27. [Google Scholar] [CrossRef] [PubMed]
| Disease | MSC Source | Administration Via | Experimental Model | Therapeutic Effect | Ref |
|---|---|---|---|---|---|
| Local administration | |||||
| Diabetic wound healing | Gingival | Topical | Diabetic rat | Promotion of healing in diabetic skin defects. | [12] |
| Synovium | Topical | Diabetic rat | Stimulation of proliferation of human dermal fibroblasts and human microvascular endothelial cells. | [13] | |
| Corneal epithelial wound | Corneal | Local | Mouse | Acceleration of corneal epithelial wound healing. | [14] |
| Traumatic and degenerative ocular disease | Bone marrow | Intravitreal injection | Rat | Promotion of retinal ganglion cells and axon regeneration survival. | [15] |
| Autistic-like behaviors | Bone marrow | Intranasal | BTBR mice | Increase of male to male social interaction and reduce repetitive behaviors. | [16] |
| Liver fibrosis | Umbilical cord | Intra-hepatic | Mouse | Decrease of surface fibrous capsules and alleviate hepatic inflammation. | [17] |
| Periodontitis | Adipose-derived | Local Injection | Rat | Increase in newly organized tissue. | [18] |
| Systemic administration | |||||
| Cutaneous wound healing | Adipose tissue | Intravenous | Mouse | Acceleration of cutaneous wound healing and stimulation of fibroblast migration and collagen synthesis. | [19] |
| Umbilical cord | Subcutaneous injection | Rat | Promotion of wound healing and angiogenesis. | [20] | |
| Adipose tissue | Intravenous injection | Mouse | Promotion of extracellular matrix reconstruction and regulation of fibroblast differentiation to mitigate scar formation. | [21] | |
| Menstrual blood-derived | Intradermic injection | Mouse | Resolution of inflammation, reepithelization accelerated by induction of M1-M2 macrophage polarization and increased neoangiogenesis. | [22] | |
| Atopic dermatitis | Adipose tissue | Intravenous and subcutaneous injection | Mouse | Decrease of clinical score, level of serum IgE, number of eosinophils in blood and infiltration of mast cells, CD86+ and CD206+ cells. Decrease of mRNA expression of pro-inflammatory cytokines. | [23] |
| Hepatic injury | Umbilical cord | Intravenous | Mouse | Reduction of oxidative stress and apoptosis. | [24] |
| Endotoxin-induced acute lung injury | Bone marrow | Intravenous | Mouse | Reduction of white blood cells and neutrophils from bronchoalveolar lavage fluid (BALF). | [25] |
| Bronchopulmo-nary dysplasia | Wharton jelly Bone marrow | Intravenous | Mouse | Amelioration of alveolar simplification, fibrosis and pulmonary vascular remodelling, reduction of pro-inflammatory M1, and increase of anti-inflammatory M2 macrophages. | [26] |
| Osteonecrosis | Synovial membrane | Intramuscular | Rat | Prevention of osteonecrosis, enhance proliferation and anti-apoptotic effects. | [27] |
| Local and systemic administration | |||||
| Pneumonia/E. coli | Bone marrow | Intratracheal Intravenous | Mouse | Reduction of lung injury, white blood cells and neutrophils in BALF. Reduction of E. coli in BALF, lung and blood. Increased survival. | [28] |
| Lung injury | Bone marrow | Intratracheal Intravenous | Mouse | Reduction of lung injury, white blood cells, neutrophils, total protein, MIP-1 and E. coli in BALF. Increase of survival. | [29] |
| Wharton jelly | Intratracheal | Mouse | Reduction of lung edema, airway resistance, pulmonary artery pressure, neutrophils in lung, and inflammatory cytokines in BALF. Increase of KGF, PGE2 and IL-10 in BALF. | [30] | |
| Lung fibrosis/Silica | Bone marrow | Intratracheal | Mouse | Reduction of calcified nodules size, hydroproline in lung, and inflammatory cells in BALF. | [31] |
| Bone marrow | Intratravenous | Mouse | Reduction of lung collagen and white blood cells in BALF. | [32] | |
| Disease | MSC Source | Administration Via | Experimental Model | Therapeutic Effect | Ref |
|---|---|---|---|---|---|
| Local administration | |||||
| Cutaneous wound healing | Bone marrow | Local | T1 diabetic rats | Acceleration of wound healing. | [33] |
| Keloid | Adipose tissue | Local | Mouse | Inhibition of proliferation and collagen synthesis of human keloid-derived fibroblast. Reduction of inflammation and fibrosis. | [34] |
| Dry eye and corneal epithelial wound | Uterine cervix | Local | Rat | Improvement in wound healing of alkali-injured corneas. Strong bactericidal effect on infected corneal contact lens | [35] |
| Rabbit | Improvement in epithelial regeneration Reduction of corneal pro-inflammatory cytokines. | [36] | |||
| Uveitis | Uterine cervix | Topical | Mouse | Reduction of inflammation, and LPS-induced pro-inflammatory cytokines. Decrease in leucocytes in aqueous humor and ocular tissues. | [37] |
| Systemic administration | |||||
| Acute liver failure | Bone marrow | Intravenous | Rat | Inhibition of liver injury biomarkers release and promotion of recovery in liver structure. | [38] |
| Multiple sclerosis | Periodontal ligament | Intravenous | Mouse | Decrease in clinical and histologic score, and modulation of inflammation, oxidative stress, and apoptotic pathways. | [39] |
| Diabetes | Adipose tissue | Intravenous | Mouse | Reverse mechanical, thermal allodynia and thermal hyperalgesia. Restoration of pro/anti-inflammatory cytokine balance. Prevention of skin innervation loss and re-establishment of Th1/Th2 balance. Recovery of kidney morphology. | [40] |
| Pneumonia/E. coli | Bone marrow | Intravenous | Rat | Increase in survival. | [41] |
| Acute kidney injury | Bone marrow | Intramuscular | Rat | Amelioration of kidney injury. | [42] |
| Myocardial infarct | Bone marrow | Intravenous and intracoronary | Porcine | Reduction of myocardial infarct size. Improvement of systolic and diastolic cardiac performance. | [43] |
| Bioactive Effects | Factors | Ref |
|---|---|---|
| Proliferation/Regeneration | FGFs, HGF, IGF-1, EGF, PDGF, VEGF, TIMP-1, TIMP-2, UPAR | [35,62,63,64] |
| Angiogenesis | FGFs, HGF, IGF-1, IL-6, MCP-1, PDGF, VEGF | [35,62,64] |
| Anti-apoptosis | FGF, IL-6, IGF-1, GM-CSF, HGF | [35,62,63,64,65] |
| Anti-fibrosis | FGFs, HGF, TIMP-1, MMPs | [35,62,64,66] |
| Chemo-attraction | CCLs, CXCLs, G-CSF, LIF, MCP-1 | [35,62,65,67] |
| Immuno-modulation | IDO, IL-10, IL-6, LIF, NT-3, PGE-2 | [37,62,67,68] |
| Anti-tumoral | FLT-3, CXC10/IP10, LAP, Light | [69] |
| Bactericidal | CXC10/IP10, CXCL8/IL8, CXCL1/GRO-7, CXCL6/GCP-2, CCL20/MIP-3, CCL5/RANTES | [35,62] |
| Antifungal | IL-6, IL-8, IL-17, IP-10, CCL-5, CXC-6, CXC-16 | [70] |
| Disease | MSC Source | MSC Features | Ref |
|---|---|---|---|
| Flattened morphology. | [74,75,76] | ||
| Increased cell senescence and apoptosis. | [77] | ||
| Impaired potential for differentiation and migration. | [78] | ||
| Systemic Lupus Erythematosus | Bone marrow | Increased activation of the p53/p21 pathway. | [79,80] |
| Increased expression of p16INK4a | [80,81] | ||
| Increased reactive oxygen species. | [80] | ||
| Alteration of expression profiles in genes related to immune function. | [74,80,82,83,84] | ||
| Idiopathic pulmonary fibrosis | Bone marrow | Mitochondrial dysfunction, with accumulation of DNA damage. Cell senescence. Decreased capacity to migrate. Increased pro-inflammatory responses. | [85] |
| Impaired differentiation and decreased proliferation. | [86,87,88,89] | ||
| Diabetes mellitus | Bone marrow and Adipose tissue | Impaired angiogenesis/vasculogenesis. | [90,91,92,93,94,95] |
| Increased pro-inflammatory cytokines. | [96] | ||
| Greater propensity to differentiate into adipocytes. | [97] | ||
| Umbilical cord | Increased pro-inflammatory cytokines. | [98] | |
| Reduced ex vivo proliferation and clonogenic potential, premature senescence, and accelerated shortening of telomere terminal restriction fragments. | [99] | ||
| Multiple sclerosis | Bone marrow | Reduced in vitro neuroprotective potential. | [100] |
| Reduced expression, activity, and secretion of key antioxidants. Increased susceptibility to nitrosative stress. | [101] | ||
| Rheumatoid arthritis | Bone marrow | Impaired proliferative potential in association with premature telomere length loss. | [73] |
| Parkinson disease | Bone marrow | Impaired differentiation, mitochondrial dysfunction and increased ROS generation and oxidative stress. | [102] |
| Amyotrophic lateral sclerosis | Bone marrow | Reduced migration. | [103] |
| Alterations in metalloproteases. | [104] | ||
| Reduced capacity of pluripotency and trophic factor secretion. | [103,105] | ||
| Psoriasis | MSCs in psoriasis plaques or from areas surrounding the psoriasic eruptions | Increased expression of inflammation and angiogenesis-related genes. | [106,107,108,109,110] |
| Myelodysplastic syndromes | Bone marrow | Altered morphology, reduced proliferative potential, p53 pathway activation, dysregulated miRNA in extracellular vesicles. | [111] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vizoso, F.J.; Eiro, N.; Costa, L.; Esparza, P.; Landin, M.; Diaz-Rodriguez, P.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cells in Homeostasis and Systemic Diseases: Hypothesis, Evidences, and Therapeutic Opportunities. Int. J. Mol. Sci. 2019, 20, 3738. https://doi.org/10.3390/ijms20153738
Vizoso FJ, Eiro N, Costa L, Esparza P, Landin M, Diaz-Rodriguez P, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cells in Homeostasis and Systemic Diseases: Hypothesis, Evidences, and Therapeutic Opportunities. International Journal of Molecular Sciences. 2019; 20(15):3738. https://doi.org/10.3390/ijms20153738
Chicago/Turabian StyleVizoso, Francisco J., Noemi Eiro, Luis Costa, Paloma Esparza, Mariana Landin, Patricia Diaz-Rodriguez, Jose Schneider, and Roman Perez-Fernandez. 2019. "Mesenchymal Stem Cells in Homeostasis and Systemic Diseases: Hypothesis, Evidences, and Therapeutic Opportunities" International Journal of Molecular Sciences 20, no. 15: 3738. https://doi.org/10.3390/ijms20153738
APA StyleVizoso, F. J., Eiro, N., Costa, L., Esparza, P., Landin, M., Diaz-Rodriguez, P., Schneider, J., & Perez-Fernandez, R. (2019). Mesenchymal Stem Cells in Homeostasis and Systemic Diseases: Hypothesis, Evidences, and Therapeutic Opportunities. International Journal of Molecular Sciences, 20(15), 3738. https://doi.org/10.3390/ijms20153738







