Potential Prognostic Markers of Acute Kidney Injury in the Early Phase of Acute Pancreatitis
Abstract
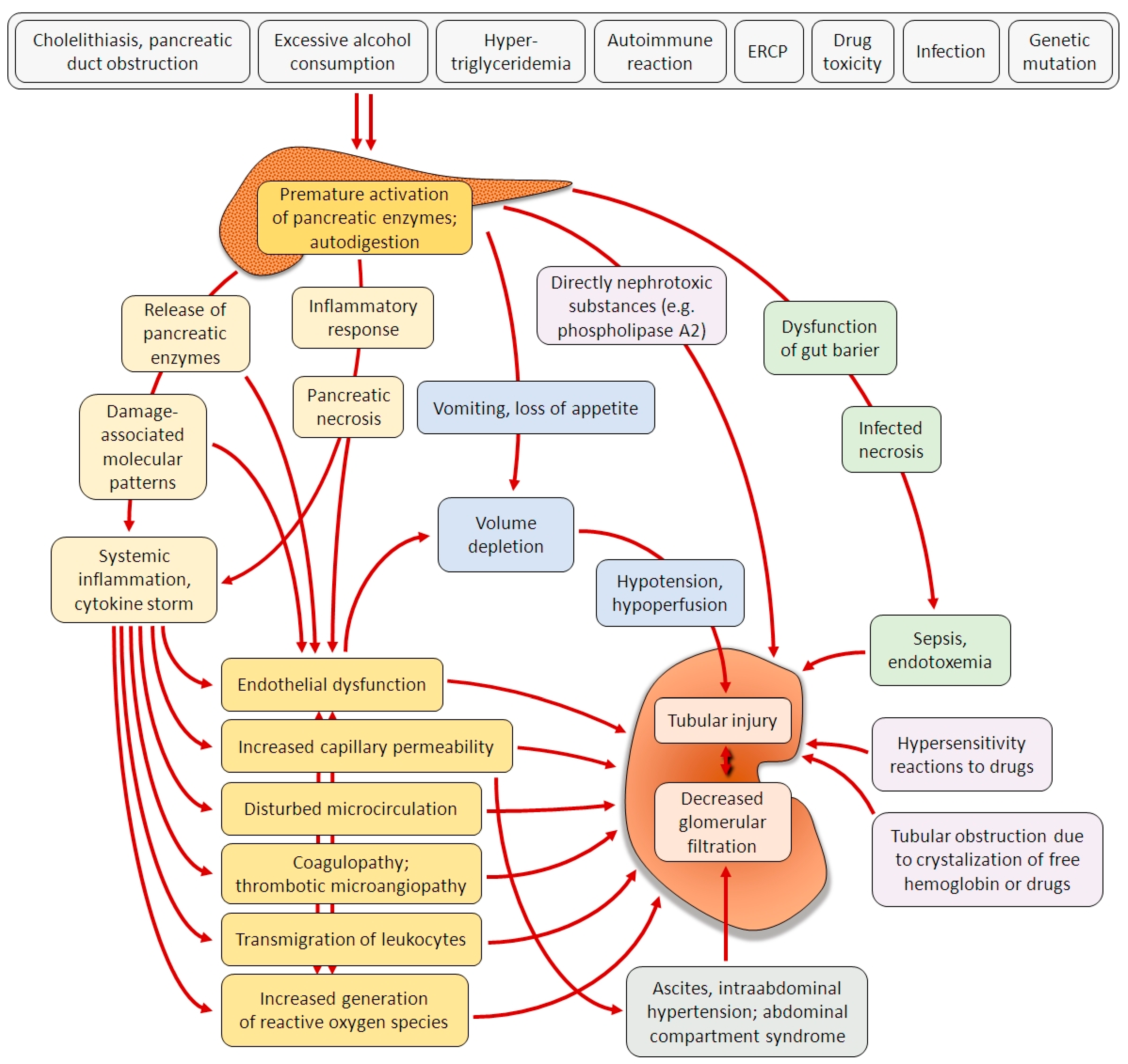
:1. Introduction
2. The Markers of Glomerular Filtration
2.1. Serum Creatinine
2.2. Serum Cystatin C
3. The Markers of Tubular Dysfunction
3.1. Neutrophil Gelatinase-Associated Lipocalin (NGAL)
3.2. Kidney Injury Molecule 1 (KIM-1)
3.3. Tissue Inhibitor Metalloproteinase-2 (TIMP-2) and Urine Insulin-Like Growth Factor-Binding Protein 7 (IGFBP7)
3.4. Interleukin 18 (IL-18)
3.5. Liver-Type Fatty Acid-Binding Protein (L-FABP)
3.6. Calprotectin
3.7. Urinary β2-Microglobulin
3.8. Monocyte Chemoattractant Protein (MCP-1)
3.9. Uromodulin
4. Other Markers Associated with Kidney Injury in AP
5. Associations between Renal Markers and AP Severity
6. Conclusions
Conflicts of Interest
References
- Beker, B.M.; Corleto, M.G.; Fieiras, C.; Musso, C.G. Novel acute kidney injury biomarkers: Their characteristics, utility and concerns. Int. Urol. Nephrol. 2018, 50, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. Acute Kidney Injury Advisory Group of the American Society of Nephrology World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.K.; McCulloch, C.E.; Dudley, R.A.; Lo, L.J.; Hsu, C. Temporal Changes in Incidence of Dialysis-Requiring AKI. J. Am. Soc. Nephrol. 2013, 24, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Park, H.; Kim, Y.; Kang, D.; Ku, H.S.; Cho, J.; Lee, J.E.; Huh, W.; Guallar, E.; Suh, G.Y.; et al. Changes in acute kidney injury epidemiology in critically ill patients: A population-based cohort study in Korea. Ann. Intensive Care 2019, 9. [Google Scholar] [CrossRef]
- Ostermann, M.; Liu, K. Pathophysiology of AKI. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef]
- Dumnicka, P.; Maduzia, D.; Ceranowicz, P.; Olszanecki, R.; Drożdż, R.; Kuśnierz-Cabala, B. The interplay between inflammation, coagulation and endothelial injury in the early phase of acute pancreatitis: Clinical implications. Int. J. Mol. Sci. 2017, 18, 354. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis - 2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.I.; Tang, Y.I.; Liu, F.; Yu, S.; Zhang, L.; Zeng, X.; Zhao, Y.; Fu, P. Effect of acute kidney injury on mortality and hospital stay in patient with severe acute pancreatitis. Nephrology (Carlton). 2015, 20, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-Y.; Lai, J.-I.; Lai, Y.-C.; Lin, P.-C.; Chang, S.-C.; Tang, G.-J. Acute renal failure in severe pancreatitis: A population-based study. Ups. J. Med. Sci. 2011, 116, 155–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykes, L.; Kalra, P.A.; Green, D. Comparison of impact on death and critical care admission of acute kidney injury between common medical and surgical diagnoses. PLoS ONE 2019, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Huang, H.-B.; Feng, G.; Cao, Y.-H.; Cheng, Q.-S.; Li, S.-H.; He, C.-Y.; Lu, W.-H.; Qin, M.-M. Baseline Serum Cystatin C Is a Potential Predictor for Acute Kidney Injury in Patients with Acute Pancreatitis. Dis. Markers 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gougol, A.; Dugum, M.; Dudekula, A.; Greer, P.; Slivka, A.; Whitcomb, D.C.; Yadav, D.; Papachristou, G.I. Clinical outcomes of isolated renal failure compared to other forms of organ failure in patients with severe acute pancreatitis. World J. Gastroenterol. 2017, 23, 5431–5437. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, P.; Crichton, S.; Lemmich Smith, J.; Morrison, D.; Atkinson, S.; Wyncoll, D.; Ostermann, M. Improved Outcome of Severe Acute Pancreatitis in the Intensive Care Unit. Crit. Care Res. Pract. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Pahwa, N.; Jain, N. Acute kidney injury in severe acute pancreatitis: An experience from a tertiary care center. Saudi J. Kidney Dis. Transpl. 2015, 26, 56–60. [Google Scholar] [PubMed]
- Párniczky, A.; Kui, B.; Szentesi, A.; Balázs, A.; Szűcs, Á.; Mosztbacher, D.; Czimmer, J.; Sarlós, P.; Bajor, J.; Gódi, S.; et al. Prospective, multicentre, nationwide clinical data from 600 cases of acute pancreatitis. PLoS ONE 2016, 11, e0165309. [Google Scholar] [CrossRef] [PubMed]
- Devani, K.; Charilaou, P.; Radadiya, D.; Brahmbhatt, B.; Young, M.; Reddy, C. Acute pancreatitis: Trends in outcomes and the role of acute kidney injury in mortality- A propensity-matched analysis. Pancreatology 2018, 18, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Manokaran, S.; Edwin, F.M.; Srinivasaprasad, N.; Suren, S. A Study of Acute Kidney Injury in Severe Acute Pancreatitis in a Tertiary Care Hospital from South India. IOSR J. Dent. Med. Sci. 2018, 17, 45–48. [Google Scholar]
- de-Madaria, E.; Banks, P.A.; Moya-Hoyo, N.; Wu, B.U.; Rey-Riveiro, M.; Acevedo-Piedra, N.G.; Martínez, J.; Lluís, F.; Sánchez-Payá, J.; Singh, V.K. Early factors associated with fluid sequestration and outcomes of patients with acute pancreatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Mao, W.; Chen, Y.; Tong, Z.; Li, G.; Zhou, J.; Ke, L.; Li, W. Aggressive Resuscitation Is Associated with the Development of Acute Kidney Injury in Acute Pancreatitis. Dig. Dis. Sci. 2019, 64, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Wu, J.; Zhang, H.; Zhou, J.; Ye, B.; Li, G.; Gao, L.; Li, X.; Ke, L.; Tong, Z.; et al. Increase in serum chloride and chloride exposure are associated with acute kidney injury in moderately severe and severe acute pancreatitis patients. Pancreatology 2019, 19, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Nadeem, A.J.; Doratotaj, B. A rare case of thrombotic microangiopathy triggered by acute pancreatitis. BMJ Case Rep. 2017, 2017, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, W.; Mori, T.; Nagahama, K.; Tamura, T. Biopsy-proven drug-induced tubulointerstitial nephritis in a patient with acute kidney injury and alcoholic severe acute pancreatitis. Case Rep. 2013, 2013, bcr2013008557. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ke, L.; Dong, J.; Ye, B.; Meng, L.; Mao, W.; Yang, Q.; Li, W.; Li, J. Significantly different clinical features between hypertriglyceridemia and biliary acute pancreatitis: A retrospective study of 730 patients from a tertiary center. BMC Gastroenterol. 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zou, L.; Shi, S.; Tong, Z.; Shen, X.; Yang, D.; Ke, L.; Li, W.; Li, J. The role of hypertriglyceridemia for acute kidney injury in the course of acute pancreatitis and an animal model. Pancreatology 2017, 17, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Petejova, N.; Martinek, A. Acute kidney injury following acute pancreatitis: A review. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2013, 157, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.; Yin, J.; Cheng, D.; Lu, Z.; Wang, N.; Wang, F.; Liang, M. Antithrombin III Attenuates AKI Following Acute Severe Pancreatitis. Shock 2018, 49, 572–579. [Google Scholar] [CrossRef]
- Nassar, T.I.; Qunibi, W.Y. AKI Associated with Acute Pancreatitis. Clin. J. Am. Soc. Nephrol. 2019. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Andreucci, M.; Faga, T.; Pisani, A.; Perticone, M.; Michael, A. The ischemic/nephrotoxic acute kidney injury and the use of renal biomarkers in clinical practice. Eur. J. Intern. Med. 2017, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Macedo, E. Urinary Biochemistry in the Diagnosis of Acute Kidney Injury. Dis. Markers 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leem, A.Y.; Park, M.S.; Park, B.H.; Jung, W.J.; Chung, K.S.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Kim, Y.S.; et al. Value of Serum Cystatin C Measurement in the Diagnosis of Sepsis-Induced Kidney Injury and Prediction of Renal Function Recovery. Yonsei Med. J. 2017, 58, 604. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zeng, Z.; Fu, C.; Zhang, S.; Cai, Y.; Chen, Z. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit. Care 2015, 19, 223. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. A Prospective Observational Study on the Predictive Value of Serum Cystatin C for Successful Weaning from Continuous Renal Replacement Therapy. Kidney Blood Press. Res. 2018, 43, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Li, M.-L.; Wang, B.; Guo, M.-X.; Zhu, R.-M. Caspase-1 inhibition alleviates acute renal injury in rats with severe acute pancreatitis. World J. Gastroenterol. 2014, 20, 10457–10463. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, J.; Wang, W.; Zhang, Z.; Li, D.; Lin, K.; Chen, Z.; Lin, W. Matrix Metalloproteinase 9 and Vasodilator-Stimulated Phosphoprotein Related to Acute Kidney Injury in Severe Acute Pancreatitis Rats. Dig. Dis. Sci. 2015, 60, 3647–3655. [Google Scholar] [CrossRef]
- Gori, E.; Pierini, A.; Lippi, I.; Boffa, N.; Perondi, F.; Marchetti, V. Urinalysis and Urinary GGT-to-Urinary Creatinine Ratio in Dogs with Acute Pancreatitis. Vet. Sci. 2019, 6, 27. [Google Scholar] [CrossRef]
- Gori, E.; Lippi, I.; Guidi, G.; Perondi, F.; Pierini, A.; Marchetti, V. Acute pancreatitis and acute kidney injury in dogs. Vet. J. 2019, 245, 77–81. [Google Scholar] [CrossRef]
- Wang, K.; Xie, S.; Xiao, K.; Yan, P.; He, W.; Xie, L. Biomarkers of Sepsis-Induced Acute Kidney Injury. Biomed. Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Rubin, J.; Han, W.; Venge, P.; Xu, S. The Origin of Multiple Molecular Forms in Urine of HNL/NGAL. Clin. J. Am. Soc. Nephrol. 2010, 5, 2229–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simsek, A.; Tugcu, V.; Tasci, A.I. New Biomarkers for the Quick Detection of Acute Kidney Injury. ISRN Nephrol. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianca, G.; Raluca, F.; Veres, M.; Orlandea, M.; Badea, J.; Hlavathy, K.; Cioc, A. Plasma Neutrophil Gelatinase Associated Lipocalin (NGAL)—Early Biomarker for Acute Kidney Injury in Critically Ill Patients. J. Crit. Care Med. 2015, 1, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Mårtensson, J.; Bellomo, R. The Rise and Fall of NGAL in Acute Kidney Injury. Blood Purif. 2014, 37, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Kari, J.A.; Shalaby, M.A.; Sofyani, K.; Sanad, A.S.; Ossra, A.F.; Halabi, R.S.; Aljuhani, M.H.; Toffaha, W.M.; Moria, F.A.; Sabry, S.; et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) and serum cystatin C measurements for early diagnosis of acute kidney injury in children admitted to PICU. World J. Pediatr. 2018, 14, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Zwiers, A.J.; de Wildt, S.N.; van Rosmalen, J.; de Rijke, Y.B.; Buijs, E.A.; Tibboel, D.; Cransberg, K. Urinary neutrophil gelatinase-associated lipocalin identifies critically ill young children with acute kidney injury following intensive care admission: A prospective cohort study. Crit. Care 2015, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Witkin, S.S. Neutrophil gelatinase-associated lipocalin and innate immune responses to bacterial infections. Med. Microbiol. Immunol. 2015, 204, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.-J.; Ahn, H.-S.; Song, J.Y.; Um, T.-H.; Cho, C.-R.; Jung, H.; Koo, H.-K.; Park, J.H.; Lee, S.-S.; et al. Is plasma neutrophil gelatinase-associated lipocalin a predictive biomarker for acute kidney injury in sepsis patients? A systematic review and meta-analysis. J. Crit. Care 2016, 33, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.S.; Mitchell, E.D.; Smith, A.F.; Cairns, D.A.; Messenger, M.; Hutchinson, M.; Wright, J.; Vinall-Collier, K.; Corps, C.; Hamilton, P.; et al. The future for diagnostic tests of acute kidney injury in critical care: Evidence synthesis, care pathway analysis and research prioritisation. Health Technol. Assess. 2018, 22, 1–274. [Google Scholar] [CrossRef]
- Siddappa, P.K.; Kochhar, R.; Sarotra, P.; Medhi, B.; Jha, V.; Gupta, V. Neutrophil gelatinase-associated lipocalin: An early biomarker for predicting acute kidney injury and severity in patients with acute pancreatitis. JGH Open 2018, 3, 105–110. [Google Scholar] [CrossRef]
- Sporek, M.; Gala-Błądzińska, A.; Dumnicka, P.; Mazur-Laskowska, M.; Kielczewski, S.; Walocha, J.; Ceranowicz, P.; Kuźniewski, M.; Mituś, J.; Kuśnierz-Cabala, B. Urine NGAL is useful in the clinical evaluation of renal function in the early course of acute pancreatitis. Folia Med. Cracov. 2016, 56, 13–25. [Google Scholar] [PubMed]
- Sporek, M.; Dumnicka, P.; Gala-Błądzińska, A.; Mazur-Laskowska, M.; Walocha, J.; Ceranowicz, P.; Warzecha, Z.; Dembiński, A.; Kuźniewski, M.; Olszanecki, R.; et al. Determination of serum neutrophil gelatinase-associated lipocalin at the early stage of acute pancreatitis. Folia Med. Cracov. 2016, 56, 5–16. [Google Scholar] [PubMed]
- Lipinski, M.; Rydzewska-Rosolowska, A.; Rydzewski, A.; Rydzewska, G. Urinary neutrophil gelatinase-associated lipocalin as an early predictor of disease severity and mortality in acute pancreatitis. Pancreas 2015, 44, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.I.; Tang, S.C.W.; Lai, K.N.; Leung, J.C.K. Kidney injury molecule-1: More than just an injury marker of tubular epithelial cells? J. Cell. Physiol. 2013, 228, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Moresco, R.N.; Bochi, G.V.; Stein, C.S.; De Carvalho, J.A.M.; Cembranel, B.M.; Bollick, Y.S. Urinary kidney injury molecule-1 in renal disease. Clin. Chim. Acta 2018, 487, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.H.; Endre, Z.H. Biomarkers in acute kidney injury (AKI). Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, S.; Pipili, C.; Grapsa, E.; Kyprianou, T.; Nanas, S. Novel Biomarkers of Acute Kidney Injury in the General Adult ICU: A Review. Ren. Fail. 2013, 35, 579–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, C.; Wang, N. Kidney injury molecule-1 in kidney disease. Ren. Fail. 2016, 38, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wang, H.; Sun, R.; Ni, Y.; Ma, L.; Xv, F.; Hu, X.; Jiang, L.; Wu, A.; Chen, X.; et al. Urinary netrin-1 and KIM-1 as early biomarkers for septic acute kidney injury. Ren. Fail. 2014, 36, 1559–1563. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Tian, L.; Xu, W.; Zhang, Z.; Wang, C.; Qi, C.; Ni, Z.; Mou, S. Diagnostic Value of Urinary Kidney Injury Molecule 1 for Acute Kidney Injury: A Meta-Analysis. PLoS ONE 2014, 9, e84131. [Google Scholar] [CrossRef]
- Ghatanatti, R.; Teli, A.; Tirkey, S.S.; Bhattacharya, S.; Sengupta, G.; Mondal, A. Role of renal biomarkers as predictors of acute kidney injury in cardiac surgery. Asian Cardiovasc. Thorac. Ann. 2014, 22, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Wasung, M.E.; Chawla, L.S.; Madero, M. Biomarkers of renal function, which and when? Clin. Chim. Acta 2015, 438, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, J.H.; Seibert, F.S.; Waldherr, S.; Bauer, F.; Tönshoff, B.; Fichtner, A.; Westhoff, T.H. Urinary calprotectin, kidney injury molecule-1, and neutrophil gelatinase-associated lipocalin for the prediction of adverse outcome in pediatric acute kidney injury. Eur. J. Pediatr. 2017, 176, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Nie, X.; Cai, B.; Tang, J.-T.; He, Y.; Miao, Q.; Song, H.-L.; Luo, T.-X.; Gao, B.-X.; Wang, L.-L.; et al. Procalcitonin levels predict acute kidney injury and prognosis in acute pancreatitis: A prospective study. PLoS ONE 2013, 8, e82250. [Google Scholar] [CrossRef] [PubMed]
- Kuśnierz-Cabala, B.; Gala-Błądzińska, A.; Mazur-Laskowska, M.; Dumnicka, P.; Sporek, M.; Matuszyk, A.; Gil, K.; Ceranowicz, P.; Walocha, J.; Kucharz, J.; et al. Serum Uromodulin Levels in Prediction of Acute Kidney Injury in the Early Phase of Acute Pancreatitis. Molecules 2017, 22, 988. [Google Scholar] [CrossRef] [PubMed]
- Sporek, M.; Dumnicka, P.; Gala-Błądzińska, A.; Ceranowicz, P.; Warzecha, Z.; Dembiński, A.; Stępień, E.; Walocha, J.; Drożdż, R.; Kuźniewski, M.; et al. Angiopoietin-2 is an early indicator of acute pancreatic-renal syndrome in patients with acute pancreatitis. Mediators Inflamm. 2016, 5780903, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dumnicka, P.; Sporek, M.; Mazur-Laskowska, M.; Ceranowicz, P.; Kuźniewski, M.; Drożdż, R.; Ambroży, T.; Olszanecki, R.; Kuśnierz-Cabala, B. Serum soluble fms-Like tyrosine kinase 1 (sFlt-1) predicts the severity of acute pancreatitis. Int. J. Mol. Sci. 2016, 17, 2038. [Google Scholar] [CrossRef]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef]
- Liu, C.; Lu, X.; Mao, Z.; Kang, H.; Liu, H.; Pan, L.; Hu, J.; Wang, L.; Zhou, F. The diagnostic accuracy of urinary [TIMP-2]·[IGFBP7] for acute kidney injury in adults: A PRISMA-compliant meta-analysis. Medicine 2017, 96, e7484. [Google Scholar] [CrossRef]
- Peng, Z.-Y.; Zhou, F.; Kellum, J.A. Cross-species validation of cell cycle arrest markers for acute kidney injury in the rat during sepsis. Intensive Care Med. Exp. 2016, 4, 12. [Google Scholar] [CrossRef]
- Honore, P.M.; Nguyen, H.B.; Gong, M.; Chawla, L.S.; Bagshaw, S.M.; Artigas, A.; Shi, J.; Joannes-Boyau, O.; Vincent, J.-L.; Kellum, J.A. Urinary Tissue Inhibitor of Metalloproteinase-2 and Insulin-Like Growth Factor-Binding Protein 7 for Risk Stratification of Acute Kidney Injury in Patients With Sepsis. Crit. Care Med. 2016, 44, 1851–1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Gong, Z.; Wu, Y.; Tian, Y.; Liao, X. Diagnostic value of urine tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for acute kidney injury: A meta-analysis. PLoS ONE 2017, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chindarkar, N.S.; Chawla, L.S.; Straseski, J.A.; Jortani, S.A.; Uettwiller-Geiger, D.; Orr, R.R.; Kellum, J.A.; Fitzgerald, R.L. Reference intervals of urinary acute kidney injury (AKI) markers [IGFBP7]∙[TIMP2] in apparently healthy subjects and chronic comorbid subjects without AKI. Clin. Chim. Acta 2016, 452, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.; Larsson, A.; Venge, P.; Bellomo, R.; Mårtensson, J. Assessment of Cell-Cycle Arrest Biomarkers to Predict Early and Delayed Acute Kidney Injury. Dis. Markers 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madro, A.; Kurzepa, J.; Celinski, K.; Slomka, M.; Czechowska, G.; Kurzepa, J.; Kazmierak, W.; Buszewicz, G.; Ciesielka, M.; Madro, R. Effects of renin-angiotensin system inhibitors on fibrosis in patients with alcoholic chronic pancreatitis. J. Physiol. Pharmacol. 2016, 67, 103–110. [Google Scholar] [PubMed]
- Kurzepa, J.; Mądro, A.; Czechowska, G.; Kurzepa, J.; Celiński, K.; Kazmierak, W.; Slomka, M. Role of MMP-2 and MMP-9 and their natural inhibitors in liver fibrosis, chronic pancreatitis and non-specific inflammatory bowel diseases. Hepatobiliary Pancreat. Dis. Int 2014, 13, 570–579. [Google Scholar] [CrossRef]
- Mikami, Y.; Dobschütz, E.V.; Sommer, O.; Wellner, U.; Unno, M.; Hopt, U.; Keck, T. Matrix metalloproteinase-9 derived from polymorphonuclear neutrophils increases gut barrier dysfunction and bacterial translocation in rat severe acute pancreatitis. Surgery 2009, 145, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Sochor, M.; Richter, S.; Schmidt, A.; Hempel, S.; Hopt, U.T.; Keck, T. Inhibition of matrix metalloproteinase-9 with doxycycline reduces pancreatitis-associated lung injury. Digestion 2009, 80, 65–73. [Google Scholar] [CrossRef]
- Wereszczynska-Siemiatkowska, U.; Siemiatkowski, A.; Swidnicka-Siergiejko, A.; Mroczko, B.; Dabrowski, A. The imbalance between matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 in acute pancreatitis. Z. Gastroenterol. 2015, 53, 199–204. [Google Scholar] [CrossRef]
- Nukarinen, E.; Lindström, O.; Kuuliala, K.; Kylänpää, L.; Pettilä, V.; Puolakkainen, P.; Kuuliala, A.; Hämäläinen, M.; Moilanen, E.; Repo, H.; et al. Association of matrix metalloproteinases -7, -8 and -9 and TIMP -1 with disease severity in acute pancreatitis. A cohort study. PLoS ONE 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Schrezenmeier, E.V.; Barasch, J.; Budde, K.; Westhoff, T.; Schmidt-Ott, K.M. Biomarkers in acute kidney injury—pathophysiological basis and clinical performance. Acta Physiol. 2017, 219, 554–572. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Craft, M.L.; Wang, P.; Wyburn, K.R.; Chen, G.; Ma, J.; Hambly, B.; Chadban, S.J. IL-18 Contributes to Renal Damage after Ischemia-Reperfusion. J. Am. Soc. Nephrol. 2008, 19, 2331–2341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Yuan, J.; Zhao, Y.; Zha, Y. Urine interleukin-18 in prediction of acute kidney injury: A systemic review and meta-analysis. J. Nephrol. 2015, 28, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, Y.; Shao, X.; Ni, Z.; Mou, S. L-FABP: A novel biomarker of kidney disease. Clin. Chim. Acta 2015, 445, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Noiri, E.; Maeda-Mamiya, R.; Ishii, T.; Negishi, K.; Hamasaki, Y.; Fujita, T.; Yahagi, N.; Koide, H.; Sugaya, T.; et al. Urinary L-type fatty acid-binding protein as a new biomarker of sepsis complicated with acute kidney injury*. Crit. Care Med. 2010, 38, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Kamijo-Ikemori, A.; Sugaya, T.; Yasuda, T.; Kimura, K. Usefulness of Urinary Biomarkers in Early Detection of Acute Kidney Injury After Cardiac Surgery in Adults. Circ. J. 2012, 76, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.; Tangri, N.; Komenda, P.; Kaushal, A.; Sood, M.; Brar, R.; Gill, K.; Walker, S.; MacDonald, K.; Hiebert, B.M.; et al. Urinary, Plasma, and Serum Biomarkers’ Utility for Predicting Acute Kidney Injury Associated With Cardiac Surgery in Adults: A Meta-analysis. Am. J. Kidney Dis. 2015, 66, 993–1005. [Google Scholar] [CrossRef]
- Doi, K.; Negishi, K.; Ishizu, T.; Katagiri, D.; Fujita, T.; Matsubara, T.; Yahagi, N.; Sugaya, T.; Noiri, E. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit*. Crit. Care Med. 2011, 39, 2464–2469. [Google Scholar] [CrossRef]
- Parr, S.K.; Clark, A.J.; Bian, A.; Shintani, A.K.; Wickersham, N.E.; Ware, L.B.; Ikizler, T.A.; Siew, E.D. Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int. 2015, 87, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Azimi, A. Could “calprotectin” and “endocan” serve as “Troponin of Nephrologists”? Med. Hypotheses 2017, 99, 29–34. [Google Scholar] [CrossRef]
- Heller, F.; Frischmann, S.; Grünbaum, M.; Zidek, W.; Westhoff, T.H. Urinary Calprotectin and the Distinction between Prerenal and Intrinsic Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2011, 6, 2347–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibert, F.S.; Pagonas, N.; Arndt, R.; Heller, F.; Dragun, D.; Persson, P.; Schmidt-Ott, K.; Zidek, W.; Westhoff, T.H. Calprotectin and neutrophil gelatinase-associated lipocalin in the differentiation of pre-renal and intrinsic acute kidney injury. Acta Physiol. 2013, 207, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Kou, H.; Chou, H.-S.; Chou, H.; Huang, S.-F.; Chang, C.-H.; Wu, C.-H.; Yu, M.-C.; Tsai, H.-I. A combination of SOFA score and biomarkers gives a better prediction of septic AKI and in-hospital mortality in critically ill surgical patients: A pilot study. World J. Emerg. Surg. 2018, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Yang, C.-H.; Yang, H.-Y.; Chen, T.-H.; Lin, C.-Y.; Chang, S.-W.; Chen, Y.-T.; Hung, C.-C.; Fang, J.-T.; Yang, C.-W.; et al. Urinary Biomarkers Improve the Diagnosis of Intrinsic Acute Kidney Injury in Coronary Care Units. Medicine 2015, 94, e1703. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, Y.; Fu, Y.; Guo, W.; Liu, G. Diagnostic and prognostic value of myeloid-related protein complex 8/14 for sepsis. Am. J. Emerg. Med. 2015, 33, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.-H.; Roumelioti, M.-E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering Beta-2 Microglobulin As a Biomarker across the Spectrum of Kidney Diseases. Front. Med. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Hossain, D.; Bostwick, D.G.; Herrera, G.A.; Zhang, P.L. Urinary β 2-Microglobulin Is a Good Indicator of Proximal Tubule Injury: A Correlative Study with Renal Biopsies. J. Biomarkers 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Du, Y.; Zappitelli, M.; Mian, A.; Bennett, M.; Ma, Q.; Devarajan, P.; Mehta, R.; Goldstein, S.L. Urinary biomarkers to detect acute kidney injury in the pediatric emergency center. Pediatr. Nephrol. 2011, 26, 267–274. [Google Scholar] [CrossRef]
- Frasquet, J.L.; Sáez, J.; Trigo, C.; Martínez, J.; Pérez-Mateo, M. Proteinuria and urinary beta 2-microglobulin as markers of tubular malfunction in the assessment of severity of acute pancreatitis. Gastroenterol. Hepatol. 2004, 27, 295–299. [Google Scholar]
- Chang, C.T.; Liao, H.Y.; Huang, W.H.; Lin, S.Y.; Tsai, T.Y.; Yang, C.Y.; Tsai, F.J.; Chen, C.J. Early prediction of severe acute pancreatitis by urinary β-2 microglobulin/saposin B peak ratios on MALDI-TOF. Clin. Chim. Acta 2015, 440, 115–122. [Google Scholar] [CrossRef]
- Shinke, H.; Masuda, S.; Togashi, Y.; Ikemi, Y.; Ozawa, A.; Sato, T.; Kim, Y.H.; Mishima, M.; Ichimura, T.; Bonventre, J.V.; et al. Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients. Cancer Chemother. Pharmacol. 2015, 76, 989–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Chi, R.; Chen, S.; Ye, H.; Yuan, J.; Wang, L.; Zhai, Y.; Gao, L.; Zhang, D.; Hu, L.; et al. Evaluation of clinically available renal biomarkers in critically ill adults: A prospective multicenter observational study. Crit. Care 2017, 21, 46. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Bertram, A.; Nadrowitz, F.; Menne, J. Monocyte chemoattractant protein-1 and the kidney. Curr. Opin. Nephrol. Hypertens. 2016, 25, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Moledina, D.G.; Isguven, S.; McArthur, E.; Thiessen-Philbrook, H.; Garg, A.X.; Shlipak, M.; Whitlock, R.; Kavsak, P.A.; Coca, S.G.; Parikh, C.R. Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI) Consortium Plasma Monocyte Chemotactic Protein-1 Is Associated With Acute Kidney Injury and Death After Cardiac Operations. Ann. Thorac. Surg. 2017, 104, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-Z.; Xiang, Y.; Chen, M.; Xian, L.-N.; Deng, X.-Y. Clinical significance of dynamic detection for serum levels of MCP-1, TNF-α and IL-8 in patients with acute pancreatitis. Asian Pac. J. Trop. Med. 2016, 9, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.G.; Pai, C.G.; Kamath, A.; Kurien, A. Monocyte chemoattractant protein-1, transforming growth factor-beta1, nerve growth factor, resistin and hyaluronic acid as serum markers: Comparison between recurrent acute and chronic pancreatitis. Hepatobiliary Pancreat. Dis. Int 2016, 15, 209–215. [Google Scholar] [CrossRef]
- Sternby, H.; Hartman, H.; Johansen, D.; Thorlacius, H.; Regnér, S. Predictive Capacity of Biomarkers for Severe Acute Pancreatitis. Eur. Surg. Res. 2016, 56, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Steubl, D.; Block, M.; Herbst, V.; Nockher, W.A.; Schlumberger, W.; Satanovskij, R.; Angermann, S.; Hasenau, A.-L.; Stecher, L.; Heemann, U.; et al. Plasma Uromodulin Correlates With Kidney Function and Identifies Early Stages in Chronic Kidney Disease Patients. Medicine 2016, 95, e3011. [Google Scholar] [CrossRef] [PubMed]
- Dumnicka, P.; Kuśnierz-Cabala, B.; Sporek, M.; Mazur-Laskowska, M.; Gil, K.; Kuźniewski, M.; Ceranowicz, P.; Warzecha, Z.; Dembiński, A.; Bonior, J.; et al. Serum concentrations of angiopoietin-2 and soluble fms-like tyrosine kinase 1 (sFlt-1) are associated with coagulopathy among patients with acute pancreatitis. Int. J. Mol. Sci. 2017, 18, 735. [Google Scholar] [CrossRef] [PubMed]
- Hoeboer, S.H.; Alberts, E.; Van den Hul, I.; Tacx, A.N.; Debets-Ossenkopp, Y.J.; Groeneveld, A.J. Old and new biomarkers for predicting high and low risk microbial infection in critically ill patients with new onset fever: A case for procalcitonin. J. Infect. 2012, 64, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Kolber, W.; Kuśnierz-Cabala, B.; Dumnicka, P.; Maraj, M.; Mazur-Laskowska, M.; Pędziwiatr, M.; Ceranowicz, P. Serum Urokinase-Type Plasminogen Activator Receptor Does Not Outperform C-Reactive Protein and Procalcitonin as an Early Marker of Severity of Acute Pancreatitis. J. Clin. Med. 2018, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Kolber, W.; Dumnicka, P.; Maraj, M.; Ku, B.; Maziarz, B.; Mazur-Laskowska, M.; Ceranowicz, P.; Michał, P.; Kuśnierz-Cabala, B.; Ceranowicz, P.; et al. Does the Automatic Measurement of Interleukin 6 Allow for Prediction of Complications during the First 48 h of Acute Pancreatitis? Int. J. Mol. Sci. 2018, 19, 1820. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.; Jaipuria, J.; Singh, M.; Chawla, A.S. Intra-abdominal pressure in the early phase of severe acute pancreatitis: Canary in a coal mine? Results from a rigorous validation protocol. Gut Liver 2013, 7, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Yang, H.-X.; Ma, C.-E. The Value of BISAP Score for Predicting Mortality and Severity in Acute Pancreatitis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0130412. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, A.L.; Barcia, A.M.; Schell, M.T.; Barber, A.; Norman, J.; Grendell, J.; Harris, H.W. Applying Ockham’s Razor to Pancreatitis Prognostication. Ann. Surg. 2006, 243, 380–388. [Google Scholar] [CrossRef] [PubMed]
- DiMagno, M.J. Clinical update on fluid therapy and nutritional support in acute pancreatitis. Pancreatology 2015, 15, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Fujinaka, H.; Xu, B.; Zhang, Y.; Magdeldin, S.; Nameta, M.; Liu, Z.; Yoshida, Y.; Yaoita, E.; Tomizawa, S.; et al. Decreased urinary calbindin 1 levels in proteinuric rats and humans with distal nephron segment injuries. Clin. Exp. Nephrol. 2014, 18, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Ariza, X.; Solà, E.; Elia, C.; Barreto, R.; Moreira, R.; Morales-Ruiz, M.; Graupera, I.; Rodríguez, E.; Huelin, P.; Solé, C.; et al. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLoS ONE 2015, 10, e0128145. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Joy, M.S.; Aleksunes, L.M. Urinary protein biomarkers of kidney injury in patients receiving cisplatin chemotherapy. Exp. Biol. Med. 2018, 243, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Mancini, V.; Galleggiante, V.; Rutigliano, M.; Vavallo, A.; Battaglia, M.; Ditonno, P. Emerging urinary markers of renal injury in obstructive nephropathy. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
| Reference | Study Design and Patients | Definition of AKI | Prevalence of AKI | Remarks |
|---|---|---|---|---|
| Lin et al., 2011 [11] | Retrospective study of 1734 patients with AP admitted to ICU | AKI was identified using the ICD-9 code 584 (AKI). | 15.05% of ICU patients with AP | Mortality was 23.76% in AP with AKI versus 8.08% in AP without AKI. |
| Pavlidis et al., 2013 [15] | Retrospective analysis of 50 patients with SAP admitted to ICU | AKI was defined according to AKIN criteria. | 54% of patients with SAP; 44% of patients with SAP required RRT | AKI more common among non-survivors (100%) than survivors (42%) |
| Zhou et al., 2015 [10] | Retrospective multi-center analysis of 414 patients with SAP admitted to ICU | AKI was defined according to AKIN criteria based on serum creatinine. | 69.3% of patients with SAP; 59.2% of patients with SAP required RRT | Mortality was 44.9% in AP with AKI versus 20.5% in AP without AKI |
| Kumar et al., 2015 [16] | Retrospective analysis of 72 patients with SAP admitted to a tertiary center | AKI was defined and classified according to the RIFLE criteria | 19.4% of patients with SAP; 13.9% of patients with SAP required RRT | Mortality was 57% in AP with AKI versus 0 in AP without AKI |
| Párniczky et al., 2016 [17] | Prospective multicenter study of 600 patients with AP (61% MAP, 30% MSAP, 9% SAP) | Renal failure as an organ complication in patients with SAP; no strict definition given | 36% of patients with SAP | Mortality was 43.8% in SAP with renal failure versus 21.4% in SAP without renal failure |
| Gougol et al., 2017 [14] | Prospective observation of 500 AP patients admitted to a tertiary center | Isolated renal failure according to the modified Marshall scoring system | Isolated renal failure reported in 15% of patients with SAP | No deaths in isolated renal failure versus 22.4% mortality in MOF |
| Devani et al., 2018 [18] | 3,466,493 patients hospitalized with AP (ICD-9 code) between 2003–2012, from Nationwide Inpatient Sample database | AKI was identified using the ICD-9 codes for AKI (584; 584.5; 584.6; 584.7; 584.8; 584.9) | Prevalence of AKI in AP nearly tripled from 4.1% in 2003 to 11.7% in 2012. Overall prevalence within the study period was 7.9%. | Mortality of patients with AKI complicating AP decreased from 17.4% in 2003 to 6.4% in 2012. |
| Chai et al., 2018 [13] | Retrospective analysis of 237 patients with AP (79% MAP, 16% MSAP, 5% SAP) | 2012 KDIGO criteria, any stage | 7.6% of all patients with AP | 50% of patients with AKI had stage 1 AKI according to 2012 KDIGO criteria |
| Manokaran et al., 2018 [19] | 100 patients with SAP from tertiary hospital | KDIGO 2012, any stage | 32% of patients with SAP | Mortality 12.5% in SAP with AKI versus 1.5% in SAP without AKI |
| Reference | Description of the Study and Results |
|---|---|
| Zhang et al., 2014 [36] | Sprague-Dawley rats with SAP was induced by retrograde infusion of 5% sodium taurocholate into the bile-pancreatic duct were treated with caspase-1/interleukin-1β-converting-enzyme inhibitor. The inhibitor attenuated intrarenal IL-1β and caspase-1 expression, the histopathologic changes in kidneys and increased serum creatinine observed in SAP. |
| Li et al., 2015 [37] | SAP was induced in Male Sprague-Dawley rats by retrograde injection of 5% sodium deoxycholate into bile-pancreatic duct. Serum creatinine and blood urea nitrogen significantly increased in rats with SAP 12 h after surgery. Histological changes in kidney tissue and injury to renal endothelial cells were most pronounced at 36-48 h post-surgery. These changes were preceded by increase in mRNA and protein expression of matrix metalloproteinase-9 (MMP-9), also in active form, and vasodilator-stimulated phosphoprotein (VASP) at 12-24 h post-surgery. |
| Wu et al., 2017 [26] | Severe hypertriglyceridemia in ApoC III transgenic mice aggravated kidney injury in the course of AP established by retrograde injection of 0.5% sodium taurocholate to pancreatic duct. ApoC III transgenic mice developed more severe pancreatic damage and more advanced histological changes in the kidneys associated with higher serum creatinine than wild type mice. |
| Kong et al., 2018 [28] | Sprague-Dawley rats with AP induced by retrograde infusion of body weight of 3.5% sodium taurocholate solution into the biliary-pancreatic duct were pretreated with antithrombin III (AT III), or AT III was administered postoperatively. Both ways of AT III administration attenuated increase in serum creatinine, renal tubular detachment, brush border loss, and necrosis of tubular cells. |
| Gori et al., 2019 [38] | The authors studied the diagnostic utility of urinalysis and urinary gamma glutamyl transpeptidase-to-urinary creatinine (GGT/Cr) in dogs with spontaneously developed AP. Non-survivors showed higher dipstick bilirubin levels and urine protein-to creatinine ratio >2 than survivors. The GGT/Cr was not useful in the prognosis of outcome. |
| Gori et al., 2019 [39] | The authors studied the prevalence of AKI complicating spontaneously developed AP in 65 dogs. Higher serum urea and creatinine and oligo- or anuria predicted death of the animals. AKI was diagnosed in 26.2% of dogs. |
| Marker | Reference | Study Design and Patients | Definition of AKI | Cut-off Value | Diagnostic Sensitivity | Diagnostic Specificity | AUC |
|---|---|---|---|---|---|---|---|
| Serum cystatin C | Chai et al., 2018 [13] | Retrospective analysis of 237 patients diagnosed with AP: 5% diagnosed with SAP; 7.6% of all AP patients diagnosed with AKI | KDIGO criteria | 1.865 mg/L | 88.9% | 100% | 0.948 (95% CI: 0.875–1.0) |
| Serum NGAL | Siddappa et al., 2018 [50] | Prospective study of 50 patients with AP admitted to tertiary center: 23 patients diagnosed with SAP, 21 with AKI | Modified Marshall scoring system and AKIN criteria | 790.9 ng/mL | 64% | 96% | 0.8 |
| Urine NGAL | 221 ng/mL | 82% | 80% | 0.9 | |||
| Serum procalcitonin | Huang et al., 2013 [64] | 305 patients with AP admitted to ICU: 52 cases of AKI | RIFLE criteria | 3.30 ng/mL | 97.2% | 92.3% | 0.986 (95% CI: 0.966–1.000) |
| Serum uromodulin | Kuśnierz-Cabala et al., 2017 [65] | Prospective study of 66 patients with AP: 5 diagnosed with SAP, 11 diagnosed with AKI | KDIGO criteria | no data | 0.684 (95% CI: 0.508–0.860) | ||
| Serum uromodulin to creatinine ratio | 0.846 (95% CI: 0.706–0.987) | ||||||
| Serum angiopoietin-2 | Sporek et al., 2016 [66] | Prospective study of 65 patients with AP: 5 diagnosed with SAP, 11 diagnosed with AKI | KDIGO criteria | Higher concentrations of angiopoietin-2 was observed in patients with AKI during first 72 h from the onset of AP. OR for AKI 1.12 (1.02–1.24) at 24 h; 1.37 (1.12–1.68) at 48 h, and 1.49 (1.17–1.90) at 72 h per 1 ng/mL increase in angiopoietin-2 | |||
| Serum soluble fms-like tyrosine kinase-1 (sFlt-1) | Dumnicka et al., 2016 [67] | Prospective study of 65 patients with AP: 5 diagnosed with SAP, 11 diagnosed with AKI | Modified Marshall scoring system | OR for renal failure at 24 h from the onset of AP symptoms 1.31 (1.06–1.63) per 10 pg/mL increase in sFlt-1 | |||
| Marker | Reference | Study Design and Patients | Severity Assessment | Cut-off Value | Diagnostic Sensitivity | Diagnostic Specificity | AUC (95% CI) |
|---|---|---|---|---|---|---|---|
| Serum NGAL | Sporek et al., 2016 [52] | Prospective observation of 65 adult patients admitted with AP; NGAL was measured at 24, 48 and 72 h from the onset of AP | Moderately severe and severe AP according to 2012 Atlanta Classification versus mild AP | 165 μg/L (at 24 h) | 63% | 80% | 0.727 (0.582–0.872) |
| 183 μg/L (at 48 h) | 90% | 72% | 0.860 (0.773–0.948) | ||||
| 182 μg/L (at 72 h) | 84% | 78% | 0.843 (0.730–0.956) | ||||
| Urine NGAL | Lipinski et al., 2015 [53] | Observational cohort study of 104 patients with acute pancreatitis | SAP according to 2012 Atlanta Classification; organ failure according to modified Marshall scoring system | Prediction of SAP: 68.9 ng/mL | 81.2% | 71.5% | 0.750 (0.622–0.890) |
| Prediction of MOF: 86.5 ng/mL | 75% | 76% | 0.870 (0.779–0.964) | ||||
| Prediction of death: 86.5 ng/mL | 75% | 74% | 0.800 (0.632–0.968) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wajda, J.; Dumnicka, P.; Maraj, M.; Ceranowicz, P.; Kuźniewski, M.; Kuśnierz-Cabala, B. Potential Prognostic Markers of Acute Kidney Injury in the Early Phase of Acute Pancreatitis. Int. J. Mol. Sci. 2019, 20, 3714. https://doi.org/10.3390/ijms20153714
Wajda J, Dumnicka P, Maraj M, Ceranowicz P, Kuźniewski M, Kuśnierz-Cabala B. Potential Prognostic Markers of Acute Kidney Injury in the Early Phase of Acute Pancreatitis. International Journal of Molecular Sciences. 2019; 20(15):3714. https://doi.org/10.3390/ijms20153714
Chicago/Turabian StyleWajda, Justyna, Paulina Dumnicka, Małgorzata Maraj, Piotr Ceranowicz, Marek Kuźniewski, and Beata Kuśnierz-Cabala. 2019. "Potential Prognostic Markers of Acute Kidney Injury in the Early Phase of Acute Pancreatitis" International Journal of Molecular Sciences 20, no. 15: 3714. https://doi.org/10.3390/ijms20153714
APA StyleWajda, J., Dumnicka, P., Maraj, M., Ceranowicz, P., Kuźniewski, M., & Kuśnierz-Cabala, B. (2019). Potential Prognostic Markers of Acute Kidney Injury in the Early Phase of Acute Pancreatitis. International Journal of Molecular Sciences, 20(15), 3714. https://doi.org/10.3390/ijms20153714








