Mast Cells May Regulate The Anti-Inflammatory Activity of IL-37
Abstract
:1. Mast Cells in Inflammation
2. IL-37 as An Anti-Inflammatory Agent
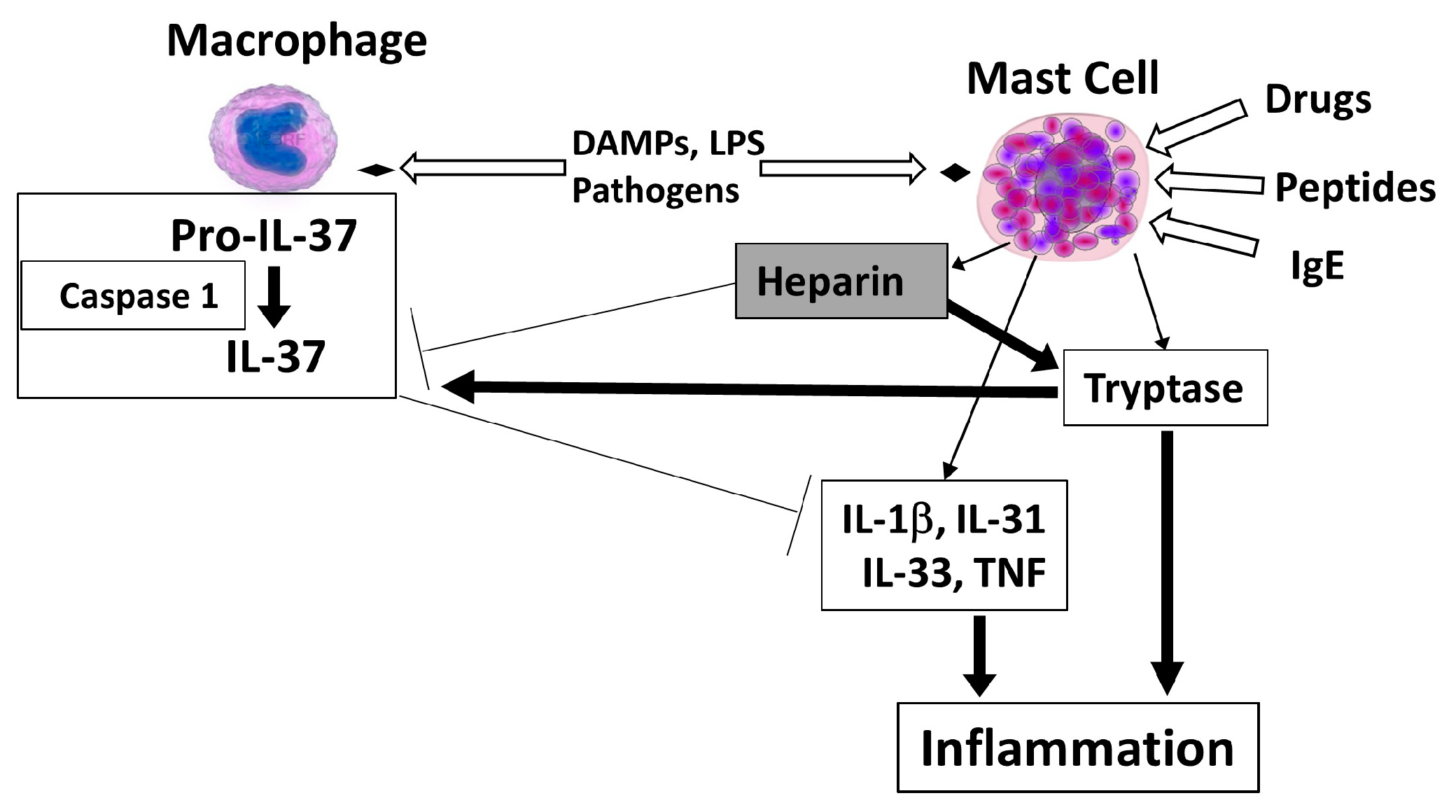
3. Mast Cell-Derived Heparin and Tryptase May Regulate IL-37
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| CRH | corticotropin releasing hormone |
| CXCL8 | chemokine (C-X-C Motif) ligand 8 |
| DAMPs | damage-associated molecular patterns |
| IL | interleukin |
| LPS | lipopolysaccharide |
| NT | neurotensin |
| PBMCs | peripheral blood-derived mononuclear cells |
| PAR | protease-activated receptors |
| SP | substance P |
| TNF | tumor necrosis factor |
References
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Valent, P.; Akin, C. Mast Cells, Mastocytosis, and Related Disorders. N. Engl. J. Med. 2015, 373, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Neuroendocrinology of mast cells: Challenges and controversies. Exp. Dermatol. 2017, 26, 751–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franza, L.; Carusi, V.; Altamura, S.; Gasbarrini, A.; Caraffa, A.; Kritas, S.K.; Ronconi, G.; Gallenga, C.E.; Di, V.F.; Pandolfi, F. Gut microbiota and immunity in common variable immunodeficiency: Crosstalk with pro-inflammatory cytokines. J. Biol. Regul. Homeost. Agents 2019, 33, 315–319. [Google Scholar] [PubMed]
- Cao, J.; Papadopoulou, N.; Kempuraj, D.; Boucher, W.S.; Sugimoto, K.; Cetrulo, C.L.; Theoharides, T.C. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J. Immunol. 2005, 174, 7665–7675. [Google Scholar] [CrossRef] [PubMed]
- Donelan, J.; Boucher, W.; Papadopoulou, N.; Lytinas, M.; Papaliodis, D.; Theoharides, T.C. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc. Natl. Acad. Sci. USA 2006, 103, 7759–7764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theoharides, T.C.; Zhang, B.; Kempuraj, D.; Tagen, M.; Vasiadi, M.; Angelidou, A.; Alysandratos, K.D.; Kalogeromitros, D.; Asadi, S.; Stavrianeas, N.; et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc. Natl. Acad. Sci. USA 2010, 107, 4448–4453. [Google Scholar] [CrossRef]
- Taracanova, A.; Tsilioni, I.; Conti, P.; Norwitz, E.R.; Leeman, S.E.; Theoharides, T.C. Substance P and IL-33 administered together stimulate a marked secretion of IL-1beta from human mast cells, inhibited by methoxyluteolin. Proc. Natl. Acad. Sci. USA 2018, 115, E9381–E9390. [Google Scholar] [CrossRef]
- Mustain, W.C.; Rychahou, P.G.; Evers, B.M. The role of neurotensin in physiologic and pathologic processes. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 75–82. [Google Scholar] [CrossRef]
- Caceda, R.; Kinkead, B.; Nemeroff, C.B. Neurotensin: Role in psychiatric and neurological diseases. Peptides 2006, 27, 2385–2404. [Google Scholar] [CrossRef]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the immune response. Cell Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Hokfelt, T.; Pernow, B.; Wahren, J. Substance P: A pioneer amongst neuropeptides. J. Intern. Med. 2001, 249, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.D.; Leeman, S.E. Neurokinin-1 receptor: Functional significance in the immune system in reference to selected infections and inflammation. Ann. N. Y. Acad. Sci. 2011, 1217, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Kalogeromitros, D. The critical role of mast cells in allergy and inflammation. Ann. N. Y. Acad. Sci. 2006, 1088, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bin, N.R.; Sugita, S. Diverse exocytic pathways for mast cell mediators. Biochem. Soc. Trans. 2018, 46, 235–247. [Google Scholar] [CrossRef]
- Gordon, J.R.; Galli, S.J. Mast cells as a source of both preformed and immunologically inducible TNF-a/cachectin. Nature 1990, 346, 274–276. [Google Scholar] [CrossRef]
- Zhang, B.; Alysandratos, K.D.; Angelidou, A.; Asadi, S.; Sismanopoulos, N.; Delivanis, D.A.; Weng, Z.; Miniati, A.; Vasiadi, M.; Katsarou-Katsari, A.; et al. Human mast cell degranulation and preformed TNF secretion require mitochondrial translocation to exocytosis sites: Relevance to atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 1522–1531. [Google Scholar] [CrossRef]
- Caraffa, A.; Conti, C.; Ovidio, D.; Gallenga, C.E.; Tettamanti, L.; Mastrangelo, F.; Ronconi, G.; Kritas, S.K.; Conti, P. New concepts in neuroinflammation: Mast cells pro-inflammatory and anti-inflammatory cytokine mediators. J. Biol. Regul. Homeost. Agents 2018, 32, 449–454. [Google Scholar]
- Gallenga, C.E.; Pandolfi, F.; Caraffa, A.; Kritas, S.K.; Ronconi, G.; Toniato, E.; Martinotti, S.; Conti, P. Interleukin-1 family cytokines and mast cells: Activation and inhibition. J. Biol. Regul. Homeost. Agents 2019, 33, 1–6. [Google Scholar]
- Theoharides, T.C.; Kempuraj, D.; Tagen, M.; Conti, P.; Kalogeromitros, D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 2007, 217, 65–78. [Google Scholar] [CrossRef]
- Beaven, M.A. Our perception of the mast cell from Paul Ehrlich to now. Eur. J. Immunol. 2009, 39, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Toniato, E.; Frydas, I.; Robuffo, I.; Ronconi, G.; Caraffa, A.; Kritas, S.K.; Conti, P. Activation and inhibition of adaptive immune response mediated by mast cells. J. Biol. Regul. Homeost. Agents 2017, 31, 543–548. [Google Scholar] [PubMed]
- Marone, G.; Galli, S.J.; Kitamura, Y. Probing the roles of mast cells and basophils in natural and acquired immunity, physiology and disease. Trends Immunol. 2002, 23, 425–427. [Google Scholar] [CrossRef]
- Gong, J.; Yang, N.S.; Croft, M.; Weng, I.C.; Sun, L.; Liu, F.T.; Chen, S.S. The antigen presentation function of bone marrow-derived mast cells is spatiotemporally restricted to a subset expressing high levels of cell surface FcepsilonRI and MHC II. BMC Immunol. 2010, 11, 34. [Google Scholar] [CrossRef]
- Carroll-Portillo, A.; Cannon, J.L.; te Riet, J.; Holmes, A.; Kawakami, Y.; Kawakami, T.; Cambi, A.; Lidke, D.S. Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J. Cell Biol. 2015, 210, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2010, 1822, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. The IL-1 family and inflammatory diseases. Clin. Exp. Rheumatol. 2002, 20, S1–S13. [Google Scholar]
- Vasiadi, M.; Therianou, A.; Sideri, K.; Smyrnioti, M.; Sismanopoulos, N.; Delivanis, D.A.; Asadi, S.; Katsarou-Katsari, A.; Petrakopoulou, T.; Theoharides, A.; et al. Increased serum CRH levels with decreased skin CRHR-1 gene expression in psoriasis and atopic dermatitis. J. Allergy Clin. Immunol. 2012, 129, 1410–1413. [Google Scholar] [CrossRef] [Green Version]
- Konnikov, N.; Pincus, S.H.; Dinarello, C.A. Elevated plasma interleukin-1 levels in humans following ultraviolet light therapy for psoriasis. J. Invest. Dermatol. 1989, 92, 235–239. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Bufler, P. Interleukin-37. Semin. Immunol. 2013, 25, 466–468. [Google Scholar] [CrossRef]
- Tettamanti, L.; Kritas, S.K.; Gallenga, C.E.; D’Ovidio, C.; Mastrangelo, F.; Ronconi, G.; Caraffa, A.; Toniato, E.; Conti, P. IL-33 mediates allergy through mast cell activation, Potential inhibitory effect of certain cytokines. J. Biol. Regul. Homeost. Agents 2018, 32, 1061–1065. [Google Scholar]
- Zhao, M.; Li, Y.; Guo, C.; Wang, L.; Chu, H.; Zhu, F.; Li, Y.; Wang, X.; Wang, Q.; Zhao, W.; et al. IL-37 isoform D downregulates pro-inflammatory cytokines expression in a Smad3-dependent manner. Cell Death Dis. 2018, 9, 582. [Google Scholar] [CrossRef]
- Pan, G.; Risser, P.; Mao, W.; Baldwin, D.T.; Zhong, A.W.; Filvaroff, E.; Yansura, D.; Lewis, L.; Eigenbrot, C.; Henzel, W.J.; et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine 2001, 13, 1–7. [Google Scholar] [CrossRef]
- Kumar, S.; Hanning, C.R.; Brigham-Burke, M.R.; Rieman, D.J.; Lehr, R.; Khandekar, S.; Kirkpatrick, R.B.; Scott, G.F.; Lee, J.C.; Lynch, F.J.; et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine 2002, 18, 61–71. [Google Scholar] [CrossRef]
- Jia, H.; Liu, J.; Han, B. Reviews of Interleukin-37: Functions, Receptors, and Roles in Diseases. Biomed. Res. Int. 2018, 2018, 3058640. [Google Scholar] [CrossRef]
- Bufler, P.; Azam, T.; Gamboni-Robertson, F.; Reznikov, L.L.; Kumar, S.; Dinarello, C.A.; Kim, S.H. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc. Natl. Acad. Sci. USA 2002, 99, 13723–13728. [Google Scholar] [CrossRef] [Green Version]
- Cavalli, G.; Justice, J.N.; Boyle, K.E.; D’Alessandro, A.; Eisenmesser, E.Z.; Herrera, J.J.; Hansen, K.C.; Nemkov, T.; Stienstra, R.; Garlanda, C.; et al. Interleukin 37 reverses the metabolic cost of inflammation, increases oxidative respiration, and improves exercise tolerance. Proc. Natl. Acad. Sci. USA 2017, 114, 2313–2318. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A.; Nold-Petry, C.; Nold, M.; Fujita, M.; Li, S.; Kim, S.; Bufler, P. Suppression of innate inflammation and immunity by interleukin-37. Eur. J. Immunol. 2016, 46, 1067–1081. [Google Scholar] [CrossRef]
- Eisenmesser, E.Z.; Gottschlich, A.; Redzic, J.S.; Paukovich, N.; Nix, J.C.; Azam, T.; Zhang, L.; Zhao, R.; Kieft, J.S.; The, E.; et al. Interleukin-37 monomer is the active form for reducing innate immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 5514–5522. [Google Scholar] [CrossRef] [Green Version]
- Ellisdon, A.M.; Nold-Petry, C.A.; D’Andrea, L.; Cho, S.X.; Lao, J.C.; Rudloff, I.; Ngo, D.; Lo, C.Y.; Soares da Costa, T.P.; Perugini, M.A.; et al. Homodimerization attenuates the anti-inflammatory activity of interleukin-37. Sci. Immunol. 2017, 2, eaaj1548. [Google Scholar] [CrossRef]
- Li, T.T.; Zhu, D.; Mou, T.; Guo, Z.; Pu, J.L.; Chen, Q.S.; Wei, X.F.; Wu, Z.J. IL-37 induces autophagy in hepatocellular carcinoma cells by inhibiting the PI3K/AKT/mTOR pathway. Mol. Immunol. 2017, 87, 132–140. [Google Scholar] [CrossRef]
- Patel, A.B.; Tsilioni, I.; Leeman, S.E.; Theoharides, T.C. Neurotensin stimulates sortilin and mTOR in human microglia inhibitable by methoxyluteolin, a potential therapeutic target for autism. Proc. Natl. Acad. Sci. USA 2016, 113, E7049–E7058. [Google Scholar] [CrossRef] [Green Version]
- Moretti, S.; Bozza, S.; Oikonomou, V.; Renga, G.; Casagrande, A.; Iannitti, R.G.; Puccetti, M.; Garlanda, C.; Kim, S.; Li, S.; et al. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 2014, 10, e1004462. [Google Scholar] [CrossRef]
- Zhang, S.R.; Nold, M.F.; Tang, S.C.; Bui, C.B.; Nold, C.A.; Arumugam, T.V.; Drummond, G.R.; Sobey, C.G.; Kim, H.A. IL-37 increases in patients after ischemic stroke and protects from inflammatory brain injury, motor impairment and lung infection in mice. Sci. Rep. 2019, 9, 6922. [Google Scholar] [CrossRef]
- Wang, Y.C.; Weng, G.P.; Liu, J.P.; Li, L.; Cheng, Q.H. Elevated serum IL-37 concentrations in patients with sepsis. Medicine 2019, 98, e14756. [Google Scholar] [CrossRef]
- Chen, B.; Huang, K.; Ye, L.; Li, Y.; Zhang, J.; Zhang, J.; Fan, X.; Liu, X.; Li, L.; Sun, J.; et al. Interleukin-37 is increased in ankylosing spondylitis patients and associated with disease activity. J. Transl. Med. 2015, 13, 36. [Google Scholar] [CrossRef]
- Zeng, Q.; Song, R.; Fullerton, D.A.; Ao, L.; Zhai, Y.; Li, S.; Ballak, D.B.; Cleveland, J.C., Jr.; Reece, T.B.; McKinsey, T.A.; et al. Interleukin-37 suppresses the osteogenic responses of human aortic valve interstitial cells in vitro and alleviates valve lesions in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 1631–1636. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Ji, L.; Wen, Z.; Zhou, Y.; Hu, D.; Li, Y.; Yu, T.; Chen, B.; Zhang, J.; Ding, L.; et al. IL-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematosus: Its correlation with disease activity. J. Transl. Med. 2014, 12, 69. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Yu, T.; Chen, B.; Zhang, J.; Huang, K.; Huang, Z. Increased expression of IL-37 in patients with Graves’ disease and its contribution to suppression of proinflammatory cytokines production in peripheral blood mononuclear cells. PLoS ONE 2014, 9, e107183. [Google Scholar] [CrossRef]
- Ye, L.; Jiang, B.; Deng, J.; Du, J.; Xiong, W.; Guan, Y.; Wen, Z.; Huang, K.; Huang, Z. IL-37 Alleviates Rheumatoid Arthritis by Suppressing IL-17 and IL-17-Triggering Cytokine Production and Limiting Th17 Cell Proliferation. J. Immunol. 2015, 194, 5110–5119. [Google Scholar] [CrossRef]
- Varvara, G.; Tettamanti, L.; Gallenga, C.E.; Caraffa, A.; D’Ovidio, C.; Mastrangelo, F.; Ronconi, G.; Kritas, S.K.; Conti, P. Stimulated mast cells release inflammatory cytokines: Potential suppression and therapeutical aspects. J. Biol. Regul. Homeost. Agents 2018, 32, 1355–1360. [Google Scholar]
- Abulkhir, A.; Samarani, S.; Amre, D.; Duval, M.; Haddad, E.; Sinnett, D.; Leclerc, J.M.; Diorio, C.; Ahmad, A. A protective role of IL-37 in cancer: A new hope for cancer patients. J. Leukoc. Biol. 2017, 101, 395–406. [Google Scholar] [CrossRef]
- Cavalli, G.; Koenders, M.; Kalabokis, V.; Kim, J.; Tan, A.C.; Garlanda, C.; Mantovani, A.; Dagna, L.; Joosten, L.A.; Dinarello, C.A. Treating experimental arthritis with the innate immune inhibitor interleukin-37 reduces joint and systemic inflammation. Rheumatology 2016, 55, 2220–2229. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Neff, C.P.; Barber, K.; Hong, J.; Luo, Y.; Azam, T.; Palmer, B.E.; Fujita, M.; Garlanda, C.; Mantovani, A.; et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc. Natl. Acad. Sci. USA 2015, 112, 2497–2502. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; mo-Aparicio, J.; Neff, C.P.; Tengesdal, I.W.; Azam, T.; Palmer, B.E.; Lopez-Vales, R.; Bufler, P.; Dinarello, C.A. Role for nuclear interleukin-37 in the suppression of innate immunity. Proc. Natl. Acad. Sci. USA 2019. [Google Scholar] [CrossRef]
- Cavalli, G.; Dinarello, C.A. Suppression of inflammation and acquired immunity by IL-37. Immunol. Rev. 2018, 281, 179–190. [Google Scholar] [CrossRef]
- Stevens, R.L.; Adachi, R. Protease-proteoglycan complexes of mouse and human mast cells and importance of their beta-tryptase-heparin complexes in inflammation and innate immunity. Immunol. Rev. 2007, 217, 155–167. [Google Scholar] [CrossRef]
- Schwartz, L.B. Tryptase, a mediator of human mast cells. J. Allergy Clin. Immunol. 1990, 86, 594–598. [Google Scholar] [CrossRef]
- Heuberger, D.M.; Schuepbach, R.A. Protease-activated receptors (PARs): Mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb. J. 2019, 17, 4. [Google Scholar] [CrossRef]
- Lefrancais, E.; Duval, A.; Mirey, E.; Roga, S.; Espinosa, E.; Cayrol, C.; Girard, J.P. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 2014, 111, 15502–15507. [Google Scholar] [CrossRef] [Green Version]
- Lefrancais, E.; Cayrol, C. Mechanisms of IL-33 processing and secretion: Differences and similarities between IL-1 family members. Eur. Cytokine Netw. 2012, 23, 120–127. [Google Scholar]
- Dinarello, C.A.; Simon, A.; van der Meer, J.W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C.; Kavalioti, M. Stress, inflammation and natural treatments. J. Biol. Regul. Homeost. Agents 2018, 32, 1345–1347. [Google Scholar]
- Mo, X.J.; Ye, X.Z.; Li, Y.P. Effects of euphorbia kansui on the serum levels of IL-6, TNF-alpha, NF-kappaB, sTNFR and IL-8 in patients with severe acute pancreatitis. J. Biol. Regul. Homeost. Agents 2019, 33, 469–475. [Google Scholar]
- Patel, A.B.; Theoharides, T.C. Methoxyluteolin Inhibits Neuropeptide-stimulated Proinflammatory Mediator Release via mTOR Activation from Human Mast Cells. J. Pharmacol. Exp. Ther. 2017, 361, 462–471. [Google Scholar] [CrossRef] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theoharides, T.C.; Tsilioni, I.; Conti, P. Mast Cells May Regulate The Anti-Inflammatory Activity of IL-37. Int. J. Mol. Sci. 2019, 20, 3701. https://doi.org/10.3390/ijms20153701
Theoharides TC, Tsilioni I, Conti P. Mast Cells May Regulate The Anti-Inflammatory Activity of IL-37. International Journal of Molecular Sciences. 2019; 20(15):3701. https://doi.org/10.3390/ijms20153701
Chicago/Turabian StyleTheoharides, Theoharis C., Irene Tsilioni, and Pio Conti. 2019. "Mast Cells May Regulate The Anti-Inflammatory Activity of IL-37" International Journal of Molecular Sciences 20, no. 15: 3701. https://doi.org/10.3390/ijms20153701
APA StyleTheoharides, T. C., Tsilioni, I., & Conti, P. (2019). Mast Cells May Regulate The Anti-Inflammatory Activity of IL-37. International Journal of Molecular Sciences, 20(15), 3701. https://doi.org/10.3390/ijms20153701




