Highly Efficient and Stable Removal of Arsenic by Live Cell Fabricated Magnetic Nanoparticles
Abstract
:1. Introduction
2. Results
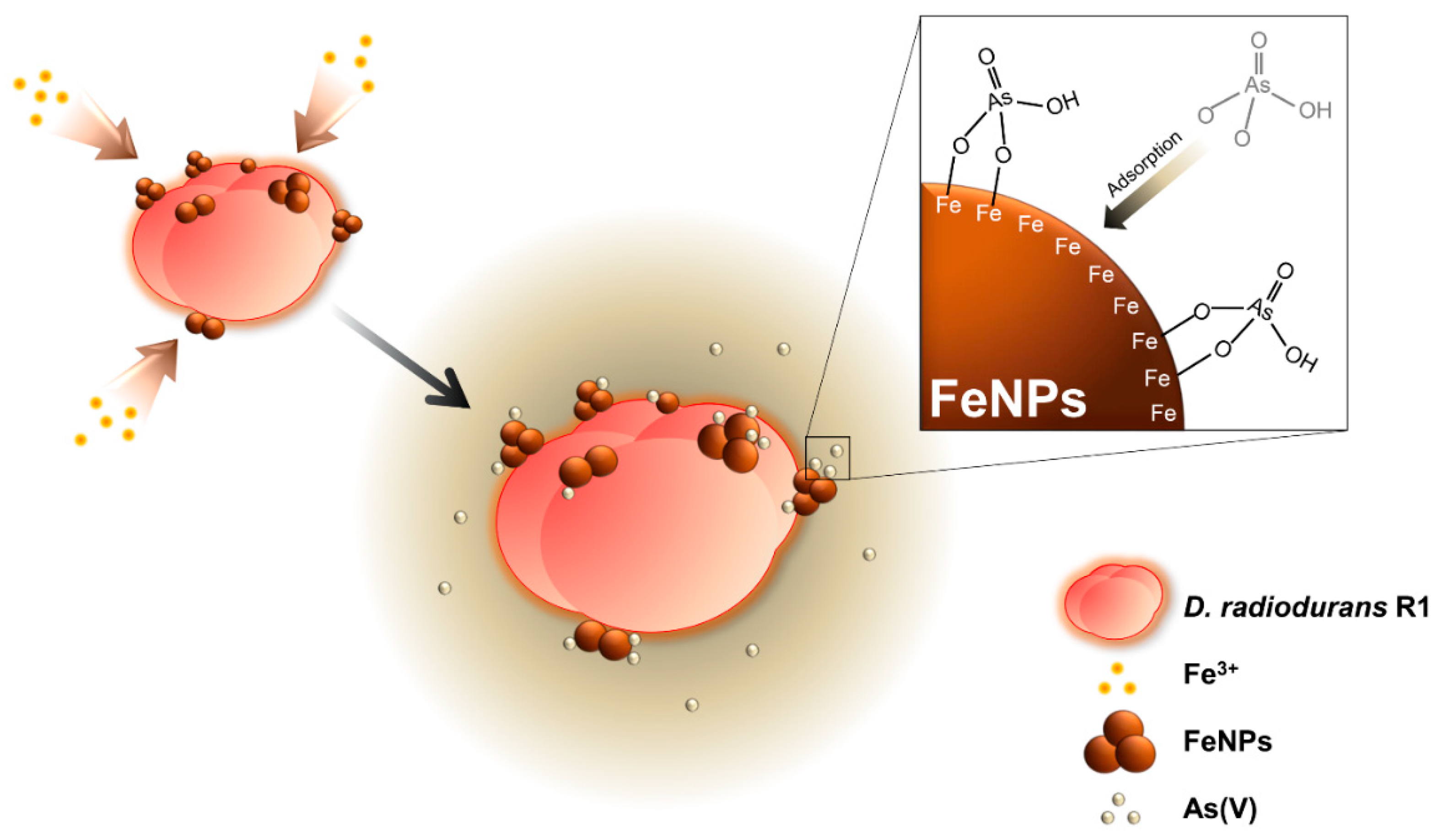
2.1. Establishment of Live Cell Fabricated Iron Nanoparticles (FeNPs)
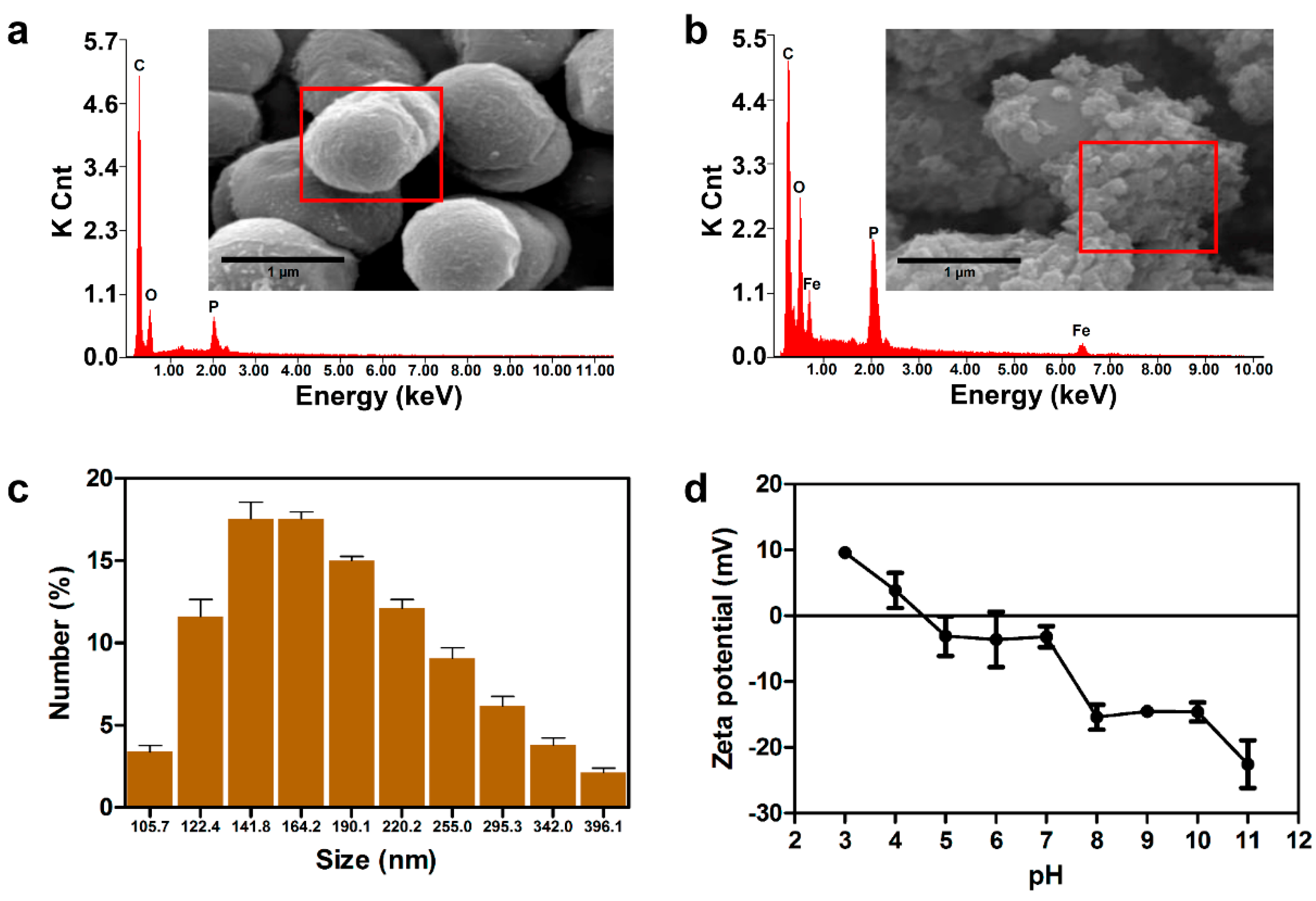
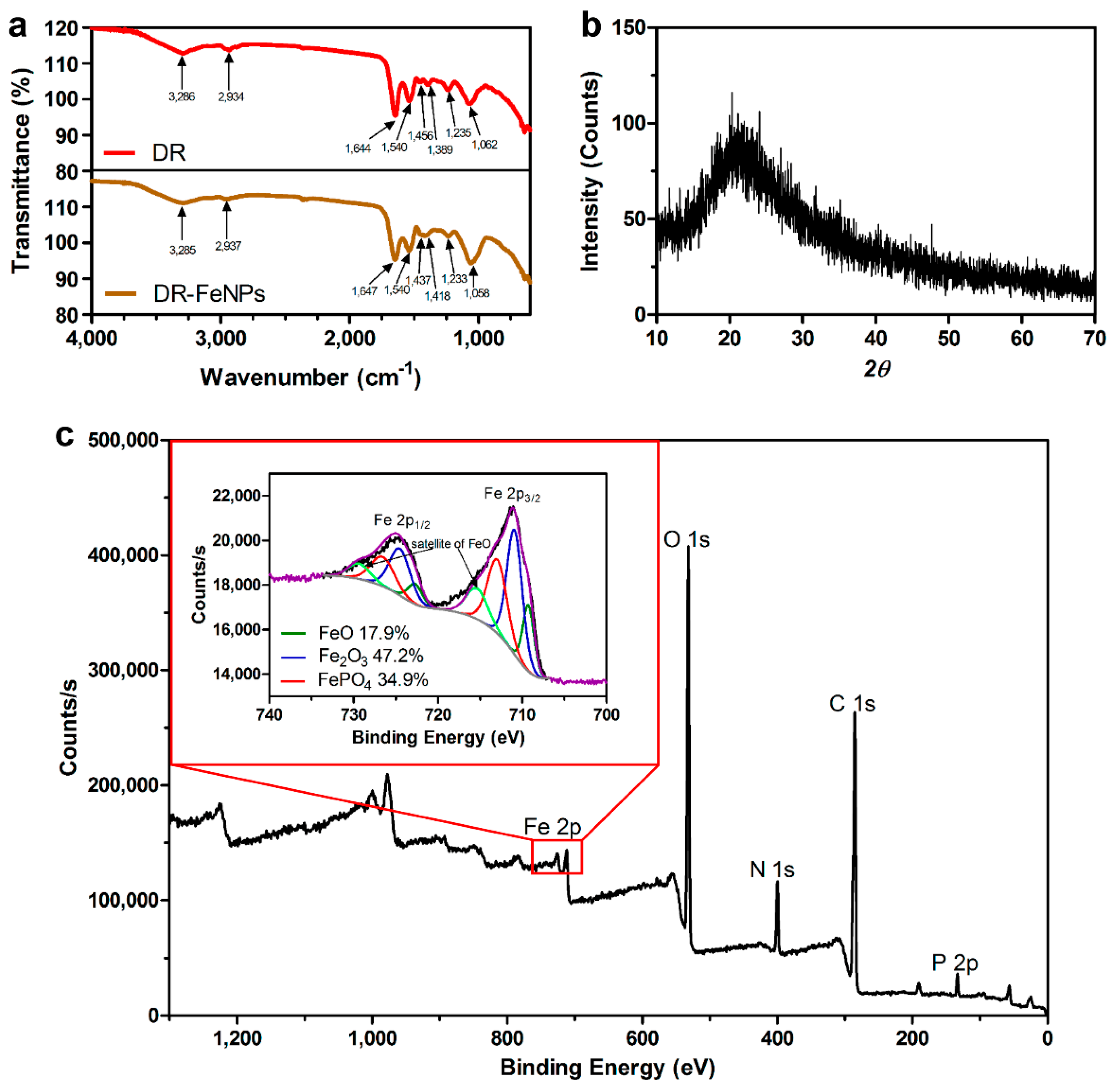
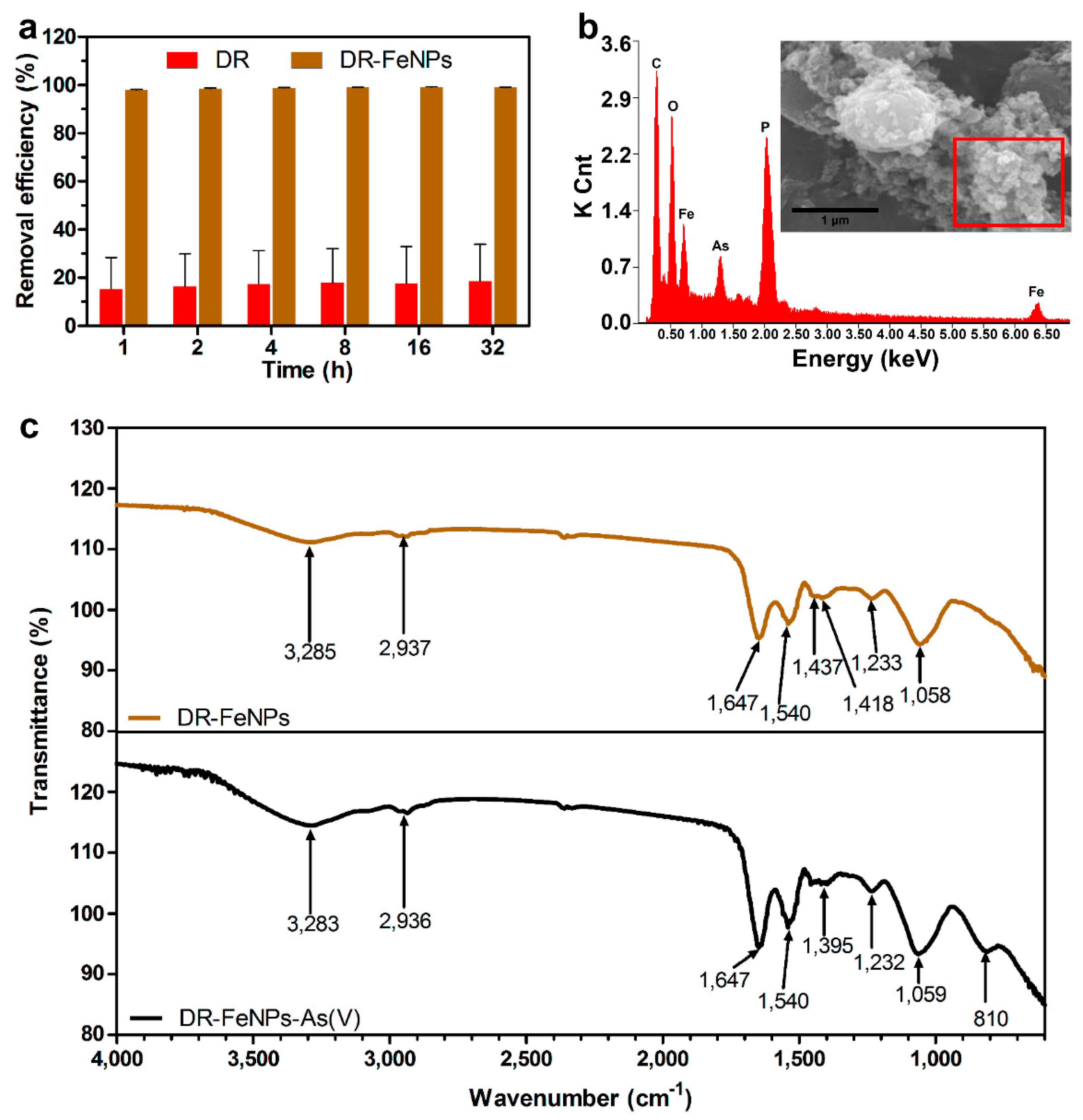
2.2. Characterization of Morphological Parameters and Composition of FeNPs
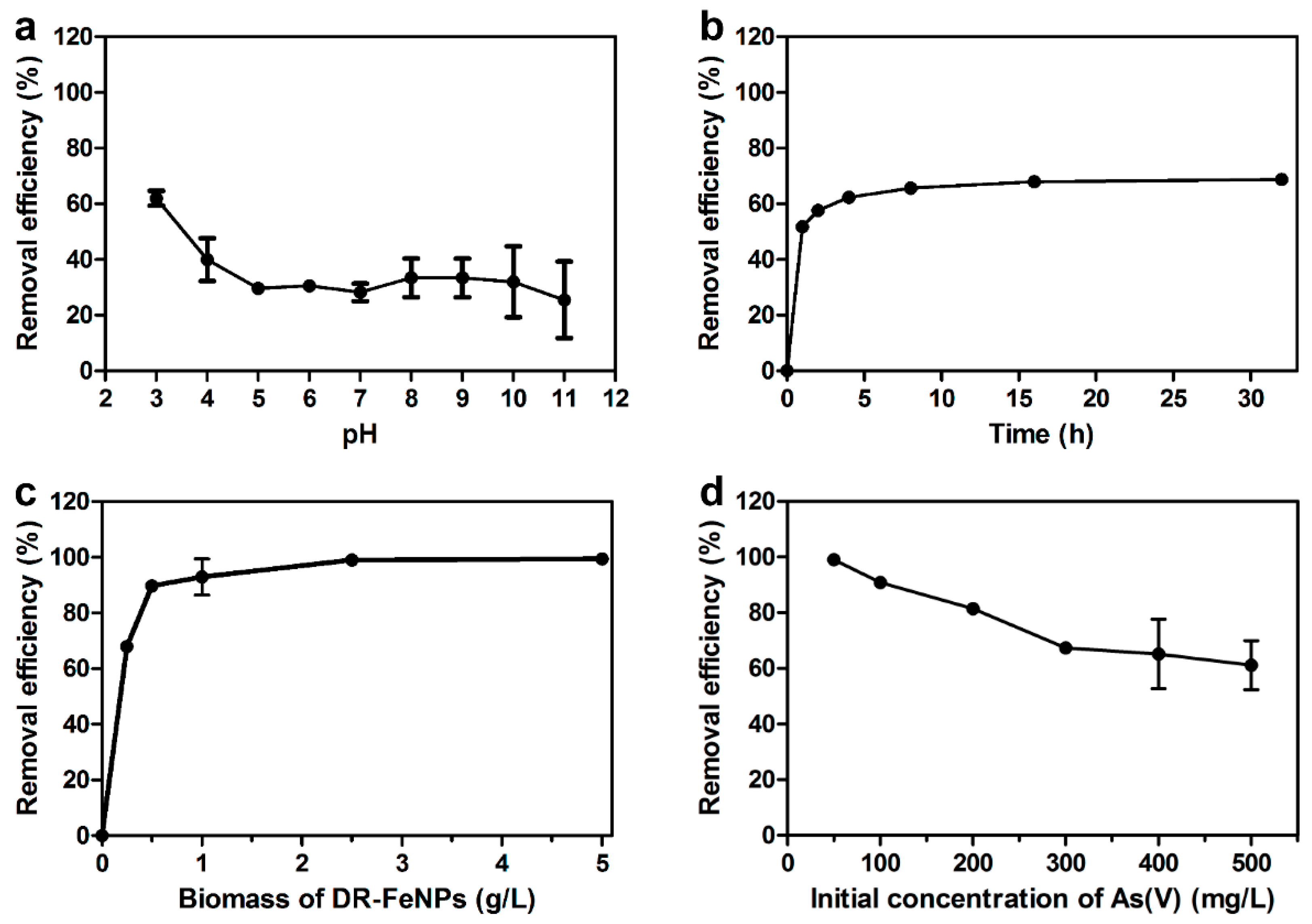
2.3. Removal of As(V) Using Iron Nanoparticle Immobilized D. radiodurans R1 (DR-FeNPs)
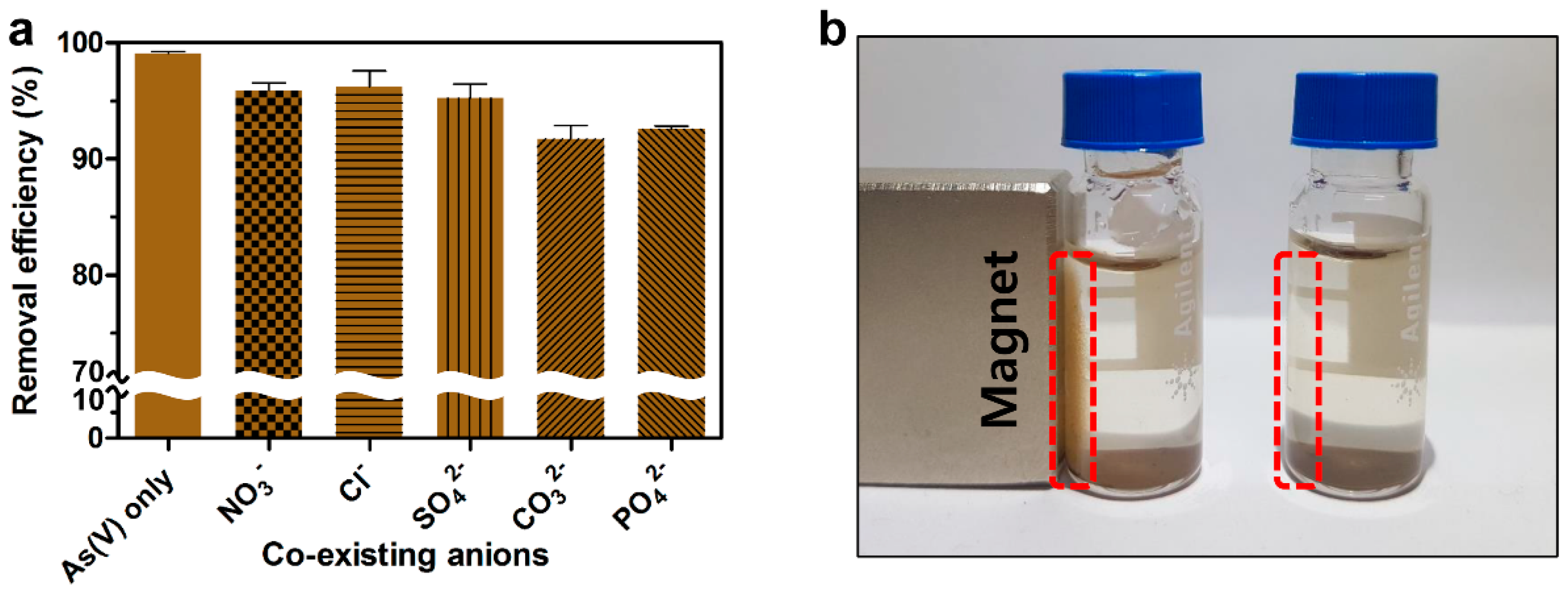
2.4. Effects of Competitive Anions on the Removal of As(V)
3. Materials and Methods
3.1. Synthesis of DR-FeNPs
3.2. Characterization of DR-FeNPs
3.3. Optimization of As(V) Adsorption Using DR-FeNPs
3.4. Remediation Procedure of As(V) Using DR-FeNPs
3.5. Analysis of the Effect of Co-Existing Ion on As(V) Adsorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DR | D. radiodurans R1 |
| DR-FeNPs | Iron nanoparticle immobilized D. radiodurans R1 |
| DR-FeNPs-As(V) | DR-FeNPs adsorbing As(V) |
| Qe | Adsorption capacity at equilibrium |
| Qt | Adsorption capacity at any time |
| Q0 | Maximum adsorption capacity |
| Ce | Concentration of metal ions in aqueous solution at equilibrium |
| KL | Langmuir adsorption constant |
| KF | Freundlich adsorption constant |
| 1/n | Adsorption intensity |
| Qexp | Adsorption capacity at equilibrium (experimental value) |
| Qcal | Adsorption capacity at equilibrium (calculation value) |
| K1 | Adsorption rate constant of the pseudo-first model |
| K2 | Adsorption rate constant of the pseudo-second model |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| SEM-EDX | Scanning electron microscopy-energy dispersive X-ray spectroscopy |
References
- Leiva, E.; Leiva-Aravena, E.; Rodríguez, C.; Serrano, J.; Vargas, I. Arsenic removal mediated by acidic pH neutralization and iron precipitation in microbial fuel cells. Sci. Total Environ. 2018, 645, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, G.; Sathishkumar, M.; Swaminathan, K. Arsenic removal from groundwater by pretreated waste tea fungal biomass. Bioresour. Technol. 2006, 97, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Kozul, C.D.; Ely, K.H.; Enelow, R.I.; Hamilton, J.W. Low-Dose Arsenic Compromises the Immune Response to Influenza A Infection in Vivo. Environ. Health Perspect. 2009, 117, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2015, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Uneyama, C.; Toda, M.; Yamamoto, M.; Morikawa, K. Arsenic in various foods: Cumulative data. Food Addit. Contam. 2007, 24, 447–534. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Kim, N.; Park, M.; Yun, Y.S.; Park, D. Removal of anionic arsenate by a PEI-coated bacterial biosorbent prepared from fermentation biowaste. Chemosphere 2019, 226, 67–74. [Google Scholar] [CrossRef]
- Tang, W.; Li, Q.; Gao, S.; Shang, J.K. Arsenic (III,V) removal from aqueous solution by ultrafine α-Fe2O3 nanoparticles synthesized from solvent thermal method. J. Hazard. Mater. 2011, 192, 131–138. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Creamer, A.E.; He, F. Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: Batch and continuous flow tests. J. Hazard. Mater. 2017, 322, 172–181. [Google Scholar] [CrossRef]
- Kim, H.A.; Choi, Y.J.; Kim, K.W.; Lee, B.T.; Ranville, J.F. Nanoparticles in the environment: Stability and toxicity. Rev. Environ. Health 2012, 27, 175–179. [Google Scholar] [CrossRef]
- Paul, B.; Parashar, V.; Mishra, A. Graphene in the Fe3O4 nano-composite switching the negative influence of humic acid coating into an enhancing effect in the removal of arsenic from water. Environ. Sci. Water Res. Technol. 2015, 1, 77–83. [Google Scholar] [CrossRef]
- Ray, P.Z.; Shipley, H.J. Inorganic nano-adsorbents for the removal of heavy metals and arsenic: A review. RSC Adv. 2015, 5, 29885–29907. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, S.Y.; Seo, T.S. In Vivo Synthesis of Nanocomposites Using the Recombinant Escherichia coli. Small 2018, 14, 1803133. [Google Scholar] [CrossRef]
- Madhavi, V.; Prasad, T.; Reddy, A.V.; Reddy, B.R.; Madhavi, G. Application of phytogenic zerovalent iron nanoparticles in the adsorption of hexavalent chromium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 17–25. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Ma, X.; Tian, B.; Li, T.; Yu, J.; Dai, S.; Weng, Y.; Hua, Y. Biosynthesis of gold nanoparticles by the extreme bacterium Deinococcus radiodurans and an evaluation of their antibacterial properties. Int. J. Nanomed. 2016, 11, 5931–5944. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Shaiwale, N.S.; Deobagkar, D.N.; Deobagkar, D.D. Synthesis and extracellular accumulation of silver nanoparticles by employing radiation-resistant Deinococcus radiodurans, their characterization, and determination of bioactivity. Int. J. Nanomed. 2015, 10, 963–974. [Google Scholar]
- Daly, M.J. Deinococcus prospects. Nat. Rev. Genet. 2009, 7, 476. [Google Scholar] [CrossRef]
- Jeong, S.W.; Shim, H.E.; Yun, S.J.; Mushtaq, S.; Choi, D.S.; Jang, B.S.; Yang, J.E.; Choi, Y.J.; Jeon, J. Efficient bioremediation of radioactive iodine using biogenic gold nanomaterial-containing radiation-resistant bacterium, Deinococcus radiodurans R1. Chem. Commun. 2017, 53, 3937–3940. [Google Scholar]
- Tc, P.; Sharma, S.K.; Kennedy, M. Development of iron oxide nanoparticle adsorbents for arsenic and fluoride removal. Desalin. Water Treat. 2017, 67, 187–196. [Google Scholar] [CrossRef]
- Lenoble, V.; Laclautre, C.; Deluchat, V.; Serpaud, B.; Bollinger, J.C. Arsenic removal by adsorption on iron(III) phosphate. J. Hazard. Mater. 2005, 123, 262–268. [Google Scholar] [CrossRef]
- Sriprang, P.; Wongnawa, S.; Sirichote, O. Amorphous titanium dioxide as an adsorbent for dye polluted water and its recyclability. J. Sol-Gel Sci. Technol. 2014, 71, 86–95. [Google Scholar] [CrossRef]
- Berg, J.M.; Romoser, A.; Banerjee, N.; Zebda, R.; Sayes, C. The relationship between pH and zeta potential of ~30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations. Nanotoxicology 2009, 3, 276–283. [Google Scholar] [CrossRef]
- Ivan, A.; Sara, A.; Mason, E.; Peter, F.; Martin, F.L.; Dusan, L. Bacterial iron-oxide nanowires from biofilm waste as a new adsorbent for the removal of arsenic from water. RSC Adv. 2017, 7, 3941–3948. [Google Scholar]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Schmitt, J.; Flemming, H.C. FTIR-spectroscopy in microbial and material analysis. Int. Biodeterior. Biodegrad. 1998, 41, 1–11. [Google Scholar] [CrossRef]
- Parikh, S.J.; Chorover, J. ATR-FTIR Spectroscopy Reveals Bond Formation During Bacterial Adhesion to Iron Oxide. Langmuir 2006, 22, 8492–8500. [Google Scholar] [CrossRef]
- Tian, B.; Li, J.; Pang, R.; Dai, S.; Li, T.; Weng, Y.; Jin, Y.; Hua, Y. Gold Nanoparticles Biosynthesized and Functionalized Using a Hydroxylated Tetraterpenoid Trigger Gene Expression Changes and Apoptosis in Cancer Cells. ACS Appl. Mater. Interfaces 2018, 10, 37353–37363. [Google Scholar] [CrossRef]
- Kumar, K.M.; Mandal, B.K.; Kumar, K.S.; Reddy, P.S.; Sreedhar, B. Biobased green method to synthesise palladium and iron nanoparticles using Terminalia chebula aqueous extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 128–133. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S.; Grosvenor, A. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Nan, C.; Lu, J.; Li, L.; Li, L.; Peng, Q.; Li, Y. Size and shape control of LiFePO4 nanocrystals for better lithium ion battery cathode materials. Nano Res. 2013, 6, 469–477. [Google Scholar] [CrossRef]
- Castro, L.; Dedryvère, R.; El Khalifi, M.; Lippens, P.E.; Bréger, J.; Tessier, C.; Gonbeau, D. The Spin-Polarized Electronic Structure of LiFePO4 and FePO4 Evidenced by in-Lab XPS. J. Phys. Chem. C 2010, 114, 17995–18000. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, X.; Jiao, J.; Li, Y.; Wei, M. Surface-Modified Biochar with Polydentate Binding Sites for the Removal of Cadmium. Int. J. Mol. Sci. 2019, 20, 1775. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Vandenbossche, M.; Jimenez, M.; Casetta, M.; Traisnel, M. Remediation of heavy metals by biomolecules: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1644–1704. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, X.Y.; Luo, T.; Jin, Z.; Sun, B.; Liu, J.H.; Huang, X.J. Necklace-like mesoporous MgO/TiO2 heterojunction structures with excellent capability for water treatment. Dalton Trans. 2014, 43, 2348–2351. [Google Scholar] [CrossRef]
- Brechbühl, Y.; Christl, I.; Elzinga, E.J.; Kretzschmar, R. Competitive sorption of carbonate and arsenic to hematite: Combined ATR-FTIR and batch experiments. J. Colloid Interface Sci. 2012, 377, 313–321. [Google Scholar] [CrossRef]
- Qiao, J.; Jiang, Z.; Sun, B.; Sun, Y.; Wang, Q.; Guan, X. Arsenate and arsenite removal by FeCl3: Effects of pH, As/Fe ratio, initial As concentration and co-existing solutes. Sep. Purif. Technol. 2012, 92, 106–114. [Google Scholar] [CrossRef]
- Li, W.; Chen, D.; Xia, F.; Tan, J.Z.; Huang, P.P.; Song, W.G.; Nursam, N.; Caruso, R.A. Extremely high arsenic removal capacity for mesoporous aluminium magnesium oxide composites. Environ. Sci. Nano 2016, 3, 94–106. [Google Scholar] [CrossRef]
- Salem Attia, T.M.; Hu, X.L.; Yin, D.Q. Synthesised magnetic nanoparticles coated zeolite (MNCZ) for the removal of arsenic (AS) from aqueous solution. J. Exp. Nanosci. 2014, 9, 551–560. [Google Scholar] [CrossRef]
- Türk, T.; Alp, I. Arsenic removal from aqueous solutions with Fe-hydrotalcite supported magnetite nanoparticle. J. Ind. Eng. Chem. 2014, 20, 732–738. [Google Scholar] [CrossRef]
- Prasad, K.S.; Gandhi, P.; Selvaraj, K. Synthesis of green nano iron particles (GnIP) and their application in adsorptive removal of As(III) and As(V) from aqueous solution. Appl. Surf. Sci. 2014, 317, 1052–1059. [Google Scholar] [CrossRef]
- Feng, L.; Cao, M.; Ma, X.; Zhu, Y.; Hu, C. Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J. Hazard. Mater. 2012, 217, 439–446. [Google Scholar] [CrossRef]

| Biomass | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| Q0 (mg/g) | KL (L/mg) | R2 | KF (L/g) | n | R2 | |
| DR-FeNPs | 172.414 | 0.176 | 0.870 | 25.146 | 1.596 | 0.961 |
| Pseudo-First Order | Qexp (mg/g) | K1 (1/h) | Qcal (mg/g) | R2 |
| 131.565 ± 3.783 | 0.197 | 32.089 | 0.984 | |
| Pseudo-Second Order | Qexp (mg/g) | K2 (g/mg·h) | Qcal (mg/g) | R2 |
| 131.565 ± 3.783 | 0.018 | 133.333 | 0.999 |
| Adsorbent | Preparation Method | Adsorption Capacity | pH | Ref |
|---|---|---|---|---|
| Magnetic nanoparticles coated zeolite | Ultra sonication | 95.6% | 2.5 | [41] |
| Fe-hydrotalcite supported mangetic nanopartilces (M-FeHT) | Co-precipitation | 104.8 μg/g | 9 | [42] |
| Green nano iron particle (GnIP) | Mint leaves extract | 94.47 mg/g | - | [43] |
| Ascrobic acid coated Fe3O4 nanoaprticle | Hydorthermal (180 °C) | 16.56 mg/g | 7 | [44] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.K.; Jeong, S.-W.; Yang, J.E.; Choi, Y.J. Highly Efficient and Stable Removal of Arsenic by Live Cell Fabricated Magnetic Nanoparticles. Int. J. Mol. Sci. 2019, 20, 3566. https://doi.org/10.3390/ijms20143566
Kim HK, Jeong S-W, Yang JE, Choi YJ. Highly Efficient and Stable Removal of Arsenic by Live Cell Fabricated Magnetic Nanoparticles. International Journal of Molecular Sciences. 2019; 20(14):3566. https://doi.org/10.3390/ijms20143566
Chicago/Turabian StyleKim, Hyo Kyeong, Sun-Wook Jeong, Jung Eun Yang, and Yong Jun Choi. 2019. "Highly Efficient and Stable Removal of Arsenic by Live Cell Fabricated Magnetic Nanoparticles" International Journal of Molecular Sciences 20, no. 14: 3566. https://doi.org/10.3390/ijms20143566
APA StyleKim, H. K., Jeong, S.-W., Yang, J. E., & Choi, Y. J. (2019). Highly Efficient and Stable Removal of Arsenic by Live Cell Fabricated Magnetic Nanoparticles. International Journal of Molecular Sciences, 20(14), 3566. https://doi.org/10.3390/ijms20143566





