Effects of Anti-Calcitonin Gene-Related Peptide for Migraines: A Systematic Review with Meta-Analysis of Randomized Clinical Trials
Abstract
1. Introduction
2. Results
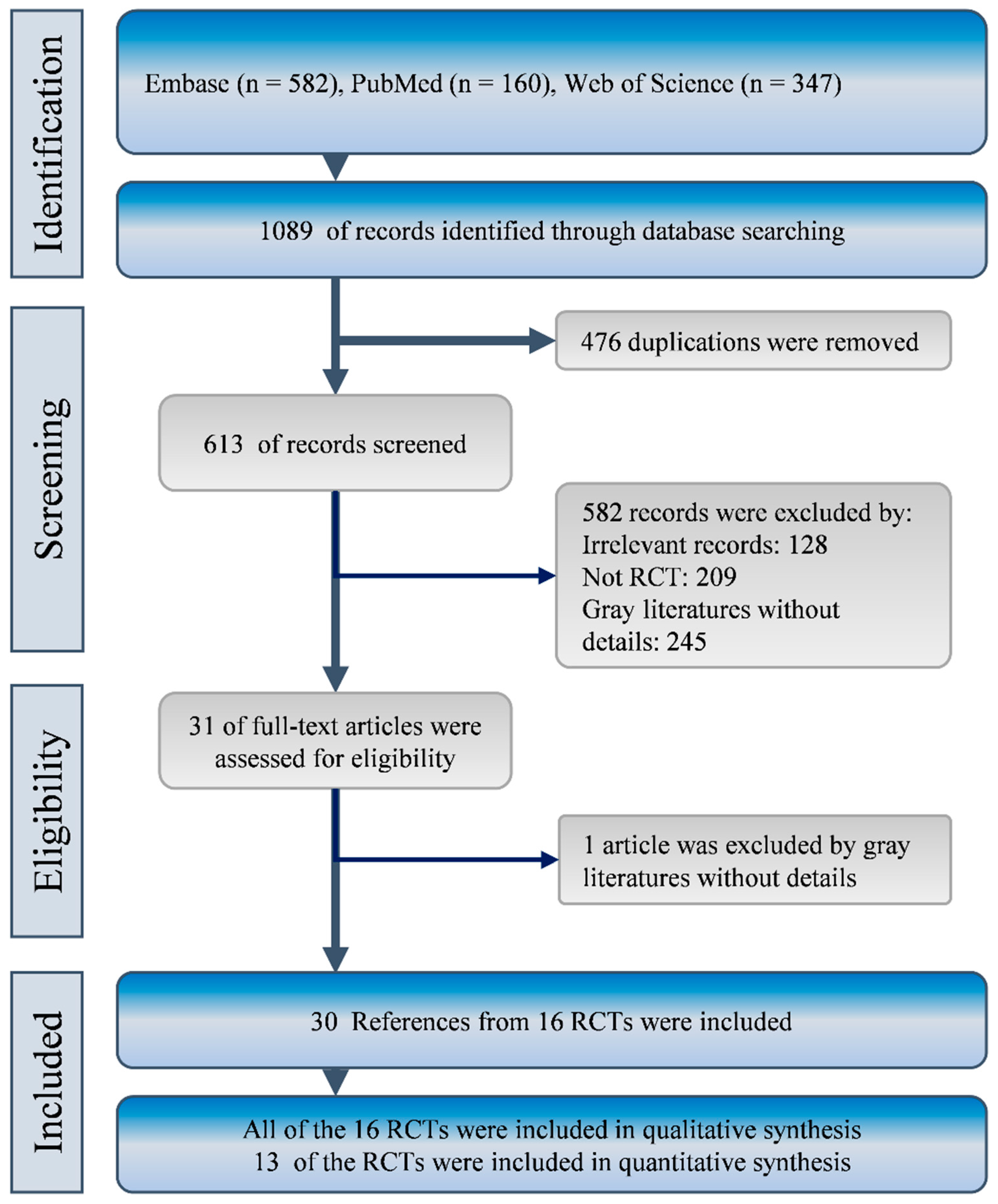
2.1. Characteristics and Quality of Included Studies
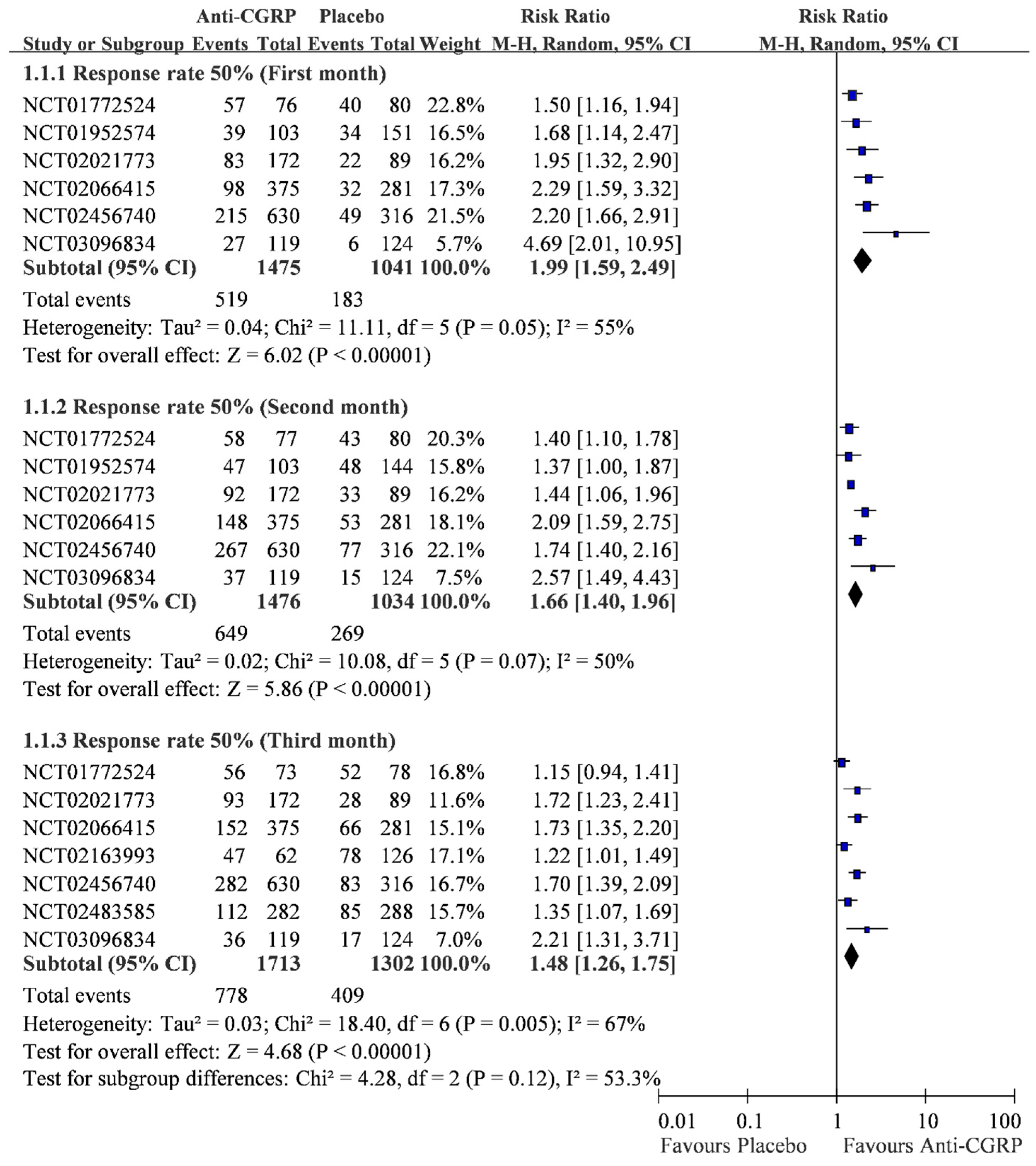
2.2. Monthly 50% Response Rate
2.3. Cumulative Response Rate within Three Months
3. Discussion
Comparison to the Previous Syntheses
4. Methods
4.1. Data Source and Search
4.2. Study Selection
4.3. Data Extraction and Quality Assessment
4.4. Data Synthesis and Analysis
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Anti-CGRP | anti-calcitonin gene-related peptide |
| CI | confidence interval |
| RCT | randomized clinical trial |
| RR | risk ratio |
| SAW | single active warming strategy |
| SUCRA | surface under the cumulative ranking curve |
References
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. Available online: https://journals.sagepub.com/doi/10.1177/0333102417738202 (accessed on 17 July 2019). [CrossRef] [PubMed]
- Saper, J.R. Diagnosis and symptomatic treatment of migraine. Headache 1997, 37, 1–14. [Google Scholar]
- Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S.; Goadsby, P.J.; Holland, P.R. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. Available online: https://www.physiology.org/doi/full/10.1152/physrev.00034.2015 (accessed on 17 July 2019).
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the global burden of disease study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Ho, T.W.; Edvinsson, L.; Goadsby, P.J. CGRP and its receptors provide new insights into migraine pathophysiology. Nat. Rev. Neurol. 2010, 6, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990, 28, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; Holland, S.; Freitag, F.; Dodick, D.W.; Argoff, C.; Ashman, E. Evidence-based guideline update: Pharmacologic treatment for episodic migraine prevention in adults: Report of the quality standards subcommittee of the american academy of neurology and the american headache society. Neurology 2012, 78, 1337–1345. [Google Scholar] [CrossRef]
- Tso, A.R.; Goadsby, P.J. Anti-CGRP Monoclonal Antibodies: The Next Era of Migraine Prevention? Curr. Treat. Options Neurol. 2017, 19, 27. [Google Scholar] [CrossRef]
- Ashina, M.; Brandes, J.L.; Katsarava, Z.; Lipton, R.B.; Pascual, J.; Palmer, K.; Desai, P.; Picard, H.; Mikol, D.D.; Lenz, R.A. Patient-reported outcomes from the arise trial: A phase 3, randomized, double-blind study of erenumab in subjects with episodic migraine. Headache 2017, 57, 192. [Google Scholar]
- Ashina, M.; Dodick, D.; Kudrow, D.; Lanteri-Minet, M.; Osipova, V.; Palmer, K.; Picard, H.; Mikol, D.; Lenz, R. A phase 3, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of erenumab in migraine prevention: Primary results of the arise trial. Eur. J. Neurol. 2017, 24, 470. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1111/ene.13368 (accessed on 17 July 2019).
- Buse, D.C.; Lipton, R.B.; Mikol, D.D.; Thach, A.V.; Desai, P.; Picard, H.; Kubo, Y.; Hareendran, A.; Kawata, A.K. Reducing the impact of migraine on functioning: Results from the strive trial: A phase 3, randomized, double-blind study of erenumab in subjects with episodic migraine. Cephalalgia 2017, 37, 195–196. [Google Scholar]
- Dodick, D.; Ashina, M.; Kudrow, D.; Lanteri-Minet, M.; Osipova, V.; Palmer, K.; Picard, H.; Mikol, D.D.; A Lenz, R. A phase 3, randomised, double-blind, placebo-controlled study to evaluate the efficacy and safety of erenumab in migraine prevention: Primary results of the arise trial. J. Neurol. Neurosurg. Psychiatry 2017, 88, e1. [Google Scholar] [CrossRef]
- Dodick, D.; Goadsby, P.; Silberstein, S.; Lipton, R.; Hirman, J.; Smith, J. Randomized, double-blind, placebo-controlled trial of ald403, an anti-cgrp peptide antibody in the prevention of chronic migraine. Neurology 2017, 88, S52–003. [Google Scholar]
- Dodick, D.W.; Ashina, M.; Kudrow, D.; Lanteri-Minet, M.; Osipova, V.; Palmer, K.; Picard, H.; Mikol, D.D.; Lenz, R.A. A phase 3, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of erenumab in migraine prevention: Primary results of the arise trial. Headache 2017, 57, 191–192. Available online: https://headachejournal.onlinelibrary.wiley.com/doi/abs/10.1111/head.13102 (accessed on 17 July 2019).
- Goadsby, P.; Reuter, U.; Bonner, J.; Broessner, G.; Hallstrom, Y.; Zhang, F.; Sapra, S.; Picard, H.; Mikol, D.; Lenz, R. Phase 3, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of erenumab (amg 334) in migraine prevention: Primary results of the strive trial. Neurology 2017, 89, e104. Available online: https://doi.org/10.1212/WNL.0000000000004380 (accessed on 17 July 2019).
- Goadsby, P.J.; Reuter, U.; Bonner, J.; Broessner, G.; Hallstrom, Y.; Zhang, F.; Sapra, S.; Picard, H.; Mikol, D.D.; Lenz, R. A Phase 3, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of erenumab (amg 334) in migraine prevention: Primary results of the strive trial. Headache 2017, 57, 128–129. Available online: https://headachejournal.onlinelibrary.wiley.com/doi/abs/10.1111/head.13102 (accessed on 17 July 2019).
- Hareendran, A.; Buse, D.C.; Lipton, R.B.; Bayliss, M.S.; Mikol, D.D.; Revicki, D.A.; Zhang, F.; Desai, P.; Picard, H.; Kawata, A.K. Reducing impaired days: Results from the strive trial, a phase 3, randomised, double-blind study of erenumab for episodic migraine. J. Headache Pain 2017, 18, 275–276. [Google Scholar]
- Goadsby, P.J.; Reuter, U.; Bonner, J.; Broessner, G.; Hallstrom, Y.; Zhang, F.; Sapra, S.; Picard, H.; Mikol, D.; Lenz, R. Phase 3, randomised, double-blind, placebo-controlled study to evaluate the efficacy and safety of erenumab (amg 334) in migraine prevention: Primary results of the strive trial. J. Neurol. Neurosurg. Psychiatry 2017, 88, e1. Available online: https://jnnp.bmj.com/content/88/5/e1.62 (accessed on 17 July 2019). [CrossRef]
- Reuter, U.; Brandes, J.; Dolezil, D.; Tepper, S.; Cheng, S.; Leonardi, D.; Lenz, R.; Mikol, D. Efficacy of erenumab (amg 334) in patients with chronic migraine in north america and europe: Subgroup analysis of a phase 2, randomised, double-blind, placebo-controlled study. Eur. J. Neurol. 2017, 24, 548. [Google Scholar]
- Smith, J.; Dodick, D.W.; Goadsby, P.J.; Silberstein, S.D.; Lipton, R.B.; Hirman, J. Randomized, double-blind, placebo-controlled trial of ald403 (eptinezumab), an anti-cgrp monoclonal antibody for the prevention of chronic migraine. Headache 2017, 57, 130. [Google Scholar]
- Stauffer, V.; Skljarevski, V.; Zhang, Q.; Ford, J.H.; Carter, J.; Aurora, S.K. The relationship between headache frequency and illness burden prior to treatment randomization in two phase 3 episodic migraine clinical trials. Headache 2017, 57, 190. [Google Scholar]
- Tepper, S.; Lipton, R.; Reuter, U.; Silberstein, S.; Stewart, W.; Leonardi, D.; Desai, P.; Cheng, S.; Mikol, D.; Lenz, R. Patient reported outcomes in patients with chronic migraine receiving placebo or erenumab (amg 334) in a phase 2, randomized, double blind study. Neurology 2017, 88, P2–167. [Google Scholar]
- Tepper, S.; Widnell, K.; Dolezil, D.; Ashina, M.; Reuter, U.; Brandes, J.L.; Silberstein, S.; Winner, P.; Leonardi, D.; Mikol, D. Evaluating the efficacy and safety of erenumab (amg 334) in chronic migraine prevention in a phase 2 randomized, double-blind, placebo-controlled study. Schmerz 2017, 31, S65. Available online: https://link.springer.com/article/10.1007/s00482-017-0249-3 (accessed on 17 July 2019).
- Tepper, S.; Widnell, K.; Dolezil, D.; Ashina, M.; Reuter, U.; Lewis Brandes, J.; Silberstein, S.; Winner, P.; Leonardi, D.; Mikol, D. Evaluating the efficacy and safety of erenumab (amg 334) in chronic migraine prevention in a phase 2 randomized, double-blind, placebo-controlled study. Neurology 2017, 88, S16. Available online: https://n.neurology.org/content/88/16_Supplement/S52.002 (accessed on 17 July 2019).
- Tepper, S.J.; Dolezil, D.; Ashina, M.; Reuter, U.; Brandes, J.L.; Silberstein, S.D.; Winner, P.; Leonardi, D.K.; Mikol, D.D. A phase 2 randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of erenumab (amg 334) in chronic migraine prevention. Headache 2017, 57, 130. [Google Scholar]
- Aurora, S.; Zhang, Q.; Stauffer, V. Galcanezumab effects in adult patients with episodic or chronic migraine are persistent: Data from three phase 3, randomized, double-blind, placebo-controlled evolve-1, evolve-2, and regain studies. Cephalalgia 2018, 38, 50–51. [Google Scholar]
- Aurora, S.K.; Zhang, Q.; Stauffer, V.L. Persistence of effect of galcanezumab in patients with episodic or chronic migraine: Phase 3, randomized, double-blind, placebocontrolled evolve-1, evolve-2 and regain studies. J. Headache Pain 2018, 19. Available online: https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-018-0951-2 (accessed on 17 July 2019).
- Aurora, S.K.; Zhang, Q.; Stauffer, V.L.; Daniele, M.I. Persistence of effect of galcanezumab in patients with episodic or chronic migraine: Phase3, randomized, double-blind, placebo-controlled evolve-1, evolve-2 and regain studies. Postgrad. Med. 2018, 130, 79–80. Available online: https://www.tandfonline.com/doi/full/10.1080/00325481.2018.1512253 (accessed on 17 July 2019).
- Depre, C.; Antalik, L.; Starling, A.; Koren, M.; Eisele, O.; Mikol, D.D. A randomised, double-blind, placebo-controlled study of erenumab safety in patients with stable angina. J. Headache Pain 2018, 19. Available online: https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-018-0900-0 (accessed on 17 July 2019).
- Depre, C.; Antalik, L.; Starling, A.J.; Koren, M.; Eisele, O.; Mikol, D. A randomized, double-blind, placebo-controlled study of erenumab safety in patients with stable angina. Headache 2018, 58, 177. Available online: https://headachejournal.onlinelibrary.wiley.com/doi/epdf/10.1111/head.13306 (accessed on 17 July 2019). [CrossRef]
- Dolezil, D.; Klatt, J.; Cheng, S.; Zhang, F.; Wen, S.; Ritter, S.; Mikol, D.D. Efficacy of erenumab in patients with chronic migraine achieving ≥50% response: Subgroup analysis of a double-blind, randomised study. J. Headache Pain 2018, 19. Available online: https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-018-0900-0 (accessed on 17 July 2019).
- Dolezil, D.; Klatt, J.; Cheng, S.; Zhang, F.; Wen, S.; RItter, S.; Mikol, D.D. Efficacy of erenumab in patients with chronic migraine achieving >50% response: Subgroup analysis of a double-blind, randomised study. Cephalalgia 2018, 38, 92–93. Available online: https://journals.sagepub.com/doi/full/10.1177/0333102418789865 (accessed on 17 July 2019).
- Lanteri-Minet, M.; Buse, D.C.; Starling, A.; Ailani, J.; Zhang, F.; Wen, S.; Bilitou, A.; Desai, P.; Cheng, S.; Klatt, J.; et al. Patient-reported outcomes in chronic migraine patients with prior prophylactic treatment failure receiving placebo or erenumab: Subgroup analysis of a pivotal randomised study. Cephalalgia 2018, 38, 49–50. Available online: https://journals.sagepub.com/doi/full/10.1177/0333102418789865 (accessed on 17 July 2019).
- Lanteri-Minet, M.; Buse, D.C.; Starling, A.J.; Ailani, J.; Zhang, F.; Wen, S.; Bilitou, A.; Desai, P.; Cheng, S.; Klatt, J.; et al. Patient-reported outcomes in chronic migraine patients with prior prophylactic treatment failure receiving placebo or erenumab: Subgroup analysis of a pivotal randomized study. Headache 2018, 58, 170–171. Available online: https://headachejournal.onlinelibrary.wiley.com/doi/epdf/10.1111/head.13306 (accessed on 17 July 2019).
- Lipton, R.B.; Saper, J.; Ashina, M.; Biondi, D.; Bhattacharya, S.; Hirman, J.; Schaeffler, B.; Cady, R. A phase 3, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of eptinezumab for the preventive treatment of chronic migraine: Results of the promise-2 (prevention of migraine via intravenous eptinezumab safety and efficacy 2) trial. Neurology 2018, 90, 2193–2194. [Google Scholar]
- Nagy, A.J.; Pearlman, E.; Ruff, D.; Day, K.; Aurora, S.K.; Rosen, N. 100% response rate to galcanezumab in patients with episodic migraine: Randomized, double-blind, placebo-controlled studies. Cephalalgia 2018, 38, 63–64. Available online: https://journals.sagepub.com/doi/full/10.1177/0333102418789865 (accessed on 17 July 2019).
- Nagy, A.J.; Pearlman, E.; Ruff, D.; Day, K.; Rosen, N. 100% response rate to galcanezumab in patients with episodic migraine: Randomized, double-blind, placebo-controlled studies. J. Headache Pain 2018, 19. Available online: https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-018-0900-0 (accessed on 17 July 2019).
- Nichols, R.M.; Ruff, D.; Pearlman, E.; Aurora, S.K. Analysis of initial non responders to galcanezumab in patients with episodic or chronic migraine: Results from the evolve-1, evolve-2, and regain randomized, double-blind, placebo-controlled trials. J. Headache Pain 2018, 19. Available online: https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-018-0900-0 (accessed on 17 July 2019).
- Dodick, D.W.; Goadsby, P.J.; Silberstein, S.D.; Lipton, R.B.; Olesen, J.; Ashina, M.; Wilks, K.; Kudrow, D.; Kroll, R.; Kohrman, B.; et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: A randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014, 13, 1100–1107. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Reuter, U.; Hallström, Y.; Broessner, G.; Bonner, J.H.; Zhang, F.; Sapra, S.; Picard, H.; Mikol, D.D.; Lenz, R.A. A Controlled Trial of Erenumab for Episodic Migraine. New Engl. J. Med. 2017, 377, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Dodick, D.W.; Silberstein, S.; Goadsby, P.J.; Reuter, U.; Ashina, M.; Saper, J.; Cady, R.; Chon, Y.; Dietrich, J.; et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 382–390. [Google Scholar] [CrossRef]
- Tepper, S.; Ashina, M.; Reuter, U.; Brandes, J.L.; Doležil, D.; Silberstein, S.; Winner, P.; Leonardi, D.; Mikol, D.; Lenz, R. Safety and efficacy of erenumab for preventive treatment of chronic migraine: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017, 16, 425–434. [Google Scholar] [CrossRef]
- Ashina, M.; Tepper, S.; Brandes, J.L.; Reuter, U.; Boudreau, G.; Dolezil, D.; Cheng, S.; Zhang, F.; Lenz, R.; Klatt, J.; et al. Efficacy and safety of erenumab (AMG334) in chronic migraine patients with prior preventive treatment failure: A subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia 2018, 38, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Dodick, D.; Goadsby, P.J.; Reuter, U.; Silberstein, S.; Zhang, F.; Gage, J.R.; Cheng, S.; Mikol, D.D.; Lenz, R.A. Erenumab (amg 334) in episodic migraine: Interim analysis of an ongoing open-label study. Neurology 2017, 89, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Ashina, M.; Brandes, J.L.; Kudrow, D.; Lantéri-Minet, M.; Osipova, V.; Palmer, K.; Picard, H.; Mikol, D.D.; A Lenz, R. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 2018, 38, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Silberstein, S.D.; E Bigal, M.; Yeung, P.P.; Goadsby, P.J.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y.; Aycardi, E. Effect of Fremanezumab Compared With Placebo for Prevention of Episodic Migraine: A Randomized Clinical Trial. JAMA 2018, 319, 1999–2008. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Dodick, D.W.; Bigal, M.E.; Yeung, P.P.; Goadsby, P.J.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y.; Aycardi, E. Fremanezumab for the Preventive Treatment of Chronic Migraine. New Engl. J. Med. 2017, 377, 2113–2122. [Google Scholar] [CrossRef]
- Li, T.; Aycardi, E.; Cohen, J.M.; Dodick, D.W.; Newman, L.C.; Bigal, M.E.; Yang, R. Fremanezumab as Add-On Treatment for Patients Treated With Other Migraine Preventive Medicines. Headache J. Head Face Pain 2017, 57, 1375–1384. [Google Scholar]
- E Bigal, M.; Edvinsson, L.; Rapoport, A.M.; Lipton, R.B.; Spierings, E.L.H.; Diener, H.-C.; Burstein, R.; Loupe, P.S.; Ma, Y.; Yang, R.; et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: A multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015, 14, 1091–1100. [Google Scholar] [CrossRef]
- Bigal, M.E.; Dodick, D.W.; Krymchantowski, A.V.; VanderPluym, J.H.; Tepper, S.J.; Aycardi, E.; Loupe, P.S.; Ma, Y.; Goadsby, P.J. Tev-48125 for the preventive treatment of chronic migraine: Efficacy at early time points. Neurology 2016, 87, 41–48. [Google Scholar] [CrossRef]
- Singh, R.B.H.; Aycardi, E.; E Bigal, M.; Loupe, P.S.; McDonald, M.; Dodick, D.W. Sustained reductions in migraine days, moderate-to-severe headache days and days with acute medication use for HFEM and CM patients taking fremanezumab: Post-hoc analyses from phase 2 trials. Cephalalgia 2018, 39, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, V.L.; Dodick, D.W.; Zhang, Q.; Carter, J.N.; Ailani, J.; Conley, R.R. Evaluation of galcanezumab for the prevention of episodic migraine: The evolve-1 randomized clinical trial. JAMA Neurol. 2018, 75, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Oakes, T.M.M.; Skljarevski, V.; Zhang, Q.; Kielbasa, W.; E Hodsdon, M.; Detke, H.C.; Camporeale, A.; Saper, J.R.; Skljarevsuki, V. Safety of galcanezumab in patients with episodic migraine: A randomized placebo-controlled dose-ranging Phase 2b study. Cephalalgia 2018, 38, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Skljarevski, V.; Matharu, M.; A Millen, B.; Ossipov, M.H.; Kim, B.-K.; Yang, J.Y. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia 2018, 38, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Goadsby, P.J.; Spierings, E.L.H.; Scherer, J.C.; Sweeney, S.P.; Grayzel, D.S. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: A phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014, 13, 885–892. [Google Scholar] [CrossRef]
- Skljarevski, V.; Oakes, T.M.; Zhang, Q.; Ferguson, M.B.; Martinez, J.; Camporeale, A.; Johnson, K.W.; Shan, Q.; Carter, J.; Schacht, A.; et al. Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: A randomized clinical trial. JAMA Neurol. 2018, 75, 187–193. [Google Scholar] [CrossRef]
- Schwedt, T.; Reuter, U.; Tepper, S.; Ashina, M.; Kudrow, D.; Broessner, G.; Boudreau, G.P.; McAllister, P.; Vu, T.; Zhang, F.; et al. Early onset of efficacy with erenumab in patients with episodic and chronic migraine. J. Headache Pain 2018, 19, 92. [Google Scholar] [CrossRef]
- Reuter, U.; Goadsby, P.J.; Lanteri-Minet, M.; Wen, S.; Hours-Zesiger, P.; Ferrari, M.D.; Klatt, J. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: A randomised, double-blind, placebo-controlled, phase 3b study. Lancet 2018, 392, 2280–2287. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Rapoport, A.M.; Loupe, P.S.; Aycardi, E.; McDonald, M.; Yang, R.; Bigal, M.E. The effect of beginning treatment with fremanezumab on headache and associated symptoms in the randomized phase 2 study of high frequency episodic migraine: Post-hoc analyses on the first 3 weeks of treatment. Headache 2019, 59, 383–393. [Google Scholar] [CrossRef]
- Rosen, N.; Pearlman, E.; Ruff, D.; Day, K.; Nagy, A.J. 100% Response Rate to Galcanezumab in Patients With Episodic Migraine: A Post Hoc Analysis of the Results From Phase 3, Randomized, Double-Blind, Placebo-Controlled EVOLVE-1 and EVOLVE-2 Studies. Headache J. Head Face Pain 2018, 58, 1347–1357. [Google Scholar] [CrossRef]
- Nichols, R.; Doty, E.; Sacco, S.; Ruff, D.; Pearlman, E.; Aurora, S.K. Analysis of Initial Nonresponders to Galcanezumab in Patients With Episodic or Chronic Migraine: Results From the EVOLVE-1, EVOLVE-2, and REGAIN Randomized, Double-Blind, Placebo-Controlled Studies. Headache J. Head Face Pain 2018, 59, 192–204. Available online: https://headachejournal.onlinelibrary.wiley.com/doi/full/10.1111/head.13443 (accessed on 17 July 2019). [CrossRef] [PubMed]
- Detke, H.C.; Goadsby, P.J.; Wang, S.; Friedman, D.I.; Selzler, K.J.; Aurora, S.K. Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled regain study. Neurology 2018, 91, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Camporeale, A.; Kudrow, D.; Sides, R.; Wang, S.; Van Dycke, A.; Selzler, K.J.; Stauffer, V.L. A phase 3, long-term, open-label safety study of Galcanezumab in patients with migraine. BMC Neurol. 2018, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Ayer, D.W.; Skljarevski, V.; Ford, J.H.; Nyhuis, A.W.; Lipton, R.B.; Aurora, S.K. Measures of Functioning in Patients With Episodic Migraine: Findings From a Double-Blind, Randomized, Placebo-Controlled Phase 2b Trial With Galcanezumab. Headache J. Head Face Pain 2018, 58, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Zhao, J.; Han, Q.; Liu, L.; Shen, X. The efficacy and safety of calcitonin gene-related peptide monoclonal antibody for episodic migraine: A meta-analysis. Neurol. Sci. 2018, 39, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, Y.; Xiong, H.; Hong, P. CGRP monoclonal antibody for preventive treatment of chronic migraine: An update of meta-analysis. Brain Behav. 2019, 9, e01215. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Wu, X.; Liu, Y.; Information, P.E.K.F.C. Calcitonin gene-related peptide monoclonal antibody for preventive treatment of episodic migraine: A meta analysis. Clin. Neurol. Neurosurg. 2017, 154, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Xing, H.; Cai, Y.; Li, B.; Wang, X.; Li, P.; Hu, X.; Chen, J. The effect and safety of monoclonal antibodies to calcitonin gene-related peptide and its receptor on migraine: A systematic review and meta-analysis. J. Headache Pain 2017, 18, 42. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, D.; Xu, D.; Liu, L. Efficacy and safety of erenumab in the preventive treatment of migraine in adults: A systematic review. Chin. J. Contemp. Neurol. Neurosurg. 2018, 18, 647–653. [Google Scholar]
- Monteith, D.; Collins, E.C.; Vandermeulen, C.; van Hecken, A.; Raddad, E.; Scherer, J.C.; Grayzel, D.; Schuetz, T.J.; de Hoon, J. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the CGRP Binding Monoclonal Antibody LY2951742 (Galcanezumab) in Healthy Volunteers. Front. Pharm. 2017, 8, 740. [Google Scholar] [CrossRef]
- Kaplon, H.; Reichert, J.M. Antibodies to watch in 2018. MABS 2018, 10, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Reuter, U. A Review of Monoclonal Antibody Therapies and Other Preventative Treatments in Migraine. Headache J. Head Face Pain 2018, 58, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Barak, O.; Weiss, S.; Rasamoelisolo, M.; Faulhaber, N.; Yeung, P.P.; Loupe, P.S.; Yoon, E.; Gandhi, M.D.; Spiegelstein, O.; Aycardi, E. A phase 1 study to assess the pharmacokinetics, safety, and tolerability of fremanezumab doses (225 mg, 675 mg and 900 mg) in Japanese and Caucasian healthy subjects. Cephalalgia 2018, 38, 1960–1971. [Google Scholar] [CrossRef] [PubMed]
- De Hoon, J.; van Hecken, A.; Vandermeulen, C.; Yan, L.; Smith, B.; Chen, J.S.; Bautista, E.; Hamilton, L.; Waksman, J.; Vu, T.; et al. Phase i, randomized, double-blind, placebo-controlled, single-dose, and multiple-dose studies of erenumab in healthy subjects and patients with migraine. Clin Pharm. 2018, 103, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-C.; Lin, Y.-S.; Chu, H.-C.; Fang, T.-C.; Wu, M.-S.; Kang, Y.-N. Association of Renal Function and Direct-Acting Antiviral Agents for HCV: A Network Meta-Analysis. J. Clin. Med. 2018, 7, 314. [Google Scholar] [CrossRef] [PubMed]
- Ku, F.-Y.; Wu, C.-C.; Hsiao, Y.-W.; Kang, Y.-N. Association of sperm source with miscarriage and take-home baby after ICSI in cryptozoospermia: A meta-analysis of testicular and ejaculated sperm. Andrology 2018, 6, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.M.; Chi, J.E.; Chang, C.C.; Kang, Y.N. Do etoricoxib and indometacin have similar effects and safety for gouty arthritis? A meta-analysis of randomized controlled trials. J. Pain Res. 2019, 12, 83–91. [Google Scholar] [CrossRef]
- Liao, A.H.-W.; Lin, Y.-C.; Bai, C.-H.; Chen, C.-Y. Optimal dose of succinylcholine for laryngeal mask airway insertion: Systematic review, meta-analysis and metaregression of randomised control trials. BMJ Open 2017, 7, e014274. [Google Scholar] [CrossRef]
- Lin, E.-Y.; Kuo, Y.-K.; Kang, Y.-N. Effects of three common lumbar interbody fusion procedures for degenerative disc disease: A network meta-analysis of prospective studies. Int. J. Surg. 2018, 60, 224–230. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]

| Trial | Area | Recruitment Duration | Medication | Patients (n) | Age | Male/Female |
|---|---|---|---|---|---|---|
| NCT01772524 [39] | USA | Jan. 28, 2013 ~ Dec. 23, 2013 | Eptinezumab 1000 mg/placebo | 163 | 18–55 | 30/133 |
| NCT02456740 [40,57] | Canada, Europe, Turkey, USA | Jul. 2015 ~ Sep. 5, 2016 | Erenumab 70 mg/140 mg/placebo | 955 | 18–65 | 141/814 |
| NCT01952574 [41,44] | Canada, Europe, USA | Aug. 6, 2013 ~ June 30, 2014 | Erenumab 7 mg/21 mg/70 mg/placebo | 483 | 18–60 | 94/389 |
| NCT02066415 [42,43,57] | Canada, Europe, USA | Apr. 3, 2014 ~ Dec. 4, 2015 | Erenumab 70 mg/140 mg/placebo | 667 | 18–65 | 115/552 |
| NCT02483585 [45] | Canada, Europe, USA | Jul. 2015 ~ Jul. 2016 | Erenumab 70 mg/placebo | 577 | 18–65 | 85/492 |
| NCT03096834 [58] | Australia, Europe | Mar. 20, 2017 ~ Oct. 27, 2017 | Erenumab 140 mg/placebo | 246 | 18–65 | 46/200 |
| NCT02629861 [46] | Canada, Europe, Russia, USA | Mar. 23, 2016 ~ Apr. 10, 2017 | Fremanezumab 225 mg monthly/ 3·225 mg single higher dose/placebo | 875 | 18–70 | 133/742 |
| NCT02621931 [47] | USA | Mar. 2016 ~ Jan. 2017 | Fremanezumab 675 mg + 2·225 mg/ 675 mg + 2·placebo/placebo | 1130 | 18–70 | 139/991 |
| NCT02021773 [48,49,50,51] | USA | Jan. 2014 ~ Dec. 2014 | Fremanezumab 900 mg/675-225 mg/placebo | 263 | 18–65 | 37/226 |
| NCT02025556 [59] | USA | Jan. 2014 ~ Jan. 2015 | Fremanezumab 675 mg/225 mg/placebo | 297 | 18–65 | 36/261 |
| NCT02614183 [52,60,61] | Canada, USA | Jan. 11, 2016 ~ Mar. 22, 2017 | Galcanezumab 120 mg/240 mg/placebo | 858 | 18–65 | 140/818 |
| NCT02163993 [53,56,64] | USA | July 7, 2014 ~ Aug. 19, 2015 | Galcanezumab 5 mg/50 mg/120 mg/ 300 mg/placebo | 410 | 18–65 | 70/340 |
| NCT01625988 [55] | USA | July 31, 2012 ~ Sep. 18, 2013 | Galcanezumab 150 mg/placebo | 217 | 18–65 | 33/184 |
| NCT02614196 [54,60,61] | Argentina, Europe, Israel, Korea, Mexico, Taiwan, USA | Jan. 2016 ~ Mar. 2017 | Galcanezumab 120 mg/240 mg/placebo | 915 | 18–65 | 134/781 |
| NCT02614261 [61,62] | Argentina, Canada, Europe, Israel, Mexico, Taiwan, USA | Jan. 2016 ~ Mar. 2017 | Galcanezumab 120 mg/240 mg/placebo | 1113 | 18–65 | 167/946 |
| NCT02614287 [63] | Canada, Europe, USA | Dec. 2015 ~ Sep. 2017 | Galcanezumab 120 mg/240 mg/placebo | 270 | 18–65 | 47/223 |
| Outcome | Effect | 95% CI 2 | Heterogeneity | Small study bias | |||||
|---|---|---|---|---|---|---|---|---|---|
| (Subset) | Study | Patients | Size 1 | Lower | Upper | I 2 (%) | p | Estimate | p |
| Response rate 50% (First month) | 3.014 | 0.119 | |||||||
| Eptinezumab | 1 | 156 | 1.5 | 1.163 | 1.935 | 0 | NE | ||
| Erenumab | 4 | 2099 | 2.206 | 1.699 | 2.866 | 40.42 | 0.169 | ||
| Frenamezumab | 1 | 261 | 1.952 | 1.316 | 2.895 | 0 | NE | ||
| Response rate 50% (Second month) | 1.649 | 0.532 | |||||||
| Eptinezumab | 1 | 157 | 1.401 | 1.102 | 1.782 | 0 | NE | ||
| Erenumab | 4 | 2092 | 1.81 | 1.459 | 2.246 | 49.38 | 0.115 | ||
| Frenamezumab | 1 | 261 | 1.443 | 1.064 | 1.956 | 0 | NE | ||
| Response rate 50% (Third month) | 3.531 | 0.147 | |||||||
| Eptinezumab | 1 | 151 | 1.151 | 0.941 | 1.408 | 0 | NE | ||
| Erenumab | 4 | 2415 | 1.628 | 1.39 | 1.908 | 31.79 | 0.222 | ||
| Frenamezumab | 1 | 261 | 1.719 | 1.228 | 2.405 | 0 | NE | ||
| Galcanezumab | 1 | 188 | 1.225 | 1.006 | 1.49 | 0 | NE | ||
| Response rate 50% (From baseline to week 12) | 1.089 | 0.544 | |||||||
| Eptinezumab | 1 | 143 | 1.86 | 1.281 | 2.703 | 0 | NE | ||
| Erenumab | 2 | 1434 | 1.555 | 0.991 | 2.438 | 83.97 | 0.013 | ||
| Frenamezumab | 4 | 2542 | 2.024 | 1.518 | 2.697 | 66.02 | 0.032 | ||
| Galcanezumab | 2 | 1287 | 1.667 | 1.403 | 1.981 | 0 | 0.435 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, I.-H.; Wu, P.-C.; Lin, E.-Y.; Chen, C.-Y.; Kang, Y.-N. Effects of Anti-Calcitonin Gene-Related Peptide for Migraines: A Systematic Review with Meta-Analysis of Randomized Clinical Trials. Int. J. Mol. Sci. 2019, 20, 3527. https://doi.org/10.3390/ijms20143527
Huang I-H, Wu P-C, Lin E-Y, Chen C-Y, Kang Y-N. Effects of Anti-Calcitonin Gene-Related Peptide for Migraines: A Systematic Review with Meta-Analysis of Randomized Clinical Trials. International Journal of Molecular Sciences. 2019; 20(14):3527. https://doi.org/10.3390/ijms20143527
Chicago/Turabian StyleHuang, I-Hsin, Po-Chien Wu, En-Yuan Lin, Chien-Yu Chen, and Yi-No Kang. 2019. "Effects of Anti-Calcitonin Gene-Related Peptide for Migraines: A Systematic Review with Meta-Analysis of Randomized Clinical Trials" International Journal of Molecular Sciences 20, no. 14: 3527. https://doi.org/10.3390/ijms20143527
APA StyleHuang, I.-H., Wu, P.-C., Lin, E.-Y., Chen, C.-Y., & Kang, Y.-N. (2019). Effects of Anti-Calcitonin Gene-Related Peptide for Migraines: A Systematic Review with Meta-Analysis of Randomized Clinical Trials. International Journal of Molecular Sciences, 20(14), 3527. https://doi.org/10.3390/ijms20143527





