Modulation of Epithelial to Mesenchymal Transition Signaling Pathways by Olea Europaea and Its Active Compounds
Abstract
1. Introduction
2. Olea Europaea
3. Epithelial-Mesenchymal Transition
3.1. Physiological EMT
3.2. Pathological EMT
3.3. Transcription Factors Influencing EMT
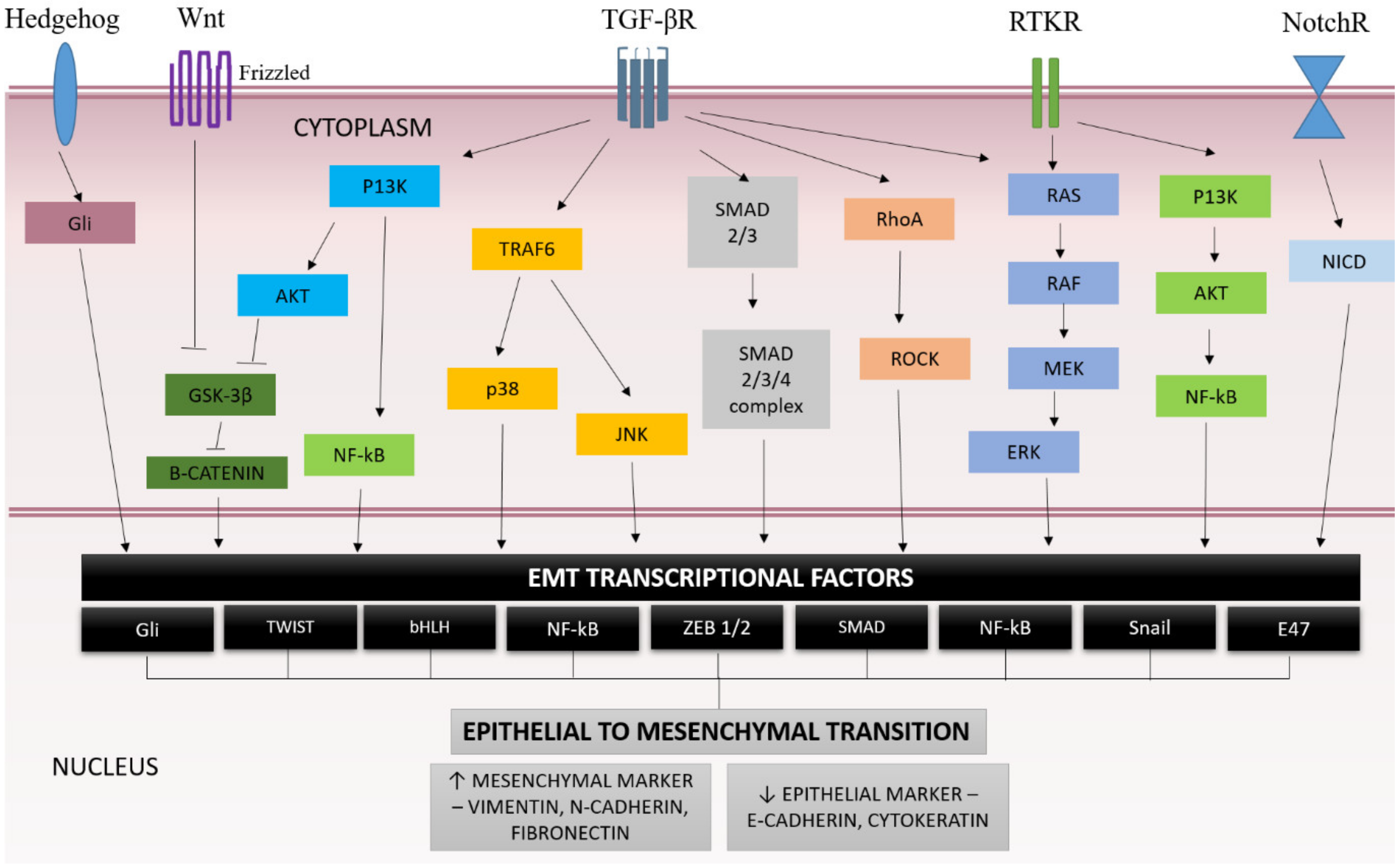
3.4. EMT Signaling Pathway
3.4.1. SMAD-Dependent Pathway
3.4.2. SMAD-Independents Pathway
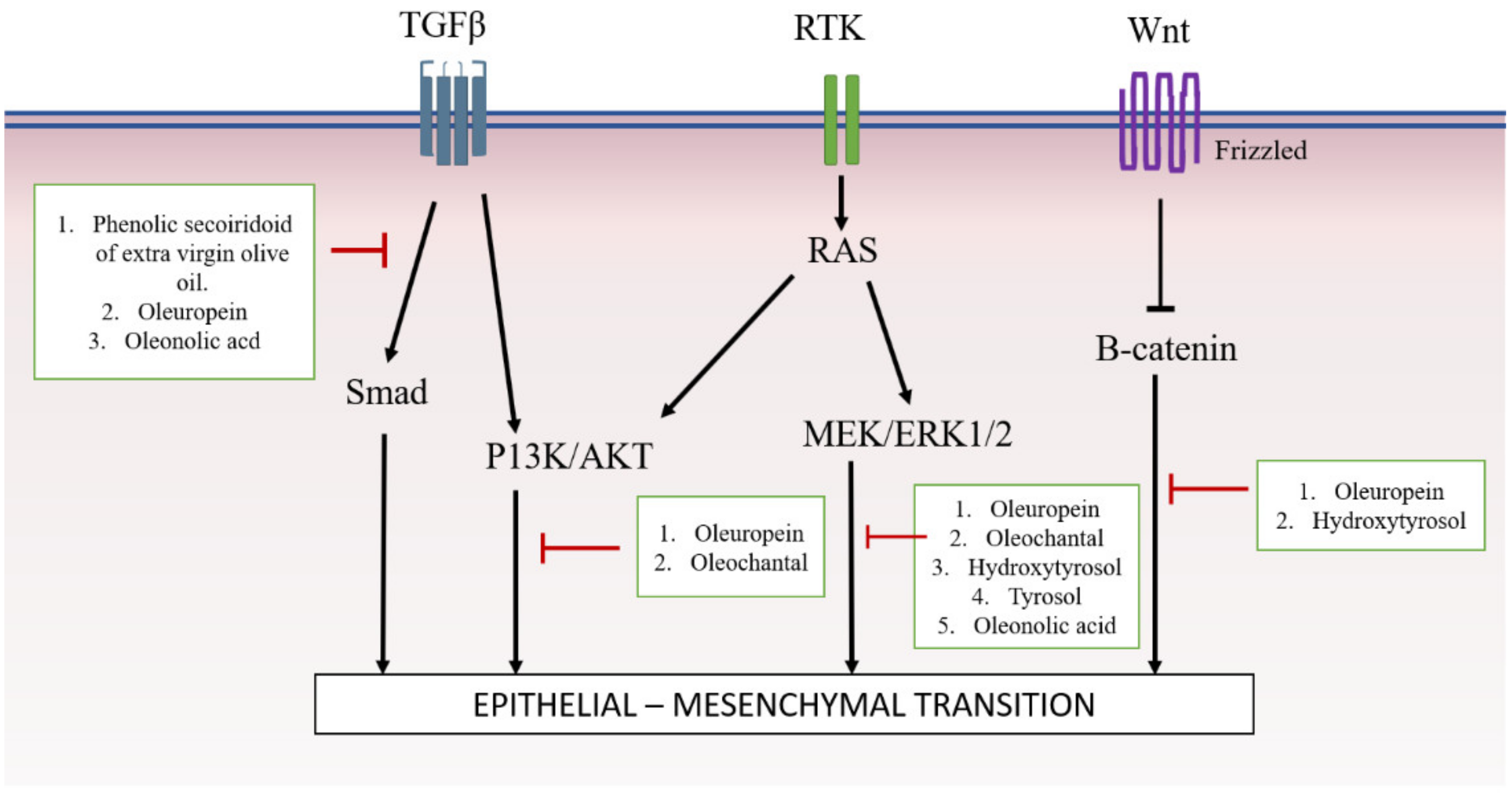
4. Involvement of Olea Europaea and Its Active Compounds in Targeting Specific Signaling Pathway
4.1. SMAD-Dependant Pathway
4.2. SMAD-Independent Pathway
4.2.1. AKT
4.2.2. MAPK/ERK
4.2.3. Wnt/β-Catenin
5. Future Direction and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal 2014, 7, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Maeng, Y.; Lee, G.; Lee, B.; Choi, S.; Kim, T.; Kim, E.K. Role of TGFBIp in Wound Healing and Mucin Expression in Corneal Epithelial Cells. Yonsei Med. J. 2017, 58, 423–431. [Google Scholar]
- Borthwick, L.A.; Gardner, A.; De Soyza, A.; Mann, D.A.; Fisher, A.J. Transforming Growth Factor-β1(TGF-β1) Driven Epithelial to Mesenchymal Transition (EMT) is Accentuated by Tumour Necrosis Factor α (TNFα) via Crosstalk Between the SMAD and NF-κB Pathways. Cancer Microenviron. 2011, 5, 45–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Granados-Principal, S.; Choi, D.S.; Brown, A.M.C.; Chang, J. The natural compound hydroxytyrosol inhibits the Wnt/EMT axis and migration of triple-negative breast cancer cells. Cancer Res. 2014, 73, 2586. [Google Scholar]
- Zhao, H.; Wu, Q.-Q.; Cao, L.-F.; Qing, H.-Y.; Zhang, C.; Chen, Y.-H.; Wang, H.; Liu, R.-R.; Xu, D.-X. Melatonin inhibits endoplasmic reticulum stress and epithelial-mesenchymal transition during bleomycin-induced pulmonary fibrosis in mice. PLoS ONE 2014, 9, e97266. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Gong, J.H.; Choi, Y.J.; Kang, M.K.; Kim, Y.H.; Kang, Y.H. Kaempferol inhibits endoplasmic reticulum stress-associated mucus hypersecretion in airway epithelial cells and ovalbumin-sensitized mice. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Lai, M. Modulation of epithelial-to-mesenchymal cancerous transition by natural products. Fitoterapia 2015, 106, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Tang, L.; Chen, H.; Wu, C.; Zhao, M.; Yang, Y.; Chen, X.; Liu, G. Resveratrol inhibits TGF-β1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology 2013, 303, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Lu, H.; Wu, C.; Liang, Y.; Wang, S.; Lin, C.; Chen, B.; Xia, P. Resveratrol inhibits epithelial-mesenchymal transition and renal fibrosis by antagonizing the hedgehog signaling pathway. Biochem. Pharmacol. 2014, 92, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M.; Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; et al. The phenolic compounds of olive oil: structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Sabir, S.M.; Ahmad, S.D.; Boligon, A.A.; Athayde, M.L.; Jabbar, A.; Qamar, I.; Khan, A. Antioxidant activities and phenolic composition of Olive (Olea europaea) leaves. Appl. Bot. Food Qual. 2015, 88, 16–21. [Google Scholar]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Covas, M.I.; Fitó, M.; Kušar, A.; Pravst, I. Health effects of olive oil polyphenols: Recent advances and possibilities for the use of health claims. Mol. Nutr. Food Res. 2013, 57, 760–771. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef] [PubMed]
- Coccia, A.; Bastianelli, D.; Mosca, L.; Monticolo, R.; Panuccio, I.; Carbone, A.; Calogero, A.; Lendaro, E. Extra virgin olive oil phenols suppress migration and invasion of T24 human bladder cancer cells through modulation of matrix metalloproteinase-2. Nutr. Cancer 2014, 66, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Busnena, B.A.; Foudah, A.I.; Melancon, T.; El Sayed, K.A. Olive secoiridoids and semisynthetic bioisostere analogues for the control of metastatic breast cancer. Bioorg. Med. Chem. 2013, 21, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.Y.; Ei Sayed, K.A. Olive phenolics as c-Met inhibitors: (-)-Oleocanthal attenuates cell proliferation, invasiveness, and tumor growth in breast cancer models. PLoS ONE 2014, 9, e97622. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M.; et al. Nutrigenomics of extra-virgin olive oil: A review. BioFactors 2017, 43, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Klen, T.J. Olive Fruit Phenols in Olive Oil Processing: The Fate and Antioxidant Potential. Ph.D. Thesis, University of Nova Gorica, Nova Gorica, Slovenia, 2014; p. 227. [Google Scholar]
- Claisseb, R.; Bellakhdar, J. Repertory of standard herbal drugs in the Moroccan pharmacopoea. J. Ethnopharmacol. 1991, 35, 123–143. [Google Scholar]
- Lawrendiadis, G. Contribution to the knowledge of the medicinal plants of Greece. Planta Med. 1960, 9, 164–169. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Factors Influencing Phenolic Compounds in Table Olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S. Quality Analysis of Extra Virgin Olive Oils—Part 6 Nutritive Benefi ts—Phenolic Compounds in Virgin Olive Oil; Agilent Technology Application Note; Agilent Technologies Deutschland GmbH: Waldbronn, Germany, 2013; publication number 5991-3801EN. [Google Scholar]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Gouvinhas, I.; Machado, N.; Sobreira, C.; Domínguez-Perles, R.; Gomes, S.; Rosa, E.; Barros, A.I.R.N.A. Critical review on the significance of Olive phytochemicals in plant physiology and human health. Molecules 2017, 22, 1986. [Google Scholar] [CrossRef]
- Garcı, M.V.; Gonza, J.; Sa, S. Anti-inflammatory, Immunomodulatory, and Prebiotic Properties of Dietary Flavonoids. In Polyphenols: Prevention and Treatment of Human Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 327–345. [Google Scholar] [CrossRef]
- Pang, K.L.; Chin, K.Y. The biological activities of oleocanthal from a molecular perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef]
- Hupin, C.; Gohy, S.; Bouzin, C.; Lecocq, M.; Polette, M.; Pilette, C. Features of mesenchymal transition in the airway epithelium from chronic rhinosinusitis. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Park, I.I.; Kang, J.J.; Shin, J.J.; Lee, H.H. Trichostatin A Inhibits Epithelial Mesenchymal Transition Induced by TGF-β1 in Airway Epithelium. PLoS ONE 2016, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sataloff, R.T. Professional Voice, Fourth Edition: The Science and Art of Clinical Care, 3-Volume Set; Plural Publishing, Incorporated: San Diego, CA, USA, 2017; ISBN 9781597567107. [Google Scholar]
- Stone, R.C.; Pastar, I.; Ojeh, N.; Chen, V.; Liu, S.; Garzon, K.I.; Tomic-canic, M. Epithelial-Mesenchymal Transition in Tissue Repair and Fibrosis. Cell Tissue Res. 2017, 365, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Yugandhar, V.G.; Clark, M.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2013, 46, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Grimm, W.A.; Garner, W.L.; Qin, L.; Travis, T.; Tan, N.; Han, Y.P. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-α through bone morphogenic protein-2. Am. J. Pathol. 2010, 176, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [PubMed]
- Ho, J.; Jun, W.; Choue, R.; Lee, J. I3C and ICZ inhibit migration by suppressing the EMT process and FAK expression in breast cancer cells. Mol. Med. Rep. 2013, 384–388. [Google Scholar] [CrossRef]
- Lo, U.G.; Lee, C.F.; Lee, M.S.; Hsieh, J.T. The role and mechanism of epithelial-to-mesenchymal transition in prostate cancer progression. Int. J. Mol. Sci. 2017, 18, 2079. [Google Scholar] [CrossRef]
- Cusimano, A.; Balasus, D.; Azzolina, A.; Augello, G.; Emma, M.R.; Di Sano, C.; Gramignoli, R.; Strom, S.C.; McCubrey, J.A.; Montalto, G.; et al. Oleocanthal exerts antitumor effects on human liver and colon cancer cells through ROS generation. Int. J. Oncol. 2017, 51, 533–544. [Google Scholar] [CrossRef]
- Zaravinos, A.; Emt, T. The Regulatory Role of MicroRNAs in EMT and Cancer. J. Oncol. 2015, 2015. [Google Scholar] [CrossRef]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; Del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, ZEB and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Teruya-Feldstein, J.; Weinberg, R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007, 449, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Allen, J.T.; Mason, R.M.; Kamimura, T.; Zhang, Z. TGF-β1 induces human alveolar epithelial to mesenchymal cell transition. Respir. Res. 2005, 15, 1–16. [Google Scholar] [CrossRef]
- Lee, C.; Lee, M.; Yoo, S.; Choi, K.; Song, J.; Jang, J.; Oh, S.; Ryu, H.; Lee, H.; Surh, Y.; et al. Magnolin inhibits cell migration and invasion by targeting the ERKs/RSK2 signaling pathway. BMC Cancer 2015, 15, 576. [Google Scholar] [CrossRef]
- Lifshitz, V.; Frenkel, D. TGF-β. In Handbook of Biologically Active Peptides; Kastin, A., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 1647–1653. [Google Scholar]
- Ji, Y.; Dou, Y.N.; Zhao, Q.W.; Zhang, J.Z.; Yang, Y.; Wang, T.; Xia, Y.F.; Dai, Y.; Wei, Z.F. Paeoniflorin suppresses TGF-β mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol. Sin. 2016, 37, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2016, 19, 156–172. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, X.-J.; Xing, J. Signal Transduction Pathways of EMT Induced by TGF-β, SHH, and WNT and Their Crosstalks. J. Clin. Med. 2016, 5, 41. [Google Scholar] [CrossRef]
- Nordin, A.; Qisya, N.; Sainik, V.; Zulfarina, M.S.; Naina-mohamed, I.; Saim, A.; Bt, R.; Idrus, H. Honey and epithelial to mesenchymal transition in wound healing: An evidence-based review. Wound Med. 2017, 18, 8–20. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, F.; Shao, J.; Wu, L.; Zhang, X.; Chen, L.; Lu, Y. Curcumin inhibits cobalt chloride-induced epithelial-to-mesenchymal transition associated with interference with TGF-β/Smad signaling in hepatocytes. Lab. Investig. 2015, 95, 1234–1245. [Google Scholar] [CrossRef]
- Juan, M.E.; Wenzel, U.; Ruiz-Gutierrez, V.; Daniel, H.; Planas, J.M. Olive Fruit Extracts Inhibit Proliferation and Induce Apoptosis in HT-29 Human Colon Cancer Cells. J. Nutr. 2006, 136, 2553–2557. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhong, W.; Zhao, J.; Zhang, H.; Zhang, Q.; Liang, Y.; Chen, S.; Liu, H.; Zong, S.; Tian, Y.; et al. Oleanolic Acid Inhibits Epithelial–Mesenchymal Transition of Hepatocellular Carcinoma by Promoting iNOS Dimerization. Mol. Cancer Ther. 2019, 18, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Choupani, J.; Alivand, M.R.; Derakhshan, S.M.; Zaeifizadeh, M.; Khaniani, M.S. Oleuropein inhibits migration ability through suppression of epithelial-mesenchymal transition and synergistically enhances doxorubicin-mediated apoptosis in MCF-7 cells. J. Cell. Physiol. 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.; Camacho-Corencia, P.; Sanchez-Rovira, P.; Vera-Ramirez, L.; Ramirez-Tortosa, M.C. Hydroxytyrosol inhibits growth and cell proliferation and promotes high expression of sfrp4 in rat mammary tumours. Mol. Nutr. Food Res. 2011, 55, S117–S126. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Martin, A.; Fernández-Arroyo, S.; Cufí, S.; Oliveras-Ferraros, C.; Lozano-Sánchez, J.; Vellón, L.; Micol, V.; Joven, J.; Segura-Carretero, A.; Menendez, J.a. Phenolic Secoiridoids in Extra Virgin Olive Oil Impede Fibrogenic and Oncogenic Epithelial-to-Mesenchymal Transition: Extra Virgin Olive Oil As a Source of Novel Antiaging Phytochemicals. Rejuvenation Res. 2012, 15, 3–21. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Yin, J.; Cheng, X.; Lu, X.; Ni, L.; Xi, Y.; Yin, G.; Lu, G.; Sun, W. Oleanolic acid attenuates TGF-β1-induced epithelial-mesenchymal transition in NRK-52E cells. BMC Complement. Altern. Med. 2018, 18, 205. [Google Scholar] [CrossRef] [PubMed]
- Lupinacci, S.; Perri, A.; Toteda, G.; Vizza, D. Olive leaf extract counteracts epithelial to mesenchymal transition process induced by peritoneal dialysis, through the inhibition of TGFβ1 signaling. Cell Biol. Toxicol. 2018, 35, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, J.; Huang, B.; Chen, A.; Li, X. Oleuropein inhibits the proliferation and invasion of glioma cells via suppression of the AKT signaling pathway. Oncol. Rep. 2016, 36, 2009–2016. [Google Scholar] [CrossRef]
- Guo, G.; Yao, W.; Zhang, Q.; Bo, Y. Oleanolic Acid Suppresses Migration and Invasion of Malignant Glioma Cells by Inactivating MAPK/ERK Signaling Pathway. PLoS ONE 2013, 8, e72079. [Google Scholar] [CrossRef]
- Acquaviva, R.; Di Giacomo, C.; Sorrenti, V.; Galvano, F.; Santangelo, R.; Cardile, V.; Gangia, S.; D’Orazio, N.; Abraham, N.G.; Vanella, L. Antiproliferative effect of oleuropein in prostate cell lines. Int. J. Oncol. 2012, 41, 31–38. [Google Scholar]
- Polini, B.; Digiacomo, M.; Carpi, S.; Bertini, S.; Gado, F.; Saccomanni, G.; Macchia, M.; Nieri, P.; Manera, C.; Fogli, S. Oleocanthal and oleacein contribute to the in vitro therapeutic potential of extra virgin oil-derived extracts in non-melanoma skin cancer. Toxicol. Vitr. 2018, 52, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Arena, C.; Carpi, S.; Polini, B.; Bertini, S.; Digiacomo, M.; Gado, F.; Saba, A.; Saccomanni, G.; Breschi, M.C.; et al. Cytotoxic Activity of Oleocanthal Isolated from Virgin Olive Oil on Human Melanoma Cells. Nutr. Cancer 2016, 68, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M. Oleocanthal Inhibits Proliferation and MIP-1α Expression in Human Multiple Myeloma Cells. Curr. Med. Chem. 2013, 2467–2475. [Google Scholar] [CrossRef]
- Hur, W.; Kim, S.W.; Lee, Y.K.; Choi, J.E.; Hong, S.W.; Song, M.J.; Bae, S.H.; Park, T.; Um, S.J.; Yoon, S.K. Oleuropein reduces free fatty acid-induced lipogenesis via lowered extracellular signal-regulated kinase activation in hepatocytes. Nutr. Res. 2012, 32, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Deiana, M.; Incani, A.; Vauzour, D.; Dessì, M.A.; Spencer, J.P.E. Hydroxytyrosol inhibits the proliferation of human colon adenocarcinoma cells through inhibition of ERK1/2 and cyclin D1. Mol. Nutr. Food Res. 2009, 53, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Maalej, A.; Bouallagui, Z.; Hadrich, F.; Isoda, H.; Sayadi, S. Assessment of Olea europaea L. fruit extracts: Phytochemical characterization and anticancer pathway investigation. Biomed. Pharmacother. 2017, 90, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, Y.; Xu, Z.; Wang, J.; Wang, F.; Wang, D.; Pan, S.; Wu, Y.; Pan, H.; Xu, D.; et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014, 347, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Meng, Q.; Han, J.; Sun, H.; Li, L.; Song, R. (-)-Oleocanthal inhibits growth and metastasis by blocking activation of STAT3 in human hepatocellular carcinoma. Oncotarget 2016, 7, 43475. [Google Scholar] [CrossRef] [PubMed]
- Giner, E.; Recio, M.C.; Ríos, J.L.; Cerdá-Nicolás, J.M.; Giner, R.M. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol. Nutr. Food Res. 2016, 60, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Grille, S.J.; Bellacosa, A.; Upson, J.; Klein-szanto, A.J.; Van Roy, F.; Lee-kwon, W.; Donowitz, M.; Tsichlis, P.N.; Larue, L. The Protein Kinase Akt Induces Epithelial Mesenchymal Transition and Promotes Enhanced Motility and Invasiveness of Squamous Cell Carcinoma Lines The Protein Kinase Akt Induces Epithelial Mesenchymal Transition and Promotes Enhanced Motility and Invasiven. Cancer Res. 2003, 63, 2172–2178. [Google Scholar] [PubMed]
| Type of Study | Disease | Pathways Involved | Subject/Cell Type/EMT Induction | Olive Product or Active Compound Concentration | Key Findings | References |
|---|---|---|---|---|---|---|
| In vitro | Breast cancer | EMT transcription factor | MCF-7 | Oleuropein (OP) [50–1200 μg/mL] |
| [58] |
| AKT MAPK | MDA-MB-231, MCF-7 and BT-474 | (-)-Oleocanthal (OC) [0–40µM] |
| [21] | ||
| Wnt | BT549 | Hydroxytyrosol [10, 25, 50, 75, and 100 μM] |
| [59] | ||
| Breast cancer and renal fibrosis | SMAD | TGFβ1 treated-MCF-7 and MDCK | Extra virgin olive oil (EVOO) [200 ng/mL] | Prevented upregulations of SMAD4, SNAIL2, TCF4, VIM, FN and SERPINE1 genes. | [60] | |
| Renal interstitial fibrosis | SMAD | Renal proximal tubular epithelial cell line (NRK-52E) | Oleanolic acid (OA) [2, 4 and 8 μM] |
| [61] | |
| Peritoneal fibrosis |
| TGFβ1-treated MeT-5A | Olive leaf extract (OLE) [high content of oleuropein] [25µg/mL] |
| [62] | |
| Malignant glioma | A KT | Human malignant glioma cell line (U251 and A172) | Oleuropein (OP) [0, 200 and 400 μM] |
| [63] | |
| MAPK/ERK | Human glioblastoma cell lines (U-87 MG, U-251 MG cells), primary glioma cells | Oleanolic acid (OA) [5, 10 and 25 mg/mL (10, 20 and 50 mM)] |
| [64] | ||
| Prostate cancer | AKT | Prostate cell lines (BPH-1, LNCaP, DU145) | Oleuropein (OP) [100–500 μM] | Downregulated pAKT, suggesting an inhibitory effect on AKT signaling | [65] | |
| Skin cancer (non-melanoma) |
| Human epidermoid carcinoma cell line (A431) and human immortalized keratinocytes (HaCat) | Oleocanthal, oleacein, tyrosol and hydroxytyrosol [1–100 μM] | Decreased expression levels of B-Raf, pAKT and pERK proteins after treatment | [66] | |
| Skin cancer (malignant melanoma) |
| Human melanoma cell line (A375) | Oleocanthal (OC) [0.01–50 µM] | Significant inhibition of ERK1/2 and AKT phosphorylation | [67] | |
| Multiple myeloma |
| Myeloma-derived cell line (ARH-77, MOPC-31C) | Oleocanthal (OC) [25 and 50 µM] |
| [68] | |
| Non-alcoholic fatty liver disease (NAFLD) |
| Free fatty acids (FFAs) induced HepG2 and FL83B | Oleuropein (OP) [10- and 50-μM] |
| [69] | |
| Colon cancer | ERK | Human colon adenocarcinoma cells (Caco-2) | Hydroxytyrosol [5.0–162.5 µM] | Strong inhibition of extracellular signal-regulated kinase (ERK)1/2 phosphorylation | [70] | |
| Colon and liver cancer |
| Liver and colon cancer cells (HepG2 and Caco-2) | Olea europaea L. fruit extracts [0–1400 mg/mL] |
| [71] | |
| In vitro and In vivo | Hepatocellular carcinoma cell (HCC) | AKT | HCC cell lines HepG2, Hep3B and SK-HEP-1 | Hydroxytyrosol [0–400 µM] | pAKT decreased | [72] |
| JAK/STAT3 | Huh-7, HepG2 and HCCLM3 | (-)-Oleocanthal (OC) [0–80 μM] |
| [73] | ||
| In vivo | Colorectal cancer |
| AOM/DSS-induced C57BL/6 mice CRC model | Oleuropein (OP) [50 or 100 mg/kg] |
| [74] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razali, R.A.; Lokanathan, Y.; Yazid, M.D.; Ansari, A.S.; Saim, A.B.; Hj Idrus, R.B. Modulation of Epithelial to Mesenchymal Transition Signaling Pathways by Olea Europaea and Its Active Compounds. Int. J. Mol. Sci. 2019, 20, 3492. https://doi.org/10.3390/ijms20143492
Razali RA, Lokanathan Y, Yazid MD, Ansari AS, Saim AB, Hj Idrus RB. Modulation of Epithelial to Mesenchymal Transition Signaling Pathways by Olea Europaea and Its Active Compounds. International Journal of Molecular Sciences. 2019; 20(14):3492. https://doi.org/10.3390/ijms20143492
Chicago/Turabian StyleRazali, Rabiatul Adawiyah, Yogeswaran Lokanathan, Muhammad Dain Yazid, Ayu Suraya Ansari, Aminuddin Bin Saim, and Ruszymah Bt Hj Idrus. 2019. "Modulation of Epithelial to Mesenchymal Transition Signaling Pathways by Olea Europaea and Its Active Compounds" International Journal of Molecular Sciences 20, no. 14: 3492. https://doi.org/10.3390/ijms20143492
APA StyleRazali, R. A., Lokanathan, Y., Yazid, M. D., Ansari, A. S., Saim, A. B., & Hj Idrus, R. B. (2019). Modulation of Epithelial to Mesenchymal Transition Signaling Pathways by Olea Europaea and Its Active Compounds. International Journal of Molecular Sciences, 20(14), 3492. https://doi.org/10.3390/ijms20143492






