Lipid Saturation and the Rheology of Human Tear Lipids
Abstract
1. Introduction
2. Results
2.1. H-NMR Spectroscopy
2.2. Effect of Saturation on Human Tear Lipid Phase Transition Parameters.
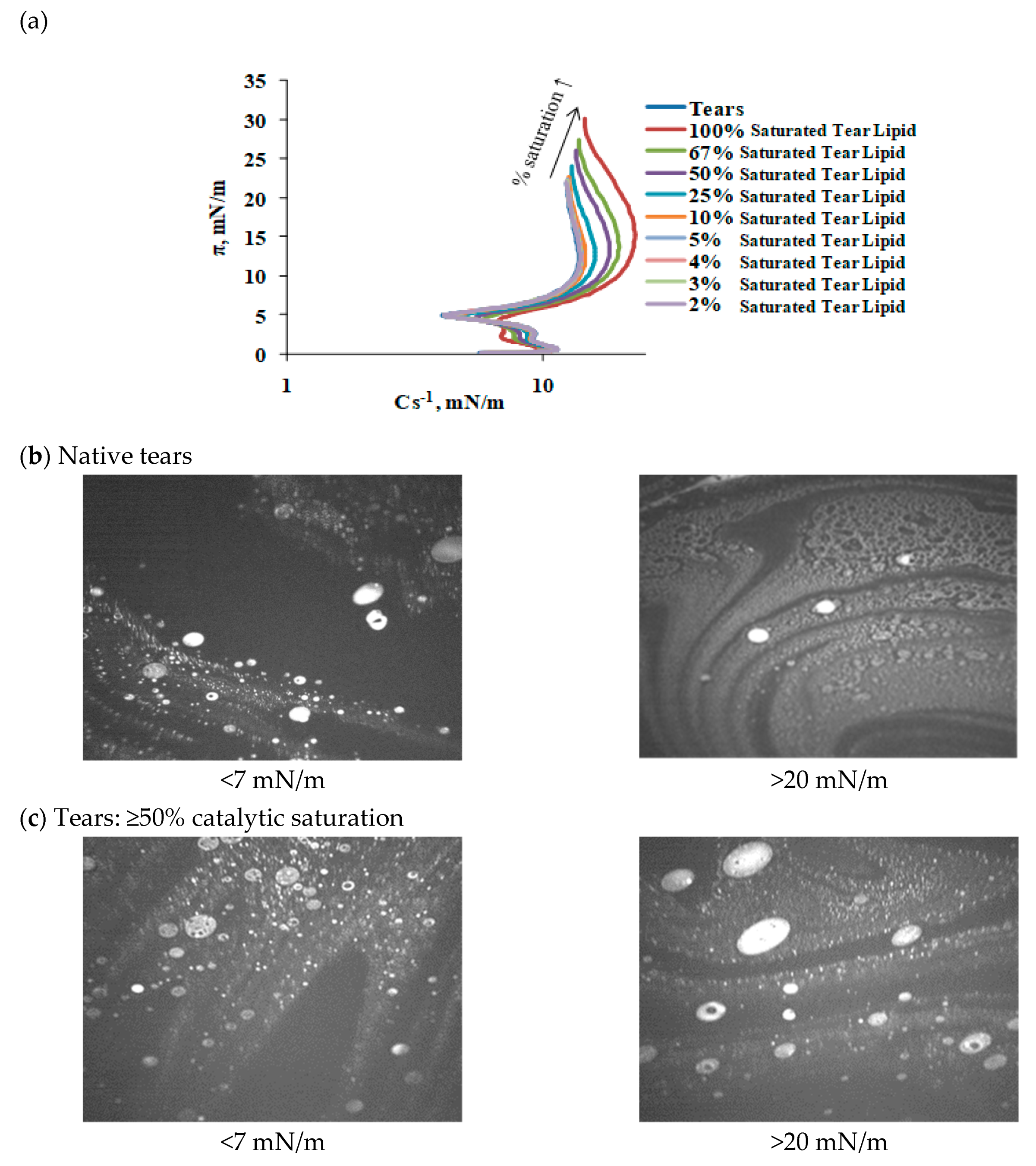
2.3. Effect of Catalytic Saturation on Human Tear Lipid Rheology
3. Discussion
Meibum Verses TL
4. Methods
4.1. Materials
4.2. Collection and Extraction of Tear Lipids
4.3. Catalytic Hydrogenation
4.4. NMR Spectroscopy
4.5. Measurement of Lipid Phase Transitions Using Fourier Transform Infrared Spectroscopy
4.6. Compression Isotherms
4.7. Stress–Relaxation Studies via the Small Deformations Method
4.8. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Tonchev, V.; Nencheva, Y.; Krastev, R. Surface relaxations as a tool to distinguish the dynamic interfacial properties of films formed by normal and diseased meibomian lipids. Soft Matter 2014, 10, 5579–5588. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Eftimov, P.; Yokoi, N. Structure-function relationship of tear film lipid layer: A contemporary perspective. Exp. Eye Res. 2017, 163, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Green-Church, K.B.; Butovich, I.; Willcox, M.; Borchman, D.; Paulsen, F.; Barabino, S.; Glasgow, B.J. The international workshop on meibomian gland dysfunction: Report of the subcommittee on tearfilm lipids and lipid-protein interactions in health and disease. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- King-Smith, P.E.; Fink, B.A.; Nichols, J.J.; Nichols, K.K.; Braun, R.J.; McFadden, G.B. The contribution of lipid layer movement to tear film thinning and breakup. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Knop, E.; Knop, N.; Schirra, F. Meibomian glands. Part II: Physiology, characteristics, distribution and function of meibomian oil. Ophthalmologe 2009, 106, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Murube, J. The origin of tears. III. The lipid component in the XIX and XX centuries. Ocul. Surf. 2012, 4, 200–209. [Google Scholar] [CrossRef]
- Pucker, A.D.; Nichols, J.J. Analysis of meibum and tear lipids. Ocul. Surf. 2012, 10, 230–250. [Google Scholar] [CrossRef]
- Brown, S.H.; Kunnen, C.M.; Duchoslav, E.; Dolla, N.K.; Kelso, M.J.; Papas, E.B.; Lazon de la Jara, P.; Willcox, M.D.; Blanksby, S.J.; Mitchell, T.W. A comparison of patient matched meibum and tear lipidomes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7417–7424. [Google Scholar] [CrossRef]
- Rantamaki, A.H.; Seppanen-Laakso, T.; Oresic, M.; Jauhiainen, M.; Holopainen, J.M. Human tear fluid lipidome: From composition to function. PLoS ONE 2011, 6, e19553. [Google Scholar] [CrossRef]
- Saville, J.T.; Zhao, Z.; Willcox, M.D.; Ariyavidana, M.A.; Blanksby, S.J.; Mitchell, T.W. Identification of phospholipids in human meibum by nano-electrospray ionisation tandem mass spectrometry. Exp. Eye Res. 2011, 92, 238–240. [Google Scholar] [CrossRef]
- Wollensak, G.; Mur, E.; Mayr, A.; Baier, G.; Gottinger, W.; Stoffler, G. Effective methods for the investigation of human tear film proteins and lipids. Graefe’s Arch. Clin. Exp. Ophthalmol 1990, 228, 78–82. [Google Scholar] [CrossRef]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Tang, D.; Ho, D.V. Spectroscopic evaluation of human tear lipids. Chem. Phys. Lipids 2007, 147, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Nagyova, B.; Tiffany, J.M. Components responsible for the surface tension of human tears. Curr. Eye Res. 1999, 19, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Yappert, M.C.; Milliner, S.E.; Smith, R.J.; Bhola, R. Confirmation of the presence of squalene in human eyelid lipid by heteronuclear single quantum correlation spectroscopy. Lipids 2013, 48, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.B.D.; Gerlach, D.; Yappert, M.C.; Whitehall, J.S. Sebum/meibum surface film interactions and phase transitional differences. Exp. Eye Res. 2016, 57, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Robosky, L.C.; Wade, K.; Woolson, D.; Baker, J.D.; Manning, M.L.; Gage, D.A.; Reily, M.D. Quantitative evaluation of sebum lipid components with nuclear magnetic resonance. J. Lipid Res. 2008, 49, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Ho, D.V. Temperature-induced conformational changes in human tear lipids hydrocarbon chains. Biopolymers 2007, 87, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D. The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul. Surf. 2019, 17, 360–364. [Google Scholar] [CrossRef]
- Mudgil, P.; Borchman, D.; Ramasubramanian, A. Insights into Tear Film Stability from Babies and Young Adults; a study of Human Meibum lipid Conformation and Rheology. Int. J. Mol. Sci. 2018, 19, 3502. [Google Scholar] [CrossRef]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Bell, J.; Wells, E.; Neravetla, S.; Greenstone, V. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3805–3817. [Google Scholar] [CrossRef]
- Foulks, G.N.; Borchman, D.; Yappert, M.C.; Kakar, S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: A comparative clinical and spectroscopic pilot study. Cornea 2013, 32, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, A.; Blackburn, R.; Sledge, S.M.; Yeo, H.; Yappert, M.C.; Gully, Z.N.; Singh, S.; Mehta, S.; Mehta, A.; Borchman, D. Structural differences in meibum from donors after hematpoietic stem cell transplantations. Cornea 2019, in press. [Google Scholar] [CrossRef]
- Ramasubramanian, A.; Borchman, D. Structural differences in meibum from teenage donors with and without dry eye induced by allogeneic hematopoietic stem cell transplantations. J. Ped. Hematol. Oncol. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C. Confirmation of changes in human meibum lipid infrared spectra with age using principal component analysis. Curr. Eye Res. 2010, 35, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp. Eye Res. 2010, 91, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Nencheva, Y.; Ramasubramanian, A.; Eftimov, P.; Yokoi, N.; Borchman, D.; Georgiev, G. Effects of lipid saturation on the surface properties of human meibum films. Int. J. Mol. Sci. 2018, 19, 2209. [Google Scholar] [CrossRef]
- Mudgil, P.; Borchman, D.; Yappert, M.C.; Duran, D.; Cox, G.W.; Smith, R.J.; Bhola, R.; Dennis, G.R.; Whitehall, J.S. Human meibum saturation and lipid order. Exp. Eye Res. 2013, 116, 79–85. [Google Scholar] [CrossRef]
- Sledge, S.; Henry, C.; Borchman, D.; Yappert, M.C.; Bhola, R.; Ramasubramanian, A.; Blackburn, R.; Austin, J.; Massey, K.; Sayied, S.; et al. Human meibum age, lipid-lipid interactions and lipid saturation in meibum from infants. Int. J. Mol. Sci. 2017, 18, 1862. [Google Scholar] [CrossRef]
- Borchman, D.; Ramasubramanian, A. Human meibum chain branching variability with age, gender and meibomian gland dysfunction. Ocul. Surf. 2019, 17, 327–335. [Google Scholar] [CrossRef]
- Borchman, D.; Cenedella, R.I.; Lamba, O.P. Role of cholesterol in the structural order of lens lipids. Exp. Eye Res. 1996, 62, 191–197. [Google Scholar] [CrossRef]
- Borchman, D.; Yappert, M.C.; Milliner, S.; Duran, D.; Cox, G.W.; Smith, R.J.; Bhola, R. 13C and 1H NMR ester region resonance assignments and the composition of human infant and child meibum. Exp. Eye Res. 2013, 112, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Tang, D.; Yappert, M.C. Lipid composition, membrane structure relationships in lens and muscle sarcoplasmic reticulum. Biospectroscopy 1999, 5, 151–167. [Google Scholar] [CrossRef]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Milliner, S.E. Changes in human meibum lipid composition with age using NMR spectroscopy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Kakar, S.; Podoll, N.; Rychwalski, P.; Schwietz, E. Physical changes in human meibum with age as measured by infrared spectroscopy. Ophthalmic Res. 2010, 44, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Ramakrishnan, V.; Collin, H.; Ramasubramanian, A. Differences in meibum and tear lipid composition and conformation. Cornea 2019, in press. [Google Scholar]
- Ferguson, S.R.; Borchman, D.; Yappert, M.C. Confirmation of the identity of the major phospholipid in human lens membranes. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1703–1706. [Google Scholar]
- Ferguson-Yankey, S.; Borchman, D.; Taylor, K.G.; DuPre, D.B.; Yappert, M.C. Conformational studies of sphingolipids by NMR spectroscopy. I. dihydrosphingomyelin. Biochim. Biophys. Acta 2000, 1467, 307–325. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Krastev, R.; Lalchev, Z. Surface chemistry study of the interactions of pharmaceutical ingredients and whole eye drops with films of human meibum. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4605–4615. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Dimitrov, T.; Andreev, K.; Krastev, R.; Lalchev, Z. Surface chemistry study of the interactions of hyaluronic acid and benzalkonium chloride with meibomian and corneal cell lipids. Soft Matter 2013, 9, 10841–10856. [Google Scholar] [CrossRef]
- Skrzypieca, M.; Georgiev, G.A.; Rojewska, M.; Prochaska, K. Interaction of polyhedral oligomericsilsesquioxanes and dipalmitoylphosphatidylcholine at the air/water interface: Thermodynamic and rheologicalstudy. Biochim. Biophys. Acta Binmembr. 2017, 1859, 1838–1850. [Google Scholar] [CrossRef]
- Davies, J.T.; Rideal, E.K. Interfacial Phenomena, 2nd ed.; Academic Press: New York, NY, USA, 1963; Volume 265, ISBN 978-0-32-316166-4. [Google Scholar]
- Flannery, B.P.; Teukolsky, S.; Press, W.H.; Vetterling, W.T. Fast Fourier Transform. In Numerical Recipes in C++; Cambridge University Press: Cambridge, UK, 2007; pp. 501–537. ISBN 978-0-521-88068-8. [Google Scholar]
- Loglio, G.; Tesei, U.; Cini, R. Viscoelastic dilatation processes of fluid/fluid interfaces: Time-domainrepresentation. Colloid Polym. Sci. 1986, 264, 712–718. [Google Scholar] [CrossRef]
- Monroy, F.; Ortega, F.; Rubio, R.G. Dilatational rheology of insoluble polymer monolayers: Poly(vinylacetate). Phys. Rev. 1998, 58, 7629–7641. [Google Scholar] [CrossRef]

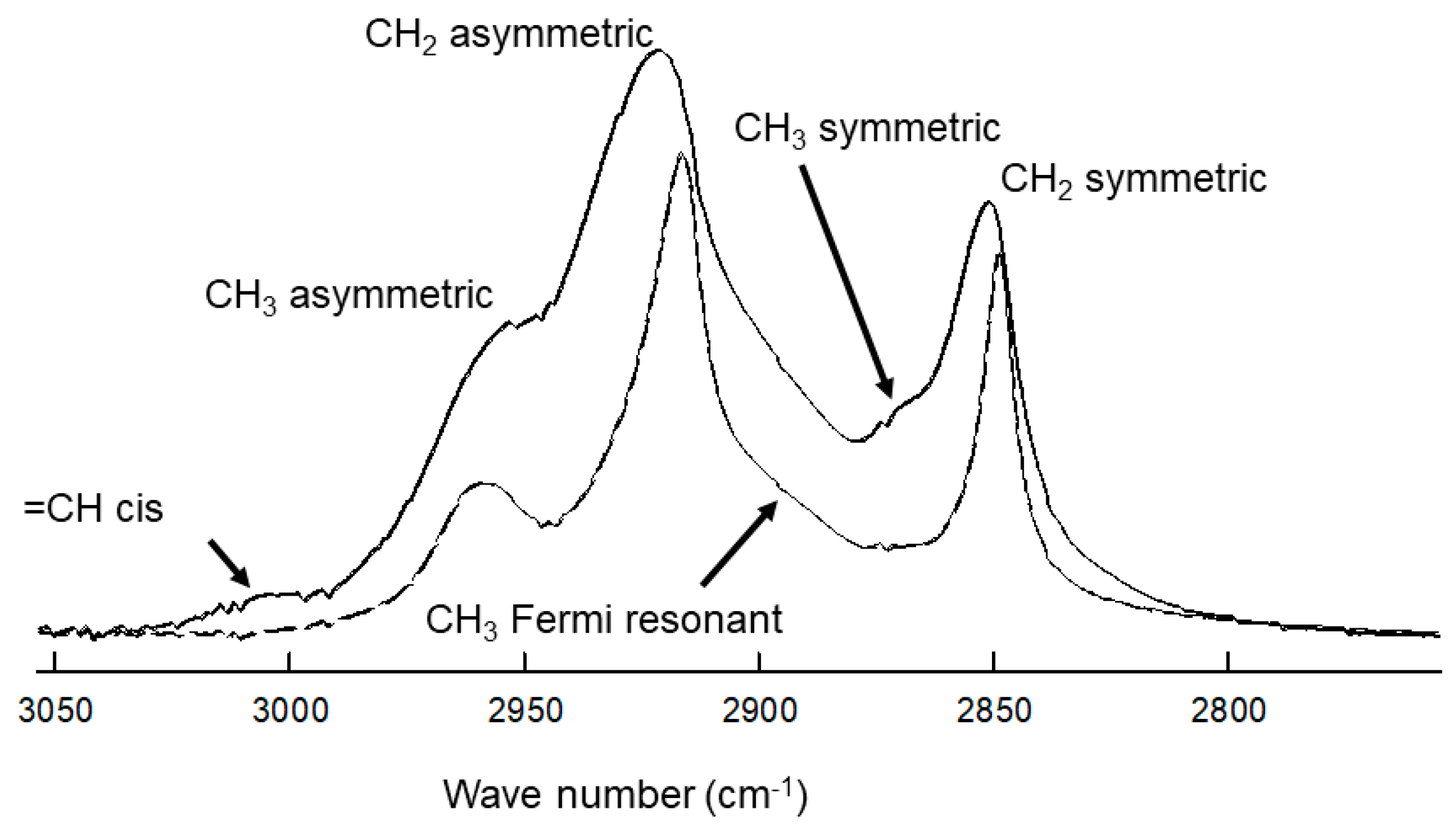
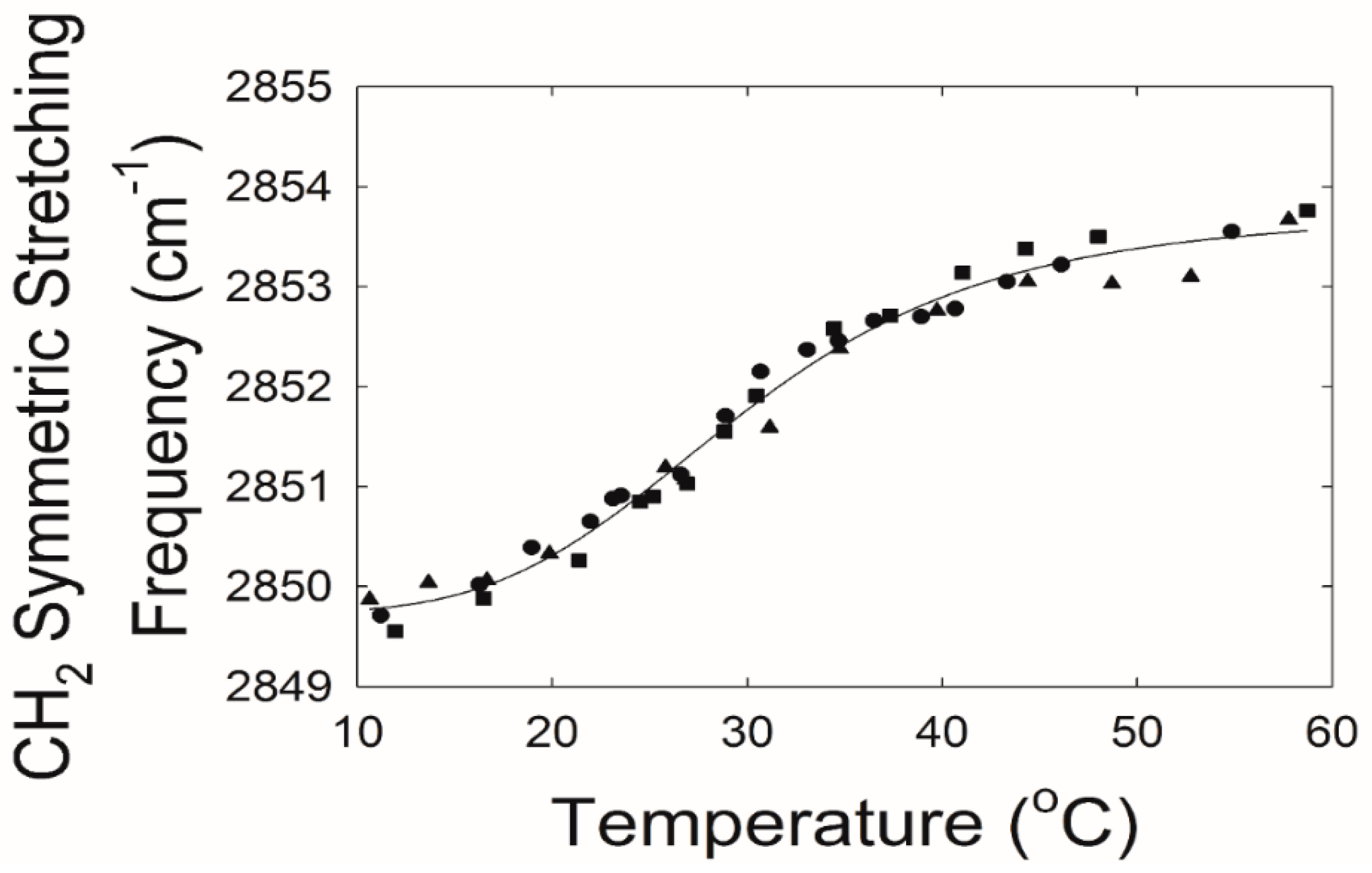
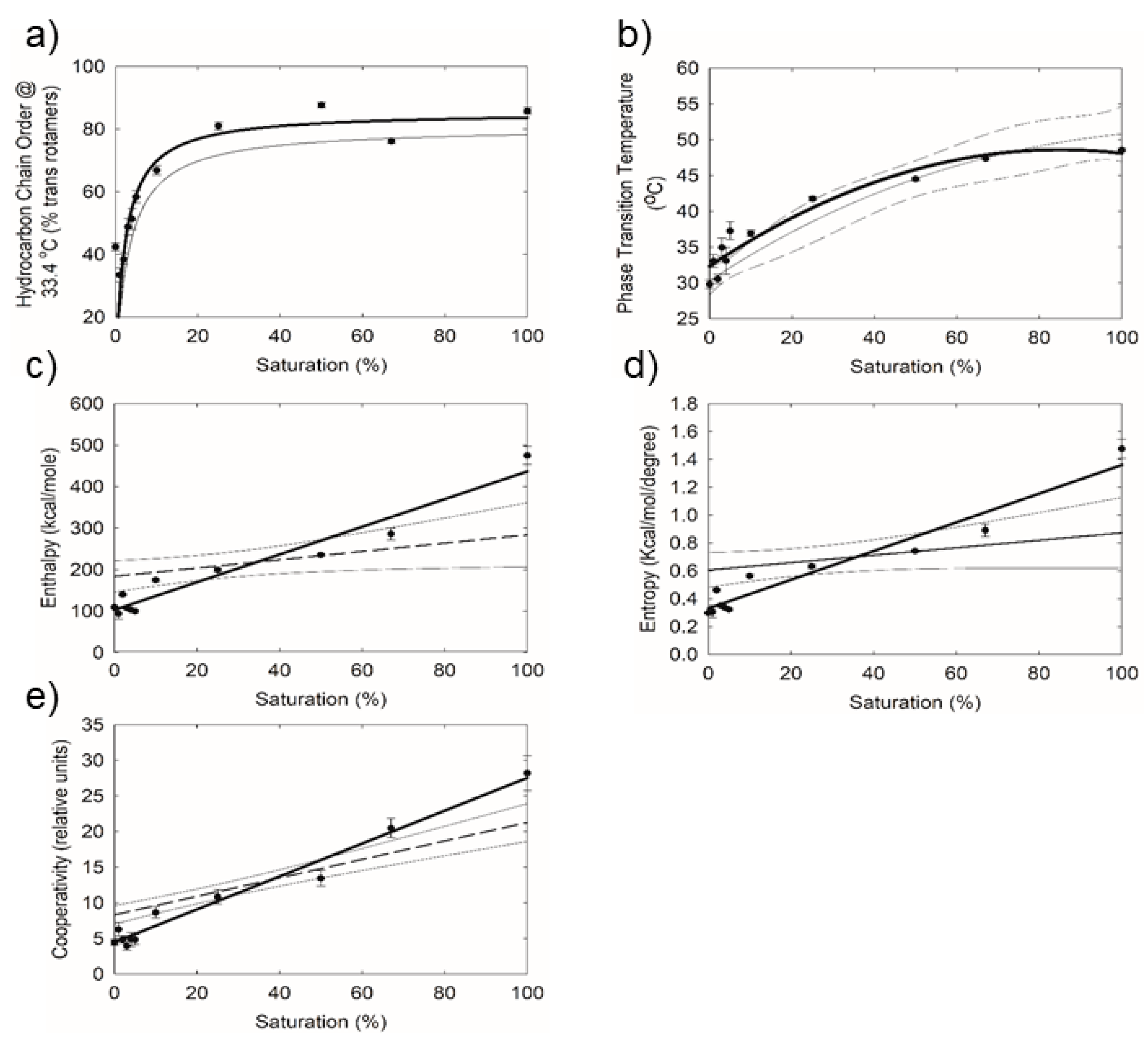
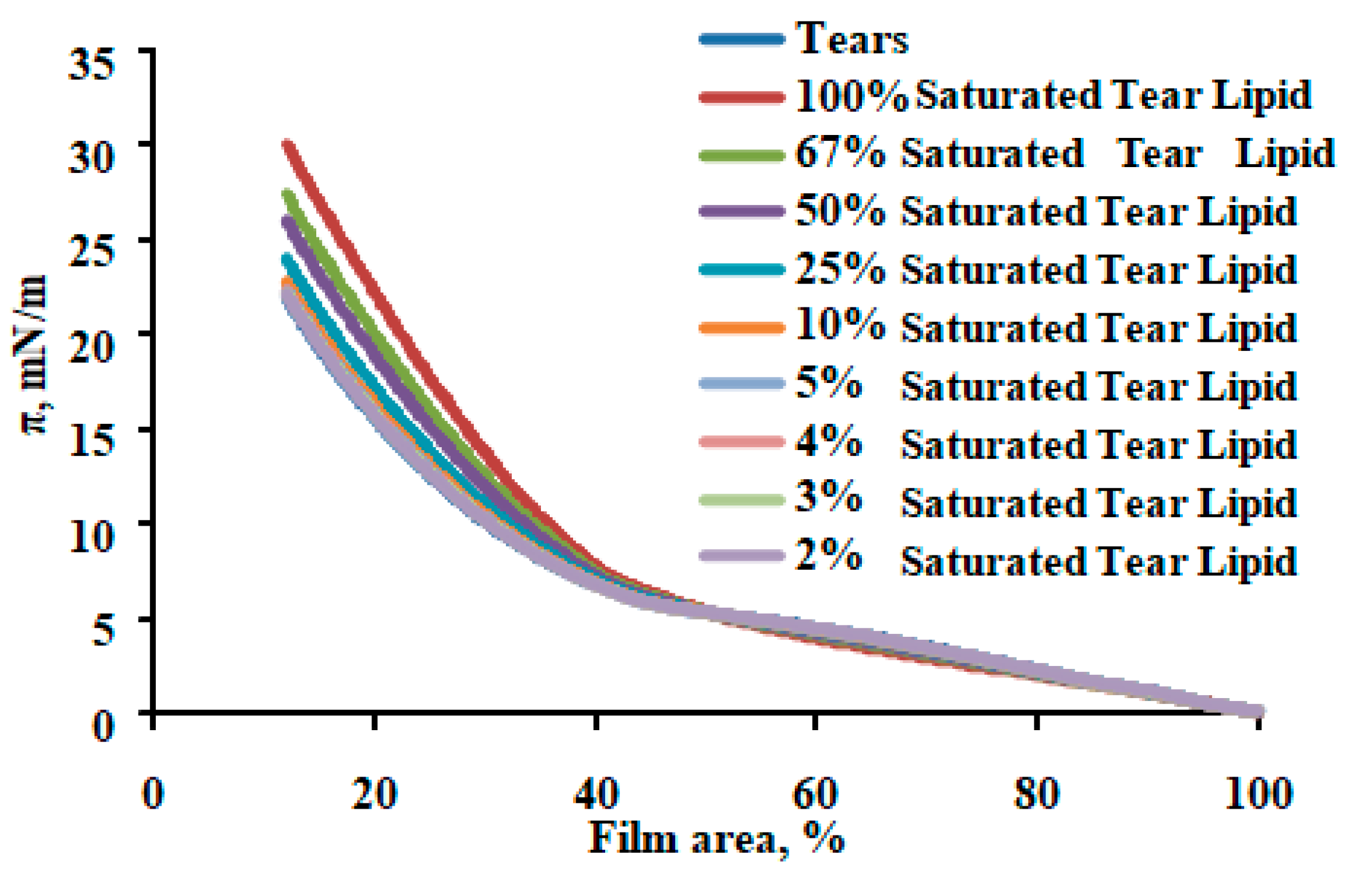


| Table Cont. | Tear Lipid from Tips | Tear Lipid from Stems | p |
|---|---|---|---|
| Minimum (cm−1) | 2849.74 ± 0.09 | 2849.5 ± 0.2 | 0.020 |
| Maximum (cm−1) | 2853.74 ± 0.13 | 2854.6 ± 0.3 | 0.013 |
| Tm (°C) | 29.8 ± 0.6 | 28.4 ± 1.0 | 0.24 |
| Hill (cooperativity) | −4.5 ± 0.4 | −4.6 ± 0.8 | 0.91 |
| Order 33.4 °C | 42.4 ± 1.3 | 32.9 ± 1.3 | >0.0001 |
| Δ Entropy (Kcal/mol) | 0.298 ± 0.004 | 0.452 ± 0.007 | >0.0001 |
| Δ Enthalpy (Kcal/mol/degree) | 90 ± 1 | 136 ± 2 | >0.0001 |
| Composition | Maxwell Rheological Model Equation |
|---|---|
| Tears | Δπ = 1.2 × 10−3exp(–t/1) + 0.65exp(–t/73) + 0.337 |
| 100%; Saturated Tear Lipid | Δπ = 7.1 × 10−2exp(–t/0.98) + 0.162exp(–t/30) + 0.75 |
| 67%; Saturated Tear Lipid | Δπ = 5.7 × 10−2exp(–t/1) + 0.162exp(–t/52) + 0.62 |
| 50%; Saturated Tear Lipid | Δπ = 2.9×10−2exp(–t/0.1) + 0.37exp(–t/43) + 0.596 |
| 25%; Saturated Tear Lipid | Δπ = 2.3×10−2exp(–t/0.1) + 0.48exp(–t/53) + 0.499 |
| 10%; Saturated Tear Lipid | Δπ = 1.9×10−3exp(–t/0.79) + 0.52exp(–t/47) + 0.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiev, G.A.; Borchman, D.; Eftimov, P.; Yokoi, N. Lipid Saturation and the Rheology of Human Tear Lipids. Int. J. Mol. Sci. 2019, 20, 3431. https://doi.org/10.3390/ijms20143431
Georgiev GA, Borchman D, Eftimov P, Yokoi N. Lipid Saturation and the Rheology of Human Tear Lipids. International Journal of Molecular Sciences. 2019; 20(14):3431. https://doi.org/10.3390/ijms20143431
Chicago/Turabian StyleGeorgiev, Georgi As., Douglas Borchman, Petar Eftimov, and Norihiko Yokoi. 2019. "Lipid Saturation and the Rheology of Human Tear Lipids" International Journal of Molecular Sciences 20, no. 14: 3431. https://doi.org/10.3390/ijms20143431
APA StyleGeorgiev, G. A., Borchman, D., Eftimov, P., & Yokoi, N. (2019). Lipid Saturation and the Rheology of Human Tear Lipids. International Journal of Molecular Sciences, 20(14), 3431. https://doi.org/10.3390/ijms20143431







