Abstract
Among nutraceuticals, phytochemical-rich compounds represent a source of naturally-derived bioactive principles, which are extensively studied for potential beneficial effects in a variety of disorders ranging from cardiovascular and metabolic diseases to cancer and neurodegeneration. In the brain, phytochemicals produce a number of biological effects such as modulation of neurotransmitter activity, growth factor induction, antioxidant and anti-inflammatory activity, stem cell modulation/neurogenesis, regulation of mitochondrial homeostasis, and counteracting protein aggregation through modulation of protein-folding chaperones and the cell clearing systems autophagy and proteasome. In particular, the ability of phytochemicals in restoring proteostasis through autophagy induction took center stage in recent research on neurodegenerative disorders such as Parkinson’s disease (PD). Indeed, autophagy dysfunctions and α-syn aggregation represent two interdependent downstream biochemical events, which concur in the parkinsonian brain, and which are targeted by phytochemicals administration. Therefore, in the present review we discuss evidence about the autophagy-based neuroprotective effects of specific phytochemical-rich plants in experimental parkinsonism, with a special focus on their ability to counteract alpha-synuclein aggregation and toxicity. Although further studies are needed to confirm the autophagy-based effects of some phytochemicals in parkinsonism, the evidence discussed here suggests that rescuing autophagy through natural compounds may play a role in preserving dopamine (DA) neuron integrity by counteracting the aggregation, toxicity, and prion-like spreading of α-syn, which remains a hallmark of PD.
1. Introduction
Nutraceuticals include a broad range of naturally occurring, though different compounds such as functional foods, fortified foods, and dietary supplements which as a common signature promote human and animal health and wellness [1,2]. Among these, dietary supplements are generally identified with herbal extracts, that is, complex mixtures of phytochemicals. These latter correspond to pharmacologically active compounds, which are also named bioactive ingredients or principles. Generally, phytochemicals are classified into major categories based on their chemical structures and characteristics. These include carbohydrates, lipids, polyphenols, terpenes, steroids, alkaloids, and other nitrogen-containing compounds [3]. Phytochemicals are widely found, either singularly or in combination, in edible plants and plant products including grains, oilseeds, beans, leaf waxes, bark, roots, spices, fruits, and vegetables with varying content and composition. In the last century, phytochemicals have become increasingly popular as potential preventive and therapeutic compounds in a variety of disorders, ranging from cancer to cardiovascular, metabolic, and neurodegenerative diseases [4,5,6,7].
Natural compounds which have been mostly investigated in experimental and clinical studies for their potential benefits in brain metabolism include curcumin (Curcuma longa), bacosides (Bacopa monnieri), catechins (Camellia sinensis), asiatic and gallic acids (Centella asiatica), withanolides (Withania somnifera, ashwagandha), and resveratrol (Vitis vinifera). Clinical studies in both healthy subjects and in patients with central nervous system (CNS) disorders such as Alzheimer’s disease (AD), dementia, and amyotrophic lateral sclerosis (ALS) provided some encouraging results indicating cognitive enhancing, anti-oxidant and anti-inflammatory effects of these phytochemicals coupled with a wide margin of tolerability [8,9,10,11,12,13,14,15,16].
However, many clinical trials have not been completed yet, especially those on Parkinson’s disease (PD), and others have yielded inconclusive results. This may be due to suboptimal phytochemical dosage, timing, or formulation, which may affect phytochemical bioavailability and accumulation in the brain at necessary concentrations for producing evident therapeutic effects [9,17,18]. Strategies aimed at overcoming such a limit include the development of nanoparticle-based formulations or concomitant supplementation with natural bioavailability-enhancing compounds such as piperine [17,19]. As documented by a vast body of experimental evidence, phytochemicals from C. longa [9,20,21], B. monnieri [22,23], C. sinensis [24], C. asiatica [25], W. somnifera, ashwagandha [26], and V. vinifera [27] are indeed able to cross the blood-brain barrier thus displaying sufficient bioavailability to yield beneficial effects in the brain.
In particular, in experimental models of PD, neuroprotective effects of curcumin (C. longa) [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54], bacosides (B. monnieri) [55,56,57,58,59,60,61,62,63,64,65,66,67,68], catechins of green tea (C. sinensis) [69,70,71,72,73,74,75,76,77,78,79,80,81], gallic and asiatic acids (C. asiatica) [82,83,84,85,86,87,88], withanolides (W. somnifera, ashwagandha) [89,90,91,92,93,94,95,96], and resveratrol (V. vinifera) [97,98,99,100,101,102,103,104,105,106,107,108,109,110,111] have been widely reported (Tables 1–6, respectively). These phytochemicals produce a number of biological effects such as modulation of dopamine (DA) metabolism and release, growth factor induction, antioxidant and anti-inflammatory activity, regulation of mitochondrial homeostasis, stem cell modulation/neurogenesis, and restoration of proteostasis through regulation of protein-folding chaperones and the cell clearing systems autophagy and proteasome [112,113,114,115,116,117,118,119,120,121,122,123,124]. As pointed by most of the past and recent discoveries in PD research, the abovementioned phytochemical-targeted processes represent key events which are altered in parkinsonism. However, when considered alone, none of these effects are expected to fully provide therapeutic efficacy in experimental parkinsonism. Indeed, PD is a multifactorial disease since different etiological (genetic and/or environmental) factors may combine to produce a chain of pathological events which tightly intermingle with each other [125,126,127,128,129,130]. These include alterations in DA metabolism and synaptic transmission, oxidative stress, mitochondrial damage, and protein aggregation. In search of convergent downstream pathways being involved in the neurobiology of PD and experimental parkinsonism, a plethora of studies indicate a key role of the cell clearing systems proteasome and autophagy [127,128,129,130,131,132,133,134,135]. In particular, autophagy is essential for DA neuronal survival being involved in the surveillance of DA release, mitochondrial homeostasis, as well as degradation of misfolded, oxidized, and aggregated proteins. The loss of autophagy in experimental models produces neurodegeneration which is reminiscent of PD [136], and autophagy dysfunctions are linked with familial PD [128]. In fact, alterations of several proteins which are encoded by PD-related genes such as alpha-synuclein (α-syn, SNCA), LRRK2, Endophilin-A, PINK1, and Parkin, may affect the autophagy machinery at various levels [125,128,129,130].
It is remarkable that several classes of phytochemicals converge to promote cell clearing systems, and mostly the autophagy machinery [133], either directly or by targeting common molecular pathways which are altered in parkinsonism. Thus, if one considers autophagy as a downstream common event in parkinsonism, the puzzling variety of effects induced by phytochemicals may turn to be only apparent, since different pieces can be cast together to converge towards autophagy activation. In fact, promoting autophagy contributes to regulating DA release, neuro-differentiation, and mitochondrial homeostasis, as well as counteracting oxidative/inflammatory toxicity and α-syn aggregation, which remains a hallmark of PD [127,129,133,137,138,139,140].
It is worth mentioning that, similar to autophagy, the ubiquitin-proteasome system is affected in DA-related CNS disorders including PD [134,135,141], and a functional interplay occurs between autophagy and the proteasome at both biochemical and morphological levels [142,143]. However, here we chose to focus on the autophagy machinery for several reasons. Although both systems are seminal for DA synaptic activity and neuronal proteostasis, autophagy degrades specific substrates such as mitochondria and large protein aggregates which cannot be processed by the proteasome. Secondly, autophagy is able to compensate for proteasome dysfunctions and to rescue DA neurons from cell death which is induced by proteasome inhibitors [128,143]. Thus, in the present review we focus on autophagy as one of the final metabolic pathways through which phytochemicals restore α-syn proteostasis to confer neuroprotection (Figure 1). This might also disclose a role of autophagy dysregulations as part of a common chain of events connecting systemic disorders with alterations of the CNS, which occurs in PD. Nonetheless, the chance that phytochemicals act at the level of the proteasome system or modify its interplay with autophagy should be constantly considered.
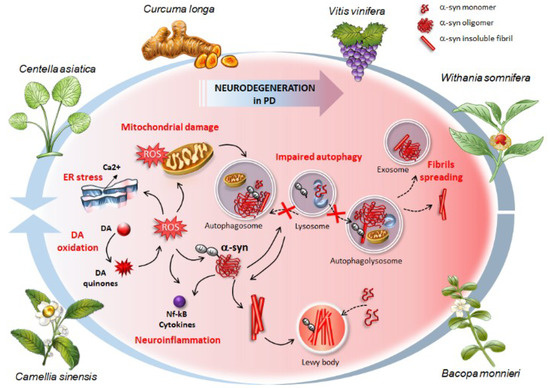
Figure 1.
The effects of phytochemical-rich plants in counteracting the cascade (plain black arrows) of molecular events, which occur in synucleinopathies and Parkinson’s disease (PD). These include (i) oxidative stress and accumulation of Reactive Oxygen Species (ROS) arising from altered dopamine (DA) metabolism (DA oxidation), (ii) endoplasmic reticulum (ER) and mitochondrial stress, (iii) structural alterations of α-syn, formation of insoluble aggregates up to Lewy bodies where native α-syn monomers are sequestered (dashed black arrows), (iv) neuroinflammation, and (v) autophagy impairment due to either altered autophagosome biogenesis or impaired fusion between lysosomes and autophagosomes (dashed black arrows). The buildup of ubiquitinated α-syn aggregates contributes to further impairing the autophagy machinery thus fueling a vicious circle where damaged autophagy substrates accumulate due to impaired clearance and turnover. This, in turn, contributes to increasing the overall vulnerability of DA neurons and promoting the spreading of α-syn (dashed black arrows). Phytochemicals from the plants represented here confer neuroprotection by preventing or reverting (blue arrows) this pathological cascade, starting from autophagy induction to inhibition of α-syn aggregation, neuroinflammation, and oxidative stress.
2. Eukaryotic Cell Clearing Pathways: A Focus on Autophagy
Eukaryotic cell clearing pathways are grouped into two main systems, which consist of the ubiquitin-proteasome and autophagy [137,140]. The latter is further distinguished into macro-autophagy (hereafter referred to as autophagy), micro-autophagy, and chaperone-mediated autophagy [140]. In addition, other terms are used to describe the clearance of specific cell compartments, which is carried out by autophagy [144]. For instance, the removal of altered mitochondria is named “mitophagy”, which does not necessarily represent a process which is purely dedicated to removing altered mitochondria. Other examples include autophagy-dependent clearance of pathogens, ribosomes, portions of endoplasmic reticulum or synaptic vesicles which are conventionally designated as “xenophagy”, “ribophagy” or “reticulophagy”, or “vesiculophagy”, respectively [129,144].
Autophagy represents a phylogenetically conserved eukaryotic degradative process which plays a crucial role in cellular homeostasis [145]. A variety of cellular components encompassing proteins, lipids, sugars, nucleic acids, whole organelles or cytoplasmic compartments are sequestered into a double-membrane nascent vacuole called phagophore, which then matures to seal in a vesicle called autophagosome [146]. Autophagy engulfment may occur either as a non-selective process or involve adaptor/receptor proteins such as SQSTM1/p62 and optineurin, which shuttle ubiquitinated cargoes to the forming autophagosome [137,145,146]. The autophagosome matures through fusion with endomembrane vesicles (late endosomes and multivesicular bodies) giving birth to the amphisome. This latter eventually fuses with the lysosome, which provides acidic hydrolases needed for the breakdown of substrates. Once engulfed within the autophagolysosome, the cargo is degraded while some metabolic by-products are recycled. A complex machinery including more than 30 autophagy-related-gene (Atg) products governs the fine steps of autophagy progression, starting from the biogenesis and maturation of autophagosomes up to the fusion with lysosomes [147,148]. One of the main mechanisms negatively regulating autophagy relies on mTOR complex1 (mTORC1)-dependent phosphorylation of Atg13 and inhibition of Atg1 (ULK1 in mammals), both belonging to a molecular complex, which is seminal for the early induction of autophagy [149]. Again, conversion of Atg8 (LC3 in mammals) into LC3I, ubiquitination-like enzymatic lipidation of LC3I into LC3II isoform, and eventually the incorporation of LC3II into the phagophore membrane are critical steps for the vacuole to expand and seal, thus allowing cytoplasmic elements to be properly engulfed. In line with this, LC3 is widely employed as a marker for monitoring autophagy at the morphological, ultrastructural, and biochemical level. Nonetheless, other several autophagy proteins ranging from Atg3 to Atg7 are key in autophagy progression, since they participate in the processing and conjugation of Atg8/LC3 to the growing autophagosome’s membrane lipids [147,148]. Moreover, several pathways besides Akt/mTOR are known to modulate autophagy. For instance, autophagy occurs upon activation of 5′ AMP-activated Protein Kinase (AMPK) or following inhibition of Glycogen Synthase Kinase 3 Beta (GSK3-β) [150]. Again, activation of the transcription factor EB (TFEB) promotes autophagy induction by acting either in cooperation with or independently of mTORC1 to regulate lysosomal activation and autophagosome-lysosome fusion [151]. Likewise, activation of the NAD-dependent deacetylase Sirtuin-1 (SIRT1) promotes autophagy via de-acetylation of Atg5, Atg7, LC3 as well as of the transcription factor forkhead box O3 (FOXO3), which, in turn, controls the expression of several pro-autophagic proteins [152].
Autophagy modulates key cell functions ranging from cell growth and metabolism to neurotransmitter release, synaptic development and plasticity, neuro-inflammation and -immunity [125,127,129,130,132,133,135,137]. This is due to the fact that autophagy regulates the turnover of key proteins and organelles which are involved in these cell processes, and again, a mutual interplay exists between autophagy machinery and secretory/trafficking pathways, heat shock protein chaperones, apoptosis, growth factors, and inflammatory cascades. In fact, various molecules such as Rab-GTPases and SNARE proteins, heat shock proteins (HSP), caspases, reactive oxygen species (ROS), neurotrophic growth factors, pro-inflammatory cytokines/transcription factors can indirectly modulate the autophagy machinery [127,130,137,153,154,155,156,157]. Thus, it is not surprising that autophagy is commonly dysregulated in a myriad of CNS disorders where a feedback loop establishes between impaired proteostasis, synaptic alterations, and oxidative/inflammatory events. In the case of PD, this is best exemplified by the fact that DA-related oxidative/inflammatory events and α-syn aggregation may converge to impair the autophagy machinery, and, in turn, impaired autophagic clearance may fuel accumulation of toxic α-syn aggregates, synaptic alterations and neurodegeneration [127,128,129,131,158,159,160]. As we shall see in the next section, autophagy is affected in both PD patients and experimental models, and promoting autophagy counteracts α-syn aggregation and rescues DA cell death in experimental parkinsonism (Section 3).
3. Autophagy Failure in Parkinson’s Disease Patients and Experimental Models
The early description of an alteration of the autophagy machinery in the brain of PD patients was carried out in the late 90s by Anglade et al. (1997) [161], who demonstrated in the Substantia Nigra pars compacta (SNpc) the concomitancy of apoptotic cells and neurons where autophagy appeared to be altered. These ultrastructural findings followed up a smoldering background, where commonalities between altered ubiquitin-dependent protein degradation and PD were already postulated by Mayer et al. [162,163]. In detail, the authors were stricken by the similarities between cell pathology developing during viral infections and neuronal inclusions observed in PD, both being cases characterized by ubiquitin-positive proteinaceous aggregates. On this basis, an altered protein degradation pathway was postulated as a common mechanism in these disorders. Indeed, alterations of autophagy machinery have been documented in the brains of patients with PD and Dementia with Lewy Bodies (DLB), featuring the occurrence of altered mitochondria within autophagy-like vacuoles, and the concomitant accumulation of LC3-II and α-syn [164,165,166,167,168]. Again, decreased levels of Atg7 along with increased levels of mTOR are detected in PD brains [169]. This occurs along with the accumulation of α-syn-filled LC3-II-positive autophagosomes, which do not co-localize with the lysosomal cathepsin D, confirming an impaired autophagy flux in PD.
The impressive insight into the genetics of PD between the end of the 90s and the first decade of 2000 led to hypotheses that autophagy failure might be a common event in PD [128]. In fact, as thoroughly reviewed elsewhere, several proteins which are coded by PARK loci-related genes play a role in autophagy machinery. Either structural changes or genetic mutations leading to a loss/gain of function of PD-related proteins such as α-syn, Synphilin, Endophilin-A, LRRK2, UCH-L1, DJ-1, Parkin, and PINK1 affect the autophagy machinery at various levels, ranging from autophagosome biogenesis to priming of aggresomes for autophagic clearance, lysosomal uptake, and degradation of substrates [125,128,129,130].
Studies on transgenic and toxin-based experimental models of parkinsonism have been seminal to confirm a key role of autophagy in the survival of DA neurons. For instance, in catecholamine-containing PC12 cell lines, the overexpression of mutant A53T human α-syn leads to cell death, which associates with impaired lysosomal degradation [170]. In detail, mutant α-syn binds to the lysosomal-associated membrane protein type 2A (LAMP-2A) to block the lysosomal uptake and inhibit both their own degradation and that of other autophagy substrates [159]. Overexpressed and mutant α-syn may also inhibit autophagy by impairing the cytosolic translocation of high mobility group box 1 (HMGB1), which blocks HMGB1-Beclin-1 binding while strengthening Beclin1-BCL2 binding [158]. As a proof of concept, when autophagy is occluded in cell lines and in cultured murine midbrain DA neurons, an accumulation of α-syn occurs [171,172,173]. Conversely, exposure to autophagy inducers such as rapamycin, a gold-standard mTORC1 inhibitor, or overexpression of Beclin-1, boosts the clearance of α-syn [158,171,173]. Again, pharmacological and genetic blockade of autophagy exacerbates DA cell loss and formation of α-syn-containing inclusions which are induced by the neurotoxic drug of abuse Methamphetamine (Meth) [143,174,175,176]. Conversely, autophagy activation is able to counteract both Meth toxicity and Meth-induced behavioral alterations [143,175,176,177]. Autophagy inhibition also exacerbates rotenone- and 6-hydroxydopamine (6-OHDA)-induced DA toxicity in vitro and in vivo [178,179], while autophagy activation protects against 6-OHDA and rotenone-induced parkinsonism [180,181]. Likewise, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced nigrostriatal damage in zebrafish is prevented by the overexpression of ATG5, which reduces the levels of α-syn and other indigested proteins while rescuing locomotor activity [182].
As demonstrated in Atg7 and Atg5-knockout (KO) mice, the presence of intracellular inclusions bearing misfolded and insoluble a-syn fibrils coupled with the degenerative and sometimes precociously lethal phenotypes, confirm the key role of constitutive autophagy in the CNS [136,183,184,185]. Remarkably, both Atg5- and Atg7-KO models fully recapitulate the severe motor impairment and neuropathology of PD patients [136,183,184,185]. In fact, the loss of autophagy in these models produces DA cell loss along with neuronal inclusions featuring protein aggregates such as α-syn, Parkin, PINK1, LRRK2, ubiquitin, and p62 [136,184]. Defective autophagy fosters protein aggregation while promoting a prion-like spreading of misfolded proteins, which is a hallmark of PD. It seems that dysfunctional autophagy due to the impaired merging of autophagosomes with endosomes and lysosomes produces an exocytotic, inter-neuronal spreading of indigested cargoes such as α-syn [186]. An impairment of the autophagy pathway is tightly intermingled with α-syn misfolding/aggregation/accumulation/spreading and, thus, with the neurobiology of PD and related “synucleinopathies” such as DLB, multisystem atrophy (MSA), pure autonomic failure (PAF), lysosomal storage diseases (LSD), and Meth abuse [127,131,187,188,189,190,191].
4. Phytochemicals: Autophagy-Based Effects and Related Potential for Alpha-Synuclein Clearance in Experimental Parkinsonism
4.1. Introduction to Phytochemicals and Rough Classification
Phytochemicals may be classified either on the basis of their chemical structure or the biological system in which they occur. This dual classification may produce some confusion since there is considerable overlap between the chemical types of phytochemicals and their biological distribution. Thus, as an in-depth classification of phytochemicals is far from the aim of this review, we limit to providing a brief overview of the main classes of phytochemicals which are found in the plants taken into account here. This is done in the attempt to roughly contextualize the distribution of different bioactive compounds in specific herbal compounds before moving to their biological effects focused on autophagy activation, α-syn clearance, and role in Parkinsonism.
Within each phytochemical category, further sub-division is based on their chemical structure. For instance, polyphenols possess multiple phenolic units in their chemical structure, thus ranging from simple molecules to highly polymerized structures. Roughly, polyphenols are classified into four major classes, that is, phenolic acids, flavonoids, lignans, and stilbenes [192]. Examples of polyphenol-rich plants we chose to examine in the present review include the turmeric C. longa containing the polyphenol curcumin, the green tea from C. sinensis containing catechins and flavonoids, C. asiatica containing gallic acids and flavonoids, and V. vinifera containing resveratrol [192,193].
Similar to polyphenols, terpenes are classified into many categories based on the number of carbon atoms and isoprene residues present in their structure, namely monoterpenes, sesquiterpenes, diterpenes, triterpenes, tetraterpenes, and polyterpenes [194]. All terpenes share a common 5-carbon unit named isoprene which has a branched carbon skeleton deriving from a basic 5-carbon unit named isopentane. Some triterpenes are steroidal in nature, and they are known as triterpenoid saponins. These correspond to tetracyclic or pentacyclic molecules. An example of bioactive tetracyclic triterpenoid saponins are bacosides, which represent the major class of nootropic phytochemicals found within B. monnieri [119]. An example of bioactive pentacyclic triterpenoid saponins are madecassosides, which are found in C. asiatica [193]. Steroidal tetracyclic molecules also occur as triterpenoid saponins, which are known as ergostane-type steroids. These are best exemplified by bioactive compounds known as withanolides, which consist of a steroid backbone bound to a lactone or one of its derivatives [195]. Withanolides and saponins are widely found in ashwagandha, which derives from W. somnifera roots [195]. Despite this rough classification, most herbal products contain both terpenoids and steroidal saponins, which indeed share many properties despite differing in their structure.
4.2. Autophagy and Alpha-Synuclein Clearance as Common Effects Induced by Phytochemicals
All the bioactive classes above-summarized feature a remarkable overlap in their neuroprotective effects, which encompass anti-oxidant and anti-inflammatory activity, mitochondrial protection, and increased neuronal lifespan. In addition, phytochemicals exert anti-fibrillogenic effects, thus counteracting aggregation of proteins such as tau, amyloid-beta, and α-syn in the brain [196] (Figure 2). Remarkably, these phytochemicals may also act as autophagy activators, which may account for some of their beneficial effects in parkinsonism, such as counteracting α-syn aggregation. Albeit being a substrate of both autophagy and proteasome, α-syn clearance is carried out by autophagy when the proteasome is impaired, suggesting that α-syn may be a preferential substrate of autophagy [171,197]. Since α-syn dynamics are tightly bound to autophagy, which, in turn, is markedly affected in PD, in the present manuscript we focus on evidence about phytochemical-induced autophagy and α-syn clearance in experimental parkinsonism.
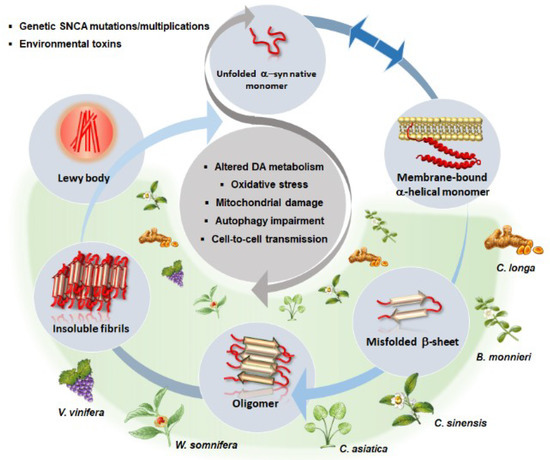
Figure 2.
A schematic overview of the beneficial effects of phytochemical-rich plants in α-syn aggregation dynamics (light grey circles), and related molecular mechanisms (central dark grey circle) occurring in PD. In a physiological state, a dynamic equilibrium (blue arrows) exists between α-syn natively unfolded monomers and membrane-bound α-helical monomers (secondary structure). Environmental toxins or mutations/multiplications within α-syn gene (SNCA) favor α-syn misfolding/overexpression and drive a pathological cascade of conversion up to insoluble fibrils and Lewy body formation. This is associated with a generalized impairment of cell homeostasis consisting of altered DA metabolism and synaptic dysfunction, oxidative stress, mitochondrial damage, autophagy impairment, and cell-to-cell spreading of misfolded and aggregated α-syn conformers. Phytochemicals found within Curcuma longa, Bacopa monnieri, Centella asiatica, Camellia sinensis, Withania somnifera and Vitis vinifera are able to reverse/prevent the pathological conversion cascade of α-syn while counteracting alterations of DA neurotransmission, oxidative stress, mitochondrial damage and autophagy impairment (green shade).
4.2.1. Curcumin from Curcuma longa
A large body of evidence converges in that curcumin may act as an autophagy inducer, which associates with various protective effects beyond the mere clearance of potentially harmful protein aggregates. For instance, curcumin promotes neurogenesis via autophagy activation [198]. In fact, in human pluripotent stem cells, curcumin upregulates neural genes along with autophagy-related genes such as Atg5, Atg8 (LC3), and Lamp1. Conversely, the inhibition of autophagy by chloroquine suppresses both autophagy and neural differentiation [198]. Furthermore, curcumin counteracts the alterations in synaptic transmission and autophagy machinery which are induced by exogenously administered misfolded proteins to cultured hippocampal neurons [199]. Again, curcumin-induced autophagy through inhibition of mTOR associates with protection from oxidative damage in several cell models [200,201].
The beneficial and neuroprotective effects of curcumin in PD experimental models have been widely demonstrated and thoroughly reviewed [112,113] (Table 1). The effects of chronic curcumin administration were recently evaluated in an animal model of PD induced by lipopolysaccharide (LPS) injection into the SN of rats [46]. Curcumin supplementation confers neuroprotection and attenuates motor deficits by preventing the LPS-induced neuro-inflammation and iron deposition in DA-containing neurons, and by promoting the anti-oxidant defense mechanisms along with preventing α-syn overexpression and aggregation [46], suggesting that curcumin holds potential as a candidate drug in the targeted therapy for synucleopathies. A number of studies aimed at enhancing the bioavailability and neuroprotective effects of curcumin also evaluated the effects of curcumin-based formulations against α-syn fibrillation and cytotoxicity. For instance, a nanoformulation consisting of amine-functionalized mesoporous silica nanoparticles of curcumin prevents α-syn fibrillation and subsequent cytotoxicity [202]. Another nanoformulation prepared with lactoferrin by sol-oil chemistry protects from rotenone-induced neurotoxicity in DA-containing cells through attenuation of oxidative stress along with a reduction of α-syn and tyrosine hydroxylase (TH) expression [203]. Similarly, curcumin-loaded polysorbate 80-modified cerasome nanoparticles alleviate MPTP-induced motor deficits in mice and confer neuroprotection by rescuing striatal DA levels and TH expression while promoting α-syn clearance [28].

Table 1.
Neuroprotective effects of curcumin in PD models.
A few studies focused specifically on the autophagy-based neuroprotective effects of curcumin. In detail, curcumin suppresses oxidative stress and neurotoxicity which are induced by the parkinsonian neurotoxins paraquat and atrazine through activation of autophagy in DA-containing SH-SY5Y cells [204,205]. Curcumin is able to modulate autophagy also via activation of TFEB to foster autophagy and lysosomal biogenesis in vitro and in vivo [206,207]. In keeping with this, it is remarkable that besides mTOR inhibitors, even compounds acting as TFEB activators protect from neurotoxicity in several experimental models, including parkinsonism [208]. In fact, curcumin confers protection and enhances DA cell survival by rescuing autophagy through TFEB activation in an MPTP-based cell model of PD [22]. Such an effect goes along with a reduction in α-syn levels [22], which is in line with several pieces of evidence indicating a role of curcumin-induced autophagy in counteracting α-syn aggregation and toxicity. For instance, curcumin rescues autophagy dysfunction which is induced by overexpression of mutated (A53T) α-syn in DA-containing SH-SY5Y cells, and such an effect is occluded by the autophagy inhibitor 3-MA. In turn, curcumin-induced activation of autophagy via mTOR inhibition reduces mutant α-syn accumulation to confer neuroprotection in DA cells [48]. Again, a nanoformulation containing curcumin and piperine with glyceryl monooleate nanoparticles efficiently crosses the blood-brain barrier in rotenone-induced mouse models of PD to attenuate oxidative stress and apoptosis while preventing α-syn oligomerization and fibrillation through induction of autophagy [209].
4.2.2. Bacosides and Bacopasides from Bacopa monnieri
B. monnieri has proven potential efficacy in both in vitro and in vivo transgenic and toxin-induced experimental parkinsonism owing to its antioxidant, anti-inflammatory and neuroprotective properties [114,119] (Table 2). As a nootropic and adaptogenic compound, B. monnieri also acts as a DA releaser, which likely underlies its ability to ameliorate locomotor activity and cognitive functions in animal models of PD [114,119]. A few recent studies suggest that B. monnieri exerts its beneficial effects through autophagy activation. In fact, B. monnieri protects against Benzo[a]pyrene-induced oxidative stress, mitochondrial damage and cytotoxicity through autophagy induction [210]. An important standpoint in this study is that B. monnieri confer cytoprotection through induction of autophagy-dependent removal of damaged mitochondria, since inhibition of autophagy by Beclin-1 KO occludes its cytoprotective effects [210]. Again, bacopasides found within B. monnieri activate autophagy to modulate stem-cell cycle and growth [211].

Table 2.
Neuroprotective effects of Bacopa monnieri in PD models.
The effects of B. monnieri were recently assessed in two Caenorhabditis elegans (C. elegans) PD models, namely a transgenic model overexpressing human α-syn, and a pharmacological model expressing green fluorescent protein (GFP) specifically in DA neurons treated with the selective neurotoxin 6-OHDA [65]. The study examined the effects of B. monnieri on α-syn aggregation in association with degeneration of DA neurons, lipids content, and longevity of the nematodes. In detail, B. monnieri prevents DA-neuron degeneration and increases lifespan in nematodes through reduction of α-syn aggregation and restoration of lipid content [65]. Studies investigating the effects of B. monnieri on α-syn aggregation and autophagy modulation specifically in parkinsonism are missing so far. However, the few available findings underlining the potential of B. monnieri as a possible anti-parkinsonian agent coupled with those demonstrating its pro-autophagic role, encourage further investigations on its autophagy-based neuroprotective effects in parkinsonism.
4.2.3. Green Tea Catechins from Camellia sinensis
C. sinensis, the most widely used plant species for green tea, is extremely rich in polyphenols including catechins and flavonoids. Green tea catechins from C. sinensis show a remarkable potential in inducing autophagy [212,213]. In detail, these polyphenols modulate autophagy through various mechanisms, including TFEB, mTOR, and 5′ AMP-activated protein kinase (AMPK) [212,213,214,215]. Intriguingly, the green tea catechin epigallocatechin gallate (EGCG) was shown to activate autophagy even through direct interaction with LC3-I protein, and to foster the exposure of its pivotal Gly-120 site to other important binding partners, thus promoting the synthesis of LC3-II [216]. EGCG also activates autophagy also via a class III histone deacetylase (HDAC) [217]. Induction of autophagy by green tea polyphenols associates with various beneficial effects ranging from neuroprotection against prion protein-induced toxicity in primary neuronal cells [217] to degradation of endotoxins, anti-inflammatory activity [218] and lipid clearance [219,220]. Again, green tea catechins prevent hypoxia-induced oxidative stress and cell death by inducing autophagy [221]. Catechins can also inhibit the growth of tumor stem cells in vitro and in vivo by inducing autophagy [222,223]. Nonetheless, the autophagy-related properties of green tea depend upon the dosage used, level of stress, and the cell models employed [212]. For instance, at low-to-moderate doses, EGCG induces autophagy to prevent apoptosis and promote cell viability, while higher concentrations of EGCG may inhibit autophagy leading to apoptosis [215,224].
Green tea polyphenols are recognized to exert powerful neuroprotective effects in both cell-based and animal models of parkinsonism owing to their ability to counteract oxidative stress, neuroinflammation, and protein aggregation, and to promote autophagy [213,225] (Table 3). For instance, green tea polyphenols activate autophagy in DA-containing SH-SY5Y cells to confer neuroprotection from the toxic herbicide atrazine [205]. Again, EGCG protects neuronal-like, catecholamine-containing PC12 cells from oxidative-radical-stress-induced toxicity through inhibition of GSK3 pathway [226], and likely, through autophagy activation. Again, in transgenic Drosophila models of PD, namely mutant LRRK2 and Parkin-null flies, EGCG protects from neurodegeneration and mitochondrial dysfunction through activation of AMPK, which is an upstream autophagy inducer [80]. Consistently, pharmacological or genetic activation of AMPK reproduces EGCG’s protective effects, while the loss of AMPK activity exacerbates Parkin-null- and mutant LRRK- induced DA neuronal loss and motor alterations [80]. Similar to parkin, AMPK is seminal to induce mitophagy, which occurs through AMPK-mediated phosphorylation of the autophagy initiator ATG1. This suggests that autophagy, and in particular mitophagy induction, may underlie the ability of EGCG to rescue from neurotoxicity which is induced by the enhanced LRRK2 kinase activity.

Table 3.
Neuroprotective effects of Camellia sinensis in PD models.
Green tea catechins, especially EGCG, also possess a remarkable potential against α-syn aggregation and fibrillation in experimental parkinsonism [70,71,196,227]. In detail, EGCG provides neuroprotection and attenuates motor abnormalities in 6-OHDA-treated parkinsonian rats, which associates with reduced α-syn expression along with decreased mTOR, AKT, and GSK3-β levels [78]. Since inhibition of the mTOR/AKT/GSK-3β axis leads to autophagy induction, it is likely that EGCG reduces α-syn levels through autophagy-dependent protein clearance. EGCG may also prevent α-syn aggregation through modulation of the hypoxia-inducible factor (HIF)-1 signaling pathway, which in turn controls oxidative and iron homeostasis, and also autophagy-dependent mitochondrial turnover [228,229]. It is worth mentioning that EGCG modulates α-syn dynamics also through conformational [196,228] or epigenetic mechanisms [230]. In particular, EGCG interferes with an early step in the aggregation cascade by binding directly to the natively unfolded α-syn to inhibit its conversion into toxic intermediates [231]. Again, EGCG converts large, mature α-syn particles into non-toxic amorphous monomers or small diffusible oligomers displaying reduced α-syn toxicity in vitro [231]. EGCG also disaggregates α-syn fibrils by preventing the amyloid formation of α-syn tandem repeat and destabilizing α-syn fibrils into soluble amorphous aggregates [232]. In detail, EGCG appears to bind directly β-sheet-rich aggregates, thus reducing the relative concentration which is required to induce conformational changes [233]. Furthermore, EGCG modulates methylation of CpG sites within the promoter region of the α-syn gene (SNCA) to regulate its expression levels in the rodent brain [230].
4.2.4. Gallic Acids, Asiatic Acids, and Madecassosides from Centella asiatica
Various in vitro and in vivo experimental studies indicate an anti-parkinsonian potential of C. asiatica (Table 4). Several bioactive compounds found within C. asiatica act as autophagy inducers, though this was mostly documented in cell-based models other than PD. For instance, madecassoside, a major bioactive component of C. asiatica, reduces oxidative stress and Ca2+ overload while attenuating subsequent mitochondrial damage through activation of autophagy [234]. Again, Asiatic acid triterpenoids found within C. asiatica downregulate stem-cell growth through inhibition of the Akt/mTOR pathway [235]. Similarly, gallic acid monophenols, which are major constituents of C. asiatica, act as autophagy inducers as shown by the increased abundance of LC3-II coupled with enhanced degradation of p62 [152]. Phytochemicals including gallic acids induce autophagy even through activation of SIRT1, which associates with decreased acetylation of cytoplasmic proteins. Conversely, administration of bafilomycin A1, which blocks late-step autophagy progression, occludes the beneficial effects of several phytochemicals including gallic acids [152].

Table 4.
Neuroprotective effects of Centella asiatica in PD models.
Studies investigating autophagy-based effects of C. asiatica specifically in PD models are still limited so far. There is some indirect evidence based on SH-SY5Y DA cell lines. Here, Asiatic acids protect from glutamate-induced excitotoxicity by decreasing apoptosis and ROS, while stabilizing mitochondrial function through activation of the autophagy inducer SIRT1 [236]. Nonetheless, C. asiatica counteracts a-syn aggregation to confer neuroprotection in several PD models. In fact, C. asiatica inhibits α-syn aggregation from monomers, the transition of oligomers to aggregates and fosters the disintegration of the preformed fibrils [237]. Such an effect may be due to gallic acids, which prevent α-syn fibril formation while stabilizing the extended, native structure of α-syn [238]. Again, they protect from α-syn-induced toxicity by disaggregating pre-formed α-syn amyloid fibrils [239]. Interestingly, at very low concentrations and similar to what reported for EGCG, gallic acid was found to bind to and stabilize soluble, non-toxic α-syn oligomers lacking β-sheet content [239]. Again, in MPTP-treated mice and in transgenic Drosophila models over-expressing human α-syn, C. asiatica increases motor ability and it protects from neurotoxicity by reducing oxidative stress, lipid peroxidation and protein carbonyl content [85,88]. Unfortunately, these studies did not specifically asses α-syn levels or autophagy status, which underlines the need for further in vivo studies aimed at clarifying whether C. asiatica exerts neuroprotection through anti-α-syn and autophagy-based effects.
4.2.5. Withanolides and Withaferin from Withania somnifera, ashwagandha
Withanolides, the biologically active steroids of ashwagandha, confer neuroprotection and improve behavioral abnormalities in experimental parkinsonism, owing to their anti-oxidant, synaptic remodeling, and nerve-regenerating properties [240,241] (Table 5). Among their various biological effects, withanolides also modulate autophagy. Withaferin A, the most investigated and major constituent of ashwagandha, induces stem-cell cycle arrest and suppresses stem-cell growth through autophagy enhancement [242]. Again, ashwagandha prevents the accumulation of misfolded proteins and exerts beneficial anti-inflammatory and immunomodulatory effects, which may be due to autophagy activation. In fact, ashwagandha prevents glial activation and phosphorylation of nuclear factor kappaB (NF-κB) while inducing autophagy to reduce disease severity in SOD1(G93A) mouse model of ALS [243]. This suggests that autophagy-based effects induced by ashwagandha may be beneficial at the early stages of neurodegeneration [243]. Nonetheless, controversial results are found in the literature concerning the autophagy-related effects of ashwagandha. In fact, some studies performed in cancer cell-lines suggest that withaferin A may act as an autophagy inhibitor, or that concomitant administration of autophagy inhibitors potentiates rather than preventing the beneficial effects of withaferin A [242,244,245,246,247,248]. These controversies may be due to several factors. Firstly, similar to that reported for other phytochemicals such as green tea catechins, the effects of withaferin upon autophagy may be dose-dependent. In fact, low doses of withaferin induce autophagy as shown by the massive accumulation of LC3II puncta coupled with progressive degradation of p62 [248]. Contrariwise, higher concentrations of withaferin may stimulate endoplasmic reticulum (ER) stress to activate pro-apoptotic proteins, which may suppress autophagy-related proteins [248]. Secondly, most of the studies investigating the effects of ashwagandha on autophagy were carried out in tumor cells, where very high, toxic concentrations of Withaferin are generally employed to induce growth arrest and sensitization to apoptosis. These considerations suggest that appropriate dosing of phytochemicals is key when investigating and interpreting potential therapeutic effects.

Table 5.
Neuroprotective effects of W. somnifera (ashwagandha) in PD models.
Despite the plethora of evidence supporting the multifold benefits of ashwagandha in experimental models of parkinsonism, only one recent study investigated the effects of withanolides specifically upon α-syn aggregation. This was carried out in stress-exposed C. elegans models expressing yellow fluorescent protein (YFP)-tagged α-syn [240]. In detail, Withanolide treatment produces a reduction of nearly 40% in α-syn levels compared with untreated animals. In withanolide-treated worms, such an effect goes along with lifespan extension, modulation of acetylcholine release, and enhancement of oxidative and thermal stress resistance [240]. Remarkably, all these beneficial effects depend on the insulin/insulin-like growth factor signaling (IIS) pathway, which is an upstream modulator of autophagy. Although the role of autophagy was not specifically investigated, it appears worthwhile to test in the future the effects of upon autophagy modulation and its potential contribution in parkinsonism and related synucleinopathies.
4.2.6. Resveratrol from Vitis vinifera
Resveratrol, a stilbene found in grapes and red wine, possesses multifold benefits including attenuation of oxidative stress, inflammation and mitochondrial impairment, modulation of stem-cell growth, neuroprotection and autophagy induction [124,249,250] (Table 6).

Table 6.
Neuroprotective effects of resveratrol in PD models.
Resveratrol-induced autophagy is associated with a variety of effects which may be relevant for PD. For instance, resveratrol-induced autophagy modulates embryonic stem-cell proliferation and pluripotency through AMPK/Ulk1 upregulation and mTORC1 suppression [251] and promotes neuronal differentiation of stem-cells as shown by increased expression of the neuro-progenitor markers Nestin, Musashi, and CD133 [252]. This latter effect occurs through SIRT1 activation, which besides AMPK/mTOR is one of the main mechanisms bridging resveratrol-induced beneficial effects and autophagy induction [100,248,253,254,255,256,257,258,259,260]. For instance, resveratrol-induced autophagy via SIRT1 exerts anti-inflammatory [253] and anti-bacterial activity [261], and it counteracts oxidative damage to promote cell viability [262,263]. Resveratrol-induced autophagy and mitophagy are associated with cytoprotection and anti-oxidant effects in a plethora of cell-based PD models, including exposure to the parkinsonian toxins atrazine and rotenone, and overexpression/exposure to misfolded peptides including mutant α-syn [102,104,105,107,205]. This is recapitulated in mice models of PD such as MPTP-induced parkinsonism, where resveratrol confers neuroprotection by preventing the loss of DA neurons and rescuing alterations in TH and DA levels while improving behavioral abnormalities through SIRT1-dependent autophagy activation [100]. Resveratrol also prevents α-syn aggregation and toxicity in both cell-based and animal models of parkinsonism [98,102,264,265]. For instance, in MPTP-treated rats, resveratrol reduces motor dysfunctions and alleviates the loss of DA neurons by counteracting apoptosis, neuroinflammation and α-syn aggregation [99]. Remarkably, a combined administration of resveratrol and L-DOPA also reduces the side effects of L-DOPA as well as the dosage of L-DOPA which is required to produce beneficial effects in MPTP-induced parkinsonism [99]. These effects are associated with an increased pAkt/Akt ratio [99]. Since Akt acts as a major upstream inhibitor of autophagy through activation of mTOR and/or inactivation of Beclin-1 [266], it is likely that the effects of resveratrol are bound to induction of autophagy. Indeed, specific autophagy-based effects of resveratrol in conferring neuroprotection through α-syn clearance have been widely reported. For instance, in PC12 cells overexpressing wild-type and mutated α-syn, and in rotenone-exposed SH-SY5Y cells, resveratrol enhances α-syn degradation by activating autophagy through the AMPK/SIRT1 signaling pathway [107]. Likewise, in MPTP-treated mice, the autophagy-based neuroprotective effects of resveratrol via induction via SIRT1-dependent LC3 de-acetylation occur along with a reduction in α-syn levels [100]. Contrariwise, an inhibitor of SIRT1 antagonizes the neuroprotective effects of resveratrol by reducing the autophagy-based degradation of α-syn [100].
From these studies, it emerges that resveratrol acts quite specifically as a powerful SIRT1 activator. In fact, when compared with other phytochemicals, resveratrol induces autophagy much more potently, in a way which is reminiscent of the gold-standard autophagy activator rapamycin [152]. Such an apparently selective SIRT1-dependent mechanism recruited by resveratrol adds on the already long lists of molecules through which phytochemicals modulate autophagy, including mTOR, AMPK, TFEB, and GSK3 (Figure 3). At the same time, these considerations remark the need for further studies aimed at disclosing yet poorly explored pathways which may be involved in the autophagy-based effects of phytochemicals. In addition to these molecular findings, ultrastructural analyses seem to confirm the key role of autophagy in resveratrol-induced beneficial effects in parkinsonism. This was shown in rats with 6-OHDA-induced parkinsonism, where resveratrol exerts neuroprotective and anti-inflammatory effects [103]. Remarkably, ultrastructural analysis of DA neurons in the SN of these rats revealed that resveratrol alleviates 6-OHDA-induced subcellular alterations which are reminiscent of autophagy failure, namely accumulation of electron-dense cytoplasmic material, accumulation of vesicles resembling stagnant autophagy-like vacuoles, and mitochondrial swelling [103]. Taken together, these studies provide compelling evidence for the key role of autophagy induction in the beneficial effects of resveratrol in parkinsonism.
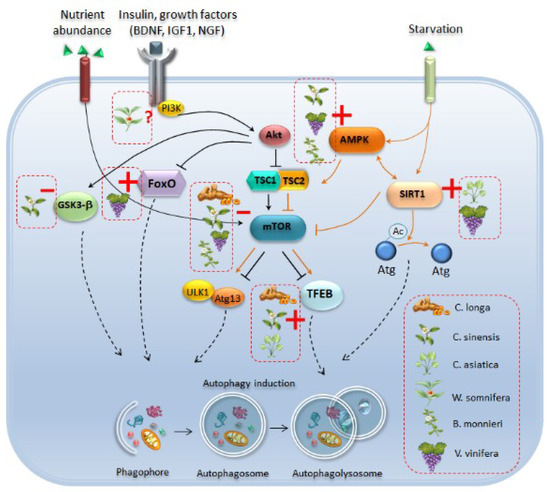
Figure 3.
Autophagy-related molecular pathways which are targeted by phytochemical-rich plants. Phytochemicals induce autophagy by acting at several molecular levels. Curcumin (C. longa), catechins of green tea (C. sinensis), resveratrol (V. vinifera) and bacosides (B. monnieri) act as mTOR inhibitors, which leads to autophagy induction either through activation of ULK1/Atg13 or transcription factor EB (TFEB). In particular, curcumin, green tea catechins, and C. asiatica activate TFEB to promote its translocation to the nucleus, and the subsequent induction of autophagy-related genes. Catechins of green tea and withanolides from W. somnifera may also activate autophagy through inhibition of Glycogen Synthase Kinase 3 Beta (GSK-3β), while resveratrol fosters the activation of the autophagy-promoting transcription factor FoxO3. Again, green tea catechins, resveratrol and B. monnieri activate autophagy through enhancement of AMP-activated Protein Kinase (AMPK), which in turn is an upstream inhibitor of mTOR and an activator of Sirtuin-1 (SIRT1). Activation of SIRT1-dependent autophagy through deacetylation of Atg proteins is mainly induced by resveratrol and C. asiatica. Again, W. somnifera may also act upstream of autophagy by modulating the IGF1-Akt axis, although a role has not been confirmed yet. Plain black arrows indicate pathways which act as upstream inhibitors of autophagy while plain orange arrows indicate pathways which promote autophagy. Dashed black arrows indicate pathways converging towards autophagy machinery. Red dashed boxes indicate the specific phytochemicals which activate autophagy by acting as inhibitors (red line) or inducers (red cross) of specific autophagy-related molecules.
5. Conclusions and Future Directions
The experimental evidence reviewed here converges in that phytochemicals such as curcumin, catechins of green tea, and resveratrol confer neuroprotection in experimental parkinsonism by fostering degradation of α-syn toxic species through activation of autophagy. For other phytochemical-rich plants such as W. somnifera, B. monnieri, and C. asiatica, the autophagy-based beneficial effects in experimental parkinsonism remain to be investigated and/or confirmed. In keeping with this, it is worth mentioning that phytochemicals may also induce autophagy indirectly. For instance, most of the compounds analyzed here, especially curcumin, EGCG, and C. asiatica extracts counteract the upregulation of pro-apoptotic molecules such as caspases and MAPK-p38, which may interact with Atg proteins to inhibit autophagy in favor of an apoptotic profile [130,157,267].
Again, neuroprotection from curcumin and B. monnieri extract associates with the activation of Nrf2, which in turn may induce mitophagy [268]. Curcumin and EGCG also decrease the activity of LRRK2, whose inhibition stimulates autophagy [269]. Phytochemicals are also able to restore DA levels and activity in experimental parkinsonism, and this may indirectly impact on autophagy through biochemical cascades arising from stimulation of specific DA receptors [270]. Other examples of target molecules through which phytochemicals may indirectly modulate autophagy include growth factors such as BDNF, pro-inflammatory factors, and epigenetic enzymes such as HDAC, which are all reported to have an effect upon the autophagy machinery [137,271,272].
Rescuing autophagy through natural compounds may play a role not only in preserving DA neuron integrity but also in counteracting the prion-like spreading of indigested α-syn, which is not limited to the CNS milieu but occurs even between distant cells operating in different organs [273]. In this scenario of multisystem interaction, neural mechanisms intermingle with immunological and neuroendocrine pathways to link emotional and cognitive centers of the brain with peripheral functions. In PD this is evident by the spreading of α-syn along the whole brain-gut-immune axis [273]. Since autophagy is seminal for both cellular and organ-level homeostasis, alterations of autophagy in PD are likely to underlie a much broader range of events featuring altered communication and spreading of abnormal signals between different systems. This is best exemplified by the concomitance between systemic disorders such as the metabolic syndrome and the occurrence of PD, where a failure of autophagy may represent a downstream systemic event occurring in and out the CNS. In fact, autophagy is seminal in modulating body and nutrient metabolism by acting either in peripheral organs or in the CNS by controlling hypothalamic energy expenditure, appetite, and body weight. Thus, targeting autophagy alterations through natural compounds possessing low side effects may be an advantageous strategy in targeting both CNS and systemic alterations, which occur in age-related and neurodegenerative disorders [274,275]. The beneficial effects of phytochemicals analyzed in the present review extend to several systemic diseases including metabolic syndrome, diabetes, cardiovascular disease, cancer, and chronic inflammation beyond neurodegeneration [116,274,276]. These considerations warrant additional studies aimed at dissecting and confirming the autophagy-based beneficial effects of phytochemicals in those CNS disorders such as PD, which are featured by alterations in the cell-clearing systems. In keeping with this, it would also be worth testing the effects of combined phytochemicals supplementations in PD models, which may disclose either synergistic or independent effects of single bioactive compounds. Further research is also needed to identify safe and effective strategies aimed at enhancing phytochemicals bioavailability. Again, well-designed clinical trials should be undertaken to identify the optimal dosage which can safely and effectively reproduce the beneficial effects observed in experimental models.
Funding
This work was funded by Ministero della Salute (Ricerca Corrente 2019).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 3-MA | 3-Methyladenine |
| 6-OHDA | 6-Hydroxydopamine |
| AD | Alzheimer’s Disease |
| ALS | Amyotrophic Lateral Sclerosis |
| AMPK | 5′ AMP-activated Protein Kinase |
| Atg | Autophagy-Related-Gene |
| BAD | Bcl-2-Associated Death Promoter |
| BDNF | Brain-Derived Neurotrophic Factor |
| CAT | Catalase |
| CNS | Central Nervous System |
| COX-2 | Cyclooxygenase-2 |
| Cyt | Cytochrome |
| DA | Dopamine |
| DAT | Dopamine Transporter |
| DLB | Dementia with Lewy Bodies |
| EGCG | Epigallocatechin Gallate |
| ER | Endoplasmic Reticulum |
| ERK | Extracellular Signal–Regulated Kinase |
| FOXO3 | Forkhead Box O3 |
| GDNF | Glial Cell Line-Derived Neurotrophic Factor |
| GFAP | Glial Fibrillary Acidic Protein |
| GFP | Green Fluorescent Protein |
| Gpx | Glutathione Peroxidase |
| GR | Glutathione Reductase |
| GSH | Glutathione |
| GSk3-β | Glycogen Synthase Kinase 3 Beta |
| GST | Glutathione S-Transferase |
| HDAC6 | Histone Deacetylase 6 |
| HIF-1 | Hypoxia-Inducible Factor 1 |
| HMGB1 | High Mobility Group Box 1 |
| IFNγ | Interferon Gamma |
| IIS | Insulin/Insulin-Like Growth Factor Signaling |
| IL-1β | Interleukine 1 Beta |
| IL-1β/a | Interleukine-1 beta/alpha |
| iNOS | inducible Nitric Oxide Synthase |
| JNK | c-Jun N-Terminal Kinase |
| LAMP-2A | Lysosomal-Associated Membrane Protein Type 2a |
| LPS | Lipopolysaccharide |
| LRRK2 | Leucine-Rich Repeat Kinase 2 |
| LSD | Lysosomal Storage Diseases |
| MALAT1 | Metastasis-Associated Lung Adenocarcinoma Transcript 1 |
| MAPK | Mitogen-Activated Protein Kinase |
| MDA | Malondialdehyde |
| Meth | Methamphetamine |
| MMP | Mitochondrial Membrane Potential |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MSA | Multisystem Atrophy |
| mTOR | Mammalian Target of Rapamycin |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate Hydrogen |
| Nf-Kb | Nuclear Factor K Beta |
| NGF | Neurotrophic Growth Factor |
| NO | Nitric Oxide |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| PAF | Pure Autonomic Failure |
| PARP | Poly (ADP-ribose) Polymerase |
| PD | Parkinson’s Disease |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha |
| PINK1 | PTEN-induced kinase 1 |
| PKC α | Protein Kinase C alpha |
| Rab GTPase | Gtp Bound Ras Proteins in Brain |
| ROS | Reactive Oxygen Species |
| SDH | Succinate Dehydrogenase |
| SIRT1 | NAD-dependent deacetylase Sirtuin-1 |
| SNARE | Soluble Nsf Attachment Protein Receptor |
| SNpc | Substantia Nigra Pars Compacta |
| SOD | Superoxide Dismutase |
| SQSTM1 | Sequestosome-1 |
| SVZ | Subventricular Zone |
| TBARS | Thiobarbituric Acid Reactive Substance |
| TFEB | Transcription Factor EB |
| TGF-b1 | Transforming Growth Factor Beta 1 |
| TH | Tyrosine Hydroxylase |
| TNFα | Tumor Necrosis Factor Alpha |
| Trk A/B | Tyrosine Receptor Kinase A/B |
| UCH-LI | Ubiquitin carboxy-terminal hydrolase L1 |
| VEGF | Vascular-Endothelial Growth Factor |
| VTA | Ventral Tegmental Area |
| YFP | Yellow Fluorescent Protein |
| α-syn | alpha-synuclein |
References
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 1, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.; Izzo, A.A. Principles of pharmacological research of nutraceuticals. Br. J. Pharmacol. 2017, 11, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Oomah, B.D. Chemistry and classification of phytochemicals. In Handbook of Plant Food Phytochemicals; Tiwari, B., Brunton, N.P., Brennan, C.S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Farooqui, T.; Madan, A.; Ong, J.H.; Ong, W.Y. Ayurvedic Medicine for the Treatment of Dementia: Mechanistic Aspects. Evid. Based Complement. Alternat. Med. 2018, 2018, 2481076. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Fornai, M.; Antonioli, L.; Blandizzi, C.; Calderone, V. Phytochemicals as Novel Therapeutic Strategies for NLRP3 Inflammasome-Related Neurological, Metabolic, and Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 2876. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, J.; Perumal, M.K.; Vallikannan, B. A critical review on anti-angiogenic property of phytochemicals. J. Nutr. Biochem. 2019, 71, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Banach, M. Botanicals and phytochemicals active on cognitive decline: The clinical evidence. Pharmacol. Res. 2018, 130, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Brondino, N.; Re, S.; Boldrini, A.; Cuccomarino, A.; Lanati, N.; Barale, F.; Politi, P. Curcumin as a therapeutic agent in dementia: A mini systematic review of human studies. Sci. World J. 2014, 2014, 174282. [Google Scholar] [CrossRef]
- Kongkeaw, C.; Dilokthornsakul, P.; Thanarangsarit, P.; Limpeanchob, N.; Norman Scholfield, C. Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. J. Ethnopharmacol. 2014, 151, 528–535. [Google Scholar] [CrossRef]
- Pase, M.P.; Kean, J.; Sarris, J.; Neale, C.; Scholey, A.B.; Stough, C. The cognitive-enhancing effects of Bacopa monnieri: A systematic review of randomized, controlled human clinical trials. J. Altern. Complement. Med. 2012, 18, 647–652. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. Alzheimer’s Disease Cooperative Study. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Bhattacharyya, S.; Bose, S. Efficacy and Safety of Ashwagandha (Withania somnifera (L.) Dunal) Root Extract in Improving Memory and Cognitive Functions. J. Diet Suppl. 2017, 14, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Bimonte, S.; Muzio, M.R.; Schiavone, V.; Cuomo, A. The efficacy of Epigallocatechin-3-gallate (green tea) in the treatment of Alzheimer’s disease: An overview of pre-clinical studies and translational perspectives in clinical practice. Infect. Agent Cancer 2017, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Chico, L.; Ienco, E.C.; Bisordi, C.; Lo Gerfo, A.; Petrozzi, L.; Petrucci, A.; Mancuso, M.; Siciliano, G. Amyotrophic Lateral Sclerosis and Oxidative Stress: A Double-Blind Therapeutic Trial After Curcumin Supplementation. CNS Neurol. Disord. Drug Targets 2018, 17, 767–779. [Google Scholar] [CrossRef]
- Singh, M.; Arseneault, M.; Sanderson, T.; Murthy, V.; Ramassamy, C. Challenges for research on polyphenols from foods in Alzheimer’s disease: Bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 2008, 56, 4855–4873. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Pereira, M.D.C.; Loureiro, J.A. Resveratrol Brain Delivery for Neurological Disorders Prevention and Treatment. Front. Pharmacol. 2018, 9, 1261. [Google Scholar] [CrossRef]
- Ji, H.F.; Shen, L. Can improving bioavailability improve the bioactivity of curcumin? Trends Pharmacol. Sci. 2014, 35, 265–266. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Rajan, K.E.; Preethi, J.; Singh, H.K. Molecular and Functional Characterization of Bacopa monniera: A Retrospective Review. Evid. Based Complement. Alternat. Med. 2015, 2015, 945217. [Google Scholar] [CrossRef] [PubMed]
- De, K.; Chandra, S.; Misra, M. Evaluation of the biological effect of brahmi (Bacopa monnieri Linn) extract on the biodistribution of technetium-99m radiopharmaceuticals. Life Sci. J. 2008, 5, 45–49. [Google Scholar]
- Suganuma, M.; Okabe, S.; Oniyama, M.; Tada, Y.; Ito, H.; Fujiki, H. Wide distribution of (-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 1998, 19, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Anukunwithaya, T.; Tantisira, M.H.; Tantisira, B.; Khemawoot, P. Pharmacokinetics of a Standardized Extract of Centella asiatica ECa 233 in Rats. Planta Med. 2017, 83, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Vareed, S.K.; Bauer, A.K.; Nair, K.M.; Liu, Y.; Jayaprakasam, B.; Nair, M.G. Blood-brain barrier permeability of bioactive withanamides present in Withania somnifera fruit extract. Phytother. Res. 2014, 28, 1260–1264. [Google Scholar] [CrossRef]
- Rege, S.D.; Geetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yan, F.; Liang, X.; Wu, M.; Shen, Y.; Chen, M.; Xu, Y.; Zou, G.; Jiang, P.; Tang, C.; et al. Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson’s disease therapy. Theranostics 2018, 8, 2264–2277. [Google Scholar] [CrossRef]
- Jinfeng, L.; Yunliang, W.; Xinshan, L.; Yutong, W.; Shanshan, W.; Peng, X.; Xiaopeng, Y.; Zhixiu, X.; Qingshan, L.; Honglei, Y.; et al. Therapeutic Effects of CUR-Activated Human Umbilical Cord Mesenchymal Stem Cells on 1-Methyl-4-phenylpyridine-Induced Parkinson’s Disease Cell Model. Biomed. Res. Int. 2016, 2016, 9140541. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, S.; Xu, B.; Zhang, R.; Xu, L. Protective effect of curcumin on dopamine neurons in Parkinson’s disease and its mechanism. J. Zhejiang Univ. Med. Sci. 2018, 47, 480–486. [Google Scholar]
- Yu, S.; Zheng, W.; Xin, N.; Chi, Z.H.; Wang, N.Q.; Nie, Y.X.; Feng, W.Y.; Wang, Z.Y. Curcumin prevents dopaminergic neuronal death through inhibition of the c-Jun N-terminal kinase pathway. Rejuvenation Res. 2010, 13, 55–64. [Google Scholar] [CrossRef]
- Pan, J.; Li, H.; Ma, J.F.; Tan, Y.Y.; Xiao, Q.; Ding, J.Q.; Chen, S.D. Curcumin inhibition of JNKs prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease through suppressing mitochondria dysfunction. Transl. Neurodegener. 2012, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- He, X.J.; Uchida, K.; Megumi, C.; Tsuge, N.; Nakayama, H. Dietary curcumin supplementation attenuates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity in C57BL mice. J. Toxicol. Pathol. 2015, 28, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.J.; Lian, Y.G.; Zhao, H.Y.; Xu, Q.L. Curcumin protects from oxidative stress and inhibits α-synuclein aggregation in MPTP induced parkinsonian mice. Int. J. Clin. Exp. Med. 2016, 9, 2654–2665. [Google Scholar]
- Wang, Y.L.; Ju, B.; Zhang, Y.Z.; Yin, H.L.; Liu, Y.J.; Wang, S.S.; Zeng, Z.L.; Yang, X.P.; Wang, H.T.; Li, J.F. Protective Effect of Curcumin Against Oxidative Stress-Induced Injury in Rats with Parkinson’s Disease Through the Wnt/β-Catenin Signaling Pathway. Cell. Physiol. Biochem. 2017, 43, 2226–2241. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Nie, Q.; Li, Z.; Du, G. Curcumin improves neurofunctions of 6-OHDA-induced parkinsonian rats. Pathol. Res. Pract. 2016, 212, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, X.X.; Jiang, H.; Xie, J.X. Curcumin attenuates 6-hydroxydopamine-induced cytotoxicity by anti-oxidation and nuclear factor-kappa B modulation in MES23.5 cells. Biochem. Pharmacol. 2009, 78, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Jaisin, Y.; Thampithak, A.; Meesarapee, B.; Ratanachamnong, P.; Suksamrarn, A.; Phivthong-Ngam, L.; Phumala-Morales, N.; Chongthammakun, S.; Govitrapong, P.; Sanvarinda, Y. Curcumin I protects the dopaminergic cell lines SH-SY5Y from 6-hydroxydopamine-induced neurotoxicity through attenuation of p53-mediated apoptosis. Neurosci. Lett. 2011, 489, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Meesarapee, B.; Thampithak, A.; Jaisin, Y.; Sanvarinda, P.; Suksamrarn, A.; Tuchinda, P.; Morales, N.P.; Sanvarinda, Y. Curcumin I mediates neuroprotective effect through attenuation of quinoprotein formation, p-p38 MAPK expression, and caspase-3 activation in 6-hydroxydopamine treated SH-SY5Y cells. Phytother. Res. 2014, 28, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.K.; Park, H.Y.; Go, J.; Kim, Y.H.; Hwang, J.H.; Choi, D.H.; Noh, J.R.; Rhee, M.; Han, P.L.; Lee, C.H.; et al. Effects of histone acetyltransferase inhibitors on L-DOPA-induced dyskinesia in a murine model of Parkinson’s disease. J. Neural Transm. 2018, 125, 1319–1331. [Google Scholar] [CrossRef]
- Ramkumar, M.; Rajasankar, S.; Gobi, V.V.; Dhanalakshmi, C.; Manivasagam, T.; Justin Thenmozhi, A.; Essa, M.M.; Kalandar, A.; Chidambaram, R. Neuroprotective effect of Demethoxycurcumin, a natural derivative of Curcumin on rotenone induced neurotoxicity in SH-SY 5Y Neuroblastoma cells. BMC Complement. Altern. Med. 2017, 17, 217. [Google Scholar] [CrossRef]
- Cui, Q.; Li, X.; Zhu, H. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Mol. Med. Rep. 2016, 13, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Khatri, D.K.; Juvekar, A.R. Neuroprotective effect of curcumin as evinced by abrogation of rotenone-induced motor deficits, oxidative and mitochondrial dysfunctions in mouse model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2016, 150–151, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, A.; Chatoui, H.; El Hiba, O.; Gamrani, H. Neuroprotective effect of curcumin-I in copper-induced dopaminergic neurotoxicity in rats: A possible link with Parkinson’s disease. Neurosci. Lett. 2017, 66, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, A.; Gamrani, H. Neuronal, astroglial and locomotor injuries in subchronic copper intoxicated rats are repaired by curcumin: A possible link with Parkinson’s disease. Acta Histochem. 2018, 120, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Nehru, B. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 2018, 26, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.S.; Boddapati, S.; Emadi, S.; Sierks, M.R. Curcumin reduces alpha-synuclein induced cytotoxicity in Parkinson’s disease cell model. BMC Neurosci. 2010, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.F.; Zhang, Y.J.; Zhou, H.Y.; Wang, H.M.; Tian, L.P.; Liu, J.; Ding, J.Q.; Chen, S.D. Curcumin ameliorates the neurodegenerative pathology in A53T α-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J. Neuroimmune Pharmacol. 2013, 8, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, Y.; Li, X.; Ross, C.A.; Smith, W.W. Curcumin protects against A53Talpha-synuclein-induced toxicity in a PC12 inducible cell model for Parkinsonism. Pharmacol. Res. 2011, 63, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Siddique, Y.H.; Naz, F.; Jyoti, S. Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson’s disease. BioMed Res. Int. 2014, 2014, 606928. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Vuu, M.D.; Huynh, M.A.; Yamaguchi, M.; Tran, L.T.; Dang, T.P.T. Curcumin Effectively Rescued Parkinson’s Disease-Like Phenotypes in a Novel Drosophila melanogaster Model with dUCH Knockdown. Oxid. Med. Cell. Longev. 2018, 2018, 2038267. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Terpstra, K.J.; Bureau, Y.; Hou, J.; Raheb, H.; Cernvosky, Z.; Badmeav, V.; Copen, J.; Husni, M.; Woodbury-Farina, M. Liposomal-formulated curcumin [Lipocurc™] targeting HDAC (histone deacetylase) prevents apoptosis and improves motor deficits in park 7 (DJ-1)-knockout rat model of Parkinson’s disease: Implications for epigenetics-based nanotechnology-driven drug platform. J. Complement. Integr. Med. 2013, 10, 75–88. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, C.; van Dyk, H.C.; Engelbrecht, L.; van der Westhuizen, F.H.; Kinnear, C.; Loos, B.; Bardien, S. Curcumin Rescues a PINK1 Knock Down SH-SY5Y Cellular Model of Parkinson’s Disease from Mitochondrial Dysfunction and Cell Death. Mol. Neurobiol. 2017, 54, 2752–2762. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, T.; Liu, Z.; Arbez, N.; Yan, J.; Moran, T.H.; Ross, C.A.; Smith, W.W. LRRK2 kinase activity mediates toxic interactions between genetic mutation and oxidative stress in a Drosophila model: Suppression by curcumin. Neurobiol. Dis. 2012, 47, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Murthy, V.; Ramassamy, C. Standardized extracts of Bacopa monniera protect against MPP+- and paraquat-induced toxicity by modulating mitochondrial activities, proteasomal functions, and redox pathways. Toxicol. Sci. 2012, 125, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Pandey, S.; Verma, R.; Ansari, J.A.; Mahdi, A.A. Comparative evaluation of extract of Bacopa monnieri and Mucuna pruriens as neuroprotectant in MPTP model of Parkinson’s disease. Indian J. Exp. Biol. 2016, 54, 758–766. [Google Scholar]
- Singh, B.; Pandey, S.; Yadav, S.K.; Verma, R.; Singh, S.P.; Mahdi, A.A. Role of ethanolic extract of Bacopa monnieri against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced mice model via inhibition of apoptotic pathways of dopaminergic neurons. Brain Res. Bull. 2017, 135, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Nellore, J.; Pauline, C.; Amarnath, K. Bacopa monnieri Phytochemicals Mediated Synthesis of Platinum Nanoparticles and Its Neurorescue Effect on 1-Methyl 4-Phenyl 1,2,3,6 Tetrahydropyridine-Induced Experimental Parkinsonism in Zebrafish. J. Neurodegener. Dis. 2013, 2013, 972391. [Google Scholar] [CrossRef]
- Hosamani, R.; Muralidhara. Prophylactic treatment with Bacopa monnieri leaf powder mitigates paraquat-induced oxidative perturbations and lethality in Drosophila melanogaster. Indian J. Biochem. Biophys. 2010, 47, 75–82. [Google Scholar]
- Singh, M.; Murthy, V.; Ramassamy, C. Neuroprotective mechanisms of the standardized extract of Bacopa monniera in a paraquat/diquat-mediated acute toxicity. Neurochem. Int. 2013, 62, 530–539. [Google Scholar] [CrossRef]
- Hosamani, R.; Krishna, G.; Muralidhara. Standardized Bacopa monnieri extract ameliorates acute paraquat-induced oxidative stress, and neurotoxicity in prepubertal mice brain. Nutr. Neurosci. 2016, 19, 434–446. [Google Scholar] [CrossRef]
- Krishna, G.; Hosamani, R.; Muralidhara. Bacopa monnieri Supplements Offset Paraquat-Induced Behavioral Phenotype and Brain Oxidative Pathways in Mice. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, S.; Fatima, M.; Mondal, A.C. Bacopa monnieri alleviates paraquat induced toxicity in Drosophila by inhibiting jnk mediated apoptosis through improved mitochondrial function and redox stabilization. Neurochem. Int. 2018, 121, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Shobana, C.; Kumar, R.R.; Sumathi, T. Alcoholic extract of Bacopa monniera Linn. protects against 6-hydroxydopamine-induced changes in behavioral and biochemical aspects: A pilot study. Cell. Mol. Neurobiol. 2012, 32, 1099–1112. [Google Scholar] [PubMed]
- Jadiya, P.; Khan, A.; Sammi, S.R.; Kaur, S.; Mir, S.S.; Nazir, A. Anti-Parkinsonian effects of Bacopa monnieri: Insights from transgenic and pharmacological Caenorhabditis elegans models of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2011, 413, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Shinomol, G.K.; Mythri, R.B.; Srinivas Bharath, M.M.; Muralidhara. Bacopa monnieri extract offsets rotenone-induced cytotoxicity in dopaminergic cells and oxidative impairments in mice brain. Cell. Mol. Neurobiol. 2012, 32, 455–465. [Google Scholar] [CrossRef]
- Hosamani, R.; Muralidhara. Neuroprotective efficacy of Bacopa monnieri against rotenone induced oxidative stress and neurotoxicity in Drosophila melanogaster. Neurotoxicology 2009, 30, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.L.; Brogan, B.; Whitworth, A.J.; Okello, E.J. Effects of five Ayurvedic herbs on locomotor behaviour in a Drosophila melanogaster Parkinson’s disease model. Phytother. Res. 2014, 28, 1789–1795. [Google Scholar] [CrossRef]
- Ye, Q.; Ye, L.; Xu, X.; Huang, B.; Zhang, X.; Zhu, Y.; Chen, X. Epigallocatechin-3-gallate suppresses 1-methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1α signaling pathway. BMC Complement. Altern. Med. 2012, 12, 82. [Google Scholar] [CrossRef]
- Levites, Y.; Weinreb, O.; Maor, G.; Youdim, M.B.H.; Mandel, S. Green tea polyphenol (-)-epigallocatechin-3gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 2001, 78, 1073–1082. [Google Scholar] [CrossRef]
- Mandel, S.; Maor, G.; Youdim, M.B. Iron and alpha-synuclein in the substantia nigra of MPTP-treated mice: Effect of neuroprotective drugs R-apomorphine and green tea polyphenol (-)-epigallocatechin-3-gallate. J. Mol. Neurosci. 2004, 24, 401–416. [Google Scholar] [CrossRef]
- Xu, Q.; Langley, M.; Kanthasamy, A.G.; Reddy, M.B. Epigallocatechin Gallate Has a Neurorescue Effect in a Mouse Model of Parkinson Disease. J. Nutr. 2017, 147, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhu, M.; Liang, Z. (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2018, 17, 4883–4888. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Perez, D.A.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Epigallocatechin-3-Gallate Protects and Prevents Paraquat-Induced Oxidative Stress and Neurodegeneration in Knockdown dj-1-β Drosophila melanogaster. Neurotox. Res. 2018, 34, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Ramirez, L.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Low doses of paraquat and polyphenols prolong life span and locomotor activity in knock-down parkin Drosophila melanogaster exposed to oxidative stress stimuli: Implication in autosomal recessive juvenile parkinsonism. Gene 2013, 512, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Levites, Y.; Youdim, M.B.; Maor, G.; Mandel, S. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-kappaB) activation and cell death by tea extracts in neuronal cultures. Biochem. Pharmacol. 2002, 63, 21–29. [Google Scholar] [CrossRef]
- Bitu Pinto, N.; da Silva Alexandre, B.; Neves, K.R.; Silva, A.H.; Leal, L.K.; Viana, G.S. Neuroprotective Properties of the Standardized Extract from Camellia sinensis (Green Tea) and Its Main Bioactive Components, Epicatechin and Epigallocatechin Gallate, in the 6-OHDA Model of Parkinson’s Disease. Evid. Based Complement. Alternat. Med. 2015, 2015, 161092. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, L.; Hu, X.; Cao, S.; Yang, J. Effects and mechanism of epigallocatechin-3-gallate on apoptosis and mTOR/AKT/GSK-3β pathway in substantia nigra neurons in Parkinson rats. Neuroreport 2019, 30, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Kamalden, T.A.; Ji, D.; Osborne, N.N. Rotenone-induced death of RGC-5 cells is caspase independent, involves the JNK and p38 pathways and is attenuated by specific green tea flavonoids. Neurochem. Res. 2012, 37, 1091–1101. [Google Scholar] [CrossRef]
- Ng, C.H.; Guan, M.S.; Koh, C.; Ouyang, X.; Yu, F.; Tan, E.K.; O’Neill, S.P.; Zhang, X.; Chung, J.; Lim, K.L. AMP kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson’s disease. J. Neurosci. 2012, 32, 14311–14317. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Jyoti, S.; Naz, F. Effect of epicatechin gallate dietary supplementation on transgenic Drosophila model of Parkinson’s disease. J. Diet Suppl. 2014, 11, 121–130. [Google Scholar] [CrossRef]
- Nataraj, J.; Manivasagam, T.; Justin Thenmozhi, A.; Essa, M.M. Neurotrophic Effect of Asiatic acid, a Triterpene of Centella asiatica Against Chronic 1-Methyl 4-Phenyl 1, 2, 3, 6-Tetrahydropyridine Hydrochloride/Probenecid Mouse Model of Parkinson’s disease: The Role of MAPK, PI3K-Akt-GSK3β and mTOR Signalling Pathways. Neurochem. Res. 2017, 42, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, M.; Goel, I.; Roy, T.; Shukla, S.D.; Khurana, S. Complete Comparison Display (CCD) evaluation of ethanol extracts of Centella asiatica and Withania somnifera shows that they can non-synergistically ameliorate biochemical and behaviouraldamages in MPTP induced Parkinson’s model of mice. PLoS ONE 2017, 12, e0177254. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.L.; Qu, R.; Zhang, J.; Li, L.F.; Ma, S.P. Neuroprotective effects of madecassoside in early stage of Parkinson’s disease induced by MPTP in rats. Fitoterapia 2013, 90, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Ponnusamy, K. Neuroprotective effect of Centella asiatica extract (CAE) on experimentally induced parkinsonism in aged Sprague-Dawley rats. J. Toxicol. Sci. 2010, 35, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, J.; Manivasagam, T.; Justin Thenmozhi, A.; Essa, M.M. Neuroprotective effect of asiatic acid on rotenone-induced mitochondrial dysfunction and oxidative stress-mediated apoptosis in differentiated SH-SYS5Y cells. Nutr. Neurosci. 2017, 20, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Teerapattarakan, N.; Benya-Aphikul, H.; Tansawat, R.; Wanakhachornkrai, O.; Tantisira, M.H.; Rodsiri, R. Neuroprotective effect of a standardized extract of Centella asiatica ECa233 in rotenone-induced parkinsonism rats. Phytomedicine 2018, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Siddique, Y.H.; Naz, F.; Jyoti, S.; Fatima, A.; Khanam, S.; Rahul Ali, F.; Mujtaba, S.F.; Faisal, M. Effect of Centella asiatica Leaf Extract on the Dietary Supplementation in Transgenic Drosophila Model of Parkinson’s Disease. Parkinson’s Dis. 2014, 2014, 262058. [Google Scholar] [CrossRef]
- Sankar, S.R.; Manivasagam, T.; Krishnamurti, A.; Ramanathan, M. The neuroprotective effect of Withania somnifera root extract in MPTP-intoxicated mice: An analysis of behavioral and biochemical variables. Cell. Mol. Biol. Lett. 2007, 12, 473–481. [Google Scholar] [CrossRef]
- Rajasankar, S.; Manivasagam, T.; Sankar, V.; Prakash, S.; Muthusamy, R.; Krishnamurti, A.; Surendran, S. Withania somnifera root extract improves catecholamines and physiological abnormalities seen in a Parkinson’s disease model mouse. J. Ethnopharmacol. 2009, 125, 369–373. [Google Scholar] [CrossRef]
- Rajasankar, S.; Manivasagam, T.; Surendran, S. Ashwagandha leaf extract: A potential agent in treating oxidative damage and physiological abnormalities seen in a mouse model of Parkinson’s disease. Neurosci. Lett. 2009, 454, 11–15. [Google Scholar] [CrossRef]
- Prakash, J.; Chouhan, S.; Yadav, S.K.; Westfall, S.; Rai, S.N.; Singh, S.P. Withania somnifera alleviates parkinsonian phenotypes by inhibiting apoptotic pathways in dopaminergic neurons. Neurochem. Res. 2014, 39, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Yadav, S.K.; Chouhan, S.; Singh, S.P. Neuroprotective role of Withania somnifera root extract in maneb-paraquat induced mouse model of parkinsonism. Neurochem. Res. 2013, 38, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Saleem, S.; Ahmad, A.S.; Ansari, M.A.; Yousuf, S.; Hoda, M.N.; Islam, F. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum. Exp. Toxicol. 2005, 24, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, M.J.; Muralidhara. Standardized extract of Withania somnifera (Ashwagandha) markedly offsets rotenone-induced locomotor deficits, oxidative impairments and neurotoxicity in Drosophila melanogaster. J. Food Sci. Technol. 2015, 52, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- De Rose, F.; Marotta, R.; Poddighe, S.; Talani, G.; Catelani, T.; Setzu, M.D.; Solla, P.; Marrosu, F.; Sanna, E.; Kasture, S.; et al. Functional and Morphological Correlates in the Drosophila LRRK2 loss-of-function Model of Parkinson’s Disease: Drug Effects of Withania somnifera (Dunal) Administration. PLoS ONE 2016, 11, e0146140. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Sui, R.; Zhang, Z. Administration of resveratrol improved Parkinson’s disease-like phenotype by suppressing apoptosis of neurons via modulating the MALAT1/miR-129/SNCA signaling pathway. J. Cell. Biochem. 2019, 120, 4942–4951. [Google Scholar] [CrossRef]
- Blanchet, J.; Longpré, F.; Bureau, G.; Morissette, M.; DiPaolo, T.; Bronchti, G.; Martinoli, M.G. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1243–1250. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, D.; Jiang, P.; Tang, X.; Lang, Q.; Yu, Q.; Zhang, S.; Che, Y.; Feng, X. Resveratrol synergizes with low doses of L-DOPA to improve MPTP-induced Parkinson disease in mice. Behav. Brain Res. 2019, 367, 10–18. [Google Scholar] [CrossRef]
- Guo, Y.J.; Dong, S.Y.; Cui, X.X.; Feng, Y.; Liu, T.; Yin, M.; Kuo, S.H.; Tan, E.K.; Zhao, W.J.; Wu, Y.C. Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of α-synuclein via SIRT1-deacetylated LC3. Mol. Nutr. Food Res. 2016, 60, 2161–2175. [Google Scholar] [CrossRef]
- Abolaji, A.O.; Adedara, A.O.; Adie, M.A.; Vicente-Crespo, M.; Farombi, E.O. Resveratrol prolongs lifespan and improves 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced oxidative damage and behavioural deficits in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2018, 503, 1042–1048. [Google Scholar] [CrossRef]
- Albani, D.; Polito, L.; Batelli, S.; De Mauro, S.; Fracasso, C.; Martelli, G.; Colombo, L.; Manzoni, C.; Salmona, M.; Caccia, S.; et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by α-synuclein or amyloid-beta (1-42) peptide. J. Neurochem. 2009, 110, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wu, Q.; Lu, Y.F.; Gong, Q.H.; Shi, J.S. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur. J. Pharmacol. 2008, 600, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.L.; Lin, K.J.; Wang, P.W.; Chuang, J.H.; Lin, H.Y.; Chen, S.D.; Chuang, Y.C.; Huang, S.T.; Tiao, M.M.; Chen, J.B.; et al. Resveratrol provides neuroprotective effects through modulation of mitochondrial dynamics and ERK1/2 regulated autophagy. Free Radic. Res. 2018, 52, 1371–1386. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Chen, S.D.; Chuang, Y.C.; Lin, H.Y.; Huang, C.R.; Chuang, J.H.; Wang, P.W.; Huang, S.T.; Tiao, M.M.; Chen, J.B.; et al. Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int. J. Mol. Sci. 2014, 15, 1625–1646. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, X.; Liu, Z.; Zhu, S.; Liu, H.; Fan, W.; Hu, Y.; Hu, T.; Yu, Y.; Li, Y.; et al. Resveratrol Suppresses Rotenone-induced Neurotoxicity Through Activation of SIRT1/Akt1 Signaling Pathway. Anat. Rec. 2018, 301, 1115–1125. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhu, J.X.; Xie, W.; Le, W.; Fan, Z.; Jankovic, J.; Pan, T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals 2011, 19, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Palle, S.; Neerati, P. Improved neuroprotective effect of resveratrol nanoparticles as evinced by abrogation of rotenone-induced behavioral deficits and oxidative and mitochondrial dysfunctions in rat model of Parkinson’s disease. Naunyn. Schmiedebergs Arch. Pharmacol. 2018, 391, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, H.H.; Zakaria, S.S.; Elbatsh, M.M.; Tahoon, N.M. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson’s disease. Chem. Biol. Interact. 2016, 251, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Yu, X.L.; Ji, M.; Liu, S.Y.; Wu, X.L.; Wang, Y.J.; Liu, R.T. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food Funct. 2018, 9, 6414–6426. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, A.; Dong, J.; Sigears, A.; Lu, B. Grape skin extract improves muscle function and extends lifespan of a Drosophila model of Parkinson’s disease through activation of mitophagy. Exp. Gerontol. 2018, 113, 10–17. [Google Scholar] [CrossRef]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Zhang, Z.R.; Zhang, M.M.; Sun, M.X.; Wang, W.W.; Xie, C.L. Neuroprotective properties of curcumin in toxin-base animal models of Parkinson’s disease: A systematic experiment literatures review. BMC Complement. Altern. Med. 2017, 17, 412. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, S.; Fatima, M.; Mondal, A.C. Important medicinal herbs in Parkinson’s disease pharmacotherapy. Biomed. Pharmacother. 2017, 92, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.K.; Rathinasamy, B.; Lohanathan, B.P.; Thiyagarajan, V.; Weng, C.F. Neuroprotective Role of Phytochemicals. Molecules 2018, 23, 2485. [Google Scholar] [CrossRef] [PubMed]
- Valero, T. Mitochondrial biogenesis: Pharmacological approaches. Curr. Pharm. Des. 2014, 20, 5507–5509. [Google Scholar] [CrossRef] [PubMed]
- Mathur, D.; Goyal, K.; Koul, V.; Anand, A. The Molecular Links of Re-Emerging Therapy: A Review of Evidence of Brahmi (Bacopa monniera). Front. Pharmacol. 2016, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Yadav, R.S. Efficacy of Natural Compounds in Neurodegenerative Disorders. Adv. Neurobiol. 2016, 12, 107–123. [Google Scholar] [CrossRef]
- Aguiar, S.; Borowski, T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013, 16, 313–326. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Bahramsoltani, R.; Abbasabadi, Z.; Braidy, N.; Nabavi, S.M. Role of green tea catechins in prevention of age-related cognitive decline: Pharmacological targets and clinical perspective. J. Cell. Physiol. 2019, 234, 2447–2459. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Gray, N.E.; Harris, C.J.; Quinn, J.F.; Soumyanath, A. Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. J. Ethnopharmacol. 2016, 180, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E. Centella asiatica (L.) Urban: From Traditional Medicine to Modern Medicine with Neuroprotective Potential. Evid. Based Complement. Alternat. Med. 2012, 2012, 946259. [Google Scholar] [CrossRef] [PubMed]
- Ur Rasheed, M.S.; Tripathi, M.K.; Mishra, A.K.; Shukla, S.; Singh, M.P. Resveratrol Protects from Toxin-Induced Parkinsonism: Plethora of Proofs Hitherto Petty Translational Value. Mol. Neurobiol. 2016, 53, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Lynch-Day, M.A.; Mao, K.; Wang, K.; Zhao, M.; Klionsky, D.J. The role of autophagy in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009357. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, S.; Ferese, R.; Biagioni, F.; Busceti, C.L.; Campopiano, R.; Griguoli, A.M.P.; Limanaqi, F.; Novelli, G.; Storto, M.; Fornai, F. The Monoamine Brainstem Reticular Formation as a Paradigm for Re-Defining Various Phenotypes of Parkinson’s Disease Owing Genetic and Anatomical Specificity. Front. Cell. Neurosci. 2017, 11, 102. [Google Scholar] [CrossRef]
- Ferrucci, M.; Pasquali, L.; Ruggieri, S.; Paparelli, A.; Fornai, F. Alpha-synuclein and autophagy as common steps in neurodegeneration. Parkinsonism Relat. Disord. 2008, 14 (Suppl. 2), S180–S184. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, L.; Ruggieri, S.; Murri, L.; Paparelli, A.; Fornai, F. Does autophagy worsen or improve the survival of dopaminergic neurons? Parkinsonism Relat. Disord. 2009, 15 (Suppl. 4), S24–S27. [Google Scholar] [CrossRef]
- Limanaqi, F.; Biagioni, F.; Gambardella, S.; Ryskalin, L.; Fornai, F. Interdependency Between Autophagy and Synaptic Vesicle Trafficking: Implications for Dopamine Release. Front. Mol. Neurosci. 2018, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Obergasteiger, J.; Frapporti, G.; Pramstaller, P.P.; Hicks, A.A.; Volta, M. A new hypothesis for Parkinson’s disease pathogenesis: GTPase-p38 MAPK signaling and autophagy as convergence points of etiology and genomics. Mol. Neurodegener. 2018, 13, 40. [Google Scholar] [CrossRef]
- Ryskalin, L.; Busceti, C.L.; Limanaqi, F.; Biagioni, F.; Gambardella, S.; Fornai, F. A Focus on the Beneficial Effects of Alpha Synuclein and a Re-Appraisal of Synucleinopathies. Curr. Protein Pept. Sci. 2018, 19, 598–611. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Bento, C.F.; Deretic, V. Therapeutic targeting of autophagy in neurodegenerative and infectious diseases. J. Exp. Med. 2015, 212, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Darley-Usmar, V.; Zhang, J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol. 2013, 2, 82–90. [Google Scholar] [CrossRef]
- Giorgi, F.S.; Bandettini di Poggio, A.; Battaglia, G.; Pellegrini, A.; Murri, L.; Ruggieri, S.; Paparelli, A.; Fornai, F. A short overview on the role of alpha-synuclein and proteasome in experimental models of Parkinson’s disease. J. Neural. Transm. Suppl. 2006, 70, 105–109. [Google Scholar]
- Limanaqi, F.; Biagioni, F.; Gaglione, A.; Busceti, C.L.; Fornai, F. A Sentinel in the Crosstalk Between the Nervous and Immune System: The (Immuno)-Proteasome. Front. Immunol. 2019, 10, 628. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Uchihara, T.; Fukuda, T.; Noda, S.; Kondo, H.; Saiki, S.; Komatsu, M.; Uchiyama, Y.; Tanaka, K.; Hattori, N. Loss of autophagy in dopaminergic neurons causes Lewy pathology and motor dysfunction in aged mice. Sci. Rep. 2018, 8, 2813. [Google Scholar] [CrossRef] [PubMed]
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Ryskalin, L.; Soldani, P.; Frati, A.; Fornai, F. Cell Clearing Systems Bridging Neuro-Immunity and Synaptic Plasticity. Int. J. Mol. Sci. 2019, 20, 2197. [Google Scholar] [CrossRef]
- Ryskalin, L.; Limanaqi, F.; Biagioni, F.; Frati, A.; Esposito, V.; Calierno, M.T.; Lenzi, P.; Fornai, F. The emerging role of m-TOR up-regulation in brain Astrocytoma. Histol. Histopathol. 2017, 32, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Ryskalin, L.; Limanaqi, F.; Frati, A.; Busceti, C.L.; Fornai, F. mTOR-Related Brain Dysfunctions in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2018, 19, 2226. [Google Scholar] [CrossRef]
- Ferrucci, M.; Biagioni, F.; Ryskalin, L.; Limanaqi, F.; Gambardella, S.; Frati, A.; Fornai, F. Ambiguous Effects of Autophagy Activation Following Hypoperfusion/Ischemia. Int. J. Mol. Sci. 2018, 19, 2756. [Google Scholar] [CrossRef]
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Ryskalin, L.; Fornai, F. The effects of proteasome on baseline and methamphetamine-dependent dopamine transmission. Neurosci. Biobehav. Rev. 2019, 102, 308–317. [Google Scholar] [CrossRef]
- Lenzi, P.; Lazzeri, G.; Biagioni, F.; Busceti, C.L.; Gambardella, S.; Salvetti, A.; Fornai, F. The Autophagoproteasome a Novel Cell Clearing Organelle in Baseline and Stimulated Conditions. Front. Neuroanat. 2016, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, G.; Biagioni, F.; Fulceri, F.; Busceti, C.L.; Scavuzzo, M.C.; Ippolito, C.; Salvetti, A.; Lenzi, P.; Fornai, F. mTOR Modulates Methamphetamine-Induced Toxicity through Cell Clearing Systems. Oxid. Med. Cell. Longev. 2018, 2018, 6124745. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K. Organellophagy: Eliminating cellular building blocks via selective autophagy. J. Cell. Biol. 2014, 205, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Tooze, S.A.; Schiavo, G. Liaisons dangereuses: Autophagy, neuronal survival and neurodegeneration. Curr. Opin. Neurobiol. 2008, 18, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy in protein and organelle turnover. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2011. [Google Scholar]
- Xie, Z.; Klionsky, D.J. Autophagosome formation: Core machinery and adaptations. Nat. Cell. Biol. 2007, 9, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2017, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Yoshino, K.; Kondo, C.; Kawamata, T.; Oshiro, N.; Yonezawa, K.; Ohsumi, Y. Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol. Cell. Biol. 2010, 30, 1049–1058. [Google Scholar] [CrossRef]
- Pasquali, L.; Busceti, C.L.; Fulceri, F.; Paparelli, A.; Fornai, F. Intracellular pathways underlying the effects of lithium. Behav. Pharmacol. 2010, 21, 473–492. [Google Scholar] [CrossRef]
- Zhou, J.; Tan, S.H.; Nicolas, V.; Bauvy, C.; Yang, N.D.; Zhang, J.; Xue, Y.; Codogno, P.; Shen, H.M. Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res. 2013, 23, 508–523. [Google Scholar] [CrossRef]
- Pietrocola, F.; Mariño, G.; Lissa, D.; Vacchelli, E.; Malik, S.A.; Niso-Santano, M.; Zamzami, N.; Galluzzi, L.; Maiuri, M.C.; Kroemer, G. Pro-autophagic polyphenols reduce the acetylation of cytoplasmic proteins. Cell Cycle 2012, 11, 3851–3860. [Google Scholar] [CrossRef]
- Janda, E.; Lascala, A.; Carresi, C.; Parafati, M.; Aprigliano, S.; Russo, V.; Savoia, C.; Ziviani, E.; Musolino, V.; Morani, F.; et al. Parkinsonian toxin-induced oxidative stress inhibits basal autophagy in astrocytes via NQO2/quinone oxidoreductase 2: Implications for neuroprotection. Autophagy 2015, 11, 1063–1080. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Zou, L.; Wu, Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 2014, 21, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Myers, O.B.; Moseley, P.L. Heat shock response and autophagy—Cooperation and control. Autophagy 2015, 11, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Tsapras, P.; Nezis, I.P. Caspase involvement in autophagy. Cell Death Differ. 2017, 24, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Lu, J.H.; Liu, L.F.; Chen, L.L.; Durairajan, S.S.; Yue, Z.; Zhang, H.Q.; Li, M. HMGB1 is involved in autophagy inhibition caused by SNCA/alpha-synuclein overexpression: A process modulated by the natural autophagy inducer corynoxine B. Autophagy 2014, 10, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M.; Stefanis, L.; Fredenburg, R.; Lansbury, P.T.; Sulzer, D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 2004, 305, 1292–1295. [Google Scholar] [CrossRef]
- Martinez-Vicente, M.; Talloczy, Z.; Kaushik, S.; Massey, A.C.; Mazzulli, J.; Mosharov, E.V.; Hodara, R.; Fredenburg, R.; Wu, D.C.; Follenzi, A.; et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J. Clin. Investig. 2008, 118, 777–788. [Google Scholar] [CrossRef]
- Anglade, P.; Vyas, S.; Javoy-Agid, F.; Herrero, M.T.; Michel, P.P.; Marquez, J.; Mouatt-Prigent, A.; Ruberg, M.; Hirsch, E.C.; Agid, Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol. Histopathol. 1997, 12, 25–31. [Google Scholar]
- Mayer, R.J.; Lowe, J.; Lennox, G.; Doherty, F.; Landon, M. Intermediate filaments and ubiquitin: A new thread in the understanding of chronic neurodegenerative diseases. Prog. Clin. Biol. Res. 1989, 317, 809–818. [Google Scholar]
- Mayer, R.J.; Lowe, J.; Lennox, G.; Landon, M.; MacLennan, K.; Doherty, F.J. Intermediate filament-ubiquitin diseases: Implications for cell sanitization. Biochem. Soc. Symp. 1989, 55, 193–201. [Google Scholar] [PubMed]
- Zhu, J.H.; Guo, F.; Shelburne, J.; Watkins, S.; Chu, C.T. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 2003, 13, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Dodiya, H.; Aebischer, P.; Olanow, C.W.; Kordower, J.H. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: Relationship to alpha-synuclein inclusions. Neurobiol. Dis. 2009, 35, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Dehay, B.; Bove, J.; Rodriguez-Muela, N.; Perier, C.; Recasens, A.; Boya, P.; Vila, M. Pathogenic lysosomal depletion in Parkinson’s disease. J. Neurosci. 2010, 30, 12535–12544. [Google Scholar] [CrossRef] [PubMed]
- Klucken, J.; Poehler, A.M.; Ebrahimi-Fakhari, D.; Schneider, J.; Nuber, S.; Rockenstein, E.; Schlötzer-Schrehardt, U.; Hyman, B.T.; McLean, P.J.; Masliah, E.; et al. Alpha-synuclein aggregation involves a bafilomycin A 1-sensitive autophagy pathway. Autophagy 2012, 8, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.M.; Chan, B.K.; Park, J.S.; Hill, K.J.; Aitken, J.B.; Cottle, L.; Farghaian, H.; Cole, A.R.; Lay, P.A.; Sue, C.M.; et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha-Synuclein externalization via exosomes. Hum. Mol. Genet. 2014, 23, 2816–2833. [Google Scholar] [CrossRef] [PubMed]
- Crews, L.; Spencer, B.; Desplats, P.; Patrick, C.; Paulino, A.; Rockenstein, E.; Hansen, L.; Adame, A.; Galasko, D.; Masliah, E. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS ONE 2010, 5, e9313. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L.; Larsen, K.E.; Rideout, H.J.; Sulzer, D.; Greene, L.A. Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J. Neurosci. 2001, 21, 9549–9560. [Google Scholar] [CrossRef]
- Webb, J.L.; Ravikumar, B.; Atkins, J.; Skepper, J.N.; Rubinsztein, D.C. Alpha-Synuclein is degraded by both autophagy and the proteasome. J. Biol. Chem. 2003, 278, 25009–25013. [Google Scholar] [CrossRef]
- Vogiatzi, T.; Xilouri, M.; Vekrellis, K.; Stefanis, L. Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J. Biol. Chem. 2008, 283, 23542–23556. [Google Scholar] [CrossRef]
- Yu, W.H.; Dorado, B.; Figueroa, H.Y.; Wang, L.; Planel, E.; Cookson, M.R.; Clark, L.N.; Duff, K.E. Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric alpha-synuclein. Am. J. Pathol. 2009, 175, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Castino, R.; Lazzeri, G.; Lenzi, P.; Bellio, N.; Follo, C.; Ferrucci, M.; Fornai, F.; Isidoro, C. Suppression of autophagy precipitates neuronal cell death following low doses of methamphetamine. J. Neurochem. 2008, 106, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Chandramani-Shivalingappa, P.; Jin, H.; Ghosh, A.; Anantharam, V.; Ali, S.; Kanthasamy, A.G.; Kanthasamy, A. Methamphetamine-induced neurotoxicity linked to ubiquitin-proteasome system dysfunction and autophagy-related changes that can be modulated by protein kinase C delta in dopaminergic neuronal cells. Neuroscience 2012, 210, 308–332. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wan, J.; Meng, J.; Banerjee, S.; Ramakrishnan, S.; Roy, S. Methamphetamine induces autophagy as a pro-survival response against apoptotic endothelial cell death through the Kappa opioid receptor. Cell Death Dis. 2014, 5, e1099. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Wu, W.R.; Lee, L.M.; Huang, P.R.; Chen, J.C. mTOR signaling in the nucleus accumbens mediates behavioral sensitization to methamphetamine. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 331–339. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yuan, W.; Li, Z.; Hou, Y.; Liu, F.; Feng, J. 6-Hydroxydopamine induces autophagic flux dysfunction by impairing transcription factor EB activation and lysosomal function in dopaminergic neurons and SH-SY5Y cells. Toxicol. Lett. 2018, 283, 58–68. [Google Scholar] [CrossRef]
- Pal, R.; Bajaj, L.; Sharma, J.; Palmieri, M.; Di Ronza, A.; Lotfi, P.; Chaudhury, A.; Neilson, J.; Sardiello, M.; Rodney, G.G. NADPH oxidase promotes Parkinsonian phenotypes by impairing autophagic flux in an mTORC1-independent fashion in a cellular model of Parkinson’s disease. Sci. Rep. 2016, 6, 22866. [Google Scholar] [CrossRef]
- Pan, T.; Rawal, P.; Wu, Y.; Xie, W.; Jankovic, J.; Le, W. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience 2009, 164, 541–551. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, J.; Zuo, Y.; Gu, Z. Rapamycin protects the mitochondria against oxidative stress and apoptosis in a rat model of Parkinson’s disease. Int. J. Mol. Med. 2013, 31, 825–832. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Chen, B.; Zhang, J.P.; Ma, Y.Y. Up-regulation of autophagy-related gene 5 (ATG5) protects dopaminergic neurons in a zebrafish model of Parkinson’s disease. J. Biol. Chem. 2017, 292, 18062–18074. [Google Scholar] [CrossRef]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006, 441, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Wang, Q.J.; Holstein, G.R.; Friedrich, V.L., Jr.; Iwata, J.; Kominami, E.; Chait, B.T.; Tanaka, K.; Yue, Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc. Natl. Acad. Sci. USA 2007, 104, 14489–14494. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Cho, E.D.; Lee, K.W.; Kim, J.H.; Cho, S.G.; Lee, S.J. Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp. Mol. Med. 2013, 45, e22. [Google Scholar] [CrossRef] [PubMed]
- Vekrellis, K.; Xilouri, M.; Emmanouilidou, E.; Rideout, H.J.; Stefanis, L. Pathological roles of α-synuclein in neurological disorders. Lancet Neurol. 2011, 10, 1015–1025. [Google Scholar] [CrossRef]
- Xilouri, M.; Brekk, O.R.; Stefanis, L. Autophagy and Alpha-Synuclein: Relevance to Parkinson’s Disease and Related Synucleopathies. Mov. Disord. 2016, 31, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Mizushima, N. Autophagy and human diseases. Cell Res. 2013, 24, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Ishikawa, T.; Michiue, T.; Li, D.R.; Zhao, D.; Oritani, S.; Zhu, B.L.; Maeda, H. Ubiquitin-immunoreactive structures in the midbrain of methamphetamine abusers. Leg. Med. 2005, 7, 144–150. [Google Scholar] [CrossRef]
- Limanaqi, F.; Gambardella, S.; Biagioni, F.; Busceti, C.L.; Fornai, F. Epigenetic Effects Induced by Methamphetamine and Methamphetamine-Dependent Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 4982453. [Google Scholar] [CrossRef]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; Dos Santos, C.N. Polyphenols Beyond Barriers: A Glimpse into the Brain. Curr. Neuropharmacol. 2017, 15, 562–594. [Google Scholar] [CrossRef]
- Lokanathan, Y.; Omar, N.; Ahmad Puzi, N.N.; Saim, A.; Hj Idrus, R. Recent Updates in Neuroprotective and Neuroregenerative Potential of Centella asiatica. Malays J. Med. Sci. 2016, 23, 4–14. [Google Scholar] [PubMed]
- Bohlmann, J.; Keeling, C.I. Terpenoid biomaterials. Plant J. 2008, 54, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Konar, A.; Kaul, S.C. Nootropic potential of Ashwagandha leaves: Beyond traditional root extracts. Neurochem. Int. 2016, 95, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Nagoor Meeran, M.F.; Azimullah, S.; Adem, A.; Sadek, B.; Ojha, S.K. Plant Extracts and Phytochemicals Targeting α-Synuclein Aggregation in Parkinson’s Disease Models. Front. Pharmacol. 2019, 9, 1555. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Wang, W.; Zhuang, J.; Zhao, Z. Proteasome inhibitor-induced autophagy in PC12 cells overexpressing A53T mutant α-synuclein. Mol. Med. Rep. 2015, 11, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Heebkaew, N.; Rujanapun, N.; Kunhorm, P.; Jaroonwitchawan, T.; Chaicharoenaudomrung, N.; Promjantuek, W.; Noisa, P. Curcumin Induces Neural Differentiation of Human Pluripotent Embryonal Carcinoma Cells through the Activation of Autophagy. Biomed. Res. Int. 2019, 2019, 4378710. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhao, Y.; Han, S.; Zhang, N.; Chen, H.; Wang, X. Autophagy-ERK1/2-Involved Disinhibition of Hippocampal Neurons Contributes to the Pre-Synaptic Toxicity Induced by Aβ42 Exposure. J. Alzheimers Dis. 2017, 59, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Pan, X.Y.; Xu, Y.; Xiao, Y.; An, Y.; Tie, L.; Pan, Y.; Li, X.J. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy 2012, 8, 812–825. [Google Scholar] [CrossRef]
- Guo, S.; Long, M.; Li, X.; Zhu, S.; Zhang, M.; Yang, Z. Curcumin activates autophagy and attenuates oxidative damage in EA. hy926 cells via the Akt/mTOR pathway. Mol. Med. Rep. 2016, 13, 2187–2193. [Google Scholar] [CrossRef]
- Taebnia, N.; Morshedi, D.; Yaghmaei, S.; Aliakbari, F.; Rahimi, F.; Arpanaei, A. Curcumin-loaded amine-functionalized mesoporous silica nanoparticles inhibit α-synuclein fibrillation and reduce its cytotoxicity-associated effects. Langmuir 2016, 32, 13394–13402. [Google Scholar] [CrossRef]
- Bollimpelli, V.S.; Kumar, P.; Kumari, S.; Kondapi, A.K. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem. Int. 2016, 95, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Jaroonwitchawan, T.; Chaicharoenaudomrung, N.; Namkaew, J.; Noisa, P. Curcumin attenuates paraquat-induced cell death in human neuroblastoma cells through modulating oxidative stress and autophagy. Neurosci. Lett. 2017, 636, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, K.; Wu, H.Y.; Wu, Y.P.; Li, B.X. Isoflavones Induce BEX2-Dependent Autophagy to Prevent ATR-Induced Neurotoxicity in SH-SY5Y Cells. Cell. Physiol. Biochem. 2017, 43, 1866–1879. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Sun, Y.R.; Peluso, I.; Zeng, Y.; Yu, X.; Lu, J.H.; Xu, Z.; Wang, M.Z.; Liu, L.F.; Huang, Y.Y.; et al. A novel curcumin analog binds to and activates TFEB in vitro and in vivo independent of MTOR inhibition. Autophagy 2016, 12, 1372–1389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Xu, J.; Lu, Y.; Jiang, J.; Wang, L.; Shen, H.M.; Xia, D. Curcumin targets the TFEB-lysosome pathway for induction of autophagy. Oncotarget 2016, 7, 75659–75671. [Google Scholar] [CrossRef] [PubMed]
- Decressac, M.; Mattsson, B.; Weikop, P.; Lundblad, M.; Jakobsson, J.; Björklund, A. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc. Natl. Acad. Sci. USA 2013, 110, E1817–E1826. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Das, M.; Tripathy, K.; Sahoo, S.K. Delivery of dual drug loaded lipid based nanoparticles across the blood-brain barrier imparts enhanced neuroprotection in a rotenone induced mouse model of Parkinson’s disease. ACS Chem. Neurosci. 2016, 7, 1658–1670. [Google Scholar] [CrossRef]
- Das, D.N.; Naik, P.P.; Nayak, A.; Panda, P.K.; Mukhopadhyay, S.; Sinha, N.; Bhutia, S.K. Bacopa monnieri-Induced Protective Autophagy Inhibits Benzo[a]pyrene-Mediated Apoptosis. Phytother. Res. 2016, 30, 1794–1801. [Google Scholar] [CrossRef]
- Smith, E.; Palethorpe, H.M.; Tomita, Y.; Pei, J.V.; Townsend, A.R.; Price, T.J.; Young, J.P.; Yool, A.J.; Hardingham, J.E. The Purified Extract from the Medicinal Plant Bacopa monnieri, Bacopaside II, Inhibits Growth of Colon Cancer Cells In Vitro by Inducing Cell Cycle Arrest and Apoptosis. Cells 2018, 7, 81. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.C.; Bartlett, J.J.; Perez, L.J.; Brunt, K.R.; Legare, J.F.; Hassan, A.; Kienesberger, P.C.; Pulinilkunnil, T. Glucolipotoxicity diminishes cardiomyocyte TFEB and inhibits lysosomal autophagy during obesity and diabetes. Biochim. Biophys. Acta 2016, 1861, 1893–1910. [Google Scholar] [CrossRef] [PubMed]
- Holczer, M.; Besze, B.; Zámbó, V.; Csala, M.; Bánhegyi, G.; Kapuy, O. Epigallocatechin-3-Gallate (EGCG) Promotes Autophagy-Dependent Survival via Influencing the Balance of mTOR-AMPK Pathways upon Endoplasmic Reticulum Stress. Oxid. Med. Cell. Longev. 2018, 2018, 6721530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, S.; Xu, J.; Li, W.; Duan, G.; Wang, H.; Yang, H.; Yang, Z.; Zhou, R. A new molecular mechanism underlying the EGCG-mediated autophagic modulation of AFP in HepG2 cells. Cell Death Dis. 2017, 8, e3160. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Moon, J.H.; Kim, S.W.; Jeong, J.K.; Nazim, U.M.; Lee, Y.J.; Seol, J.W.; Park, S.Y. EGCG-mediated autophagy flux has a neuroprotection effect via a class III histone deacetylase in primary neuron cells. Oncotarget 2015, 6, 9701–9717. [Google Scholar] [CrossRef]
- Li, W.; Zhu, S.; Li, J.; Assa, A.; Jundoria, A.; Xu, J.; Fan, S.; Eissa, N.T.; Tracey, K.J.; Sama, A.E.; et al. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem. Pharmacol. 2011, 81, 1152–1163. [Google Scholar] [CrossRef]
- Kim, H.S.; Montana, V.; Jang, H.J.; Parpura, V.; Kim, J.A. Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: A potential role for reducing lipid accumulation. J. Biol. Chem. 2013, 288, 22693–22705. [Google Scholar] [CrossRef]
- Zhou, J.; Farah, B.L.; Sinha, R.A.; Wu, Y.; Singh, B.K.; Bay, B.H.; Yang, C.S.; Yen, P.M. Epigallocatechin-3-Gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS ONE 2014, 9, e87161. [Google Scholar] [CrossRef]
- Chen, C.M.; Wu, C.T.; Yang, T.H.; Chang, Y.A.; Sheu, M.L.; Liu, S.H. Green Tea Catechin Prevents Hypoxia/Reperfusion-Evoked Oxidative Stress-Regulated Autophagy-Activated Apoptosis and Cell Death in Microglial Cells. J. Agric. Food Chem. 2016, 64, 4078–4085. [Google Scholar] [CrossRef]
- Wei, R.; Mao, L.; Xu, P.; Zheng, X.; Hackman, R.M.; Mackenzie, G.G.; Wang, Y. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct. 2018, 9, 5682–5696. [Google Scholar] [CrossRef]
- Grube, S.; Ewald, C.; Kögler, C.; Lawson McLean, A.; Kalff, R.; Walter, J. Achievable Central Nervous System Concentrations of the Green Tea Catechin EGCG Induce Stress in Glioblastoma Cells in Vitro. Nutr. Cancer 2018, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Sakagami, H. Induction of apoptosis by epigallocatechin gallate and autophagy inhibitors in a mouse macrophage-like cell line. Anticancer Res. 2008, 28, 1713–1718. [Google Scholar] [PubMed]
- Renaud, J.; Nabavi, S.F.; Daglia, M.; Nabavi, S.M.; Martinoli, M.G. Epigallocatechin-3-Gallate, a Promising Molecule for Parkinson’s Disease? Rejuvenation Res. 2015, 18, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.H.; Kim, S.H.; Kwon, H.; Park, Y.; Kim, K.S.; Song, C.W.; Kim, J.; Kim, M.H.; Yu, H.J.; Henkel, J.S.; et al. Epigallocatechin gallate protects nerve growth factor differentiated PC12 cells from oxidative-radical-stress-induced apoptosis through its effect on phosphoinositide 3-kinase/Akt and glycogen synthase kinase-3. Brain Res. Mol. Brain. Res. 2003, 118, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Šneideris, T.; Baranauskiene, L.; Cannon, J.G.; Rutkiene, R.; Meškys, R.; Smirnovas, V. Looking for a generic inhibitor of amyloid-like fibril formation among flavone derivatives. Peer J. 2015, 3, e1271. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, O.; Mandel, S.; Youdim, M.B.H.; Amit, T. Targeting dysregulation of brain iron homeostasis in Parkinson’s disease by iron chelators. Free Radic. Biol. Med. 2013, 62, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bosch-Marce, M.; Shimoda, L.A.; Tan, Y.S.; Baek, J.H.; Wesley, J.B.; Gonzalez, F.J.; Semenza, G.L. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008, 283, 10892–91003. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, N.; Meeker, H.C.; Brown, W.T. Novel Epigenetic Regulation of Alpha-Synuclein Expression in Down Syndrome. Mol. Neurobiol. 2016, 53, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef]
- Bae, S.Y.; Kim, S.; Hwang, H.; Kim, H.K.; Yoon, H.C.; Kim, J.H.; Lee, S.; Kim, T.D. Amyloid formation and disaggregation of α-synuclein and its tandem repeat (α-TR). Biochem. Biophys. Res. Commun. 2010, 400, 531–536. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, S.; Shi, D.; Bai, Q.; Liu, H.; Yao, X. Influence of EGCG on α-synuclein (αS) aggregation and identification of their possible binding mode: A computational study using molecular dynamics simulation. Chem. Biol. Drug Des. 2018, 91, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Gong, Q.; Xiong, X.; Sun, L.; Zhao, W.; Zhu, W.; Lu, Y. Protective effect of madecassoside on H2O2-induced oxidative stress and autophagy activation in human melanocytes. Oncotarget 2017, 8, 51066–51075. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Cao, Q.X.; Zhai, F.R.; Yang, S.Q.; Zhang, H.X. Asiatic acid exerts anticancer potential in human ovarian cancer cells via suppression of PI3K/Akt/mTOR signalling. Pharm. Biol. 2016, 54, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.F.; Xiong, Y.Y.; Liu, J.K.; Qian, J.J.; Zhu, L.; Gao, J. Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharmacol. Sin. 2012, 33, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, R.; Vasudevaraju, P.; Indi, S.S.; Sambasiva Rao, K.R.; Rao, K.S. In vitro evidence that an aqueous extract of Centella asiatica modulates α-synuclein aggregation dynamics. J. Alzheimers Dis. 2014, 39, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Carver, J.A.; Calabrese, A.N.; Pukala, T.L. Gallic acid interacts with α-synuclein to prevent the structural collapse necessary for its aggregation. Biochim. Biophys. Acta 2014, 1844, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Ardah, M.T.; Paleologou, K.E.; Lv, G.; Abul Khair, S.B.; Kazim, A.S.; Minhas, S.T.; Al-Tel, T.H.; Al-Hayani, A.A.; Haque, M.E.; Eliezer, D.; et al. Structure activity relationship of phenolic acid inhibitors of α-synuclein fibril formation and toxicity. Front. Aging Neurosci. 2014, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Akhoon, B.A.; Pandey, S.; Tiwari, S.; Pandey, R. Withanolide A offers neuroprotection, ameliorates stress resistance and prolongs the life expectancy of Caenorhabditis elegans. Exp. Gerontol. 2016, 78, 47–56. [Google Scholar] [CrossRef]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br. J. Pharmacol. 2005, 144, 961–971. [Google Scholar] [CrossRef]
- Okamoto, S.; Tsujioka, T.; Suemori, S.; Kida, J.; Kondo, T.; Tohyama, Y.; Tohyama, K. Withaferin A suppresses the growth of myelodysplasia and leukemia cell lines by inhibiting cell cycle progression. Cancer Sci. 2016, 107, 1302–1314. [Google Scholar] [CrossRef]
- Dutta, K.; Patel, P.; Julien, J.P. Protective effects of Withania somnifera extract in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2018, 309, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, F.; Jiang, J.; Sun, C.; Zhong, Q.; Shen, M.; Wang, X.; Tian, R.; Shi, C.; Xu, M.; et al. Simultaneous inhibition of the ubiquitin-proteasome system and autophagy enhances apoptosis induced by ER stress aggravators in human pancreatic cancer cells. Autophagy 2016, 12, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; De, S.; Mukherjee, S.; Das, S.; Ghosh, A.N.; Sengupta, S.B. Withaferin A induced impaired autophagy and unfolded protein response in human breast cancer cell-lines MCF-7 and MDA-MB-231. Toxicol. In Vitro 2017, 44, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Muniraj, N.; Siddharth, S.; Nagalingam, A.; Walker, A.; Woo, J.; Gyorffy, B.; Gabrielson, E.; Saxena, N.K.; Sharma, D. Withaferin A inhibits lysosomal activity to block autophagic flux and induces apoptosis via energetic impairment in breast cancer cells. Carcinogenesis 2019, in press. [Google Scholar] [CrossRef]
- Siddharth, S.; Muniraj, N.; Saxena, N.K.; Sharma, D. Concomitant Inhibition of Cytoprotective Autophagy Augments the Efficacy of Withaferin A in Hepatocellular Carcinoma. Cancers 2019, 11, 453. [Google Scholar] [CrossRef]
- Rah, B.; ur Rasool, R.; Nayak, D.; Yousuf, S.K.; Mukherjee, D.; Kumar, L.D.; Goswami, A. PAWR-mediated suppression of BCL2 promotes switching of 3-azido withaferin A (3-AWA)-induced autophagy to apoptosis in prostate cancer cells. Autophagy 2015, 11, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef]
- Park, D.; Jeong, H.; Lee, M.N.; Koh, A.; Kwon, O.; Yang, Y.R.; Noh, J.; Suh, P.G.; Park, H.; Ryu, S.H. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 2016, 6, 21772. [Google Scholar] [CrossRef]
- Suvorova, I.I.; Knyazeva, A.R.; Petukhov, A.V.; Aksenov, N.D.; Pospelov, VA. Resveratrol enhances pluripotency of mouse embryonic stem cells by activating AMPK/Ulk1 pathway. Cell Death Discov. 2019, 5, 61. [Google Scholar] [CrossRef]
- Joe, I.S.; Jeong, S.G.; Cho, G.W. Resveratrol-induced SIRT1 activation promotes neuronal differentiation of human bone marrow mesenchymal stem cells. Neurosci. Lett. 2015, 584, 97–102. [Google Scholar] [CrossRef]
- Yang, Q.B.; He, Y.L.; Zhong, X.W.; Xie, W.G.; Zhou, J.G. Resveratrol ameliorates gouty inflammation via upregulation of sirtuin 1 to promote autophagy in gout patients. Inflammopharmacology 2019, 27, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Cao, N.; Li, Z.; Han, J.; Li, L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. Onco Targets Ther. 2018, 11, 7777–7786. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, X. Resveratrol alleviates early brain injury following subarachnoid hemorrhage: Possible involvement of the AMPK/SIRT1/autophagy signaling pathway. Biol. Chem. 2018, 399, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, Z.; Wang, Y.; Hou, Y.; Li, L.; Zhao, J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int. Immunopharmacol. 2017, 50, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Bai, L.; Lu, W.; Gao, Y.; Bi, Y.; Lv, G. Regulation of autophagy by AMP-activated protein kinase/sirtuin 1 pathway reduces spinal cord neurons damage. Iran. J. Basic Med. Sci. 2017, 20, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, S.; Gao, K.; Zhou, Z.; Wang, C.; Shen, Z.; Guo, Y.; Li, Z.; Wan, Z.; Liu, C.; et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience 2017, 348, 241–251. [Google Scholar] [CrossRef]
- Deng, H.; Mi, M.T. Resveratrol Attenuates Aβ25-35 Caused Neurotoxicity by Inducing Autophagy Through the TyrRS-PARP1-SIRT1 Signaling Pathway. Neurochem. Res. 2016, 41, 2367–2379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, X.; Zhu, W.; Liu, Z.; Liu, H.; Zhou, Y.; Cao, Y.; Liu, C.; Xie, Y. Resveratrol Enhances Autophagic Flux and Promotes Ox-LDL Degradation in HUVECs via Upregulation of SIRT1. Oxid. Med. Cell. Longev. 2016, 2016, 7589813. [Google Scholar] [CrossRef]
- Al Azzaz, J.; Rieu, A.; Aires, V.; Delmas, D.; Chluba, J.; Winckler, P.; Bringer, M.A.; Lamarche, J.; Vervandier-Fasseur, D.; Dalle, F.; et al. Resveratrol-Induced Xenophagy Promotes Intracellular Bacteria Clearance in Intestinal Epithelial Cells and Macrophages. Front. Immunol. 2019, 9, 3149. [Google Scholar] [CrossRef]
- Du, L.; Chen, E.; Wu, T.; Ruan, Y.; Wu, S. Resveratrol attenuates hydrogen peroxide-induced aging through upregulation of autophagy in human umbilical vein endothelial cells. Drug Des. Devel. Ther. 2019, 13, 747–755. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, T.; Li, W.; Gao, N.; Zhang, T. Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol. Lett. 2018, 282, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Sivanesam, K.; Andersen, N.H. Modulating the Amyloidogenesis of α-Synuclein. Curr. Neuropharmacol. 2016, 14, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kang, S.J.; Poncz, M.; Song, K.J.; Park, K.S. Resveratrol protects SH-SY5Y neuroblastoma cells from apoptosis induced by dopamine. Exp. Mol. Med. 2007, 39, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.C.; Wei, Y.; An, Z.; Zou, Z.; Xiao, G.; Bhagat, G.; White, M.; Reichelt, J.; Levine, B. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 2012, 338, 956–959. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; She, H.; Zhang, T.; Xu, H.; Cheng, L.; Yepes, M.; Zhao, Y.; Mao, Z. p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. J. Cell Biol. 2018, 217, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yang, S.; Li, S.; Hang, C. Melatonin Upregulates Nuclear Factor Erythroid-2 Related Factor 2 (Nrf2) and Mediates Mitophagy to Protect Against Early Brain Injury After Subarachnoid Hemorrhage. Med. Sci. Monit. 2018, 24, 6422–6430. [Google Scholar] [CrossRef]
- Manzoni, C.; Mamais, A.; Dihanich, S.; Abeti, R.; Soutar, M.P.M.; Plun-Favreau, H.; Giunti, P.; Tooze, S.A.; Bandopadhyay, R.; Lewis, P.A. Inhibition of LRRK2 kinase activity stimulates macroautophagy. Biochim. Biophys. Acta 2013, 1833, 2900–2910. [Google Scholar] [CrossRef]
- Wang, D.; Ji, X.; Liu, J.; Li, Z.; Zhang, X. Dopamine Receptor Subtypes Differentially Regulate Autophagy. Int. J. Mol. Sci. 2018, 19, 1540. [Google Scholar] [CrossRef]
- Chen, A.; Xiong, L.J.; Tong, Y.; Mao, M. Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol. Med. Rep. 2013, 8, 1011–1016. [Google Scholar] [CrossRef]
- Pandey, U.B.; Nie, Z.; Batlevi, Y.; McCray, B.A.; Ritson, G.P.; Nedelsky, N.B.; Schwartz, S.L.; DiProspero, N.A.; Knight, M.A.; Schuldiner, O.; et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 2007, 447, 859–863. [Google Scholar] [CrossRef]
- Natale, G.; Pasquali, L.; Paparelli, A.; Fornai, F. Parallel manifestations of neuropathologies in the enteric and central nervous systems. Neurogastroenterol. Motil. 2011, 23, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Chen, Z.; Leng, S.X. Connection between Systemic Inflammation and Neuroinflammation Underlies Neuroprotective Mechanism of Several Phytochemicals in Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 1972714. [Google Scholar] [CrossRef] [PubMed]
- Akintunde, J.K.; Farouk, A.A.; Mogbojuri, O. Metabolic treatment of syndrome linked with Parkinson’s disease and hypothalamus pituitary gonadal hormones by turmeric curcumin in Bisphenol-A induced neuro-testicular dysfunction of wistar rat. Biochem. Biophys. Rep. 2018, 17, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. Biomed. Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).