Carcinoembryonic Cell Adhesion-Related Molecule 2 Regulates Insulin Secretion and Energy Balance
Abstract
1. General Introduction
2. Gene Structure of CEACAM2
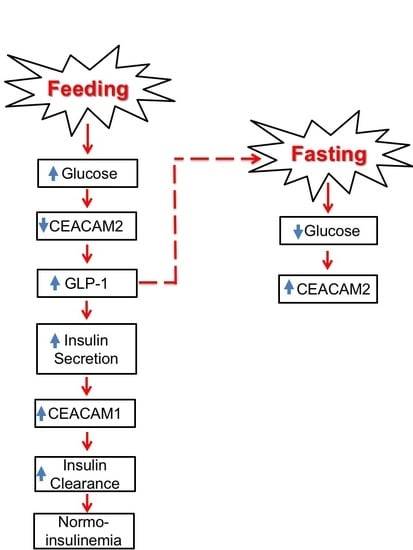
3. Role of CEACAM2 in Insulin Secretion
4. Role of CEACAM2 in Insulin Clearance
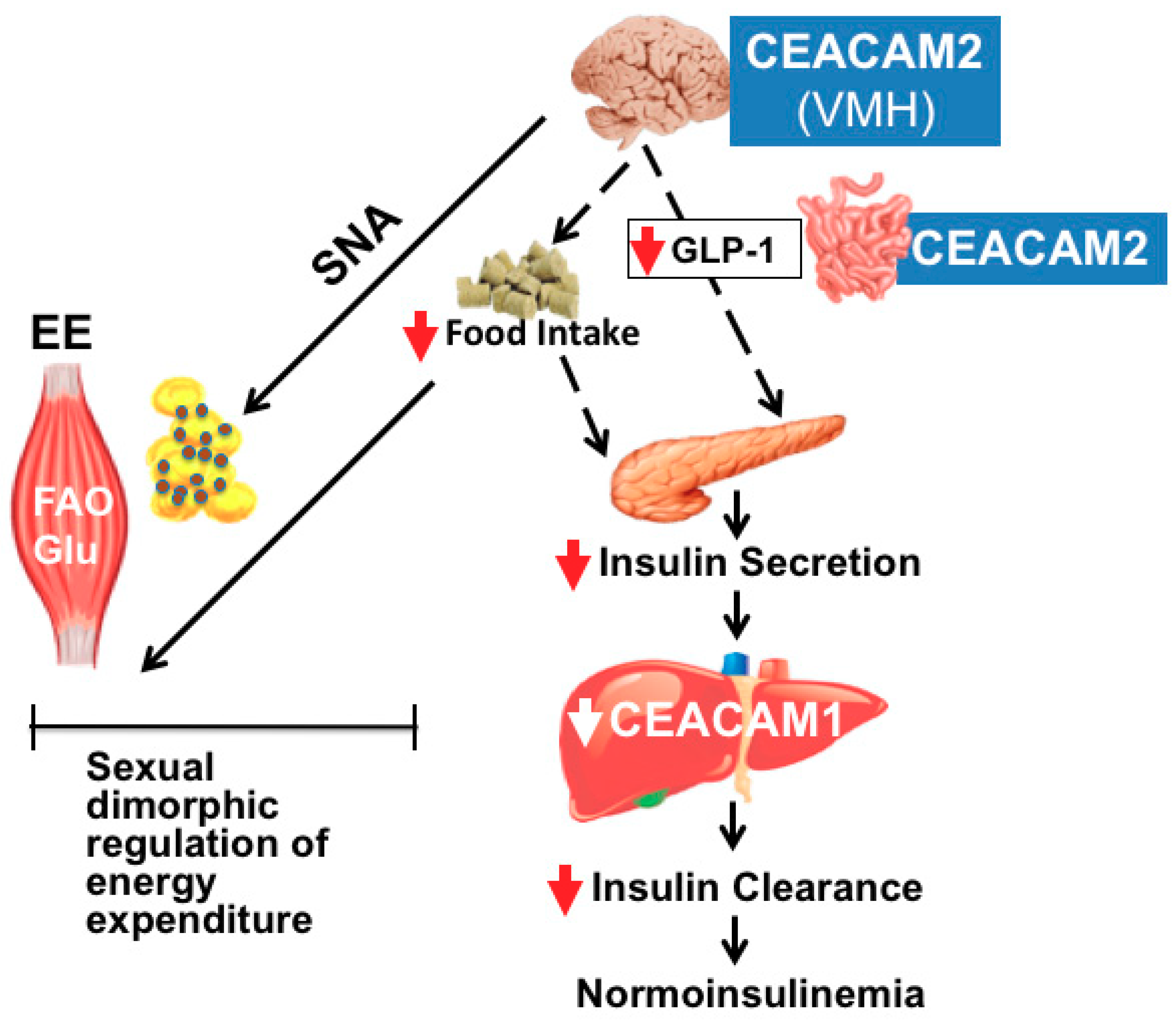
5. Role of CEACAM2 in Food Intake: Effect on Insulin Action
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horst, A.K.; Najjar, S.M.; Wagener, C.; Tiegs, G. Ceacam1 in liver injury, metabolic and immune regulation. Int. J. Mol. Sci. 2018, 19, 3110. [Google Scholar] [CrossRef]
- Najjar, S.; Boisclair, Y.; Nabih, Z.; Philippe, N.; Imai, Y.; Suzuki, Y.; Suh, D.; Ooi, G. Cloning and characterization of a functional promoter of the rat pp120 gene, encoding a substrate of the insulin receptor tyrosine kinase. J. Biol. Chem. 1996, 271, 8809–8817. [Google Scholar] [CrossRef]
- Ramakrishnan, S.K.; Khuder, S.S.; Al-Share, Q.Y.; Russo, L.; Abdallah, S.L.; Patel, P.R.; Heinrich, G.; Muturi, H.T.; Mopidevi, B.R.; Oyarce, A.M.; et al. Pparalpha (peroxisome proliferator-activated receptor alpha) activation reduces hepatic ceacam1 protein expression to regulate fatty acid oxidation during fasting-refeeding transition. J. Biol. Chem. 2016, 291, 8121–8129. [Google Scholar] [CrossRef]
- Dery, K.J.; Silver, C.; Yang, L.; Shively, J.E. Interferon regulatory factor 1 and a variant of heterogeneous nuclear ribonucleoprotein l coordinately silence the gene for adhesion protein ceacam1. J. Biol. Chem. 2018, 293, 9277–9291. [Google Scholar] [CrossRef]
- Heinrich, G.; Ghadieh, H.E.; Ghanem, S.S.; Muturi, H.T.; Rezaei, K.; Al-Share, Q.Y.; Bowman, T.A.; Zhang, D.; Garofalo, R.S.; Yin, L.; et al. Loss of hepatic ceacam1: A unifying mechanism linking insulin resistance to obesity and non-alcoholic fatty liver disease. Front. Endocrinol. (Lausanne) 2017, 8, 8. [Google Scholar] [CrossRef]
- Najjar, S.M.; Perdomo, G. Hepatic insulin clearance: Mechanism and physiology. Physiology (Bethesda) 2019, 34, 198–215. [Google Scholar] [CrossRef]
- Gray-Owen, S.D.; Blumberg, R.S. Ceacam1: Contact-dependent control of immunity. Nat. Rev. Immunol. 2006, 6, 433–446. [Google Scholar] [CrossRef]
- Han, E.; Phan, D.; Lo, P.; Poy, M.N.; Behringer, R.; Najjar, S.M.; Lin, S.H. Differences in tissue-specific and embryonic expression of mouse ceacam1 and ceacam2 genes. Biochem. J. 2001, 355, 417–423. [Google Scholar] [CrossRef]
- Salaheldeen, E.; Kurio, H.; Howida, A.; Iida, H. Molecular cloning and localization of a ceacam2 isoform, ceacam2-l, expressed in spermatids in mouse testis. Mol. Reprod. Dev. 2012, 79, 843–852. [Google Scholar] [CrossRef]
- Salaheldeen, E.; Howida, A.; Wakayama, T.; Iida, H. Ceacam2-l on spermatids interacts with poliovirus receptor on sertoli cells in mouse seminiferous epithelium. J. Histochem. Cytochem. 2014, 62, 632–644. [Google Scholar] [CrossRef]
- Alshahrani, M.M.; Yang, E.; Yip, J.; Ghanem, S.S.; Abdallah, S.L.; deAngelis, A.M.; O’Malley, C.J.; Moheimani, F.; Najjar, S.M.; Jackson, D.E. Ceacam2 negatively regulates hemi (itam-bearing) gpvi and clec-2 pathways and thrombus growth in vitro and in vivo. Blood 2014, 124, 2431–2441. [Google Scholar] [CrossRef]
- Gupta, S.; Yan, Y.; Malhotra, D.; Liu, J.; Xie, Z.; Najjar, S.M.; Shapiro, J.I. Ouabain and insulin induce sodium pump endocytosis in renal epithelium. Hypertension 2012, 59, 665–672. [Google Scholar] [CrossRef]
- Ghanem, S.S.; Heinrich, G.; Lester, S.G.; Pfeiffer, V.; Bhattacharya, S.; Patel, P.R.; DeAngelis, A.M.; Dai, T.; Ramakrishnan, S.K.; Smiley, Z.N.; et al. Increased glucose-induced secretion of glucagon-like peptide-1 in mice lacking the carcinoembryonic antigen-related cell adhesion molecule 2 (ceacam2). J. Biol. Chem. 2016, 291, 980–988. [Google Scholar] [CrossRef]
- Ghanem, S.S.; Muturi, H.T.; DeAngelis, A.M.; Hu, J.; Kulkarni, R.N.; Heinrich, G.; Najjar, S.M. Age-dependent insulin resistance in male mice with null deletion of the carcinoembryonic antigen-related cell adhesion molecule 2 gene. Diabetologia 2017, 60, 1751–1760. [Google Scholar] [CrossRef]
- Heinrich, G.; Ghosh, S.; Deangelis, A.M.; Schroeder-Gloeckler, J.M.; Patel, P.R.; Castaneda, T.R.; Jeffers, S.; Lee, A.D.; Jung, D.Y.; Zhang, Z.; et al. Carcinoembryonic antigen-related cell adhesion molecule 2 controls energy balance and peripheral insulin action in mice. Gastroenterology 2010, 139, 644–652. [Google Scholar] [CrossRef]
- Patel, P.R.; Ramakrishnan, S.K.; Kaw, M.K.; Raphael, C.K.; Ghosh, S.; Marino, J.S.; Heinrich, G.; Lee, S.J.; Bourey, R.E.; Hill, J.W.; et al. Increased metabolic rate and insulin sensitivity in male mice lacking the carcinoembryonic antigen-related cell adhesion molecule 2. Diabetologia 2012, 55, 763–772. [Google Scholar] [CrossRef]
- Nedellec, P.; Dveksler, G.S.; Daniels, E.; Turbide, C.; Chow, B.; Basile, A.A.; Holmes, K.V.; Beauchemin, N. Bgp2, a new member of the carcinoembryonic antigen-related gene family, encodes an alternative receptor for mouse hepatitis viruses. J. Virol. 1994, 68, 4525–4537. [Google Scholar]
- Najjar, S.M.; Accili, D.; Philippe, N.; Jernberg, J.; Margolis, R.; Taylor, S.I. Pp120/ecto-atpase, an endogenous substrate of the insulin receptor tyrosine kinase, is expressed as two variably spliced isoforms. J. Biol. Chem. 1993, 268, 1201–1206. [Google Scholar]
- Robitaille, J.; Izzi, L.; Daniels, E.; Zelus, B.; Holmes, K.V.; Beauchemin, N. Comparison of expression patterns and cell adhesion properties of the mouse biliary glycoproteins bbgp1 and bbgp2. Eur. J. Biochem. 1999, 264, 534–544. [Google Scholar] [CrossRef]
- Zebhauser, R.; Kammerer, R.; Eisenried, A.; McLellan, A.; Moore, T.; Zimmermann, W. Identification of a novel group of evolutionarily conserved members within the rapidly diverging murine cea family. Genomics 2005, 86, 566–580. [Google Scholar] [CrossRef]
- DeAngelis, A.M.; Heinrich, G.; Dai, T.; Bowman, T.A.; Patel, P.R.; Lee, S.J.; Hong, E.G.; Jung, D.Y.; Assmann, A.; Kulkarni, R.N.; et al. Carcinoembryonic antigen-related cell adhesion molecule 1: A link between insulin and lipid metabolism. Diabetes 2008, 57, 2296–2303. [Google Scholar] [CrossRef]
- D’Alessio, D. Is glp-1 a hormone: Whether and when? J. Diabetes Investig. 2016, 7 (Suppl. 1), 50–55. [Google Scholar] [CrossRef]
- Reimann, F.; Gribble, F.M. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes 2002, 51, 2757–2763. [Google Scholar] [CrossRef]
- Kedees, M.H.; Guz, Y.; Grigoryan, M.; Teitelman, G. Functional activity of murine intestinal mucosal cells is regulated by the glucagon-like peptide-1 receptor. Peptides 2013, 48, 36–44. [Google Scholar] [CrossRef]
- Habib, A.M.; Richards, P.; Rogers, G.J.; Reimann, F.; Gribble, F.M. Co-localisation and secretion of glucagon-like peptide 1 and peptide yy from primary cultured human l cells. Diabetologia 2013, 56, 1413–1416. [Google Scholar] [CrossRef]
- Seino, Y.; Yabe, D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: Incretin actions beyond the pancreas. J. Diabetes Investig. 2013, 4, 108–130. [Google Scholar] [CrossRef]
- Drucker, D.J. Deciphering metabolic messages from the gut drives therapeutic innovation: The 2014 banting lecture. Diabetes 2015, 64, 317–326. [Google Scholar] [CrossRef][Green Version]
- Sidhu, S.S.; Thompson, D.G.; Warhurst, G.; Case, R.M.; Benson, R.S. Fatty acid-induced cholecystokinin secretion and changes in intracellular ca2+ in two enteroendocrine cell lines, stc-1 and glutag. J. Physiol. 2000, 528, 165–176. [Google Scholar] [CrossRef]
- Reimann, F.; Maziarz, M.; Flock, G.; Habib, A.M.; Drucker, D.J.; Gribble, F.M. Characterization and functional role of voltage gated cation conductances in the glucagon-like peptide-1 secreting glutag cell line. J. Physiol. 2005, 563, 161–175. [Google Scholar] [CrossRef]
- Nadkarni, P.; Chepurny, O.G.; Holz, G.G. Regulation of glucose homeostasis by glp-1. Prog. Mol. Biol. Transl. Sci. 2014, 121, 23–65. [Google Scholar]
- Levin, B.E.; Routh, V.H.; Kang, L.; Sanders, N.M.; Dunn-Meynell, A.A. Neuronal glucosensing: What do we know after 50 years? Diabetes 2004, 53, 2521–2528. [Google Scholar] [CrossRef]
- Thorens, B. Central control of glucose homeostasis: The brain--endocrine pancreas axis. Diabetes Metab. 2010, 36 (Suppl. 3), S45–S49. [Google Scholar] [CrossRef]
- Osundiji, M.A.; Lam, D.D.; Shaw, J.; Yueh, C.Y.; Markkula, S.P.; Hurst, P.; Colliva, C.; Roda, A.; Heisler, L.K.; Evans, M.L. Brain glucose sensors play a significant role in the regulation of pancreatic glucose-stimulated insulin secretion. Diabetes 2012, 61, 321–328. [Google Scholar] [CrossRef]
- Polidori, D.C.; Bergman, R.N.; Chung, S.T.; Sumner, A.E. Hepatic and extrahepatic insulin clearance are differentially regulated: Results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes 2016, 65, 1556–1564. [Google Scholar] [CrossRef]
- Satin, L.S.; Butler, P.C.; Ha, J.; Sherman, A.S. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol. Asp. Med. 2015, 42, 61–77. [Google Scholar] [CrossRef]
- Mohamad, M.; Mitchell, S.J.; Wu, L.E.; White, M.Y.; Cordwell, S.J.; Mach, J.; Solon-Biet, S.M.; Boyer, D.; Nines, D.; Das, A.; et al. Ultrastructure of the liver microcirculation influences hepatic and systemic insulin activity and provides a mechanism for age-related insulin resistance. Aging Cell 2016, 15, 706–715. [Google Scholar] [CrossRef]
- Duckworth, W.C.; Bennett, R.G.; Hamel, F.G. Insulin degradation: Progress and potential. Endocrinol. Rev. 1998, 19, 608–624. [Google Scholar]
- Barrett, E.J.; Eggleston, E.M.; Inyard, A.C.; Wang, H.; Li, G.; Chai, W.; Liu, Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009, 52, 752–764. [Google Scholar] [CrossRef]
- Kolka, C.M.; Bergman, R.N. The barrier within: Endothelial transport of hormones. Physiology (Bethesda) 2012, 27, 237–247. [Google Scholar] [CrossRef]
- Lee, W.L.; Klip, A. Endothelial transcytosis of insulin: Does it contribute to insulin resistance? Physiology (Bethesda) 2016, 31, 336–345. [Google Scholar] [CrossRef]
- Tokarz, V.L.; MacDonald, P.E.; Klip, A. The cell biology of systemic insulin function. J. Cell Biol. 2018, 217, 2273–2289. [Google Scholar] [CrossRef]
- Al-Share, Q.Y.; DeAngelis, A.M.; Lester, S.G.; Bowman, T.A.; Ramakrishnan, S.K.; Abdallah, S.L.; Russo, L.; Patel, P.R.; Kaw, M.K.; Raphael, C.K.; et al. Forced hepatic overexpression of ceacam1 curtails diet-induced insulin resistance. Diabetes 2015, 64, 2780–2790. [Google Scholar] [CrossRef]
- Formisano, P.; Najjar, S.M.; Gross, C.N.; Philippe, N.; Oriente, F.; Kern-Buell, C.L.; Accili, D.; Gorden, P. Receptor-mediated internalization of insulin. Potential role of pp120/ha4, a substrate of the insulin receptor kinase. J. Biol. Chem. 1995, 270, 24073–24077. [Google Scholar] [CrossRef]
- Poy, M.N.; Yang, Y.; Rezaei, K.; Fernstrom, M.A.; Lee, A.D.; Kido, Y.; Erickson, S.K.; Najjar, S.M. Ceacam1 regulates insulin clearance in liver. Nat. Genet. 2002, 30, 270–276. [Google Scholar] [CrossRef]
- Ghadieh, H.E.; Muturi, H.T.; Najjar, S.M. Exenatide prevents diet-induced hepatocellular injury in a ceacam1-dependent mechanism. J. Diabetes Treat. 2017, 2017. [Google Scholar] [CrossRef]
- Pan, W.W.; Myers, M.G., Jr. Leptin and the maintenance of elevated body weight. Nat. Rev. Neurosci. 2018, 19, 95–105. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Cota, D.; Barrera, J.G.; Seeley, R.J. Leptin in energy balance and reward: Two faces of the same coin? Neuron 2006, 51, 678–680. [Google Scholar] [CrossRef]
- Bingham, N.C.; Anderson, K.K.; Reuter, A.L.; Stallings, N.R.; Parker, K.L. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 2008, 149, 2138–2148. [Google Scholar] [CrossRef]
- Wang, H.; Knaub, L.A.; Jensen, D.R.; Young Jung, D.; Hong, E.G.; Ko, H.J.; Coates, A.M.; Goldberg, I.J.; de la Houssaye, B.A.; Janssen, R.C.; et al. Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes 2009, 58, 116–124. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef]
- Toda, C.; Shiuchi, T.; Lee, S.; Yamato-Esaki, M.; Fujino, Y.; Suzuki, A.; Okamoto, S.; Minokoshi, Y. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes 2009, 58, 2757–2765. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Badman, M.K.; Flier, J.S. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology 2007, 132, 2103–2115. [Google Scholar] [CrossRef]
- Flak, J.N.; Myers, M.G., Jr. Minireview: Cns mechanisms of leptin action. Mol. Endocrinol. 2016, 30, 3–12. [Google Scholar] [CrossRef]
- Hinoi, E.; Gao, N.; Jung, D.Y.; Yadav, V.; Yoshizawa, T.; Kajimura, D.; Myers, M.G., Jr.; Chua, S.C., Jr.; Wang, Q.; Kim, J.K.; et al. An osteoblast-dependent mechanism contributes to the leptin regulation of insulin secretion. Ann N. Y. Acad Sci 2009, 1173 (Suppl. 1), E20–E30. [Google Scholar] [CrossRef]
- Yalamanchi, S.V.; Stewart, K.J.; Ji, N.; Golden, S.H.; Dobs, A.; Becker, D.M.; Vaidya, D.; Kral, B.G.; Kalyani, R.R. The relationship of fasting hyperglycemia to changes in fat and muscle mass after exercise training in type 2 diabetes. Diabetes Res. Clin. Pract. 2016, 122, 154–161. [Google Scholar] [CrossRef]
- Ghadieh, H.E.; Russo, L.; Muturi, H.T.; Ghanem, S.S.; Manaserh, I.H.; Noh, H.L.; Suk, S.; Kim, J.K.; Hill, J.W.; Najjar, S.M. Hyperinsulinemia drives hepatic insulin resistance in male mice with liver-specific ceacam1 deletion independently of lipolysis. Metabolism 2019, 93, 33–43. [Google Scholar] [CrossRef]
- Hue, L.; Taegtmeyer, H. The randle cycle revisited: A new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E578–E591. [Google Scholar] [CrossRef]
- Titchenell, P.M.; Quinn, W.J.; Lu, M.; Chu, Q.; Lu, W.; Li, C.; Chen, H.; Monks, B.R.; Chen, J.; Rabinowitz, J.D.; et al. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Cell Metab. 2016, 23, 1154–1166. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Leibel, R.L. Adaptive thermogenesis in humans. Int. J. Obes. 2010, 34 (Suppl. 1), S47–S55. [Google Scholar] [CrossRef]
- de Jesus, L.A.; Carvalho, S.D.; Ribeiro, M.O.; Schneider, M.; Kim, S.W.; Harney, J.W.; Larsen, P.R.; Bianco, A.C. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J. Clin. Investig. 2001, 108, 1379–1385. [Google Scholar] [CrossRef]
- Plum, L.; Rother, E.; Munzberg, H.; Wunderlich, F.T.; Morgan, D.A.; Hampel, B.; Shanabrough, M.; Janoschek, R.; Konner, A.C.; Alber, J.; et al. Enhanced leptin-stimulated pi3k activation in the cns promotes white adipose tissue transdifferentiation. Cell Metab. 2007, 6, 431–445. [Google Scholar] [CrossRef]
- Saito, M.; Minokoshi, Y.; Shimazu, T. Brown adipose tissue after ventromedial hypothalamic lesions in rats. Am. J. Physiol. Endocrinol. Metab. 1985, 248, E20–E25. [Google Scholar] [CrossRef]
- King, B.M. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol. Behav. 2006, 87, 221–244. [Google Scholar] [CrossRef]
- Obici, S.; Feng, Z.; Karkanias, G.; Baskin, D.G.; Rossetti, L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat. Neurosci. 2002, 5, 566–572. [Google Scholar] [CrossRef]
- Shin, A.C.; Filatova, N.; Lindtner, C.; Chi, T.; Degann, S.; Oberlin, D.; Buettner, C. Insulin receptor signaling in pomc, but not agrp, neurons controls adipose tissue insulin action. Diabetes 2017, 66, 1560–1571. [Google Scholar] [CrossRef]
- Plum, L.; Schubert, M.; Bruning, J.C. The role of insulin receptor signaling in the brain. Trends Endocrinol. Metab. 2005, 16, 59–65. [Google Scholar] [CrossRef]
- Erion, K.A.; Corkey, B.E. Hyperinsulinemia: A cause of obesity? Curr. Obes. Rep. 2017, 6, 178–186. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. Srebps: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Loftus, T.M.; Jaworsky, D.E.; Frehywot, G.L.; Townsend, C.A.; Ronnett, G.V.; Lane, M.D.; Kuhajda, F.P. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 2000, 288, 2379–2381. [Google Scholar] [CrossRef]
- Cha, S.H.; Hu, Z.; Chohnan, S.; Lane, M.D. Inhibition of hypothalamic fatty acid synthase triggers rapid activation of fatty acid oxidation in skeletal muscle. Proc. Natl. Acad. Sci. USA 2005, 102, 14557–14562. [Google Scholar] [CrossRef]
- Chakravarthy, M.V.; Zhu, Y.; Lopez, M.; Yin, L.; Wozniak, D.F.; Coleman, T.; Hu, Z.; Wolfgang, M.; Vidal-Puig, A.; Lane, M.D.; et al. Brain fatty acid synthase activates pparalpha to maintain energy homeostasis. J.Clin. Investig. 2007, 117, 2539–2552. [Google Scholar] [CrossRef]
- Najjar, S.M.; Yang, Y.; Fernstrom, M.A.; Lee, S.J.; Deangelis, A.M.; Rjaily, G.A.; Al-Share, Q.Y.; Dai, T.; Miller, T.A.; Ratnam, S.; et al. Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab. 2005, 2, 43–53. [Google Scholar] [CrossRef]
- Najjar, S.M.; Russo, L. Ceacam1 loss links inflammation to insulin resistance in obesity and non-alcoholic steatohepatitis (nash). Semin. Immunopathol. 2014, 36, 55–71. [Google Scholar] [CrossRef]
- Barrera, J.G.; Sandoval, D.A.; D’Alessio, D.A.; Seeley, R.J. Glp-1 and energy balance: An integrated model of short-term and long-term control. Nat. Rev. Endocrinol. 2011, 7, 507–516. [Google Scholar] [CrossRef]
- Haque, M.S.; Minokoshi, Y.; Hamai, M.; Iwai, M.; Horiuchi, M.; Shimazu, T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes 1999, 48, 1706–1712. [Google Scholar] [CrossRef]
- Shiuchi, T.; Haque, M.S.; Okamoto, S.; Inoue, T.; Kageyama, H.; Lee, S.; Toda, C.; Suzuki, A.; Bachman, E.S.; Kim, Y.B.; et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009, 10, 466–480. [Google Scholar] [CrossRef]
- Heinrich, G.; Russo, L.; Castaneda, T.R.; Pfeiffer, V.; Ghadieh, H.E.; Ghanem, S.S.; Wu, J.; Faulkner, L.D.; Ergun, S.; McInerney, M.F.; et al. Leptin resistance contributes to obesity in mice with null mutation of carcinoembryonic antigen-related cell adhesion molecule 1. J. Biol. Chem. 2016, 291, 11124–11132. [Google Scholar] [CrossRef]
- Russo, L.; Muturi, H.T.; Ghadieh, H.E.; Ghanem, S.S.; Bowman, T.A.; Noh, H.L.; Dagdeviren, S.; Dogbey, G.Y.; Kim, J.K.; Heinrich, G.; et al. Liver-specific reconstitution of ceacam1 reverses the metabolic abnormalities caused by its global deletion in male mice. Diabetologia 2017, 60, 2463–2474. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salaheldeen, E.; Jaume, A.; Michael Najjar, S. Carcinoembryonic Cell Adhesion-Related Molecule 2 Regulates Insulin Secretion and Energy Balance. Int. J. Mol. Sci. 2019, 20, 3231. https://doi.org/10.3390/ijms20133231
Salaheldeen E, Jaume A, Michael Najjar S. Carcinoembryonic Cell Adhesion-Related Molecule 2 Regulates Insulin Secretion and Energy Balance. International Journal of Molecular Sciences. 2019; 20(13):3231. https://doi.org/10.3390/ijms20133231
Chicago/Turabian StyleSalaheldeen, Elsaid, Alexa Jaume, and Sonia Michael Najjar. 2019. "Carcinoembryonic Cell Adhesion-Related Molecule 2 Regulates Insulin Secretion and Energy Balance" International Journal of Molecular Sciences 20, no. 13: 3231. https://doi.org/10.3390/ijms20133231
APA StyleSalaheldeen, E., Jaume, A., & Michael Najjar, S. (2019). Carcinoembryonic Cell Adhesion-Related Molecule 2 Regulates Insulin Secretion and Energy Balance. International Journal of Molecular Sciences, 20(13), 3231. https://doi.org/10.3390/ijms20133231