Age-Associated mRNA and miRNA Expression Changes in the Blood-Brain Barrier
Abstract
1. Introduction
2. Results
2.1. Age-Associated Gene Expression Changes in the BBB
2.2. Pathway Enrichment Reveals Age-Related Changes in Genes Associated with DNA Binding and Apoptosis/Autophagy
2.3. Age-Associated miRNA Expression Changes in the Ageing BBB
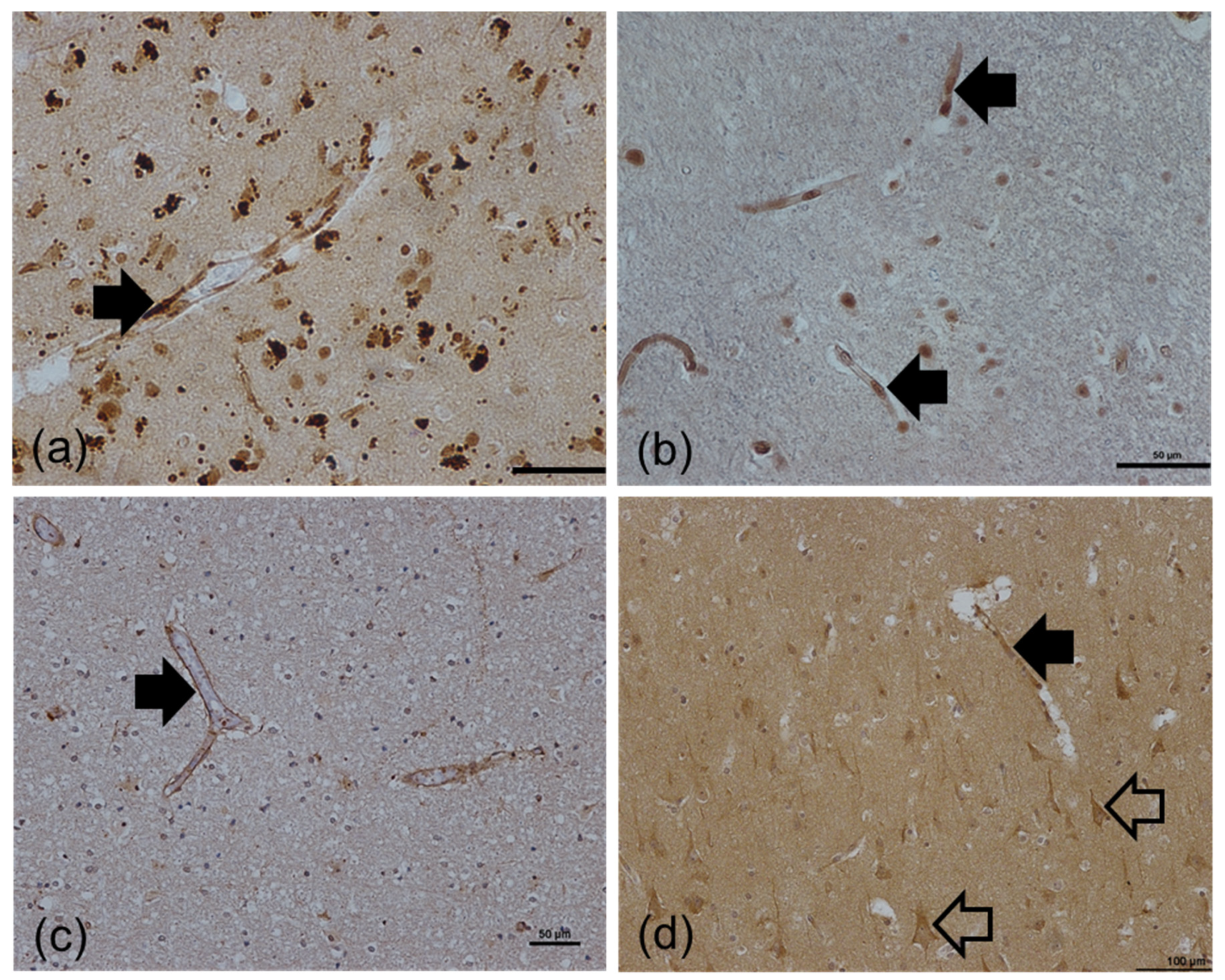
2.4. qPCR and Immunohistochemistry Validation of Age-Associated Candidate Gene Expression Changes
3. Discussion
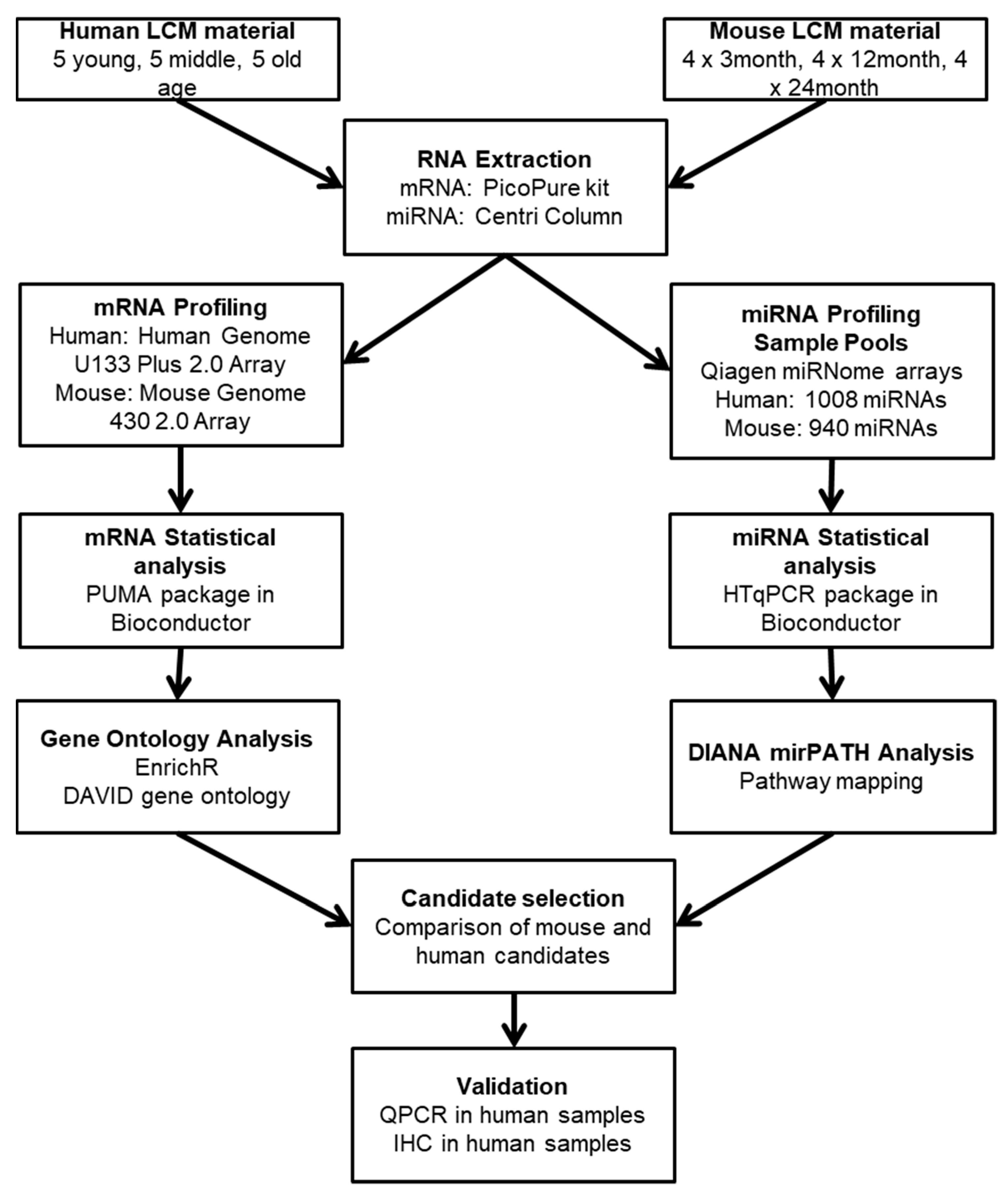
4. Materials and Methods
4.1. Study Cohort
4.2. Laser Capture Microdissection
4.3. Total RNA Extraction
4.4. mRNA Expression Profiling
4.5. miRNA Expression Profiling
4.6. Data Analysis: mRNA
4.7. Data Analysis: miRNA
4.8. Quantitative PCR
4.9. Immunohistochemistry
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC-HRP | Avidin-biotinylated complex-horse radish peroxidase |
| AD | Alzheimer’s disease |
| ARHGAP42 | Rho GTPase-activating protein 42 |
| ARSA | arylsufatase A |
| ATF | Activating Transcription Factor |
| BAD | BCL2 associated agonist of cell death |
| BBB | blood-brain barrier |
| BCL2L11 | BCL2-like 11 |
| BECN1 | beclin 1 |
| BID | BH3 interacting domain death agonist |
| CDC42BPB | CDC42 binding protein kinase-β |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| CTNNB1 | catenin beta 1 |
| DAVID | Database for Annotation Visualisation and Integrated Discovery |
| DSCAM | Down syndrome cell adhesion molecule |
| EBF1 | early B-Cell Factor 1 |
| ERCC1 | excision repair cross-complementation group 1 |
| ERLEC1 | endoplasmic reticulum lectin 1 |
| FC | fold change |
| FLI1 | friend leukemia integration 1 transcription facto |
| FOX | Forkhead family of transcription factors |
| GEO | Gene Expression Omnibus |
| GFAP | glial fibrillary acidic protein |
| GRIN2C | glutamate ionotropic receptor NMDA type subunit 2C |
| HTT | huntingtin |
| IKZF1 | Ikaros family zinc finger protein 1 |
| IPPLR | improved probability of positive log ratio |
| KPNA1 | karyopherin subunit alpha 1 |
| LCM | laser capture microdissection |
| MAFF | v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog F |
| MIAT | myocardial infarction associated transcript |
| miRNA | microRNA |
| MMGMOS | multi-chip modified gamma model for oligonucleotide signal |
| mRNA | messenger RNA |
| mTOR | mechanistic target of rapamycin |
| NFIB | nuclear factor I B |
| NFL | neurofilament light |
| NVU | neurovascular unit |
| PBS | phosphate buffered saline |
| PHF20L1 | PHD finger protein 20 like 1 |
| PPOX | protoporphyrinogen oxidase |
| PPP3CC | protein phosphatase 3 catalytic subunit gamma |
| R3HDM1 | R3H domain containing 1 |
| RBBP4 | histone-binding protein RBBP4 |
| RB1CC1 | RB1-inducible coiled-coil 1 |
| RCOR3 | REST Corepressor 3 |
| SNAI2 | Snail family transcriptional repressor 2 |
| SPTBN1 | spectrin β, non-erythrocytic 1 |
| TCF7L1 | transcription factor 7-like 1 |
| TP53 | tumor protein p53 |
| vWF | von Willibrand factor |
| ZFHX4 | zinc finger homeobox 4 |
| ZKSCAN3 | zinc finger with KRAB and SCAN domains 3 |
| ZNF | zinc finger protein |
References
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Erdo, F.; Denes, L.; de Lange, E. Age-associated physiological and pathological changes at the blood-brain barrier: A review. J. Cereb. Blood Flow Metab. 2017, 37, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Bors, L.; Toth, K.; Toth, E.Z.; Bajza, A.; Csorba, A.; Szigeti, K.; Mathe, D.; Perlaki, G.; Orsi, G.; Toth, G.K.; et al. Age-dependent changes at the blood-brain barrier. A Comparative structural and functional study in young adult and middle aged rats. Brain Res. Bull. 2018, 139, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Erdo, F.; Trapp, T.; Mies, G.; Hossmann, K.A. Immunohistochemical analysis of protein expression after middle cerebral artery occlusion in mice. Acta Neuropathol. 2004, 107, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Zipser, B.D.; Johanson, C.E.; Gonzalez, L.; Berzin, T.M.; Tavares, R.; Hulette, C.M.; Vitek, M.P.; Hovanesian, V.; Stopa, E.G. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging 2007, 28, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; McLarnon, J.G. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J. Cell. Mol. Med. 2009, 13, 2911–2925. [Google Scholar] [CrossRef] [PubMed]
- Viggars, A.P.; Wharton, S.B.; Simpson, J.E.; Matthews, F.E.; Brayne, C.; Savva, G.M.; Garwood, C.; Drew, D.; Shaw, P.J.; Ince, P.G. Alterations in the blood brain barrier in ageing cerebral cortex in relationship to Alzheimer-type pathology: A study in the MRC-CFAS population neuropathology cohort. Neurosci. Lett. 2011, 505, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef] [PubMed]
- van de Haar, H.J.; Burgmans, S.; Jansen, J.F.; van Osch, M.J.; van Buchem, M.A.; Muller, M.; Hofman, P.A.; Verhey, F.R.; Backes, W.H. Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology 2016, 281, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Iturria-Medina, Y.; Sotero, R.C.; Toussaint, P.J.; Mateos-Perez, J.M.; Evans, A.C.; Alzheimer’s Disease Neuroimaging, I. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 2016, 7, 11934. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, M.; Dickstein, D.L.; Carlow, D.A.; Jefferies, W.A. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation 2003, 10, 463–470. [Google Scholar] [PubMed]
- de la Torre, J.C.; Mussivand, T. Can disturbed brain microcirculation cause Alzheimer’s disease? Neurol. Res. 1993, 15, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lee, H.G.; Perry, G.; Smith, M.A. Alzheimer disease, the two-hit hypothesis: An update. Biochim. Biophys. Acta 2007, 1772, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Lu, T.; Pan, Y.; Kao, S.Y.; Li, C.; Kohane, I.; Chan, J.; Yankner, B.A. Gene regulation and DNA damage in the ageing human brain. Nature 2004, 429, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Cribbs, D.H.; Coleman, P.D.; Rogers, J.; Head, E.; Kim, R.; Beach, T.; Miller, C.; Troncoso, J.; Trojanowski, J.Q.; et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. USA 2008, 105, 15605–15610. [Google Scholar] [CrossRef]
- Colantuoni, C.; Lipska, B.K.; Ye, T.; Hyde, T.M.; Tao, R.; Leek, J.T.; Colantuoni, E.A.; Elkahloun, A.G.; Herman, M.M.; Weinberger, D.R.; et al. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature 2011, 478, 519–523. [Google Scholar] [CrossRef]
- Lu, T.; Aron, L.; Zullo, J.; Pan, Y.; Kim, H.; Chen, Y.; Yang, T.H.; Kim, H.M.; Drake, D.; Liu, X.S.; et al. REST and stress resistance in ageing and Alzheimer’s disease. Nature 2014, 507, 448–454. [Google Scholar] [CrossRef]
- Chen, C.Y.; Logan, R.W.; Ma, T.; Lewis, D.A.; Tseng, G.C.; Sibille, E.; McClung, C.A. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc. Natl. Acad. Sci. USA 2016, 113, 206–211. [Google Scholar] [CrossRef] [PubMed]
- McPherson, C.A.; Aoyama, M.; Harry, G.J. Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: Differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain Behav. Immun. 2011, 25, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zaidi, A.; Pal, R.; Garrett, A.S.; Braceras, R.; Chen, X.W.; Michaelis, M.L.; Michaelis, E.K. Genomic and biochemical approaches in the discovery of mechanisms for selective neuronal vulnerability to oxidative stress. BMC Neurosci. 2009, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Alldred, M.J.; Lee, S.H.; Petkova, E.; Ginsberg, S.D. Expression profile analysis of hippocampal CA1 pyramidal neurons in aged Ts65Dn mice, a model of Down syndrome (DS) and Alzheimer’s disease (AD). Brain Struct. Funct. 2015, 220, 2983–2996. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.E.; Ince, P.G.; Minett, T.; Matthews, F.E.; Heath, P.R.; Shaw, P.J.; Goodall, E.; Garwood, C.J.; Ratcliffe, L.E.; Brayne, C.; et al. Neuronal DNA damage response-associated dysregulation of signalling pathways and cholesterol metabolism at the earliest stages of Alzheimer-type pathology. Neuropathol. Appl. Neurobiol. 2016, 42, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.E.; Ince, P.G.; Shaw, P.J.; Heath, P.R.; Raman, R.; Garwood, C.J.; Gelsthorpe, C.; Baxter, L.; Forster, G.; Matthews, F.E.; et al. Microarray analysis of the astrocyte transcriptome in the aging brain: Relationship to Alzheimer’s pathology and APOE genotype. Neurobiol. Aging 2011, 32, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Teale, J.M. Transcriptome analysis of the ependymal barrier during murine neurocysticercosis. J. Neuroinflamm. 2012, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Teale, J.M. Changes in gene expression of pial vessels of the blood brain barrier during murine neurocysticercosis. PLoS Negl. Trop. Dis. 2013, 7, e2099. [Google Scholar] [CrossRef]
- Pen, A.; Moreno, M.J.; Martin, J.; Stanimirovic, D.B. Molecular markers of extracellular matrix remodeling in glioblastoma vessels: Microarray study of laser-captured glioblastoma vessels. Glia 2007, 55, 559–572. [Google Scholar] [CrossRef]
- Dieterich, L.C.; Mellberg, S.; Langenkamp, E.; Zhang, L.; Zieba, A.; Salomaki, H.; Teichert, M.; Huang, H.; Edqvist, P.H.; Kraus, T.; et al. Transcriptional profiling of human glioblastoma vessels indicates a key role of VEGF-A and TGFbeta2 in vascular abnormalization. J. Pathol. 2012, 228, 378–390. [Google Scholar] [CrossRef]
- Cunnea, P.; McMahon, J.; O’Connell, E.; Mashayekhi, K.; Fitzgerald, U.; McQuaid, S. Gene expression analysis of the microvascular compartment in multiple sclerosis using laser microdissected blood vessels. Acta Neuropathol. 2010, 119, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.W.; Wayland, M.; Lan, M.; Ryan, M.; Giger, T.; Lockstone, H.; Wuethrich, I.; Mimmack, M.; Wang, L.; Kotter, M.; et al. The cerebral microvasculature in schizophrenia: A laser capture microdissection study. PLoS ONE 2008, 3, e3964. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Harries, L.W. MicroRNAs as Mediators of the Ageing Process. Genes 2014, 5, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Lu, J.; Manaenko, A.; Qi, X.; Tang, J.; Mei, Q.; Xia, Y.; Hu, Q. MicroRNA-132 attenuates cerebral injury by protecting blood-brain-barrier in MCAO mice. Exp. Neurol. 2019, 316, 12–19. [Google Scholar] [CrossRef]
- Burek, M.; Konig, A.; Lang, M.; Fiedler, J.; Oerter, S.; Roewer, N.; Bohnert, M.; Thal, S.C.; Blecharz-Lang, K.G.; Woitzik, J.; et al. Hypoxia-Induced MicroRNA-212/132 Alter Blood-Brain Barrier Integrity Through Inhibition of Tight Junction-Associated Proteins in Human and Mouse Brain Microvascular Endothelial Cells. Transl. Stroke Res. 2019. [Google Scholar] [CrossRef]
- Goodall, E.F.; Wang, C.; Simpson, J.E.; Baker, D.J.; Drew, D.R.; Heath, P.R.; Saffrey, M.J.; Romero, I.A.; Wharton, S.B. Age-associated changes in the blood-brain barrier: Comparative studies in human and mouse. Neuropathol. Appl. Neurobiol. 2018, 44, 328–340. [Google Scholar] [CrossRef]
- Cooper-Knock, J.; Kirby, J.; Ferraiuolo, L.; Heath, P.R.; Rattray, M.; Shaw, P.J. Gene expression profiling in human neurodegenerative disease. Nat. Rev. Neurol. 2012, 8, 518–530. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Zahn, J.M.; Poosala, S.; Owen, A.B.; Ingram, D.K.; Lustig, A.; Carter, A.; Weeraratna, A.T.; Taub, D.D.; Gorospe, M.; Mazan-Mamczarz, K.; et al. AGEMAP: A gene expression database for aging in mice. PLoS Genet. 2007, 3, e201. [Google Scholar] [CrossRef]
- Carney, E.F. Hypertension: Role of ARHGAP42 in hypertension. Nat. Rev. Nephrol. 2017, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.L.; Fu, Z.X.; Zhang, B.H.; Jia, Y.J. Hippocampal overexpression of Down syndrome cell adhesion molecule in amyloid precursor protein transgenic mice. Braz. J. Med. Biol. Res. 2017, 50, e6049. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wade, P.; Mandell, K.J.; Akyildiz, A.; Parkos, C.A.; Mrsny, R.J.; Nusrat, A. Raf 1 represses expression of the tight junction protein occludin via activation of the zinc-finger transcription factor slug. Oncogene 2007, 26, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Xiang, R.L.; Cong, X.; Zhang, Y.; Li, J.; Yi, X.; Park, K.; Han, J.Y.; Wu, L.L.; Yu, G.Y. Claudin-3 is required for modulation of paracellular permeability by TNF-alpha through ERK1/2/slug signaling axis in submandibular gland. Cell. Signal. 2015, 27, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, K.; Konishi, H.; Arima, C.; Tomida, S.; Takeuchi, T.; Shimada, Y.; Yatabe, Y.; Mitsudomi, T.; Osada, H.; Takahashi, T. Novel metastasis-related gene CIM functions in the regulation of multiple cellular stress-response pathways. Cancer Res. 2010, 70, 9949–9958. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, P.S.; Ugale, R.R.; Yilmazer-Hanke, D.; Stairs, D.J.; Dravid, S.M. GluN2C/GluN2D subunit-selective NMDA receptor potentiator CIQ reverses MK-801-induced impairment in prepulse inhibition and working memory in Y-maze test in mice. Br. J. Pharmacol. 2014, 171, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschella, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef]
- Minster, R.L.; Sanders, J.L.; Singh, J.; Kammerer, C.M.; Barmada, M.M.; Matteini, A.M.; Zhang, Q.; Wojczynski, M.K.; Daw, E.W.; Brody, J.A.; et al. Genome-Wide Association Study and Linkage Analysis of the Healthy Aging Index. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1003–1008. [Google Scholar] [CrossRef]
- Hillary, R.F.; FitzGerald, U. A lifetime of stress: ATF6 in development and homeostasis. J. Biomed. Sci. 2018, 25, 48. [Google Scholar] [CrossRef]
- Atkin, J.D.; Farg, M.A.; Walker, A.K.; McLean, C.; Tomas, D.; Horne, M.K. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol. Dis. 2008, 30, 400–407. [Google Scholar] [CrossRef]
- Wruck, W.; Schroter, F.; Adjaye, J. Meta-Analysis of Transcriptome Data Related to Hippocampus Biopsies and iPSC-Derived Neuronal Cells from Alzheimer’s Disease Patients Reveals an Association with FOXA1 and FOXA2 Gene Regulatory Networks. J. Alzheimers Dis. 2016, 50, 1065–1082. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Becanovic, K.; Desplats, P.A.; Spencer, B.; Hill, A.M.; Connolly, C.; Masliah, E.; Leavitt, B.R.; Thomas, E.A. Forkhead box protein p1 is a transcriptional repressor of immune signaling in the CNS: Implications for transcriptional dysregulation in Huntington disease. Hum. Mol. Genet. 2012, 21, 3097–3111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamano, T.; Hayashi, K.; Shirafuji, N.; Nakamoto, Y. The Implications of Autophagy in Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Rohn, T.T.; Wirawan, E.; Brown, R.J.; Harris, J.R.; Masliah, E.; Vandenabeele, P. Depletion of Beclin-1 due to proteolytic cleavage by caspases in the Alzheimer’s disease brain. Neurobiol. Dis. 2011, 43, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.C.; Oliveira, C.R.; Pereira, C.F.; Cardoso, S.M. Loss of proteostasis induced by amyloid beta peptide in brain endothelial cells. Biochim. Biophys. Acta 2014, 1843, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Huang, Y.; Chen, S.D.; Halliday, G. Immunohistochemical evidence for macroautophagy in neurones and endothelial cells in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2010, 36, 312–319. [Google Scholar] [CrossRef]
- Garwood, C.J.; Simpson, J.E.; Al Mashhadi, S.; Axe, C.; Wilson, S.; Heath, P.R.; Shaw, P.J.; Matthews, F.E.; Brayne, C.; Ince, P.G.; et al. DNA damage response and senescence in endothelial cells of human cerebral cortex and relation to Alzheimer’s neuropathology progression: A population-based study in the Medical Research Council Cognitive Function and Ageing Study (MRC-CFAS) cohort. Neuropathol. Appl. Neurobiol. 2014, 40, 802–814. [Google Scholar] [CrossRef]
- Rajewsky, N. microRNA target predictions in animals. Nat. Genet. 2006, 38, S8–S13. [Google Scholar] [CrossRef]
- Sierksma, A.; Lu, A.; Salta, E.; Vanden Eynden, E.; Callaerts-Vegh, Z.; D’Hooge, R.; Blum, D.; Buee, L.; Fiers, M.; De Strooper, B. Deregulation of neuronal miRNAs induced by amyloid-beta or TAU pathology. Mol. Neurodegener. 2018, 13, 54. [Google Scholar] [CrossRef]
- Tili, E.; Mezache, L.; Michaille, J.J.; Amann, V.; Williams, J.; Vandiver, P.; Quinonez, M.; Fadda, P.; Mikhail, A.; Nuovo, G. microRNA 155 up regulation in the CNS is strongly correlated to Down’s syndrome dementia. Ann. Diagn. Pathol. 2018, 34, 103–109. [Google Scholar] [CrossRef]
- Arena, A.; Iyer, A.M.; Milenkovic, I.; Kovacs, G.G.; Ferrer, I.; Perluigi, M.; Aronica, E. Developmental Expression and Dysregulation of miR-146a and miR-155 in Down’s Syndrome and Mouse Models of Down’s Syndrome and Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 14, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Guedes, J.R.; Custodia, C.M.; Silva, R.J.; de Almeida, L.P.; Pedroso de Lima, M.C.; Cardoso, A.L. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer’s disease triple transgenic mouse model. Hum. Mol. Genet. 2014, 23, 6286–6301. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ramirez, M.A.; Wu, D.; Pryce, G.; Simpson, J.E.; Reijerkerk, A.; King-Robson, J.; Kay, O.; de Vries, H.E.; Hirst, M.C.; Sharrack, B.; et al. MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J. 2014, 28, 2551–2565. [Google Scholar] [CrossRef] [PubMed]
- Barker, K.R.; Lu, Z.; Kim, H.; Zheng, Y.; Chen, J.; Conroy, A.L.; Hawkes, M.; Cheng, H.S.; Njock, M.S.; Fish, J.E.; et al. miR-155 Modifies Inflammation, Endothelial Activation and Blood-Brain Barrier Dysfunction in Cerebral Malaria. Mol. Med. 2017, 23, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Bhardwaj, A.; Arora, S.; Tyagi, N.; Singh, S.; Andrews, J.; McClellan, S.; Wang, B.; Singh, A.P. MicroRNA-345 induces apoptosis in pancreatic cancer cells through potentiation of caspase-dependent and -independent pathways. Br. J. Cancer 2015, 113, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Shiu, T.Y.; Huang, S.M.; Shih, Y.L.; Chu, H.C.; Chang, W.K.; Hsieh, T.Y. Hepatitis C virus core protein down-regulates p21(Waf1/Cip1) and inhibits curcumin-induced apoptosis through microRNA-345 targeting in human hepatoma cells. PLoS ONE 2013, 8, e61089. [Google Scholar] [CrossRef] [PubMed]
- Freiesleben, S.; Hecker, M.; Zettl, U.K.; Fuellen, G.; Taher, L. Analysis of microRNA and Gene Expression Profiles in Multiple Sclerosis: Integrating Interaction Data to Uncover Regulatory Mechanisms. Sci. Rep. 2016, 6, 34512. [Google Scholar] [CrossRef] [PubMed]
- Xi, T.; Jin, F.; Zhu, Y.; Wang, J.; Tang, L.; Wang, Y.; Liebeskind, D.S.; Scalzo, F.; He, Z. MiR-27a-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. J. Biol. Chem. 2018. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, M.; Wang, Y.; Liu, A.; Lv, M.; Li, Y.; Yang, X.; Wu, Z. Neuroprotective effects of miR-27a against traumatic brain injury via suppressing FoxO3a-mediated neuronal autophagy. Biochem. Biophys. Res. Commun. 2017, 482, 1141–1147. [Google Scholar] [CrossRef]
- Urena-Peralta, J.R.; Alfonso-Loeches, S.; Cuesta-Diaz, C.M.; Garcia-Garcia, F.; Guerri, C. Deep sequencing and miRNA profiles in alcohol-induced neuroinflammation and the TLR4 response in mice cerebral cortex. Sci. Rep. 2018, 8, 15913. [Google Scholar] [CrossRef]
- Roitbak, T.; Bragina, O.; Padilla, J.L.; Pickett, G.G. The role of microRNAs in neural stem cell-supported endothelial morphogenesis. Vasc. Cell 2011, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Bernstock, J.D.; Lee, Y.J.; Peruzzotti-Jametti, L.; Southall, N.; Johnson, K.R.; Maric, D.; Volpe, G.; Kouznetsova, J.; Zheng, W.; Pluchino, S.; et al. A novel quantitative high-throughput screen identifies drugs that both activate SUMO conjugation via the inhibition of microRNAs 182 and 183 and facilitate neuroprotection in a model of oxygen and glucose deprivation. J. Cereb. Blood Flow Metab. 2016, 36, 426–441. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, P.; Lacorte, E.; Feligioni, M.; Mayer, F.; Crestini, A.; Piccolo, L.; Bacigalupo, I.; Filareti, M.; Ficulle, E.; Confaloni, A.; et al. MicroRNAs and Mild Cognitive Impairment: A systematic review. Ageing Res. Rev. 2019, 50, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Ventorp, F.; Barzilay, R.; Erhardt, S.; Samuelsson, M.; Traskman-Bendz, L.; Janelidze, S.; Weizman, A.; Offen, D.; Brundin, L. The CD44 ligand hyaluronic acid is elevated in the cerebrospinal fluid of suicide attempters and is associated with increased blood-brain barrier permeability. J. Affect. Disord. 2016, 193, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Su, E.J.; Cao, C.; Fredriksson, L.; Nilsson, I.; Stefanitsch, C.; Stevenson, T.K.; Zhao, J.; Ragsdale, M.; Sun, Y.Y.; Yepes, M.; et al. Microglial-mediated PDGF-CC activation increases cerebrovascular permeability during ischemic stroke. Acta Neuropathol. 2017, 134, 585–604. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.E.; Wharton, S.B.; Heath, P.R. Immuno-Laser-Capture Microdissection for the Isolation of Enriched Glial Populations from Frozen Post-Mortem Human Brain. Methods Mol. Biol. 2018, 1723, 273–284. [Google Scholar] [PubMed]
- Horvath, S.; Dong, J. Geometric interpretation of gene coexpression network analysis. PLoS Comput. Biol. 2008, 4, e1000117. [Google Scholar] [CrossRef]
- Wu, X.; Dewey, T.G. From microarray to biological networks: Analysis of gene expression profiles. Methods Mol. Biol. 2006, 316, 35–48. [Google Scholar]
- Hu, W.; Park, C.Y. Measuring microRNA expression in mouse hematopoietic stem cells. Methods Mol. Biol. 2014, 1185, 121–140. [Google Scholar]
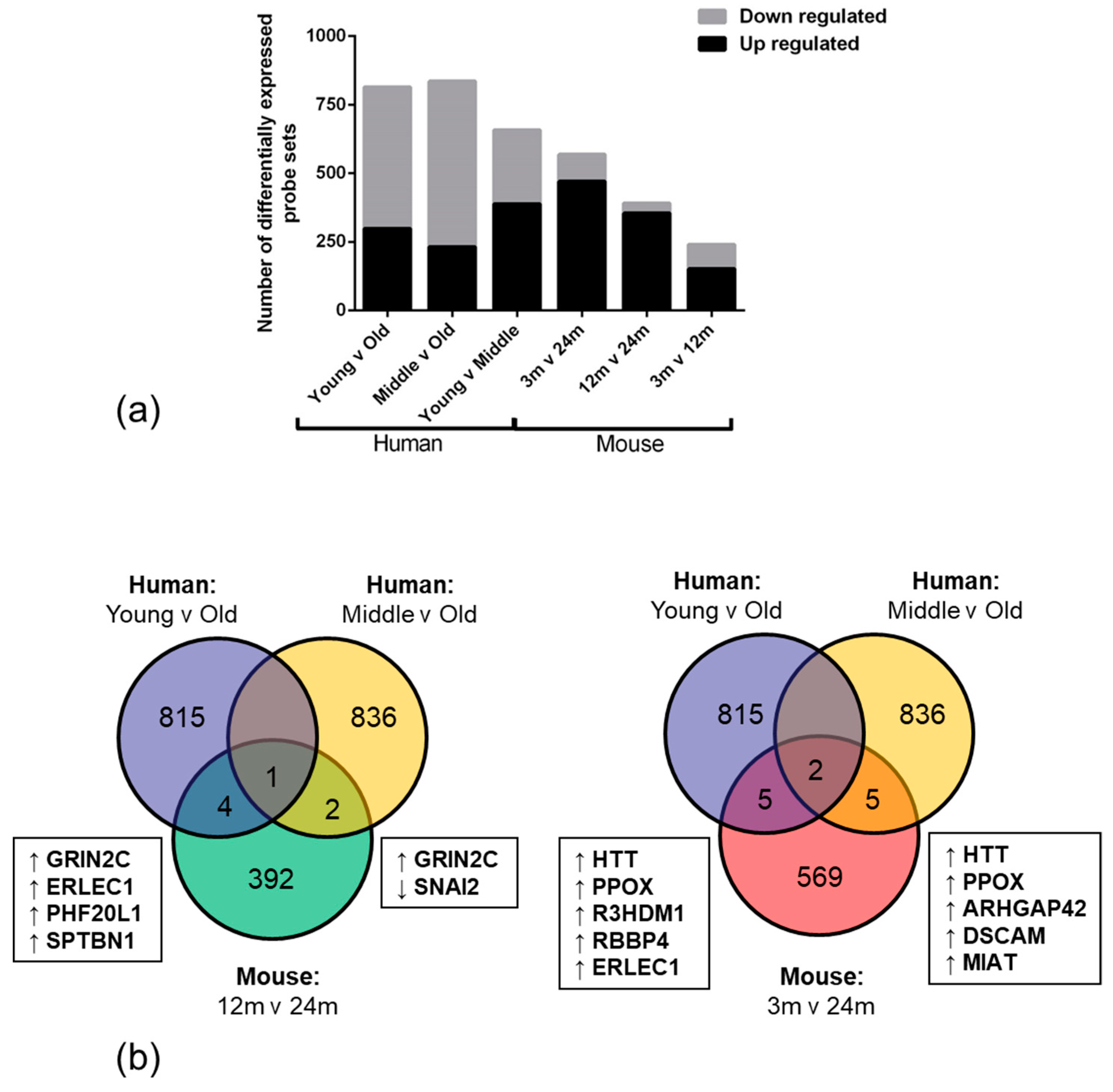
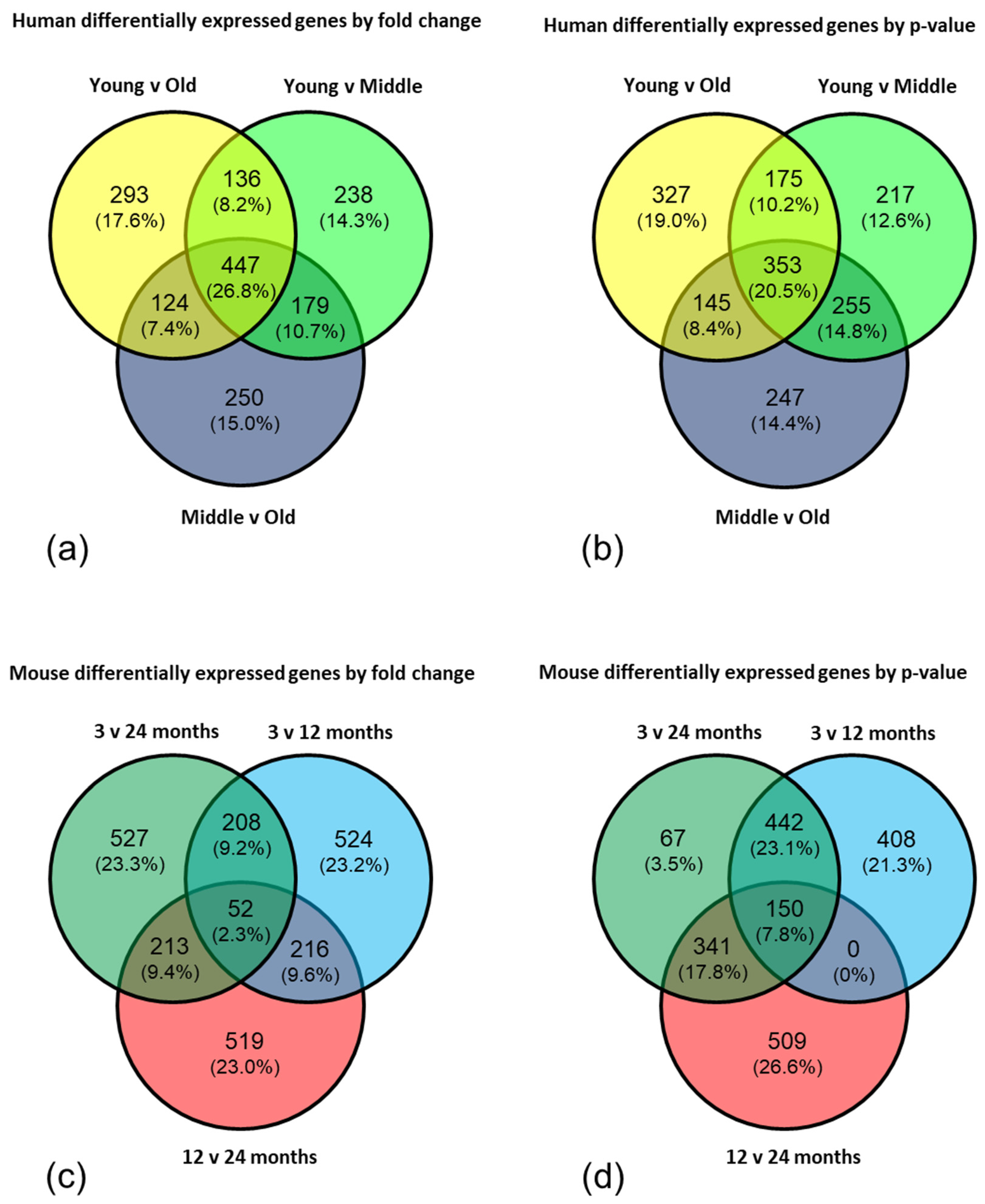
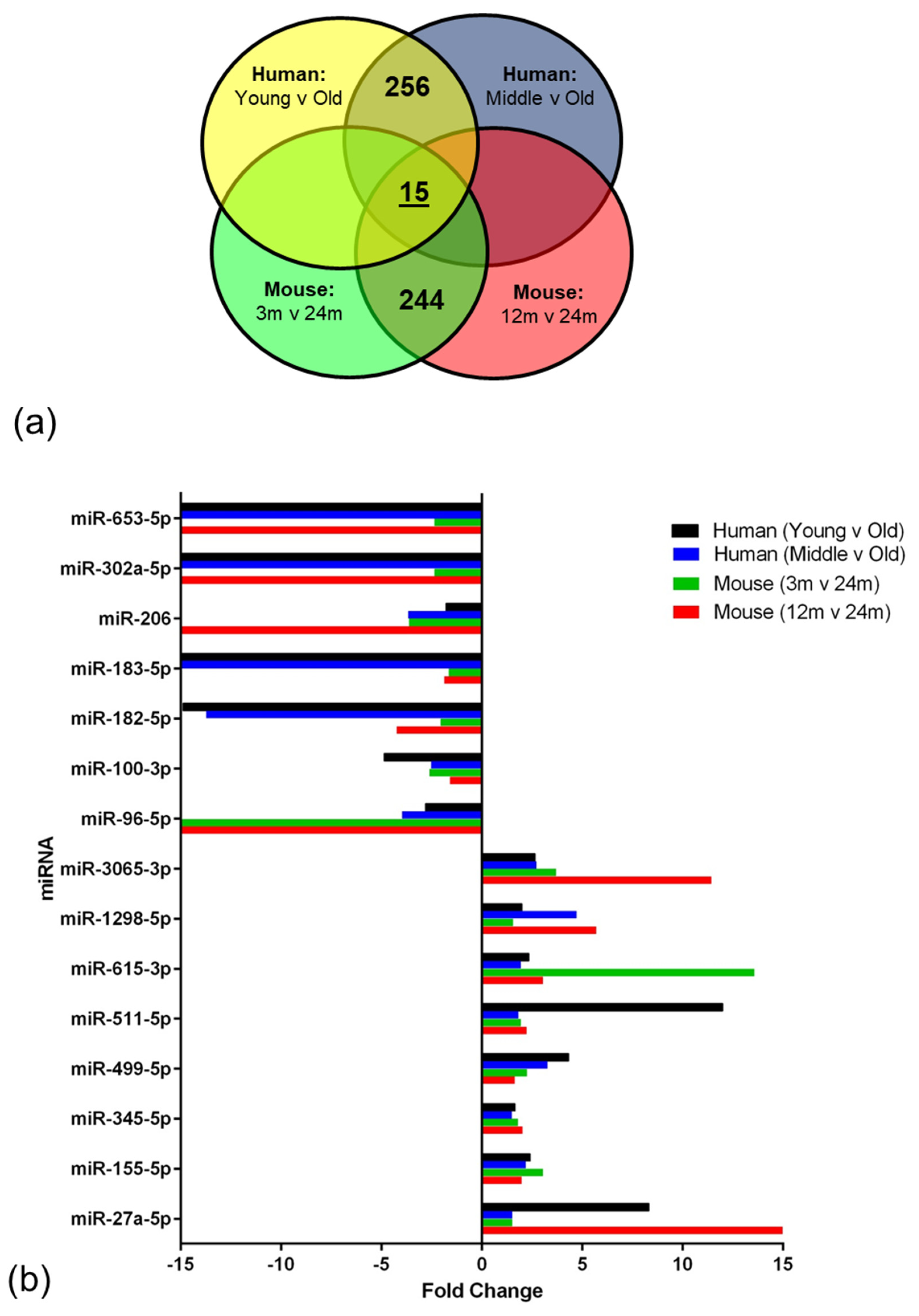
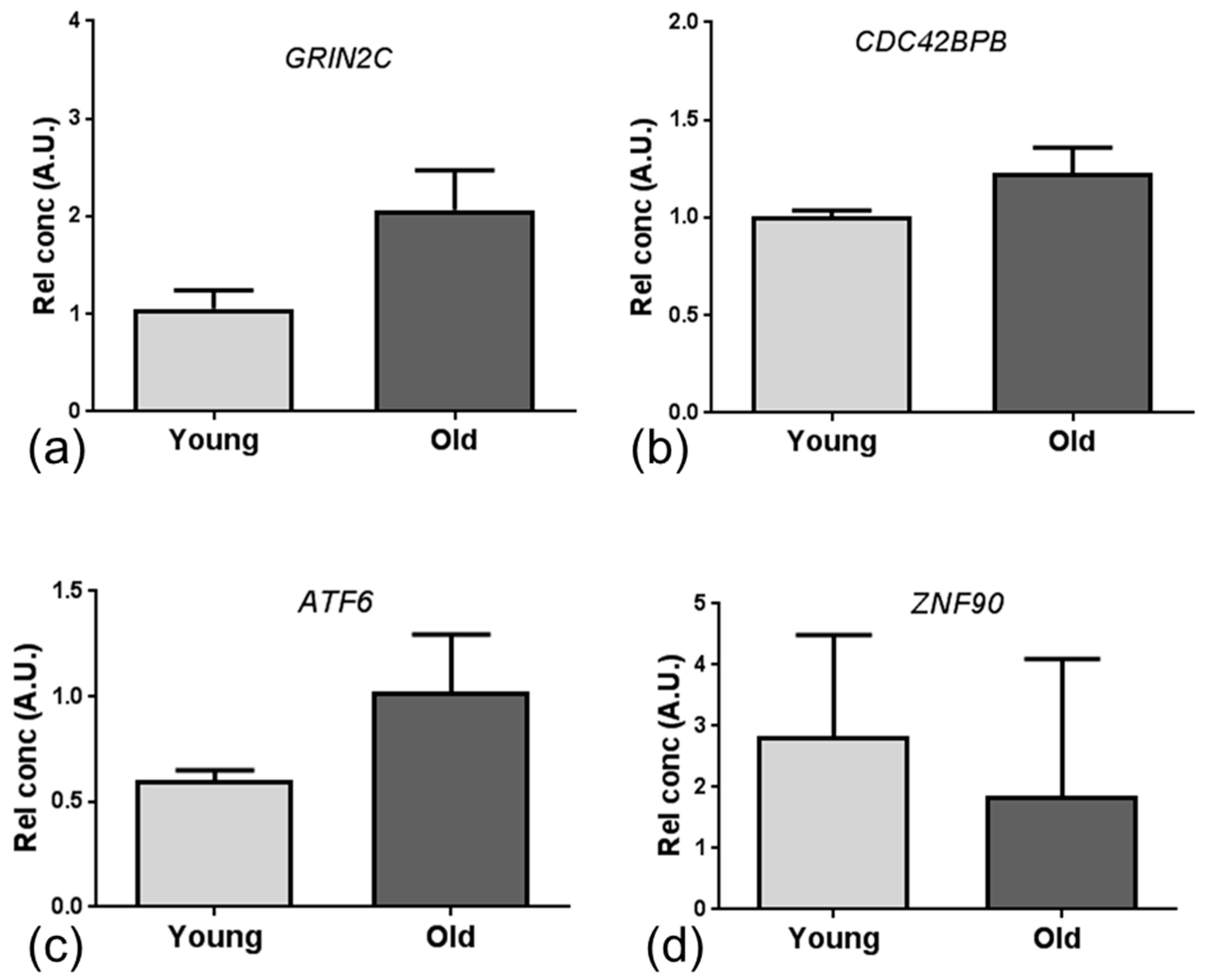

| Dataset | Cluster | No. Genes | Enrichment Score |
|---|---|---|---|
| Human (sorted by FC) | DNA binding | 6 | 1.88 |
| Kelch-like | 5 | 1.73 | |
| tRNA modification | 4 | 1.46 | |
| Human (sorted by p-value) | DNA binding | 5 | 1.45 |
| Apoptosis | 3 | 1.32 | |
| Mouse (sorted by p-value) | GTP binding | 7 | 2.32 |
| Armadillo-like | 4 | 1.81 | |
| Ion channel binding | 3 | 1.76 |
| miRNA Gene Target | 1298-5p | 653-5p | 511-3p | 505-5p | 345-5p | 302a-5p | 224-5p | 205-5p | 182-5p | 181c-3p | 155-5p | 100-3p | 27a-5p | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NFIB | 9 | |||||||||||||
| ZNF704 | 7 | |||||||||||||
| EBF1 | 6 | |||||||||||||
| FOXP1 | 6 | |||||||||||||
| IKZF1 | 5 | |||||||||||||
| ZFHX4 | 4 | |||||||||||||
| FLI1 | 4 | |||||||||||||
| ATF6 | 4 | |||||||||||||
| HNRNPD | 4 | |||||||||||||
| MAFF | 4 | |||||||||||||
| RCOR3 | 3 | |||||||||||||
| ZKSCAN3 | 3 | |||||||||||||
| ZNF514 | 3 | |||||||||||||
| FOXA1 | 2 | |||||||||||||
| ZNF268 | 2 | |||||||||||||
| ZNF90 | 2 | |||||||||||||
| TP53 | 2 | |||||||||||||
| RAD51D | 2 | |||||||||||||
| ERCC1 | 2 | |||||||||||||
| ZNF600 | 2 | |||||||||||||
| FOXR2 | 1 | |||||||||||||
| TCF7L1 | 1 |
| Age Group | Age | Gender | PMD | pH | Cause of Death | Neuropathology |
|---|---|---|---|---|---|---|
| Young | 20 | F | 71 | 6.5 | SBL | None |
| 24 | F | 47 | 6.4 | SBL | Small vessel disease | |
| 25 | M | 53 | 6.4 | SBL | None | |
| 29 | M | 44 | 6.5 | SBL | Focal Tau | |
| 30 | M | 71 | 6.4 | NK | None | |
| Middle aged | 44 | M | 47 | 6.3 | Drug overdose | None |
| 48 | M | 72 | 6.3 | CAD | None | |
| 50 | M | 45 | 6.3 | IHD/CAD | None | |
| 52 | M | 91 | 6.4 | Road traffic collision | None | |
| 57 | M | 66 | 6.5 | IHD/CAD | None | |
| Old | 71 | F | 41 | 6.5 | IHD | Mild and focal vascular tau. Mild amyloid tangles and plaques |
| 74 | M | 46 | 6.3 | Pulmonary thromboembolism | None | |
| 74 | M | 66 | 6.3 | IHD/CAD | Mild tau tangles, plaques and threads. | |
| 75 | M | 78 | 6.4 | IHD/CAD | Mild tau tangles and threads | |
| 79 | F | 45 | 6.3 | IHD/CAD | Venous collagenosis and small vessel disease |
| Gene | Sequence | |
|---|---|---|
| ATF6 | Probe | 5′-FAM/CATTCCTCCACCTCCTTGTCAGCC-3′ |
| Primer 1 | 5′-CTTGGTCCTTTCTACTTCATGTCT-3′ | |
| Primer 2 | 5′-CCCTGATGGTGCTAACTGAA-3′ | |
| CDC42BPB | Probe | 5′FAM/ACAAAGAGCCTGATTCGGACTCCAC-3′ |
| Primer 1 | 5′-GGAGCTATTCGATGGAGTTGAG-3′ | |
| Primer 2 | 5′-GAACAAGCCCTACATCTCGTG-3′ | |
| GRIN2C | Probe | 5′-FAM/ AAGGCATCCAGCTTCCCCATCTT-3′ |
| Primer 1 | 5′-TTGAGGA -3′AGCAGCATCATAG-3′ | |
| Primer 2 | 5′-CGCAGTAACTACCGTGACAT-3′ | |
| ZNF90 | Probe | 5′-FAM/AGCTGTGGATCTCCCAATACCTGC-3′ |
| Primer 1 | 5′-GGCCACATCTCTAAATTCCAATG-3′ | |
| Primer 2 | 5′-CTTAGCTGCTTCGTGTCTTCT-3′ | |
| ACTB | Probe | 5′-FAM-CCATGTACGTTGCTATCCAGGCTGT-3′ |
| Primer 1 | 5′-CCAGTGGTACGGCCAGA-3′ | |
| Primer 2 | 5′-GCGAGAAGATGACCCAGAT-3′ |
| Antibody | Isotype | Dilution | Antigen Retrieval | Supplier |
|---|---|---|---|---|
| Beclin-1 | Rabbit IgG | 1:250 (60 min, RT) | PC EDTA | AbCam |
| p53 | Mouse IgG | 1:50 (o/n, 4 °C) | PC EDTA | Santa Cruz |
| RB1CC1 | Rabbit IgG | 1:100 (60 min, RT) | PC TSC | Sigma Aldrich |
| SLUG | Rabbit IgG | 1:200 (60 min, RT) | MW TSC | AbCam |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodall, E.F.; Leach, V.; Wang, C.; Cooper-Knock, J.; Heath, P.R.; Baker, D.; Drew, D.R.; Saffrey, M.J.; Simpson, J.E.; Romero, I.A.; et al. Age-Associated mRNA and miRNA Expression Changes in the Blood-Brain Barrier. Int. J. Mol. Sci. 2019, 20, 3097. https://doi.org/10.3390/ijms20123097
Goodall EF, Leach V, Wang C, Cooper-Knock J, Heath PR, Baker D, Drew DR, Saffrey MJ, Simpson JE, Romero IA, et al. Age-Associated mRNA and miRNA Expression Changes in the Blood-Brain Barrier. International Journal of Molecular Sciences. 2019; 20(12):3097. https://doi.org/10.3390/ijms20123097
Chicago/Turabian StyleGoodall, Emily F., Vicki Leach, Chunfang Wang, Johnathan Cooper-Knock, Paul R. Heath, David Baker, David R. Drew, M. Jill Saffrey, Julie E. Simpson, Ignacio A. Romero, and et al. 2019. "Age-Associated mRNA and miRNA Expression Changes in the Blood-Brain Barrier" International Journal of Molecular Sciences 20, no. 12: 3097. https://doi.org/10.3390/ijms20123097
APA StyleGoodall, E. F., Leach, V., Wang, C., Cooper-Knock, J., Heath, P. R., Baker, D., Drew, D. R., Saffrey, M. J., Simpson, J. E., Romero, I. A., & Wharton, S. B. (2019). Age-Associated mRNA and miRNA Expression Changes in the Blood-Brain Barrier. International Journal of Molecular Sciences, 20(12), 3097. https://doi.org/10.3390/ijms20123097





