Carbonic Anhydrase as a Biomarker of Global and Local Impacts: Insights from Calcifying Animals
Abstract
1. Carbonic Anhydrase and Its Biological Function
1.1. Carbonic Anhydrase
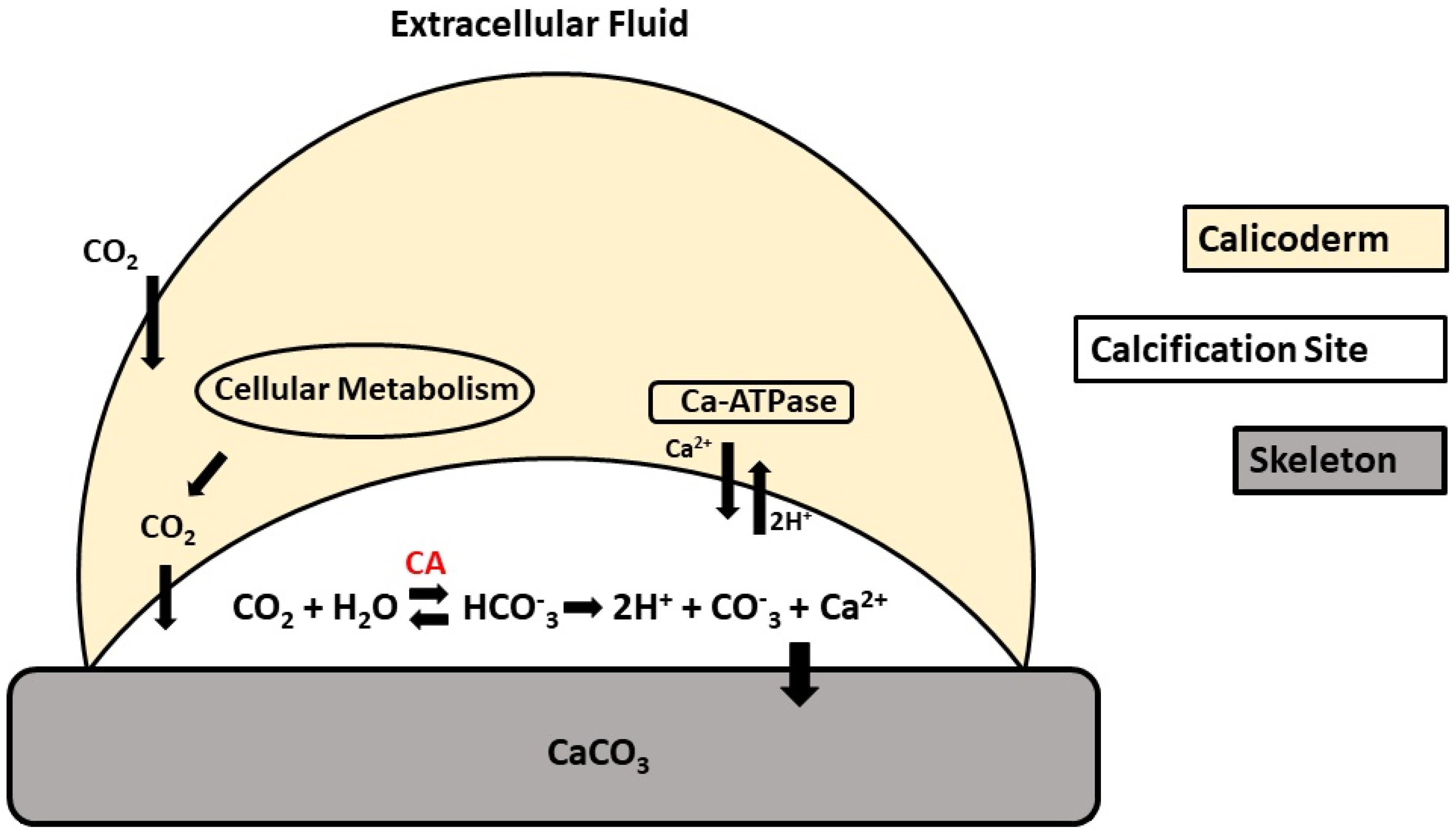
1.2. Biological Function
2. Untangling Global and Local Impacts
2.1. Global Impacts
2.2. Local Impacts
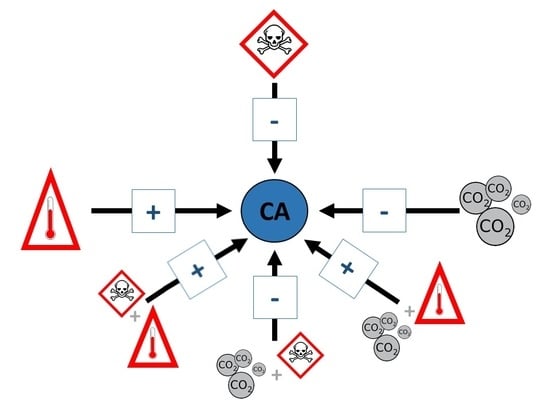
2.3. Tangled Effects: The Role of Interactions
3. Bioindicators and Biomarkers
3.1. Bioindicators
3.2. Biomarkers
4. Carbonic Anhydrase as a Biomarker in Calcifying Organisms
4.1. Global Impacts: Effects of Water Acidification
4.2. Global Impacts: Effects of Warming
4.3. Local Impacts: Effects of Environmental Pollution
4.4. Combined Impacts: The Role of Interactions
5. Corals in the Spotlight of Biomonitoring Programs: Combining Carbonic Anhydrase Assessment with Specific Organismal Analyses
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tripp, B.C.; Smith, K.S.; Ferry, J.G. Carbonic Anhydrase: New Insights for an Ancient Enzyme. J. Biol. Chem. 2001, 276, 48615–48618. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, N.; Jackson, D.J.; Marie, B.; Ramos-Silva, P.; Marin, F. Carbonic anhydrase and metazoan biocalcification: A focus on molluscs. Key Eng. Mater. 2016, 672, 151–157. [Google Scholar] [CrossRef]
- Christianson, D.W.; Fierke, C.A. Carbonic Anhydrase: Evolution of the zinc binding site by nature and by design. Acc. Chem. Res. 1996, 29, 331–339. [Google Scholar] [CrossRef]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- Gilmour, K.M.; Perry, S.F. Carbonic anhydrase and acid-base regulation in fish. J. Exp. Biol. 2009, 212, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Monserrat, J.M.; Martínez, P.E.; Geracitano, L.A.; Amado, L.L.; Martins, C.M.G.; Pinho, G.L.L.; Chaves, I.S.; Ferreira-Cravo, M.; Ventura-Lima, J.; Bianchini, A. Pollution biomarkers in estuarine animals: Critical review and new perspectives. Comp. Biochem. Physiol. C 2007, 146, 221–234. [Google Scholar] [CrossRef]
- Henry, R.P.; Cameron, J.N. The distribution and partial characterization of carbonic anhydrase in selected aquatic and terrestrial decapod crustaceans. J. Exp. 1982, 221, 309–321. [Google Scholar] [CrossRef]
- Henry, R.P. The role of carbonic anhydrase in blood ion and acid-base regulation. Am. Zool. 1984, 24, 241–251. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Grosell, M. Intestinal anion exchange in marine fish osmoregulation. J. Exp. Biol. 2006, 209, 2813–2827. [Google Scholar] [CrossRef]
- Grosell, M.; Genz, J.; Taylor, J.R.; Perry, S.F.; Gilmour, K.M. The involvement of H+-ATPase and carbonic anhydrase in intestinal HCO3− secretion in seawater acclimated rainbow trout. J. Exp. Biol. 2009, 212, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M.; Laliberte, C.N.; Wood, S.; Jensen, F.B.; Wood, C.M. Intestinal HCO3− secretion in marine teleost fish: Evidence for an apical rather than a basolateral Cl−/HCO3− exchanger. Fish. Physiol. Biochem. 2001, 24, 81–95. [Google Scholar] [CrossRef]
- Grosell, M.; Wood, C.M.; Wilson, R.W.; Bury, N.R.; Hogstrand, C.; Rankin, C.; Jensen, F.B. Bicarbonate secretion plays a role in chloride and water absorption of the European flounder intestine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R936–R946. [Google Scholar] [CrossRef] [PubMed]
- Genz, J.; Taylor, J.R.; Grosell, M. Effects of salinity on intestinal bicarbonate secretion and compensatory regulation of acid–base balance in Opsanus Beta. J. Exp. Biol. 2008, 211, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Burnett, L.E. CO2 excretion across isolated perfused crab gills: Facilitation by carbonic anhydrase. Am. Zool. 1984, 24, 253–264. [Google Scholar] [CrossRef]
- McMahon, B.R.; Burnett, L.E.; Defur, P.L. Carbon dioxide excretion and carbonic anhydrase function in the red rock crab, Cancer productus. J. Comp. Physiol. 1984, 154, 371–383. [Google Scholar] [CrossRef]
- Weihrauch, D.; Wilkie, M.P.; Walsh, P.J. Ammonia and urea transporters in gills of fish and aquatic crustaceans. J. Exp. Biol. 2009, 212, 1716–1730. [Google Scholar] [CrossRef] [PubMed]
- Lonnerholm, C. Pulmonary carbonic anhydrase in the human, monkey and rat. J. Appl. Physiol. 1982, 52, 352–356. [Google Scholar] [CrossRef]
- Karlmark, B.B.; Agerup, B.; Wistrand, P.J. Renal proximal tubular acidification. Role of brush-border and cytoplasmic carbonic anhydrase. Acta Physiol. Scud 1979, 106, 145–150. [Google Scholar] [CrossRef]
- Elder, J.A.; Lehninger, A.L. Respiration-dependent transport of carbon dioxide into rat liver mitochondria. Biochemistry 1973, 12, 976–982. [Google Scholar] [CrossRef]
- Simone, G.; Supuran, C.T. Antiobesity carbonic anhydrase inhibitors. Curr. Top. Med. Chem. 2007, 7, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Colombo-Pallotta, M.F.; Rodríguez-Román, A.; Iglesias-Prieto, R. Calcification in bleached and unbleached Montastraea faveolata: Evaluating the role of oxygen and glycerol. Coral Reefs 2010, 29, 899–907. [Google Scholar] [CrossRef]
- Wild, C.; Hoegh-Guldberg, O.; Naumann, M.S.; Colombo-Pallotta, M.F.; Ateweberhan, M.; Fitt, W.K.; Iglesiasprieto, R.; Palmer, C.; Bythell, J.C.; Ortiz, J.C.; et al. Climate change impedes scleractinian corals as primary reef ecosystem engineers. Mar. Freshw. Res. 2011, 62, 205–215. [Google Scholar] [CrossRef]
- Allemand, D.; Ferrier-Pagès, C.; Furla, P.; Houlbrèque, F.; Puverel, S.; Reynaud, S.; Tambutté, É.; Tambutté, S.; Zoccola, D. Biomineralisation in reef-building corals: From molecular mechanisms to environmental control. Gen. Palaeont. 2004, 3, 453–467. [Google Scholar] [CrossRef]
- Al-Horani, F.A.; Al-Moghrabi, S.M.; De Beer, D. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 2003, 142, 419–426. [Google Scholar] [CrossRef]
- Zoccola, D.; Tambutte, E.; Kulhanek, E.; Puverel, S.; Scimeca, J.C.; Allemand, D.; Tambutte, S. Molecular cloning and localization of a PMCA P-type calcium ATPase from the coral Stylophora Pist. Biochim. Biophys. Acta-Biomembr. 2004, 1663, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, A.; Moya, A.; Tambutté, S.; Allemand, D.; Supuran, C.T.; Zoccola, D. Carbonic anhydrases in anthozoan corals e a review. Bioorg. Med. Chem. 2013, 21, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Sandeman, I.M. Light driven lipid peroxidation of coral membranes and a suggested role in calcification. Rev. Biol. Trop. 2008, 56, 1–9. [Google Scholar] [CrossRef][Green Version]
- Pedrozo, H.A.; Schwartz, Z.; Dean, D.D.; Wiederhold, M.L.; Boyan, B.D. Regulation of statoconia mineralization in Aplysia californica in vitro. Connect. Tissue Res. 1996, 35, 317–323. [Google Scholar] [CrossRef]
- Ebanks, S.C.; O’Donnell, M.J.; Grosell, M. Acquisition of Ca2+ and HCO3−/CO3− for shell formation in embryos of the common pond snail Lymnaea stagnalis. J. Comp. Physiol. B 2010, 180, 953–965. [Google Scholar] [CrossRef]
- Gaume, B.M.; Fouchereau-Peron, A.; Badou, M.N.; Helléouet, S.; Auzoux-Bordenave, S. Biomineralization markers during early shell formation in the European abalone Haliotis tuberculata, Linnaeus. Mar. Biol. 2011, 158, 341–353. [Google Scholar] [CrossRef]
- Jones, W.C.; Ledger, P.W. The effect of diamox and various concentrations of calcium on spicule secretion in the calcareous sponge Sycon Cibiatum. Comp. Biochem. Physiol. 1986, 84, 149–158. [Google Scholar] [CrossRef]
- Lucas, J.M.; Knapp, L.W. A physiological evalution of carbon sources for calcification in the octocoral Leptogorgia virgulata (Lamarck). J. Exp. Biol. 1997, 200, 2653–2662. [Google Scholar] [PubMed]
- Marangoni, L.F.B.; Calderon, E.N.; Marques, J.A.; Pereira, C.M.; Duarte, G.A.S.; Castro, C.B.; Bianchini, A. Effects of CO2-driven acidification of seawater on the calcification process in the calcareous hydrozoan Millepora alcicornis (Linnaeus, 1758). Coral Reefs 2017, 36, 1133–1141. [Google Scholar] [CrossRef]
- Kingsley, R.; Watabe, N. Role of carbonic anhydrase in calcification in the gorgonian Leptogorgia Virgulata. J. Exp. Zool. 1987, 241, 171–180. [Google Scholar] [CrossRef]
- Giraud, M.M. Carbonic anhydrase activity in the integument of the crab Carcinus maenas during the intermolt cycle. Comp. Biochem. Physiol. 1981, 69, 381–387. [Google Scholar] [CrossRef]
- Giraud-Guille, M.M. Calcification initiation sites in the crab cuticle: The interprismatic septa. An ultrastructural cytochemical study. Cell Tissue Res. 1984, 236, 413–420. [Google Scholar] [CrossRef]
- Okazaki, M. Carbonic anhydrase in the calcareous red alga, Serraticardia Maxima. Bot. Mar. 1972, 15, 133–138. [Google Scholar] [CrossRef]
- Zilberberg, C.; Abrantes, D.P.; Marques, J.A.; Machado, L.F.; Marangoni, L.F.B. Conhecendo os Recifes Brasileiros: Rede de Pesquisas Coral Vivo; Museu Nacional: Rio de Janeiro, UFRJ, Brasil, 2016; 360p. [Google Scholar]
- IPCC. The Fifth Assessment Report of the Intergovernmental Panel on Climate. 2014. Available online: https://www.ipcc.ch/assessment-report/ar5/ (accessed on 20 February 2019).
- Uprety, D.C.; Reddy, V.R.; Mura, J.D. Greenhouse Gases: A Historical Perspective. In Climate Change and Agriculture; Springer: Singapore, 2019; pp. 31–41. [Google Scholar]
- Rodhe, H. A comparison of the contribution of various gases to the greenhouse effect. Science 1990, 248, 1217–1219. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Gattuso, J.P. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef]
- Meinshausen, M.; Meinshausen, N.; Hare, W.; Raper, S.C.; Frieler, K.; Knutti, R.; Allen, M.R. Greenhouse-gas emission targets for limiting global warming to 2 C. Nature 2009, 458, 1158. [Google Scholar] [CrossRef]
- Cook, J.; Oreskes, N.; Doran, P.T.; Anderegg, W.R.; Verheggen, B.; Maibach, E.W.; Nuccitelli, D. Consensus on consensus: A synthesis of consensus estimates on human-caused global warming. Environ. Res. Lett. 2016, 11, 048002. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57. [Google Scholar] [CrossRef]
- Baroiller, J.F.; D’Cotta, H.; Saillant, E. Environmental effects on fish sex determination and differentiation. Sex. Dev. 2009, 3, 118–135. [Google Scholar] [CrossRef]
- Inazawa, J.; Hattori, R.S.; Oura, M.; Yokota, M.; Strüssmann, C.A. Temperature effects on sex differentiation of the reciprocal hybrids of Odontesthes bonariensis and Odontesthes hatcheri (Atherinopsidae). Aquac. Res. 2011, 42, 746–753. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanism and Process in Physiological Evolution; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Cherkasov, A.S.; Biswas, P.K.; Ridings, D.M.; Ringwood, A.H.; Sokolova, I.M. Effects of acclimation temperature and cadmium exposure on cellular energy budgets in a marine mollusk Crassostrea virginica: Linking cellular and mitochondrial responses. J. Exp. Biol. 2006, 209, 1274–1284. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2013, 166, 237–243. [Google Scholar] [CrossRef]
- Fonseca, J.S.; Marangoni, L.F.; Marques, J.A.; Bianchini, A. Effects of increasing temperature alone and combined with copper exposure on biochemical and physiological parameters in the zooxanthellate scleractinian coral Mussismilia Harttii. Aquat. Toxicol. 2017, 190, 121–132. [Google Scholar] [CrossRef]
- Wang, J.; Dong, B.; Yu, Z.X.; Yao, C.L. The impact of acute thermal stress on green mussel Perna viridis: Oxidative damage and responses. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2018, 222, 7–15. [Google Scholar] [CrossRef]
- Zafalon-Silva, B.; Zebral, Y.D.; Bianchini, A.; Da Rosa, C.E.; Marins, L.F.; Colares, E.P.; Robaldo, R.B. Erythrocyte nuclear abnormalities and leukocyte profile in the Antarctic fish Notothenia coriiceps after exposure to short-and long-term heat stress. Pol. Biol. 2017, 40, 1755–1760. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Zebral, Y.D.; Roza, M.; da Silva Fonseca, J.; Costa, P.G.; Oliveira, C.S.; Zocke, T.G.; Bianchini, A. Waterborne copper is more toxic to the killifish Poecilia vivipara in elevated temperatures: Linking oxidative stress in the liver with reduced organismal thermal performance. Aquat. Toxicol. 2019, 209, 142–149. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Bradbury, R.H.; Sala, E.; Hughes, T.P.; Bjorndal, K.A.; Cooke, R.G.; Warner, R.R. Global trajectories of the long-term decline of coral reef ecosystems. Science 2003, 301, 955–958. [Google Scholar] [CrossRef]
- Magrin, G.O.; Marengo, J.A.; Boulanger, J.-P.; Buckeridge, M.S.; Castellanos, E.; Poveda, G.; Scarano, F.R.; Vicuña, S. 2014: Central and South America. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part. B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Barros, V.R., Field, C.B., Dokken, D.J., Mastrandrea, M.D., Mach, K.J., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1499–1566. [Google Scholar]
- Wilkinson, C.R. Global and local threats to coral reef functioning and existence: Review and predictions. Mar. Freshw. Res. 1999, 50, 867–878. [Google Scholar] [CrossRef]
- Borgå, K. Ecotoxicology: Bioaccumulation. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 346–348. [Google Scholar]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Lewitus, A. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Goldscheider, N. Delineation of spring protection zones. In Groundwater Hydrology of Springs; Elsevier Butterworth-Heinemann: Oxford, UK, 2010; pp. 305–338. [Google Scholar]
- Carpenter, K.E.; Abrar, M.; Aeby, G.; Aronson, R.B.; Banks, S.; Bruckner, A.; Edgar, G.J. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 2008, 321, 560–563. [Google Scholar] [CrossRef]
- Ban, S.S.; Graham, N.A.; Connolly, S.R. Evidence for multiple stressor interactions and effects on coral reefs. Glob. Chang. Biol. 2014, 20, 681–697. [Google Scholar] [CrossRef]
- Knowlton, N.; Jackson, J.B.C. Shifting Baselines, Local Impacts, and Global Change on Coral Reefs. PLoS Biol. 2008, 6, e54. [Google Scholar] [CrossRef]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 345, 101–118. [Google Scholar]
- Rushton, S.P.; Luff, M.L.; Eyre, M.D. Effect of pasture improvement and management on the ground beetle and spider communities of upland grasslands. J. Appl. Ecol. 1989, 26, 489–503. [Google Scholar] [CrossRef]
- Rushton, S.P.; Eyre, M.D.; Luff, M.L. The effects of management on the occurrence of some ground beetle species in grassland. In The Role of Ground Beetles in Ecological and Environmental Studies; Stork, N.E., Ed.; Intercept: Andover, UK, 1990; pp. 209–216. [Google Scholar]
- Abensperg, T.M.; Smith, G.T.; Arnold, G.W.; Steven, D.E. The effects of habitat fragmentation and livestock-grazing on animal communities in remnants of gimlet Eucalyptus salubris woodland in the Western Australian wheatbelt. I. Arthropods. J. Appl. Ecol. 1996, 33, 1281–1303. [Google Scholar] [CrossRef]
- Vieira, C.E.D.; Costa, P.G.; Caldas, S.S.; Tesser, M.E.; Risso, W.E.; Escarrone, A.L.V.; Reis Martinez, C.B. An integrated approach in subtropical agro-ecosystems: Active biomonitoring, environmental contaminants, bioaccumulation, and multiple biomarkers in fish. Sci. Total Environ. 2019, 666, 508–524. [Google Scholar] [CrossRef]
- Marques, D.S.; Costa, P.G.; Souza, G.M.; Cardozo, J.G.; Barcarolli, I.F.; Bianchini, A. Selection of biochemical and physiological parameters in the croaker Micropogonias furnieri as biomarkers of chemical contamination in estuaries using a generalized additive model (GAM). Sci. Total Environ. 2019, 647, 1456–1467. [Google Scholar] [CrossRef]
- Acott, C. JS Haldane, JBS Haldane, L Hill and A Siebe: A brief resume of their lives. South Pac. Underw. Med. Soc. J. 1999, 29, 161–165. [Google Scholar]
- Withrow, S.J.; Vail, D.M. Withrow and MacEwen’s Small Animal Clinical Oncology, 4th ed.; Elsevier: Philadelphia, PA, USA, 2007; pp. 73–74. [Google Scholar]
- Rainio, J.; Niemelä, J. Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Cooper, T.F.; Gilmour, J.P.; Fabricius, K.E. Bioindicators of changes in water quality on coral reefs: Review and recommendations for monitoring programmes. Coral Reefs 2009, 28, 589–606. [Google Scholar] [CrossRef]
- Lambeck, R.J. Focal species: A multi-species umbrella for nature conservation. Conserv. Biol. 1997, 11, 849–856. [Google Scholar] [CrossRef]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The role of biomarkers in the assessment of aquatic ecosystem health. Integr. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Erroi, E.; Giordano, M.E.; Schettino, T. Potential application of carbonic anhydrase activity in bioassay and biomarker studies. Chem. Ecol. 2006, 22, S119–S125. [Google Scholar] [CrossRef]
- Kawahata, H.; Fujita, K.; Iguchi, A.; Inoue, M.; Iwasaki, S.; Kuroyanagi, A.; Toyofuku, T. Perspective on the response of marine calcifiers to global warming and ocean acidification—Behavior of corals and foraminifera in a high CO2 world “hot house”. Prog. Earth Planet. Sci. 2019, 6, 5. [Google Scholar] [CrossRef]
- Vogel, N.; Meyer, F.W.; Wild, C.; Uthicke, S. Decreased light availability can amplify negative impacts of ocean acidification on calcifying coral reef organisms. Mar. Ecol. Prog. Ser. 2015, 521, 49–61. [Google Scholar] [CrossRef]
- Spalding, C.; Finnegan, S.; Fischer, W.W. Energetic costs of calcification under ocean acidification. Glob. Biogeochem. Cycles 2017, 31, 866–877. [Google Scholar] [CrossRef]
- Hofmann, L.C.; Straub, S.; Bischof, K. Elevated CO2 levels affect the activity of nitrate reductase and carbonic anhydrase in the calcifying rhodophyte Corallina officinalis. J. Exp. Bot. 2013, 64, 899–908. [Google Scholar] [CrossRef]
- Marangoni, L.F.B.; Pinto, M.M.D.A.N.; Marques, J.A.; Bianchini, A. Copper exposure and seawater acidification interaction: Antagonistic effects on biomarkers in the zooxanthellate scleractinian coral Mussismilia harttii. Aquat. Toxicol. 2019, 206, 123–133. [Google Scholar] [CrossRef]
- Prazeres, M.; Uthicke, S.; Pandolfi, J.M. Ocean acidification induces biochemical and morphological changes in the calcification process of large benthic foraminifera. Proc. R. Soc. B 2015, 282. [Google Scholar] [CrossRef]
- Moya, A.; Huisman, L.; Ball, E.E.; Hayward, D.C.; Grasso, L.C.; Chua, C.M.; Woo, H.N.; Gattuso, J.P.; ForêT, S.; Miller, D.J. Whole transcriptome analysis of the coral Acropora millepora reveals complex responses to CO2-driven acidification during the initiation of calcification. Mol. Ecol. 2012, 21, 2440–2454. [Google Scholar] [CrossRef]
- Zoccola, D.; Innocenti, A.; Bertucci, A.; Tambutté, E.; Supuran, C.; Tambutté, S. Coral carbonic anhydrases: Regulation by ocean acidification. Mar. Drugs 2016, 14, 109. [Google Scholar] [CrossRef]
- Richier, S.; Fiorini, S.; Kerros, M.E.; Von Dassow, P.; Gattuso, J.P. Response of the calcifying coccolithophore Emiliania huxleyi to low pH/high pCO2: From physiology to molecular level. Mar. Biol. 2011, 158, 551–560. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Jia, Z.; Qiu, L.; Wang, L.; Zhang, A.; Song, L. A carbonic anhydrase serves as an important acid-base regulator in pacific oyster Crassostrea gigas exposed to elevated CO2: Implication for physiological responses of mollusk to ocean acidification. Mar. Biotechnol. 2017, 19, 22–35. [Google Scholar] [CrossRef]
- Carreiro-Silva, M.; Cerqueira, T.; Godinho, A.; Caetano, M.; Santos, R.S.; Bettencourt, R. Molecular mechanisms underlying the physiological responses of the cold-water coral Desmophyllum dianthus to ocean acidification. Coral Reefs 2014, 33, 465–476. [Google Scholar] [CrossRef]
- Vidal-Dupiol, J.; Zoccola, D.; Tambutté, E.; Grunau, C.; Cosseau, C.; Smith, K.M.; Freitag, M.; Dheilly, N.M.; Allemand, D.; Tambutté, S. Genes related to ion-transport and energy production are upregulated in response to CO2-driven pH decrease in corals: New insights from transcriptome analysis. PLoS ONE 2013, 8, e58652. [Google Scholar] [CrossRef]
- Sun, T.; Tang, X.; Zhou, B.; Wang, Y. Comparative studies on the effects of seawater acidification caused by CO2 and HCl enrichment on physiological changes in Mytilus edulis. Chemosphere 2016, 144, 2368–2376. [Google Scholar] [CrossRef]
- Fitzer, S.C.; Phoenix, V.R.; Cusack, M.; Kamenos, N.A. Ocean acidification impacts mussel control on biomineralisation. Sci. Rep. 2014, 4, 6218. [Google Scholar] [CrossRef]
- Moreira, A.; Figueira, E.; Soares, A.M.; Freitas, R. The effects of arsenic and seawater acidification on antioxidant and biomineralization responses in two closely related Crassostrea species. Sci. Total Environ. 2016, 545, 569–581. [Google Scholar] [CrossRef]
- Edge, S.E.; Morgan, M.B.; Gleason, D.F.; Snell, T.W. Development of a coral cDNA array to examine gene expression profiles in Montastraea faveolata exposed to environmental stress. Mar. Pollut. Bull. 2005, 51, 507–523. [Google Scholar] [CrossRef]
- Ogawa, D.; Bobeszko, T.; Ainsworth, T.; Leggat, W. The combined effects of temperature and CO 2 lead to altered gene expression in Acropora aspera. Coral Reefs 2013, 32, 895–907. [Google Scholar] [CrossRef]
- Hoadley, K.D.; Pettay, D.T.; Grottoli, A.G.; Cai, W.J.; Melman, T.F.; Schoepf, V.; Matsui, Y. Physiological response to elevated temperature and pCO2 varies across four Pacific coral species: Understanding the unique host+ symbiont response. Sci. Rep. 2015, 5, 18371. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Dickinson, G.H.; Matoo, O.B.; Bagwe, R.; Dickinson, A.; Beniash, E.; Sokolova, I.M. Interactive effects of elevated temperature and CO2 levels on energy metabolism and biomineralization of marine bivalves Crassostrea virginica and Mercenaria mercenaria. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2013, 166, 101–111. [Google Scholar] [CrossRef]
- Pepper, I.L.; Brusseau, M.L.; Gerba, C.P. Environmental and Pollution Science, 3rd ed.; Academic Press/Elsevier: San Diego, CA, USA, 2019. [Google Scholar]
- El-Gendy, K.S.; Radwan, M.A.; Gad, A.F.; Khamis, A.E.; Eshra, E.S.H. Physiological traits of land snails Theba pisanaas simple endpoints to assess the exposure to some pollutants. Environ. Sci. Pollut. Res. 2019, 1–9. [Google Scholar]
- Santini, O.; Chahbane, N.; Vasseur, P.; Frank, H. Effects of low-level copper exposure on Ca2+-ATPase and carbonic anhydrase in the freshwater bivalve Anodonta anatina. Toxicol. Environ. Chem. 2011, 93, 1826–1837. [Google Scholar] [CrossRef]
- Bielmyer, G.K.; Grosell, M.; Bhagooli, R.; Baker, A.C.; Langdon, C.; Gillette, P.; Capo, T.R. Differential effects of copper on three species of scleractinian corals and their algal symbionts (Symbiodinium spp.). Aquat. Toxicol. 2010, 97, 125–133. [Google Scholar] [CrossRef]
- Fonseca, S.J.; de Barros Marangoni, L.F.; Marques, J.A.; Bianchini, A. Carbonic anhydrase activity as a potential biomarker for acute exposure to copper in corals. Chemosphere 2019, 227, 598–605. [Google Scholar] [CrossRef]
- Caricato, R.; Lionetto, M.G.; Dondero, F.; Viarengo, A.; Schettino, T. Carbonic anhydrase activity in Mytilus galloprovincialis digestive gland: Sensitivity to heavy metal exposure. Comp. Biochem. Physiol. C 2010, 152, 241–247. [Google Scholar]
- Balbi, T.; Camisassi, G.; Montagna, M.; Fabbri, R.; Franzellitti, S.; Carbone, C.; Canesi, L. Impact of cationic polystyrene nanoparticles (PS-NH2) on early embryo development of Mytilus galloprovincialis: Effects on shell formation. Chemosphere 2017, 186, 1–9. [Google Scholar] [CrossRef]
- Capolupo, M.; Franzellitti, S.; Valbonesi, P.; Lanzas, C.S.; Fabbri, E. Uptake and transcriptional effects of polystyrene microplastics in larval stages of the Mediterranean mussel Mytilus galloprovincialis. Environ. Pollut. 2018, 241, 1038–1047. [Google Scholar] [CrossRef]
- Santos, M.B.; Neto, I.E.M.; de Souza Melo, S.R.C.; Amado, E.M. Hemolymph and gill carbonic anhydrase are more sensitive to aquatic contamination than mantle carbonic anhydrase in the mangrove oyster Crassostrea rhizophorae. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2017, 201, 19–25. [Google Scholar] [CrossRef]
- Azevedo-Linhares, M.; Freire, C. Evaluation of impacted Brazilian estuaries using the native oyster Crassostrea rhizophorae: Branchial carbonic anhydrase as a biomarker. Ecotoxicol. Environ. Saf. 2015, 122, 483–489. [Google Scholar] [CrossRef]
- Bielmyer-Fraser, G.K.; Patel, P.; Capo, T.; Grosell, M. Physiological responses of corals to ocean acidification and copper exposure. Mar. Pollut. Bull. 2018, 133, 781–790. [Google Scholar] [CrossRef]
- Kaniewska, P.; Chan, C.K.K.; Kline, D.; Ling, E.Y.S.; Rosic, N.; Edwards, D.; Dove, S. Transcriptomic changes in coral holobionts provide insights into physiological challenges of future climate and ocean change. PLoS ONE 2015, 10, e0139223. [Google Scholar] [CrossRef]
- Obura, D.O. Can differential bleaching and mortality among coral species offer useful indicators for assessment and management of reefs under stress? Bull. Mar. Sci. 2001, 69, 421–442. [Google Scholar]
- Siebeck, U.E.; Marshall, N.J.; Klueter, A.; Hoegh-Guldberg, O. Monitoring coral bleaching using a colour reference card. Coral Reefs 2006, 25, 453–460. [Google Scholar] [CrossRef]
- Bythell, J.C.; Brown, B.E.; Kirkwood, T.B. Do reef corals age? Biol. Rev. 2018, 93, 1192–1202. [Google Scholar] [CrossRef]
- Davies, P. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 1989, 101, 389–395. [Google Scholar] [CrossRef]
- Chisholm, J.R.M.; Gattuso, J.P. Validation of the alkalinity anomaly technique for investigating calcification and photosynthesis in coral reef communities. Limnol. Oceanogr. 1991, 36, 1232–1239. [Google Scholar] [CrossRef]
- Yao, W.; Byrne, R.H. Simplified seawater alkalinity analysis: Use of linear array spectrometers. Deep. Sea. Res. Part. 1 Oceanogr. Res. Pap. 1998, 45, 1383–1392. [Google Scholar] [CrossRef]
- Lough, J.M. Coral calcification from skeletal records revisited. Mar. Ecol. Prog. Ser. 2008, 373, 257–264. [Google Scholar] [CrossRef]
- De’ath, G.; Lough, J.M.; Fabricius, K.E. Declining coral calcification on the Great Barrier Reef. Science 2009, 323, 116–119. [Google Scholar] [CrossRef]
- Cantin, N.E.; Cohen, A.L.; Karnauskas, K.B.; Tarrant, A.M.; McCorkle, D.C. Ocean warming slows coral growth in the central Red Sea. Science 2010, 329, 322–325. [Google Scholar] [CrossRef]
- Crook, E.D.; Cohen, A.L.; Rebolledo-Vieyra, M.; Hernandez, L.; Paytan, A. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc. Natl. Acad. Sci. USA 2013, 110, 11044–11049. [Google Scholar] [CrossRef]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Claar, D.C. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef]
- Stanley, G.D. Photosymbiosis and the evolution of modern coral reefs. Science 2006, 312, 857–858. [Google Scholar] [CrossRef]
- Yellowlees, D.; Rees, T.A.V.; Leggat, W. Metabolic interactions between algal symbionts and invertebrate hosts. Plant. Cell. Environ. 2008, 31, 679–694. [Google Scholar] [CrossRef]
- Baird, A.H.; Marshall, P.A. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002, 237, 133–141. [Google Scholar] [CrossRef]
- Meehan, W.J.; Ostrander, G.K. Coral bleaching: A potential biomarker of environmental stress. J. Toxicol. Environ. HealthPart. A Curr. Issues 1997, 50, 529–552. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zebral, Y.D.; da Silva Fonseca, J.; Marques, J.A.; Bianchini, A. Carbonic Anhydrase as a Biomarker of Global and Local Impacts: Insights from Calcifying Animals. Int. J. Mol. Sci. 2019, 20, 3092. https://doi.org/10.3390/ijms20123092
Zebral YD, da Silva Fonseca J, Marques JA, Bianchini A. Carbonic Anhydrase as a Biomarker of Global and Local Impacts: Insights from Calcifying Animals. International Journal of Molecular Sciences. 2019; 20(12):3092. https://doi.org/10.3390/ijms20123092
Chicago/Turabian StyleZebral, Yuri Dornelles, Juliana da Silva Fonseca, Joseane Aparecida Marques, and Adalto Bianchini. 2019. "Carbonic Anhydrase as a Biomarker of Global and Local Impacts: Insights from Calcifying Animals" International Journal of Molecular Sciences 20, no. 12: 3092. https://doi.org/10.3390/ijms20123092
APA StyleZebral, Y. D., da Silva Fonseca, J., Marques, J. A., & Bianchini, A. (2019). Carbonic Anhydrase as a Biomarker of Global and Local Impacts: Insights from Calcifying Animals. International Journal of Molecular Sciences, 20(12), 3092. https://doi.org/10.3390/ijms20123092