Anti-Obesity Effects of Dietary Calcium: The Evidence and Possible Mechanisms
Abstract
1. The Current Situation of Obesity and Its Adverse Effects
2. The Anti-Obesity Effects of Calcium Supplementation
2.1. Inhibition of Adipogenic Differentiation by Calcium in Cell Models
2.2. Anti-Obesity Effects of Dietary Calcium in Animals
2.3. Anti-Obesity Effects of Dietary Calcium in Humans
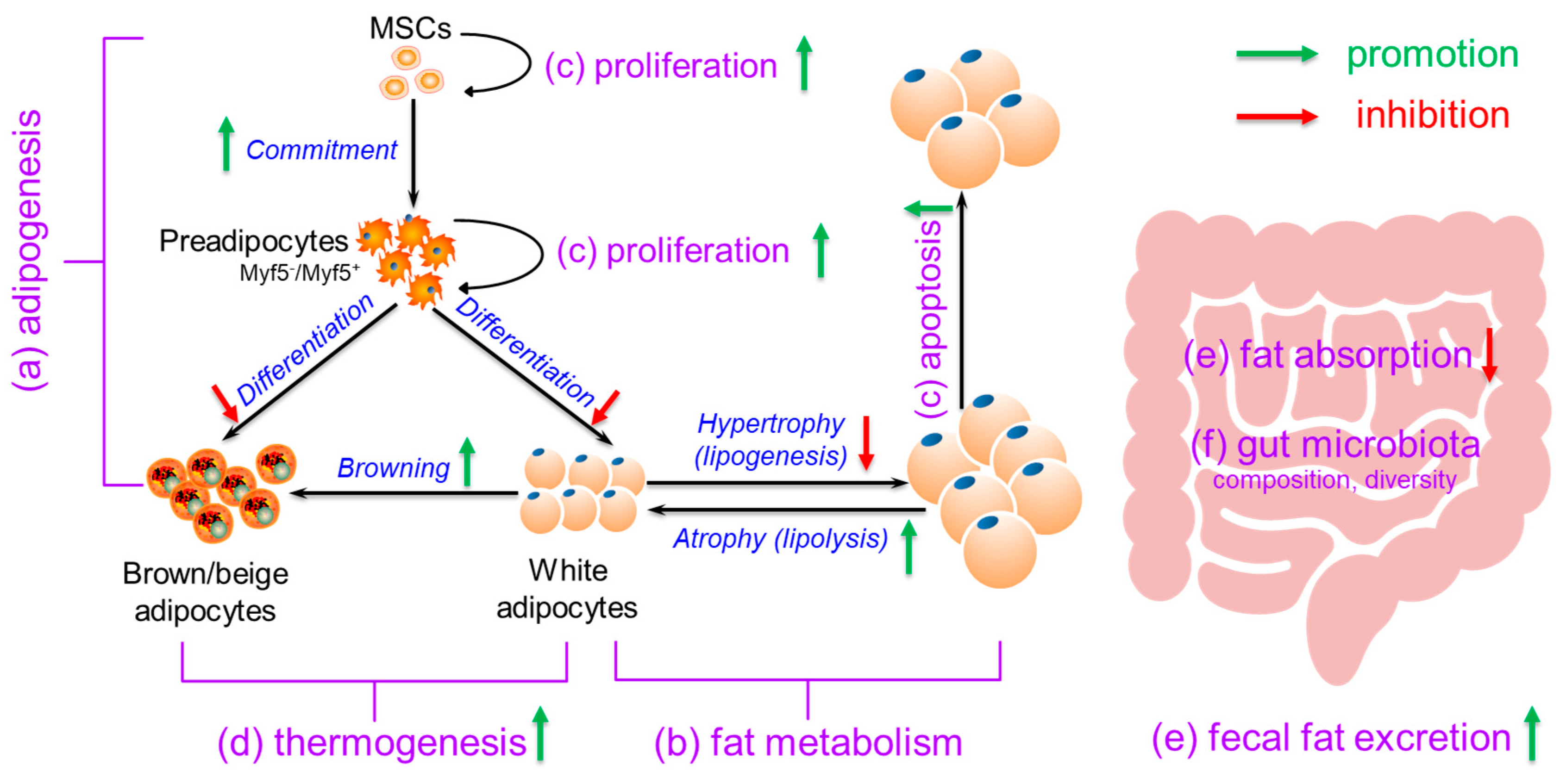
3. Possible Mechanisms for Calcium’s Anti-Obesity Effects
3.1. Effects of Calcium on Adipogenesis
3.2. Effects of Calcium on Fat Metabolism
3.3. Effects of Calcium on Adipocyte (Precursor) Proliferation and Apoptosis
3.4. Effects of Calcium on Thermogenesis
3.5. Effects of Calcium on Fat Absorption and Fecal Fat Excretion
3.6. Effects of Calcium on Gut Microbiota
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BAT | brown adipose tissue |
| iBAT | interscapular brown adipose tissue |
| [Ca2+]i | intracellular calcium |
| [Ca2+]o | extracellular calcium |
| CaSR | calcium-sensing receptor |
| C/EBPα | CCAAT/enhancer binding protein α |
| FAS | fatty acid synthetase |
| HSL | hormone sensitive lipase |
| LPL | lipoprotein lipase |
| MSCs | mesenchymal stem cells |
| pBMSCs | porcine bone marrow mesenchymal stem cells |
| PGC1-α | peroxisome proliferator-activated receptor coactivator 1α |
| PPARγ | peroxisome proliferator activated receptor γ |
| PRDM16 | PR domain zinc-finger protein 16 |
| T2DM | type 2 diabetes mellitus |
| TG | Triglyceride |
| TRPV2 | transient receptor potential vanilloid 2 |
| UCP1 | uncoupling protein 1 |
| VAT | visceral adipose tissue |
| WAT | white adipose tissue |
| eWAT | epididymal white adipose tissue |
| iWAT | inguinal white adipose tissue |
References
- Collaboration, N.C.D.R.F. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar]
- Ludwig, D.S. Epidemic childhood obesity: Not yet the end of the beginning. Pediatrics 2018, 141, e20174078. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Skinner, A.C.; Ravanbakht, S.N.; Skelton, J.A.; Perrin, E.M.; Armstrong, S.C. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 2018, 141, e20173459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Wang, Z.; Du, W.; Su, C.; Zhang, J.; Jiang, H.; Jia, X.; Huang, F.; Ouyang, Y.; et al. Prevalence and stabilizing trends in overweight and obesity among children and adolescents in China, 2011–2015. BMC Public Health 2018, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.H.; Min, J.; Xue, H.; Du, S.; Xu, F.; Wang, H.; Wang, Y. What factors may contribute to sex differences in childhood obesity prevalence in China? Public Health Nutr. 2018, 21, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, J.; Farr, O.; Perakakis, N.; Ghaly, W.; Mantzoros, C. Obesity as a disease. Med. Clin. N. Am. 2018, 102, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical Problems Caused by Obesity. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.V.; Ruotolo, G.; Robbins, D.C. Obesity and dyslipidemia. Endocrinol. Metab. Clin. N. Am. 2003, 32, 855–867. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, V.G.; Aroor, A.R.; Sowers, J.R. The pathophysiology of hypertension in patients with obesity. Nat. Rev. Endocrinol. 2014, 10, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef]
- Mandviwala, T.; Khalid, U.; Deswal, A. Obesity and Cardiovascular Disease: A Risk Factor or a Risk Marker? Curr. Atheroscler. Rep. 2016, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Milic, S.; Lulic, D.; Stimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef]
- Dimitriadis, G.K.; Barber, T.M. Obesity-related metabolic and reproductive dysfunction: Variations between the sexes. Expert Rev. Endocrinol. Metab. 2016, 11, 387–393. [Google Scholar] [CrossRef]
- Kahn, B.E.; Brannigan, R.E. Obesity and male infertility. Curr. Opin. Urol. 2017, 27, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.E.; Blackburn, O.A.; Marchildon, F.; Cohen, P. Insights into the Link between Obesity and Cancer. Curr. Obes. Rep. 2017, 6, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi, Z.; Khazaei, M.; Moodi, M.; Chamani, E. From obesity to cancer: A review on proposed mechanisms. Cell Biochem. Funct. 2016, 34, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Fallah-Fini, S.; Adam, A.; Cheskin, L.J.; Bartsch, S.M.; Lee, B.Y. The Additional Costs and Health Effects of a Patient Having Overweight or Obesity: A Computational Model. Obesity 2017, 25, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.D.; Basu, A. Estimating the Medical Care Costs of Obesity in the United States: Systematic Review, Meta-Analysis, and Empirical Analysis. Value Health 2016, 19, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Caterson, I.; Seidell, J.C.; James, W.P. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004, 7, 123–146. [Google Scholar] [PubMed]
- Koliaki, C.; Spinos, T.; Spinou, M.; Brinia Mu, E.; Mitsopoulou, D.; Katsilambros, N. Defining the Optimal Dietary Approach for Safe, Effective and Sustainable Weight Loss in Overweight and Obese Adults. Healthcare 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Zulet, M.A.; Moreno-Aliaga, M.J.; Alfredo Martínez, J. Dietary determinants of fat mass and body composition. In Adipose Tissue Biology; Symonds, M.E., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 319–382. [Google Scholar]
- Lai, C.S.; Wu, J.C.; Pan, M.H. Molecular mechanism on functional food bioactives for anti-obesity. Curr. Opin. Food Sci. 2015, 2, 9–13. [Google Scholar] [CrossRef]
- Ye, J.; Ai, W.; Zhang, F.; Zhu, X.; Shu, G.; Wang, L.; Gao, P.; Xi, Q.; Zhang, Y.; Jiang, Q.; et al. Enhanced Proliferation of Porcine Bone Marrow Mesenchymal Stem Cells Induced by Extracellular Calcium is Associated with the Activation of the Calcium-Sensing Receptor and ERK Signaling Pathway. Stem Cells Int. 2016, 2016, 6570671. [Google Scholar] [CrossRef]
- Goudarzi, F.; Mohammadalipour, A.; Khodadadi, I.; Karimi, S.; Mostoli, R.; Bahabadi, M.; Goodarzi, M.T. The Role of Calcium in Differentiation of Human Adipose-Derived Stem Cells to Adipocytes. Mol. Biotechnol. 2018, 60, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Pramme-Steinwachs, I.; Jastroch, M.; Ussar, S. Extracellular calcium modulates brown adipocyte differentiation and identity. Sci. Rep. 2017, 7, 8888. [Google Scholar] [CrossRef] [PubMed]
- Pannu, P.K.; Calton, E.K.; Soares, M.J. Calcium and Vitamin D in Obesity and Related Chronic Disease. Adv. Food Nutr. Res. 2016, 77, 57–100. [Google Scholar] [PubMed]
- Onakpoya, I.J.; Perry, R.; Zhang, J.; Ernst, E. Efficacy of calcium supplementation for management of overweight and obesity: Systematic review of randomized clinical trials. Nutr. Rev. 2011, 69, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.O.; Huggins, C.E.; Wattanapenpaiboon, N.; Nowson, C.A. Effect of increasing dietary calcium through supplements and dairy food on body weight and body composition: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.S.; Qahiz, N.M.A. Can Dietary Calcium Consumption be Beneficial in Body Weight Loss Regimen? Merit Res. J. Med. Med. Sci. 2016, 4, 282–289. [Google Scholar]
- Chaturvedi, R.; Singh, N. Role of calcium in obesity: Does it help? CIBTech J. Zool. 2013, 2, 10–16. [Google Scholar]
- Barba, G.; Russo, P. Dairy foods, dietary calcium and obesity: A short review of the evidence. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Ping-Delfos, W.C.S.; Ghanbari, M.H. Calcium and vitamin D for obesity: A review of randomized controlled trials. Eur. J. Clin. Nutr. 2011, 65, 994–1004. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Stern, J.H.; Scherer, P.E. The cell biology of fat expansion. J. Cell Biol. 2015, 208, 501–512. [Google Scholar] [CrossRef]
- Jensen, B.; Farach-Carson, M.C.; Kenaley, E.; Akanbi, K.A. High extracellular calcium attenuates adipogenesis in 3T3-L1 preadipocytes. Exp. Cell Res. 2004, 301, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Qi, R.; Wang, L.; Yan, J.; Wang, Y. p38 MAPK regulates calcium signal-mediated lipid accumulation through changing VDR expression in primary preadipocytes of mice. Mol. Biol. Rep. 2012, 39, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, L.; Yan, J.; Liu, S. Calcium ameliorates obesity induced by high-fat diet and its potential correlation with p38 MAPK pathway. Mol. Biol. Rep. 2012, 39, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Halvorsen, Y.D.; Ellis, P.N.; Wilkison, W.O.; Zemel, M.B. Role of intracellular calcium in human adipocyte differentiation. Physiol. Genom. 2000, 3, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ye, J.; Meng, Y.; Ai, W.; Su, H.; Zheng, J.; Liu, F.; Zhu, X.; Wang, L.; Gao, P.; et al. Calcium Supplementation Enhanced Adipogenesis and Improved Glucose Homeostasis Through Activation of Camkii and PI3K/Akt Signaling Pathway in Porcine Bone Marrow Mesenchymal Stem Cells (pBMSCs) and Mice Fed High Fat Diet (HFD). Cell. Physiol. Biochem. 2018, 51, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Parra, P.; Laraichi, S.; Serra, F.; Palou, A. Calcium supplementation modulates gut microbiota in a prebiotic manner in dietary obese mice. Mol. Nutr. Food Res. 2016, 60, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N.; Song, Q. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol. Nutr. Food Res. 2014, 58, 1342–1348. [Google Scholar] [CrossRef]
- Conceicao, E.P.; Moura, E.G.; Manhaes, A.C.; Carvalho, J.C.; Nobre, J.L.; Oliveira, E.; Lisboa, P.C. Calcium reduces vitamin D and glucocorticoid receptors in the visceral fat of obese male rats. J. Endocrinol. 2016, 230, 263–274. [Google Scholar] [CrossRef]
- Quitete, F.T.; Nobre, J.L.; Peixoto-Silva, N.; de Moura, E.G.; Lisboa, P.C.; de Oliveira, E. Anti-obesogenic effects of calcium prevent changes in the GLP-1 profile in adult rats primed by early weaning. Mol. Nutr. Food Res. 2015, 59, 773–783. [Google Scholar] [CrossRef]
- Conceicao, E.P.S.; Moura, E.G.; Oliveira, E.; Guarda, D.S.; Figueiredo, M.S.; Quitete, F.T.; Calvino, C.; Miranda, R.A.; Mathias, P.C.F.; Manhaes, A.C.; et al. Dietary calcium supplementation in adult rats reverts brown adipose tissue dysfunction programmed by postnatal early overfeeding. J. Nutr. Biochem. 2017, 39, 117–125. [Google Scholar] [CrossRef]
- Li, P.; Fan, C.; Lu, Y.; Qi, K. Effects of calcium supplementation on body weight: A meta-analysis. Am. J. Clin. Nutr. 2016, 104, 1263–1273. [Google Scholar] [CrossRef]
- Heaney, R.P.; Davies, K.M.; Barger-Lux, M.J. Calcium and weight: Clinical studies. J. Am. Coll. Nutr. 2002, 21, 152S–155S. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. The national academies collection: Reports funded by national institutes of health. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press (US) National Academy of Sciences: Washington, DC, USA, 2011. [Google Scholar]
- McGuire, S. U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv. Nutr. 2011, 2, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Guenther, P.M.; Subar, A.F.; Kirkpatrick, S.I.; Dodd, K.W. Americans do not meet federal dietary recommendations. J. Nutr. 2010, 140, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, J.L.; Castro, V.M.; Moore, C.E.; Kaplan, L.M. Calcium and vitamin D supplementation is associated with decreased abdominal visceral adipose tissue in overweight and obese adults. Am. J. Clin. Nutr. 2012, 95, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Winzenberg, T.; Shaw, K.; Fryer, J.; Jones, G. Calcium supplements in healthy children do not affect weight gain, height, or body composition. Obesity 2007, 15, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell. Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Al-Musharaf, S.; Al-Othman, A.; Al-Daghri, N.M.; Krishnaswamy, S.; Yusuf, D.S.; Alkharfy, K.M.; Al-Saleh, Y.; Al-Attas, O.S.; Alokail, M.S.; Moharram, O.; et al. Vitamin D deficiency and calcium intake in reference to increased body mass index in children and adolescents. Eur. J. Pediatr. 2012, 171, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Reid, I.R. Should we prescribe calcium or vitamin D supplements to treat or prevent osteoporosis? Climacteric 2015, 18 (Suppl. 2), 22–31. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Yeum, K.J.; Park, S.J.; Choi, B.; Joo, N.S. Dietary calcium and Framingham Risk Score in vitamin D deficient male (KNHANES 2009-2011). Yonsei Med. J. 2015, 56, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Nappo, A.; Sparano, S.; Intemann, T.; Kourides, Y.A.; Lissner, L.; Molnar, D.; Moreno, L.A.; Pala, V.; Sioen, I.; Veidebaum, T.; et al. Dietary calcium intake and adiposity in children and adolescents: Cross-sectional and longitudinal results from IDEFICS/I.Family cohort. Nutr. Metab. Cardiovasc. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.G.; Costa, J.D.A.; Alfenas, R.D.C.G. Dietary calcium from dairy, body composition and glycaemic control in patients with type 2 diabetes pursuing an energy restricted diet: A parallel group randomised clinical trial. Int. Dairy J. 2017, 73, 50–56. [Google Scholar] [CrossRef]
- Rautiainen, S.; Wang, L.; Lee, I.M.; Manson, J.E.; Buring, J.E.; Sesso, H.D. Dairy consumption in association with weight change and risk of becoming overweight or obese in middle-aged and older women: A prospective cohort study. Am. J. Clin. Nutr. 2016, 103, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.M.; McMahon, D.J.; Laughlin, A.; Hanson, C.; Desmangles, J.C.; Begley, M.; Schwartz, M. The effect of increasing dairy calcium intake of adolescent girls on changes in body fat and weight. Am. J. Clin. Nutr. 2017, 105, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Cho, W. The Consumption of Dairy Products Is Associated with Reduced Risks of Obesity and Metabolic Syndrome in Korean Women but not in Men. Nutrients 2017, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.; Padez, C.; Mourao, I.; Rosado, V. Dietary calcium and body mass index in Portuguese children. Eur. J. Clin. Nutr. 2005, 59, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Smith, U.; Kahn, B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Int. Med. 2016, 280, 465–475. [Google Scholar] [CrossRef]
- Hashimoto, R.; Katoh, Y.; Nakamura, K.; Itoh, S.; Iesaki, T.; Daida, H.; Nakazato, Y.; Okada, T. Enhanced accumulation of adipocytes in bone marrow stromal cells in the presence of increased extracellular and intracellular [Ca (2) (+)]. Biochem. Biophys. Res. Commun. 2012, 423, 672–678. [Google Scholar] [CrossRef]
- Hashimoto, R.; Katoh, Y.; Miyamoto, Y.; Itoh, S.; Daida, H.; Nakazato, Y.; Okada, T. Increased extracellular and intracellular Ca (2) (+) lead to adipocyte accumulation in bone marrow stromal cells by different mechanisms. Biochem. Biophys. Res. Commun. 2015, 457, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Dry, H.; Jorgenson, K.; Ando, W.; Hart, D.A.; Frank, C.B.; Sen, A. Effect of calcium on the proliferation kinetics of synovium-derived mesenchymal stromal cells. Cytotherapy 2013, 15, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Meng, Q.X.; Lu, L.; Cui, Z.L.; Ren, L.P. The effect of calcium propionate supplementation on performance, meat quality, and mRNA expression of finishing steers fed a high-concentrate diet. J. Anim. Feed Sci. 2015, 24, 100–106. [Google Scholar] [CrossRef]
- Zemel, M.B. Regulation of adiposity and obesity risk by dietary calcium: Mechanisms and implications. J. Am. Coll. Nutr. 2002, 21, 146s–151s. [Google Scholar] [CrossRef] [PubMed]
- Maus, M.; Cuk, M.; Patel, B.; Lian, J.; Ouimet, M.; Kaufmann, U.; Yang, J.; Horvath, R.; Hornig-Do, H.T.; Chrzanowska-Lightowlers, Z.M.; et al. Store-Operated Ca (2+) Entry Controls Induction of Lipolysis and the Transcriptional Reprogramming to Lipid Metabolism. Cell Metab. 2017, 25, 698–712. [Google Scholar] [CrossRef]
- Sandeep, D.; Dipayan, C. Role of Low Calcium and High Calcium Diet on Adipocyte Metabolism with Respect to Serum Parathyroid Hormone (PTH) Levels in Male Wistar Rats. Indian J. Physiol. Pharmacol. 2017, 61, 430–439. [Google Scholar]
- Pesarini, J.R.; Oliveira, E.J.T.; Pessatto, L.R.; Rabacow, A.P.M.; Camassola, M.; Dos Santos, B.P.; de Barros, M.E.; Cantero, W.B.; Antoniolli-Silva, A.; Oliveira, R.J. Calcitriol combined with calcium chloride causes apoptosis in undifferentiated adipose tissue-derived human mesenchymal stem cells, but this effect decreases during adipogenic differentiation. Biomed. Pharmacother. 2018, 108, 914–924. [Google Scholar] [CrossRef]
- Rocha, G.; Villalobos, E.; Fuentes, C.; Villarroel, P.; Reyes, M.; Diaz, X.; Mattar, P.; Cifuentes, M. Preadipocyte proliferation is elevated by calcium sensing receptor activation. Mol. Cell. Endocrinol. 2015, 412, 251–256. [Google Scholar] [CrossRef]
- Aguirre, A.; González, A.; Planell, J.; Engel, E. Extracellular calcium modulates in vitro bone marrow-derived Flk-1+ CD34+ progenitor cell chemotaxis and differentiation through a calcium-sensing receptor. Biochem. Biophys. Res. Commun. 2010, 393, 156–161. [Google Scholar] [CrossRef]
- Liu, Y.K.; Lu, Q.Z.; Pei, R.; Ji, H.J.; Zhou, G.S.; Zhao, X.L.; Tang, R.K.; Zhang, M. The effect of extracellular calcium and inorganic phosphate on the growth and osteogenic differentiation of mesenchymal stem cells in vitro: Implication for bone tissue engineering. Biomed. Mater. 2009, 4, 025004. [Google Scholar] [CrossRef]
- Lin, T.M.; Tsai, J.L.; Lin, S.D.; Lai, C.S.; Chang, C.C. Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev. 2005, 14, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N. 1,25-Dihydroxyvitamin D3 induces Ca2+-mediated apoptosis in adipocytes via activation of calpain and caspase-12. Biochem. Biophys. Res. Commun. 2009, 384, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Chehab, T.; Bultynck, G.; Parys, J.B.; Rietdorf, K. The regulation of autophagy by calcium signals: Do we have a consensus? Cell Calcium 2018, 70, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Mattar, P.; Bravo-Sagua, R.; Tobar, N.; Fuentes, C.; Troncoso, R.; Breitwieser, G.; Lavandero, S.; Cifuentes, M. Autophagy mediates calcium-sensing receptor-induced TNFalpha production in human preadipocytes. Biochim. Biophys. Acta. Mol. Basis Dis. 2018, 1864, 3585–3594. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Pathak, K.; Calton, E.K. Calcium and vitamin D in the regulation of energy balance: Where do we stand? Int. J. Mol. Sci. 2014, 15, 4938–4945. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Veech, R.L. Brown and Brite: The Fat Soldiers in the Anti-obesity Fight. Front. Physiol. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Kissig, M.; Shapira, S.N.; Seale, P. SnapShot: Brown and Beige Adipose Thermogenesis. Cell 2016, 166, 258. [Google Scholar] [CrossRef]
- Cohen, P.; Spiegelman, B.M. Brown and Beige Fat: Molecular Parts of a Thermogenic Machine. Diabetes 2015, 64, 2346–2351. [Google Scholar] [CrossRef]
- Zhang, F.; Su, H.; Song, M.; Zheng, J.; Liu, F.; Yuan, C.; Fu, Q.; Chen, S.; Zhu, X.; Wang, L.; et al. Calcium Supplementation Alleviates High-Fat Diet-Induced Estrous Cycle Irregularity and Subfertility Associated with Concomitantly Enhanced Thermogenesis of Brown Adipose Tissue and Browning of White Adipose Tissue. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef]
- Sun, W.; Uchida, K.; Suzuki, Y.; Zhou, Y.; Kim, M.; Takayama, Y.; Takahashi, N.; Goto, T.; Wakabayashi, S.; Kawada, T.; et al. Lack of TRPV2 impairs thermogenesis in mouse brown adipose tissue. EMBO Rep. 2016, 17, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Uchida, K.; Tominaga, M. TRPV2 regulates BAT thermogenesis and differentiation. Channels 2017, 11, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Golic, I.; Velickovic, K.; Markelic, M.; Stancic, A.; Jankovic, A.; Vucetic, M.; Otasevic, V.; Buzadzic, B.; Korac, B.; Korac, A. Calcium-induced alteration of mitochondrial morphology and mitochondrial-endoplasmic reticulum contacts in rat brown adipocytes. Eur. J. Histochem. 2014, 58, 2377. [Google Scholar] [CrossRef] [PubMed]
- Parra, P.; Bruni, G.; Palou, A.; Serra, F. Dietary calcium attenuation of body fat gain during high-fat feeding in mice. J. Nutr. Biochem. 2008, 19, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Yuangklang, C.; Wensing, T.; Van den Broek, L.; Jittakhot, S.; Beynen, A.C. Fat digestion in veal calves fed milk replacers low or high in calcium and containing either casein or soy protein isolate. J. Dairy Sci. 2004, 87, 1051–1056. [Google Scholar] [CrossRef]
- Xu, C.; Wensing, T.; Beynen, A.C. Effects of high calcium intake on fat digestion and bile acid excretion in feces of veal calves. J. Dairy Sci. 1998, 81, 2173–2177. [Google Scholar] [CrossRef]
- Strauss, E.W. Effects of calcium and magnesium ions upon fat absorption by sacs of everted hamster intestine. Gastroenterology 1977, 73, 421–424. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Flatt, W.P.; Huth, P.J.; Harris, R.B. High dietary calcium reduces body fat content, digestibility of fat, and serum vitamin D in rats. Obes. Res. 2003, 11, 387–394. [Google Scholar] [CrossRef]
- Ayala-Bribiesca, E.; Turgeon, S.L.; Pilon, G.; Marette, A.; Britten, M. Postprandial lipemia and fecal fat excretion in rats is affected by the calcium content and type of milk fat present in Cheddar-type cheeses. Food Res. Int. 2018, 107, 589–595. [Google Scholar] [CrossRef]
- Shahkhalili, Y.; Murset, C.; Meirim, I.; Duruz, E.; Guinchard, S.; Cavadini, C.; Acheson, K. Calcium supplementation of chocolate: Effect on cocoa butter digestibility and blood lipids in humans. Am. J. Clin. Nutr. 2001, 73, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, R.; Lorenzen, J.K.; Toubro, S.; Krog-Mikkelsen, I.; Astrup, A. Effect of short-term high dietary calcium intake on 24-h energy expenditure, fat oxidation, and fecal fat excretion. Int. J. Obes. 2005, 29, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Bendsen, N.T.; Hother, A.L.; Jensen, S.K.; Lorenzen, J.K.; Astrup, A. Effect of dairy calcium on fecal fat excretion: A randomized crossover trial. Int. J. Obes. 2008, 32, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.; Lorenzen, J.K.; Svith, C.R.; Bartels, E.M.; Melanson, E.L.; Saris, W.H.; Tremblay, A.; Astrup, A. Effect of calcium from dairy and dietary supplements on faecal fat excretion: A meta-analysis of randomized controlled trials. Obes. Rev. 2009, 10, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Juul, S.R.; Sorensen, K.V.; Lorenzen, J.K.; Astrup, A. Supplementation with dairy calcium and/or flaxseed fibers in conjunction with orlistat augments fecal fat excretion without altering ratings of gastrointestinal comfort. Nutr. Metab. 2017, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Welberg, J.W.; Monkelbaan, J.F.; de Vries, E.G.; Muskiet, F.A.; Cats, A.; Oremus, E.T.; Boersma-van Ek, W.; van Rijsbergen, H.; van der Meer, R.; Mulder, N.H.; et al. Effects of supplemental dietary calcium on quantitative and qualitative fecal fat excretion in man. Ann. Nutr. Metab. 1994, 38, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Denke, M.A.; Fox, M.M.; Schulte, M.C. Short-term dietary calcium fortification increases fecal saturated fat content and reduces serum lipids in men. J. Nutr. 1993, 123, 1047–1053. [Google Scholar]
- Vinarova, L.; Vinarov, Z.; Tcholakova, S.; Denkov, N.D.; Stoyanov, S.; Lips, A. The mechanism of lowering cholesterol absorption by calcium studied by using an in vitro digestion model. Food Funct. 2016, 7, 151–163. [Google Scholar] [CrossRef]
- Torcello-Gomez, A.; Boudard, C.; Mackie, A.R. Calcium Alters the Interfacial Organization of Hydrolyzed Lipids during Intestinal Digestion. Langmuir 2018, 34, 7536–7544. [Google Scholar] [CrossRef]
- Dao, M.C.; Clement, K. Gut microbiota and obesity: Concepts relevant to clinical care. Eur. J. Int. Med. 2018, 48, 18–24. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Di Sarno, R.; Giorgio, V.; Miele, L. Gut microbiota, obesity and metabolic disorders. Minerva Gastroenterol. Dietol. 2017, 63, 337–344. [Google Scholar] [PubMed]
- Gerard, P. Gut microbiota and obesity. Cell. Mol. Life Sci. CMLS 2016, 73, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Al-Assal, K.; Martinez, A.C.; Torrinhas, R.S.; Cardinelli, C.; Waitzberg, D. Gut microbiota and obesity. Clin. Nutr. Exp. 2018, 20, 60–64. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Bervoets, L.; Van Hoorenbeeck, K.; Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.; Andersen, A.D.; Hermann-Bank, M.L.; Stagsted, J.; Boye, M. The effect of high-fat diet on the composition of the gut microbiota in cloned and non-cloned pigs of lean and obese phenotype. Gut Microb. 2013, 4, 371–381. [Google Scholar] [CrossRef]
- Dror, T.; Dickstein, Y.; Dubourg, G.; Paul, M. Microbiota manipulation for weight change. Microb. Pathog. 2017, 106, 146–161. [Google Scholar] [CrossRef]
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Can We Prevent Obesity-Related Metabolic Diseases by Dietary Modulation of the Gut Microbiota? Adv. Nutr. 2016, 7, 90–101. [Google Scholar] [CrossRef]
- Zhang, N.; Ju, Z.; Zuo, T. Time for food: The impact of diet on gut microbiota and human health. Nutrion 2018, 51–52, 80–85. [Google Scholar] [CrossRef]
- Gomes, J.M.; Costa, J.A.; Alfenas, R.C. Could the beneficial effects of dietary calcium on obesity and diabetes control be mediated by changes in intestinal microbiota and integrity? Br. J. Nutr. 2015, 114, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhang, F.; Song, M.; Liu, F.; Zhu, X.; Shu, G.; Wang, L.; Gao, P.; Jiang, Q.; Wang, S. Effects of calcium chloride supplementation in drinking water on fat deposition and intestinal flora in mice fed with high-fat diet. J. South China Agric. Univ. 2019, 40, 1–5. [Google Scholar]
- Metzler-Zebeli, B.U.; Zijlstra, R.T.; Mosenthin, R.; Ganzle, M.G. Dietary calcium phosphate content and oat beta-glucan influence gastrointestinal microbiota, butyrate-producing bacteria and butyrate fermentation in weaned pigs. FEMS Microbiol. Ecol. 2011, 75, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Borda-Molina, D.; Vital, M.; Sommerfeld, V.; Rodehutscord, M.; Camarinha-Silva, A. Insights into Broilers’ Gut Microbiota Fed with Phosphorus, Calcium, and Phytase Supplemented Diets. Front. Microbiol. 2016, 7, 2033. [Google Scholar] [CrossRef] [PubMed]
- Dastar, B.; Khosravi, A.; Boldajie, F.; Ghoorchi, T. Effect of calcium with and without probiotic, lactose, or both on organ and body weights, immune response and caecal microbiota in moulted laying hens. J. Anim. physiol. Anim. Nutr. 2016, 100, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Anuta, J.D.; Buentello, A.; Patnaik, S.; Lawrence, A.L.; Mustafa, A.; Hume, M.E.; Gatlin, D.M.; Kemp, M.C. Effect of Dietary Supplementation of Acidic Calcium Sulfate (Vitoxal) on Growth, Survival, Immune Response and Gut Microbiota of the Pacific White Shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2011, 42, 834–844. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Ye, J.; Zhu, X.; Wang, L.; Gao, P.; Shu, G.; Jiang, Q.; Wang, S. Anti-Obesity Effects of Dietary Calcium: The Evidence and Possible Mechanisms. Int. J. Mol. Sci. 2019, 20, 3072. https://doi.org/10.3390/ijms20123072
Zhang F, Ye J, Zhu X, Wang L, Gao P, Shu G, Jiang Q, Wang S. Anti-Obesity Effects of Dietary Calcium: The Evidence and Possible Mechanisms. International Journal of Molecular Sciences. 2019; 20(12):3072. https://doi.org/10.3390/ijms20123072
Chicago/Turabian StyleZhang, Fenglin, Jingjing Ye, Xiaotong Zhu, Lina Wang, Ping Gao, Gang Shu, Qingyan Jiang, and Songbo Wang. 2019. "Anti-Obesity Effects of Dietary Calcium: The Evidence and Possible Mechanisms" International Journal of Molecular Sciences 20, no. 12: 3072. https://doi.org/10.3390/ijms20123072
APA StyleZhang, F., Ye, J., Zhu, X., Wang, L., Gao, P., Shu, G., Jiang, Q., & Wang, S. (2019). Anti-Obesity Effects of Dietary Calcium: The Evidence and Possible Mechanisms. International Journal of Molecular Sciences, 20(12), 3072. https://doi.org/10.3390/ijms20123072





