The Role of Serine-Threonine Protein Phosphatase PP2A in Plant Oxidative Stress Signaling—Facts and Hypotheses
Abstract
1. Introduction
- (i)
- Plasma membrane-associated NADPH oxidases (respiratory burst oxidase homologs, RBOHs). These will produce superoxide radicals during the formation of NADP+, which are then reduced to H2O2 by SOD. The nature and localization of this SOD isoform was controversial for a long time [7]. More recent research shows it is Cu/Zn SOD [8].
- (ii)
- For chloroplasts, the thylakoid membrane associated photosynthetic electron transport chain produces singlet oxygen at PSII and superoxide both at PSII and PSI (the latter by the Mehler reaction). Superoxide anion is then scavenged to H2O2 by Cu/ZnSOD and FeSOD. Hydrogen peroxide is then reduced by APX and the ascorbate-glutathione cycle.
- (iii)
- In mitochondria, superoxide is produced at complexes I (NADH dehydrogenase) and III (ubisemiquinone) of the inner membrane and scavenged to H2O2 by MnSOD. Components of the ascorbate-glutathione cycle from both the intermembrane space and matrix scavenge hydrogen-peroxide.
- (iv)
- (v)
- In the cytosol, superoxide anions produced by diverse metabolic processes are scavenged by Cu/ZnSOD and then by the ascorbate-glutathione cycle.
- (vi)
- Cell wall/ apoplastic space. ROS producing and scavenging directly at the level of cell wall is not well known, but in general terms of apoplast, ROS produced mainly by the activity of NADPH oxidases will lead ultimately to a systemic oxidative burst by their cell-to-cell spread as seen during pathogen attack. During ozone exposure, the ROS produced will be used for reducing ascorbate to dehydroascorbate (DHO) that regulates intracellular ROS signaling [11]. Moreover, class III peroxidases that are anchored to the cell wall, may also contribute to apoplastic H2O2 generation [12].
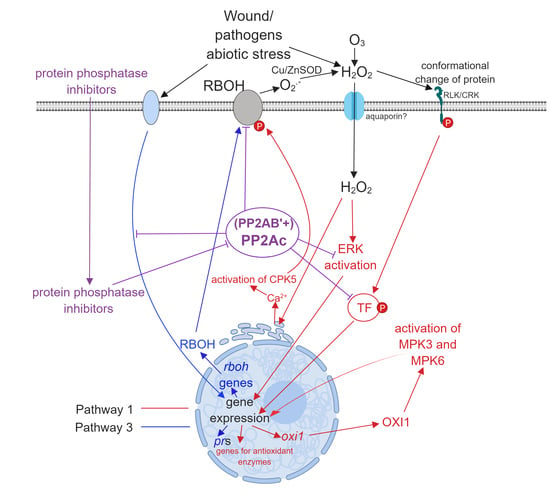
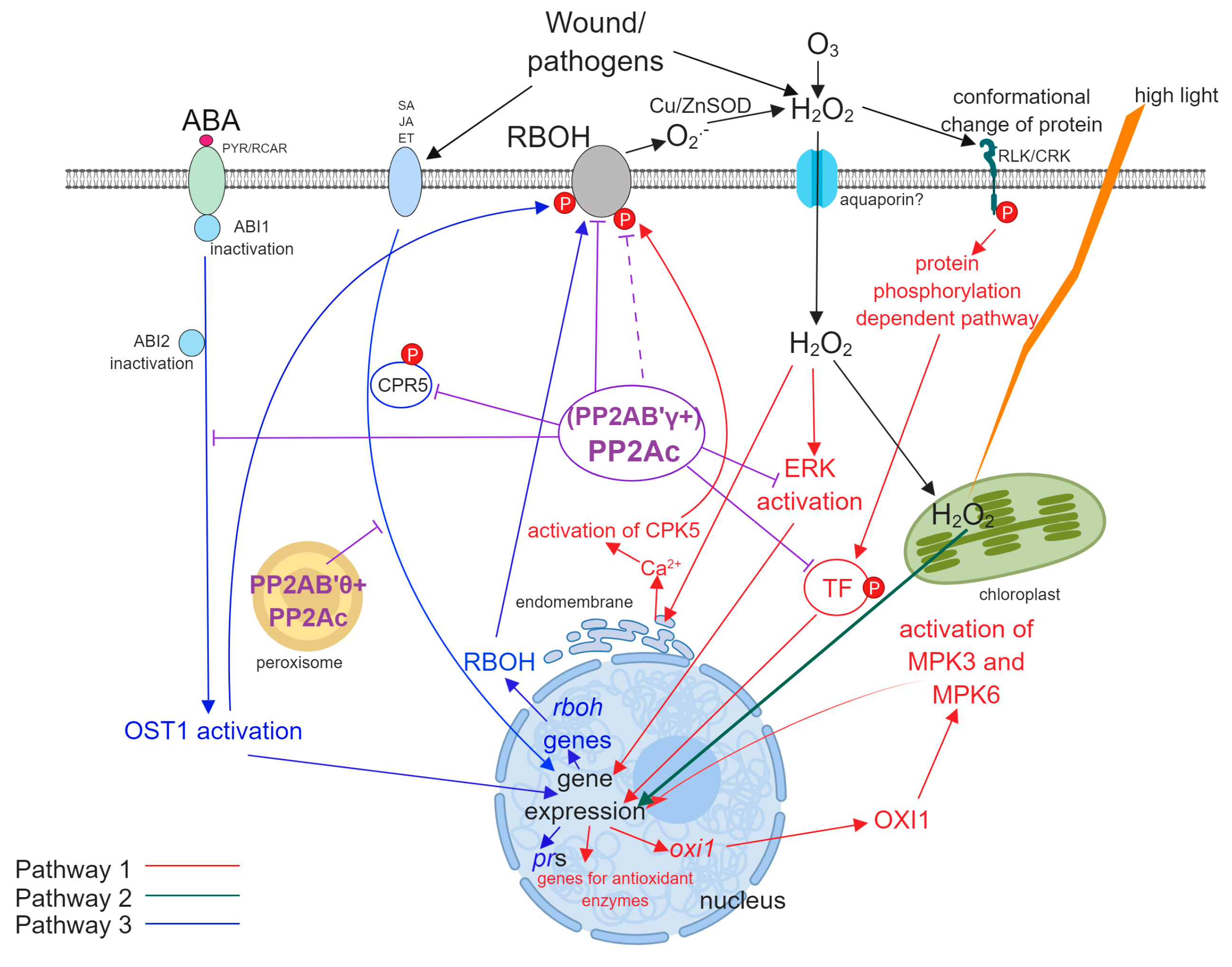
- (i)
- Pathway 1. External ROS, mainly superoxide and H2O2 produced by RBHOs trigger signal transduction pathways that alter gene expression leading to oxidative stress responses (see references [2,13,14] for an examples). External ROS may arise by the “ROS wave mechanism”: the activity of RBOH results in the production of extracellular ROS or pathogens induce the formation of intracellular ROS that is released and stimulates ROS formation in the neighboring cells [15].
- (ii)
- Pathway 2. Intracellular ROS generated by distinct cell compartments (see above) is extensively studied and this can trigger signaling pathways as well.
- (iii)
- Pathway 3. From the perspective of the present review, this is probably the most exciting pathway. Here, environmental stresses induce signal transduction pathways that lead to ROS generation by induction of the expression of ROS generating enzymes, then ROS generated by this mechanism induces further intracellular changes involving Pathways 1 and 2 (see reference [16] for an example). ABA is a well-known mediator of intracellular H2O2 generation in guard cells [13] and Pathway 3 has a crucial role in this (Subchapter 3.3.).
2. Types of Serine-Threonine Protein Phosphatases and ROS-Related Effects of PP2A Inhibitors in Higher Plants
2.1. Overview of Serine-Threonine Phosphatases with Special Emphasis on PP2A
2.2. How PP2A Inhibitors Influence ROS Production and Signaling in Plants?
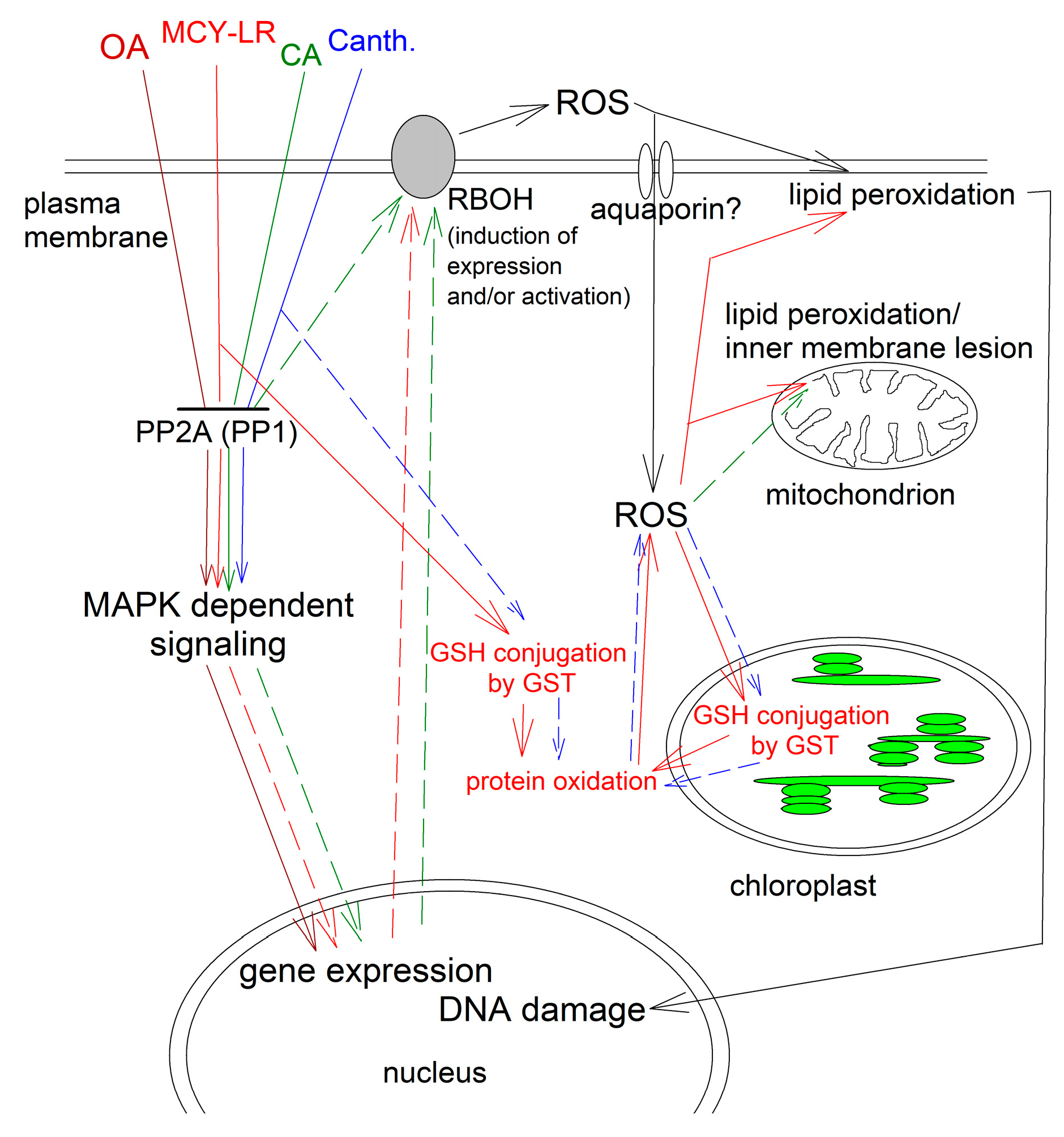
2.2.1. Microcystin-LR
- (i)
- Mechanism 1. MCY-LR generates ROS directly, without the involvement of protein phosphatases. The most probable pathway is the formation of MCY-LR-glutathione conjugates catalyzed by glutathione-S-transferase (GST) leading to the decrease of reduced glutathione (GSH) levels. This leads to oxidative stress –ROS production- by inducing an imbalance in the cell redox state, leading to lipid peroxidation, cytoskeletal disruption, mitochondrial damage, nuclear DNA strand breaks. Oxidative stress modulates expression and activities of antioxidant enzymes as well [56,57]. Based on assays of MCY-glutathione conjugate levels/ GST activities and of antioxidant enzyme activities, this model was proposed in plants by Pflugmacher [58,59]. Related to this, the level of lipid peroxidation as proven by the elevation of malondialdehyde level, increased in MCY-LR treated pepper fruits and Arabidopsis seedlings [53,60]. It is worth mentioning that MCY-LR can bind purified catalase that changes its activity [61], suggesting another direct effect of the inhibitor on oxidative stress.
- (ii)
- Mechanism 2. MCY-LR induces signaling pathways leading to ROS production without evidence for the involvement of the protein phosphatase PP2A. Related to this, the inhibitor modulated Ca2+ dependent signaling pathways involving calcium calmodulin dependent multifunctional protein kinase II (CaMKII) to produce ROS [62]. Similar pathway has not been proven for plants.
- (iii)
- Mechanism 3. MCY-LR induces signaling pathways, leading to ROS production with proven or possible involvement of PP2A. MCY-LR induces cytoskeletal disruption via a MAPK28 pathway that can be modulated both by protein phosphorylation and oxidative stress in the nervous system, however the mechanisms of the connection between these two cellular processes were not clearly elucidated [63]. For Brassica rapa, MCY-LR induced ROS formation by NO formation which is known to release Ca2+ at the intracellular level. Since NADPH oxidase expression is protein phosphorylation dependent, this probably initiates a phosphorylation dependent cascade, leading to the regulation of NADPH oxidase which is responsible for ROS production [64]. Gehringer [65] raised the possibility that the inhibition of PP2A by MCY-LR activates MAPKs and among other subcellular changes, this might lead to the production of ROS. MCY-LR prevents dephosphorylation of the multifunctional Ca2+/calmodulin-dependent protein kinase (CaMKII) at Thr286, leading to its activation and finally, cell death of rat hepatocytes [66]. These data and hypotheses raise the possibility of the usefulness of MCY-LR as a tool for further study of phosphatase mediated ROS production in plants.
2.2.2. Calyculin A and Cantharidin
2.2.3. Okadaic Acid
2.2.4. Lessons from the Effects of PP2A Inhibitors
3. The Involvement of PP2A in Oxidative Stress Pathways
3.1. Pathway 1
3.2. Pathway 2
3.3. Pathway 3
3.4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APX | ascorbate peroxidase |
| CA | calyculin A |
| CAT | catalase |
| GST | glutathione S-transferase |
| MAPK | mitogen activated protein kinase |
| MCY-LR | microcystin-LR |
| OA | okadaic acid |
| PR | pathogenesis response |
| RBOH | respiratory burst oxidase homologue, plasma membrane NADPH oxidase |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
References
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Uemura, M.; Bailey-Serres, J.; Bray, E.A.; Weretilnyk, E. Responses to Abiotic Stress. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley & Sons: Oxford, UK, 2015; pp. 1085–1094. ISBN 978-0-470-71422-5. [Google Scholar]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hammond-Kosack, K.E.; Jones, J.D.G. Responses to plant pathogens. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley & Sons: Oxford, UK, 2015; pp. 984–1050. ISBN 978-0-470-71422-5. [Google Scholar]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Fluhr, R. Production of Reactive Oxygen Species by Plant NADPH Oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Feierabend, J. Catalases in plants: Molecular and functional properties and role in stress defense. In Antioxidants and Reactive Oxygen Species in Plants; Smirnoff, N., Ed.; Blackwell Pub: Oxford, UK, 2005; pp. 101–140. ISBN 978-1-4051-2529-1. [Google Scholar]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Paul Bolwell, G. Reactive oxygen species and their role in plant defense and cell wall metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Hancock, J.; Desikan, R.; Harrison, J.; Bright, J.; Hooley, R.; Neill, S. Doing the unexpected: Proteins involved in hydrogen peroxide perception. J. Exp. Bot. 2006, 57, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Mittler, R. ROS-induced ROS release in plant and animal cells. Free Radic. Biol. Med. 2018, 122, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ohura, I.; Kawakita, K.; Yokota, N.; Fujiwara, M.; Shimamoto, K.; Doke, N.; Yoshioka, H. Calcium-Dependent Protein Kinases Regulate the Production of Reactive Oxygen Species by Potato NADPH Oxidase. Plant Cell 2007, 19, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Wrzaczek, M.; Hirt, H. Plant MAP kinase pathways: How many and what for? Biol. Cell 2001, 93, 81–87. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Shi, Y. Serine/Threonine Phosphatases: Mechanism through Structure. Cell 2009, 139, 468–484. [Google Scholar] [CrossRef] [PubMed]
- Bheri, M.; Pandey, G.K. Protein phosphatases meet reactive oxygen species in plant signaling networks. Environ. Exp. Bot. 2019, 161, 26–40. [Google Scholar] [CrossRef]
- Luan, S. Protein Phosphatases in Plants. Annu. Rev. Plant Biol. 2003, 54, 63–92. [Google Scholar] [CrossRef]
- You, J.; Zong, W.; Hu, H.; Li, X.; Xiao, J.; Xiong, L. A STRESS-RESPONSIVE NAC1-Regulated Protein Phosphatase Gene Rice Protein Phosphatase18 Modulates Drought and Oxidative Stress Tolerance through Abscisic Acid-Independent Reactive Oxygen Species Scavenging in Rice. Plant Physiol. 2014, 166, 2100–2114. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Y.; Luo, L.; Peck, S.C. Central Roles and Regulatory Mechanisms of Dual-Specificity MAPK Phosphatases in Developmental and Stress Signaling. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Uhrig, R.G.; Labandera, A.-M.; Moorhead, G.B. Arabidopsis PPP family of serine/threonine protein phosphatases: Many targets but few engines. Trends Plant Sci. 2013, 18, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Farkas, I.; Dombrádi, V.; Miskei, M.; Szabados, L.; Koncz, C. Arabidopsis PPP family of serine/threonine phosphatases. Trends Plant Sci. 2007, 12, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, C.; Azimzadeh, J.; Pastuglia, M.; Bellini, C.; Grandjean, O.; Bouchez, D. The Arabidopsis TONNEAU2 Gene Encodes a Putative Novel Protein Phosphatase 2A Regulatory Subunit Essential for the Control of the Cortical Cytoskeleton. Plant Cell 2002, 14, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Spinner, L.; Gadeyne, A.; Belcram, K.; Goussot, M.; Moison, M.; Duroc, Y.; Eeckhout, D.; Winne, N.D.; Schaefer, E.; Slijke, E.V.D.; et al. A protein phosphatase 2A complex spatially controls plant cell division. Nat. Commun. 2013, 4, 1863. [Google Scholar] [CrossRef] [PubMed]
- Virshup, D.M.; Shenolikar, S. From Promiscuity to Precision: Protein Phosphatases Get a Makeover. Mol. Cell 2009, 33, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.; Wrzaczek, M.; Scharte, J.; Tikkanen, M.; Konert, G.; Rahikainen, M.; Holmström, M.; Hiltunen, H.-M.; Rips, S.; Sipari, N.; et al. Regulatory Subunit B′γ of Protein Phosphatase 2A Prevents Unnecessary Defense Reactions under Low Light in Arabidopsis. Plant Physiol. 2011, 156, 1464–1480. [Google Scholar] [CrossRef] [PubMed]
- Skottke, K.R.; Yoon, G.M.; Kieber, J.J.; DeLong, A. Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms. PLoS Genet. 2011, 7, e1001370. [Google Scholar] [CrossRef]
- Blakeslee, J.J.; Zhou, H.-W.; Heath, J.T.; Skottke, K.R.; Barrios, J.A.R.; Liu, S.-Y.; DeLong, A. Specificity of RCN1-Mediated Protein Phosphatase 2A Regulation in Meristem Organization and Stress Response in Roots. Plant Physiol. 2008, 146, 539–553. [Google Scholar] [CrossRef]
- Luo, J.; Shen, G.; Yan, J.; He, C.; Zhang, H. AtCHIP functions as an E3 ubiquitin ligase of protein phosphatase 2A subunits and alters plant response to abscisic acid treatment. Plant J. 2006, 46, 649–657. [Google Scholar] [CrossRef]
- Trotta, A.; Konert, G.; Rahikainen, M.; Aro, E.-M.; Kangasjärvi, S. Knock-down of protein phosphatase 2A subunit B’γ promotes phosphorylation of CALRETICULIN 1 in Arabidopsis thaliana. Plant Signal. Behav. 2011, 6, 1665–1668. [Google Scholar] [CrossRef][Green Version]
- Li, S.; Mhamdi, A.; Trotta, A.; Kangasjärvi, S.; Noctor, G. The protein phosphatase subunit PP2A-B′γ is required to suppress day length-dependent pathogenesis responses triggered by intracellular oxidative stress. New Phytol. 2014, 202, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Konert, G.; Trotta, A.; Kouvonen, P.; Rahikainen, M.; Durian, G.; Blokhina, O.; Fagerstedt, K.; Muth, D.; Corthals, G.L.; Kangasjärvi, S. Protein phosphatase 2A (PP2A) regulatory subunit B′γ interacts with cytoplasmic ACONITASE 3 and modulates the abundance of AOX1A and AOX1D in Arabidopsis thaliana. New Phytol. 2015, 205, 1250–1263. [Google Scholar] [CrossRef] [PubMed]
- Konert, G.; Rahikainen, M.; Trotta, A.; Durian, G.; Salojärvi, J.; Khorobrykh, S.; Tyystjärvi, E.; Kangasjärvi, S. Subunits B′γ and B′ζ of protein phosphatase 2A regulate photo-oxidative stress responses and growth in Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 2641–2651. [Google Scholar] [CrossRef] [PubMed]
- Kataya, A.R.; Heidari, B.; Lillo, C. Protein phosphatase 2A regulatory subunits affecting plant innate immunity, energy metabolism, and flowering time—Joint functions among B’η subfamily members. Plant Signal. Behav. 2015, 10, e1026024. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Anderson, J.C.; del Pozo, O.; Gu, Y.-Q.; Tang, X.; Martin, G.B. Silencing of subfamily I of protein phosphatase 2A catalytic subunits results in activation of plant defense responses and localized cell death. Plant J. 2004, 38, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Y.; Su, Z.; Lv, L.; Zhang, Z. Silencing of the Wheat Protein Phosphatase 2A Catalytic Subunit TaPP2Ac Enhances Host Resistance to the Necrotrophic Pathogen Rhizoctonia cerealis. Front. Plant Sci. 2018, 9, 1437. [Google Scholar] [CrossRef] [PubMed]
- Pernas, M.; García-Casado, G.; Rojo, E.; Solano, R.; Sánchez-Serrano, J.J. A protein phosphatase 2A catalytic subunit is a negative regulator of abscisic acid signalling1. Plant J. 2007, 51, 763–778. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, C.; MacKintosh, R.W. Inhibitors of protein kinases and phosphatases. Trends Biochem. Sci. 1994, 19, 444–448. [Google Scholar] [CrossRef]
- MacKintosh, C.; Diplexcito, J. CHAPTER 102—Naturally Occurring Inhibitors of Protein Serine/Threonine Phosphatases. In Handbook of Cell Signaling; Bradshaw, R.A., Dennis, E.A., Eds.; Academic Press: Burlington, UK, 2003; pp. 607–611. ISBN 978-0-12-124546-7. [Google Scholar]
- Máthé, C.; Beyer, D.; M-Hamvas, M.; Vasas, G. The Effects of Microcystins (Cyanobacterial Heptapeptides) on the Eukaryotic Cytoskeletal System. Mini Rev. Med. Chem. 2016, 1063–1077. [Google Scholar] [CrossRef]
- Erdodi, F.; Toth, B.; Hirano, K.; Hirano, M.; Hartshorne, D.J.; Gergely, P. Endothall thioanhydride inhibits protein phosphatases-1 and -2A in vivo. Am. J. Physiol.-Cell Physiol. 1995, 269, C1176–C1184. [Google Scholar] [CrossRef]
- Ayaydin, F.; Vissi, E.; Mészáros, T.; Miskolczi, P.; Kovács, I.; Fehér, A.; Dombrádi, V.; Erdödi, F.; Gergely, P.; Dudits, D. Inhibition of serine/threonine-specific protein phosphatases causes premature activation of cdc2MsF kinase at G2/M transition and early mitotic microtubule organisation in alfalfa. Plant J. 2000, 23, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Biotechnol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Garda, T.; Kónya, Z.; Freytag, C.; Erdődi, F.; Gonda, S.; Vasas, G.; Szücs, B.; M-Hamvas, M.; Kiss-Szikszai, A.; Vámosi, G.; et al. Allyl-Isothiocyanate and Microcystin-LR Reveal the Protein Phosphatase Mediated Regulation of Metaphase-Anaphase Transition in Vicia faba. Front. Plant Sci. 2018, 9, 1823. [Google Scholar] [CrossRef] [PubMed]
- Sents, W.; Ivanova, E.; Lambrecht, C.; Haesen, D.; Janssens, V. The biogenesis of active protein phosphatase 2A holoenzymes: A tightly regulated process creating phosphatase specificity: The biogenesis of PP2A. FEBS J. 2013, 280, 644–661. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Lin, S.; Wang, B.; Xing, M.; Guo, Z.; Xu, L. MCLR-induced PP2A inhibition and subsequent Rac1 inactivation and hyperphosphorylation of cytoskeleton-associated proteins are involved in cytoskeleton rearrangement in SMMC-7721 human liver cancer cell line. Chemosphere 2014, 112, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Han, F.X.; Wang, F.; Zhang, H.; Shi, Z. Accumulation and phytotoxicity of microcystin-LR in rice (Oryza sativa). Ecotoxicol. Environ. Saf. 2012, 76, 193–199. [Google Scholar] [CrossRef]
- Garda, T.; Kónya, Z.; Tándor, I.; Beyer, D.; Vasas, G.; Erdődi, F.; Vereb, G.; Papp, G.; Riba, M.; M-Hamvas, M.; et al. Microcystin-LR induces mitotic spindle assembly disorders in Vicia faba by protein phosphatase inhibition and not reactive oxygen species induction. J. Plant Physiol. 2016, 199, 1–11. [Google Scholar] [CrossRef]
- Jia, Y.; Li, H.; Qu, Y.; Chen, W.; Song, L. Phytotoxicity, bioaccumulation and potential risks of plant irrigations using cyanobloom-loading freshwater. Sci. Total Environ. 2018, 624, 704–712. [Google Scholar] [CrossRef]
- M.-Hamvas, M.; Máthé, C.; Vasas, G.; Jámbrik, K.; Papp, M.; Beyer, D.; Mészáros, I.; Borbély, G. Cylindrospermopsin and microcystin-LR alter the growth, development and peroxidase enzyme activity of white mustard (Sinapis alba L.) seedlings, a comparative analysis. Acta Biol. Hung. 2010, 61, 35–48. [Google Scholar] [CrossRef]
- Pflugmacher, S. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat. Toxicol. 2004, 70, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xie, P. Mechanisms of Microcystin-induced Cytotoxicity and Apoptosis. Mini-Rev. Med. Chem. 2016, 16, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Valério, E.; Vasconcelos, V.; Campos, A. New Insights on the Mode of Action of Microcystins in Animal Cells—A Review. Mini Rev. Med. Chem. 2016, 16, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S. Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environ. Toxicol. 2002, 17, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S. Biotransformation of Microcystins in Eukaryotic Cells—Possible Future Research Directions. Mini Rev. Med. Chem. 2016, 16, 1078–1083. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Kiprovski, B.; Malenčić, D.; Važić, T.; Nybom, S.; Meriluoto, J.; Svirčev, Z. Microcystin accumulation and potential effects on antioxidant capacity of leaves and fruits of Capsicum annuum. J. Toxicol. Environ. Health A 2017, 80, 145–154. [Google Scholar] [CrossRef]
- Hu, Y.; Da, L. Insights into the selective binding and toxic mechanism of microcystin to catalase. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 121, 230–237. [Google Scholar] [CrossRef]
- Li, T.; Ying, L.; Wang, H.; Li, N.; Fu, W.; Guo, Z.; Xu, L. Microcystin-LR Induces Ceramide to Regulate PP2A and Destabilize Cytoskeleton in HEK293 Cells. Toxicol. Sci. 2012, 128, 147–157. [Google Scholar] [CrossRef]
- Meng, G.; Sun, Y.; Fu, W.; Guo, Z.; Xu, L. Microcystin-LR induces cytoskeleton system reorganization through hyperphosphorylation of tau and HSP27 via PP2A inhibition and subsequent activation of the p38 MAPK signaling pathway in neuroendocrine (PC12) cells. Toxicology 2011, 290, 218–229. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, Y.M.; Zhang, H.Q.; Shi, Z.Q. Nitrate Reductase-Dependent Nitric Oxide Production Is Involved in Microcystin-LR-Induced Oxidative Stress in Brassica rapa. Water Air Soil Pollut. 2012, 223, 4141–4152. [Google Scholar] [CrossRef]
- Gehringer, M.M. Microcystin-LR and okadaic acid-induced cellular effects: A dualistic response. FEBS Lett. 2004, 557, 1–8. [Google Scholar] [CrossRef]
- Fladmark, K.E.; Brustugun, O.T.; Mellgren, G.; Krakstad, C.; Bøe, R.; Vintermyr, O.K.; Schulman, H.; Døskeland, S.O. Ca2+/Calmodulin-dependent Protein Kinase II Is Required for Microcystin-induced Apoptosis. J. Biol. Chem. 2002, 277, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Máthé, C.; Beyer, D.; Erdődi, F.; Serfőző, Z.; Székvölgyi, L.; Vasas, G.; M-Hamvas, M.; Jámbrik, K.; Gonda, S.; Kiss, A. Microcystin-LR induces abnormal root development by altering microtubule organization in tissue-cultured common reed (Phragmites australis) plantlets. Aquat. Toxicol. 2009, 92, 122–130. [Google Scholar] [CrossRef]
- Máthé, C.; Vasas, G.; Borbély, G.; Erdődi, F.; Beyer, D.; Kiss, A.; Surányi, G.; Gonda, S.; Jámbrik, K.; M-Hamvas, M. Histological, cytological and biochemical alterations induced by microcystin-LR and cylindrospermopsin in white mustard (Sinapis alba L.) seedlings. Acta Biol. Hung. 2013, 64, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Favre, B.; Turowski, P.; Hemmings, B.A. Differential Inhibition and Posttranslational Modification of Protein Phosphatase 1 and 2A in MCF7 Cells Treated with Calyculin-A, Okadaic Acid, and Tautomycin. J. Biol. Chem. 1997, 272, 13856–13863. [Google Scholar] [CrossRef] [PubMed]
- duBell, W.H.; Gigena, M.S.; Guatimosim, S.; Long, X.; Lederer, W.J.; Rogers, T.B. Effects of PP1/PP2A inhibitor calyculin A on the E-C coupling cascade in murine ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H38–H48. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Low, P.S. Role of phosphorylation in elicitation of the oxidative burst in cultured soybean cells. Proc. Natl. Acad. Sci. USA 1995, 92, 4120–4123. [Google Scholar] [CrossRef]
- Cazalé, A.-C.; Droillard, M.-J.; Wilson, C.; Heberle-Bors, E.; Barbier-Brygoo, H.; Laurière, C. MAP kinase activation by hypoosmotic stress of tobacco cell suspensions: Towards the oxidative burst response? Plant J. 1999, 19, 297–307. [Google Scholar] [CrossRef]
- Felix, G.; Regenass, M.; Spanu, P.; Boller, T. The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labeling with [33P] phosphate. Proc. Natl. Acad. Sci. USA 1994, 91, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kawasaki, T.; Wong, H.L.; Suharsono, U.; Hirano, H.; Shimamoto, K. Hyperphosphorylation of a Mitochondrial Protein, Prohibitin, Is Induced by Calyculin A in a Rice Lesion-Mimic Mutant cdr1. Plant Physiol. 2003, 132, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kawasaki, T.; Henmi, K.; Shii, K.; Kodama, O.; Satoh, H.; Shimamoto, K. Lesion mimic mutants of rice with alterations in early signaling events of defense. Plant J. 1999, 17, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, Y.; Kaya, H.; Hiraoka, G.; Yumoto, F.; Kimura, S.; Kadota, Y.; Hishinuma, H.; Senzaki, E.; Yamagoe, S.; Nagata, K.; et al. Synergistic Activation of the Arabidopsis NADPH Oxidase AtrbohD by Ca2+ and Phosphorylation. J. Biol. Chem. 2008, 283, 8885–8892. [Google Scholar] [CrossRef] [PubMed]
- Fagerholm, A.E.; Habrant, D.; Koskinen, A.M.P. Calyculins and Related Marine Natural Products as Serine- Threonine Protein Phosphatase PP1 and PP2A Inhibitors and Total Syntheses of Calyculin A, B, and C. Mar. Drugs 2010, 8, 122–172. [Google Scholar] [CrossRef] [PubMed]
- Bajsa, J.; Pan, Z.; Duke, S.O. Cantharidin, a protein phosphatase inhibitor, strongly upregulates detoxification enzymes in the Arabidopsis proteome. J. Plant Physiol. 2015, 173, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tsukitani, Y.; John, P.C.L. Mitotic Arrest in Tobacco Caused by the Phosphoprotein Phosphatase Inhibitor Okadaic Acid. Plant Cell Physiol. 1992, 33, 677–688. [Google Scholar]
- Baharians, Z.; Schönthal, A.H. Autoregulation of protein phosphatase type 2A expression. J. Biol. Chem. 1998, 273, 19019–19024. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.K.; Tamogami, S.; Iwahashi, H.; Agrawal, V.P.; Rakwal, R. Transient regulation of jasmonic acid-inducible rice MAP kinase gene (OsBWMK1) by diverse biotic and abiotic stresses. Plant Physiol. Biochem. 2003, 41, 355–361. [Google Scholar] [CrossRef]
- Carmody, M.; Waszczak, C.; Idänheimo, N.; Saarinen, T.; Kangasjärvi, J. ROS signalling in a destabilised world: A molecular understanding of climate change. J. Plant Physiol. 2016, 203, 69–83. [Google Scholar] [CrossRef]
- Bourdais, G.; Burdiak, P.; Gauthier, A.; Nitsch, L.; Salojärvi, J.; Rayapuram, C.; Idänheimo, N.; Hunter, K.; Kimura, S.; Merilo, E.; et al. Large-Scale Phenomics Identifies Primary and Fine-Tuning Roles for CRKs in Responses Related to Oxidative Stress. PLoS Genet. 2015, 11, e1005373. [Google Scholar] [CrossRef]
- Rentel, M.C.; Lecourieux, D.; Ouaked, F.; Usher, S.L.; Petersen, L.; Okamoto, H.; Knight, H.; Peck, S.C.; Grierson, C.S.; Hirt, H.; et al. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 2004, 427, 858. [Google Scholar] [CrossRef]
- Samuel, M.A.; Miles, G.P.; Ellis, B.E. Ozone treatment rapidly activates MAP kinase signalling in plants. Plant J. 2000, 22, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.; Kandlbinder, A.; Golldack, D.; DIETZ, K.-J. Oxidative stress and ozone: Perception, signalling and response. Plant Cell Environ. 2005, 28, 1012–1020. [Google Scholar] [CrossRef]
- Shapiguzov, A.; Vainonen, J.; Wrzaczek, M.; Kangasjärvi, J. ROS-talk–how the apoplast, the chloroplast, and the nucleus get the message through. Front. Plant Sci. 2012, 3, 292. [Google Scholar] [CrossRef]
- Shumbe, L.; Chevalier, A.; Legeret, B.; Taconnat, L.; Monnet, F.; Havaux, M. Singlet Oxygen-Induced Cell Death in Arabidopsis under High-Light Stress Is Controlled by OXI1 Kinase. Plant Physiol. 2016, 170, 1757–1771. [Google Scholar] [CrossRef] [PubMed]
- Meinhard, M.; Rodriguez, P.L.; Grill, E. The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta 2002, 214, 775–782. [Google Scholar] [CrossRef]
- Meinhard, M.; Grill, E. Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. FEBS Lett. 2001, 508, 443–446. [Google Scholar] [CrossRef]
- Sierla, M.; Waszczak, C.; Vahisalu, T.; Kangasjärvi, J. Reactive Oxygen Species in the Regulation of Stomatal Movements. Plant Physiol. 2016, 171, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. The Roles of ROS and ABA in Systemic Acquired Acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Mustilli, A.-C.; Merlot, S.; Vavasseur, A.; Fenzi, F.; Giraudat, J. Arabidopsis OST1 Protein Kinase Mediates the Regulation of Stomatal Aperture by Abscisic Acid and Acts Upstream of Reactive Oxygen Species Production. Plant Cell 2002, 14, 3089–3099. [Google Scholar] [CrossRef] [PubMed]
- Kesarwani, M.; Yoo, J.; Dong, X. Genetic Interactions of TGA Transcription Factors in the Regulation of Pathogenesis-Related Genes and Disease Resistance in Arabidopsis. Plant Physiol. 2007, 144, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.-H.; Saijo, Y.; Mauch, S.; Biskup, C.; Bieri, S.; Keller, B.; Seki, H.; Ülker, B.; Somssich, I.E.; Schulze-Lefert, P. Nuclear Activity of MLA Immune Receptors Links Isolate-Specific and Basal Disease-Resistance Responses. Science 2007, 315, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Adachi, H.; Nakano, T.; Miyagawa, N.; Asai, S.; Ishihama, N.; Yoshioka, M. Hierarchical regulation of NADPH oxidase by protein kinases in plant immunity. Physiol. Mol. Plant Pathol. 2016, 95, 20–26. [Google Scholar] [CrossRef]
- Kwak, J.M.; Moon, J.-H.; Murata, Y.; Kuchitsu, K.; Leonhardt, N.; DeLong, A.; Schroeder, J.I. Disruption of a Guard Cell–Expressed Protein Phosphatase 2A Regulatory Subunit, RCN1, Confers Abscisic Acid Insensitivity in Arabidopsis. Plant Cell 2002, 14, 2849–2861. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Wojtaszek, P. Mechanisms for the generation of reactive oxygen species in plant defense—A broad perspective. Physiol. Mol. Plant Pathol. 1997, 51, 347–366. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive Oxygen Species Signaling in Response to Pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef]
| Subunit | Gene/Mutant Name | Organism | Function: Activation/Inactivation of PP2A/C (for the A and B Subunits) and Physiological Consequence | Ref. |
|---|---|---|---|---|
| PP2A/Aα | rcn1 | Arabidopsis thaliana | Activates PP2Ac that will inhibit ET biosynthesis and regulate auxin transport to promote normal root elongation growth. It confers ABA sensitivity. Loss of function of rcn1 promotes ET signaling and leads to the induction of defence genes following necrotroph infection that leads to ROS production. | [30,31] |
| PP2A/A3 | pp2a/a3 | Arabidopsis thaliana | Interacts with the E3 ubiquitin ligase AtCHIP, which will increase PP2A catalytic activity that modulates responses to dark and cold treatments as well as ABA sensitivity. AtCHIP overexpressing plants are cold sensitive. | [32] |
| PP2A/B′γ | pp2a-b’γ | Arabidopsis thaliana | Activation of PP2A. Pathogenesis response(PR), usually induced by SA or JA, involves inactivation of PP2A e.g., by inactivation of this regulatory subunit, that increases the phosphorylation state of CONSTITUTIVE EXPRESSION OF PR GENES5 (CPR5), leading to H2O2 production and induction of PR related gene expression via DNA demethylation. B’γ is also important in the regulation of peroxisomal serine:glyoxylate aminotransferase (SGAT) activity, expression and RBOH activation, expression of a GST isoform and of APX2. It has probably an indirect role in the regulation of ET biosynthesis. | [29,33,34,35] |
| PP2A/B′γ and PP2A/B′ζ | pp2a-b’γ and pp2a-b’ ζ | Arabidopsis thaliana | Activate PP2A, keep stress tolerance enzymes at low level under normal (non-stressed) conditions. PP2A-B′γ negatively regulates the mitochondrial alternative oxidases AOX1A and AOX1D | [35,36] |
| PP2A/B’θ | b’θ-1 | Arabidopsis thaliana | Activates PP2A, localized to peroxisomes and negatively regulates plant immunity responses. | [37] |
| PP2A/C | PP2Ac | Nicotiana benthamiana | Inhibition of PR under normal (non-stressed) conditions. During pathogen infection, its gene is probably silenced, conferring resistance to bacterial and fungal pathogens. | [38] |
| PP2A/C, family II | TaPP2Ac | wheat | Decreases expression of CAT, APX2 and PR2. Infection with the fungal pathogen Rhizoctonia cerealis and H2O2 increases its expression. | [39] |
| PP2A/C | pp2ac-2 | Arabidopsis thaliana | The loss of function mutant phenotype can partially suppress the ABA-insensitive phenotype of the abi1-1 gain of function mutant - which shows increased PP2C activity. | [40] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Máthé, C.; Garda, T.; Freytag, C.; M-Hamvas, M. The Role of Serine-Threonine Protein Phosphatase PP2A in Plant Oxidative Stress Signaling—Facts and Hypotheses. Int. J. Mol. Sci. 2019, 20, 3028. https://doi.org/10.3390/ijms20123028
Máthé C, Garda T, Freytag C, M-Hamvas M. The Role of Serine-Threonine Protein Phosphatase PP2A in Plant Oxidative Stress Signaling—Facts and Hypotheses. International Journal of Molecular Sciences. 2019; 20(12):3028. https://doi.org/10.3390/ijms20123028
Chicago/Turabian StyleMáthé, Csaba, Tamás Garda, Csongor Freytag, and Márta M-Hamvas. 2019. "The Role of Serine-Threonine Protein Phosphatase PP2A in Plant Oxidative Stress Signaling—Facts and Hypotheses" International Journal of Molecular Sciences 20, no. 12: 3028. https://doi.org/10.3390/ijms20123028
APA StyleMáthé, C., Garda, T., Freytag, C., & M-Hamvas, M. (2019). The Role of Serine-Threonine Protein Phosphatase PP2A in Plant Oxidative Stress Signaling—Facts and Hypotheses. International Journal of Molecular Sciences, 20(12), 3028. https://doi.org/10.3390/ijms20123028