Molybdenum-Induced Effects on Nitrogen Metabolism Enzymes and Elemental Profile of Winter Wheat (Triticum aestivum L.) Under Different Nitrogen Sources
Abstract
1. Introduction
2. Results
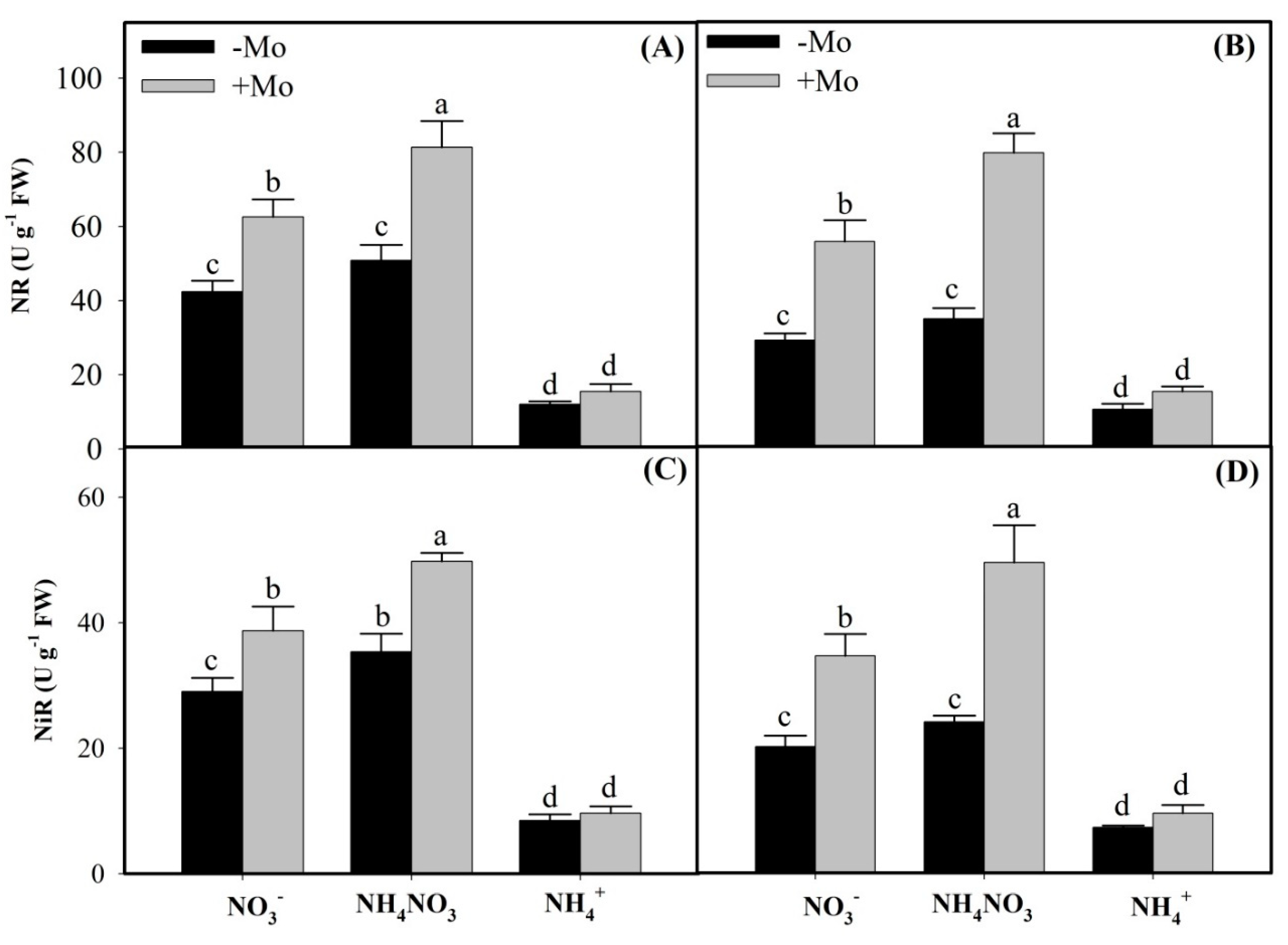
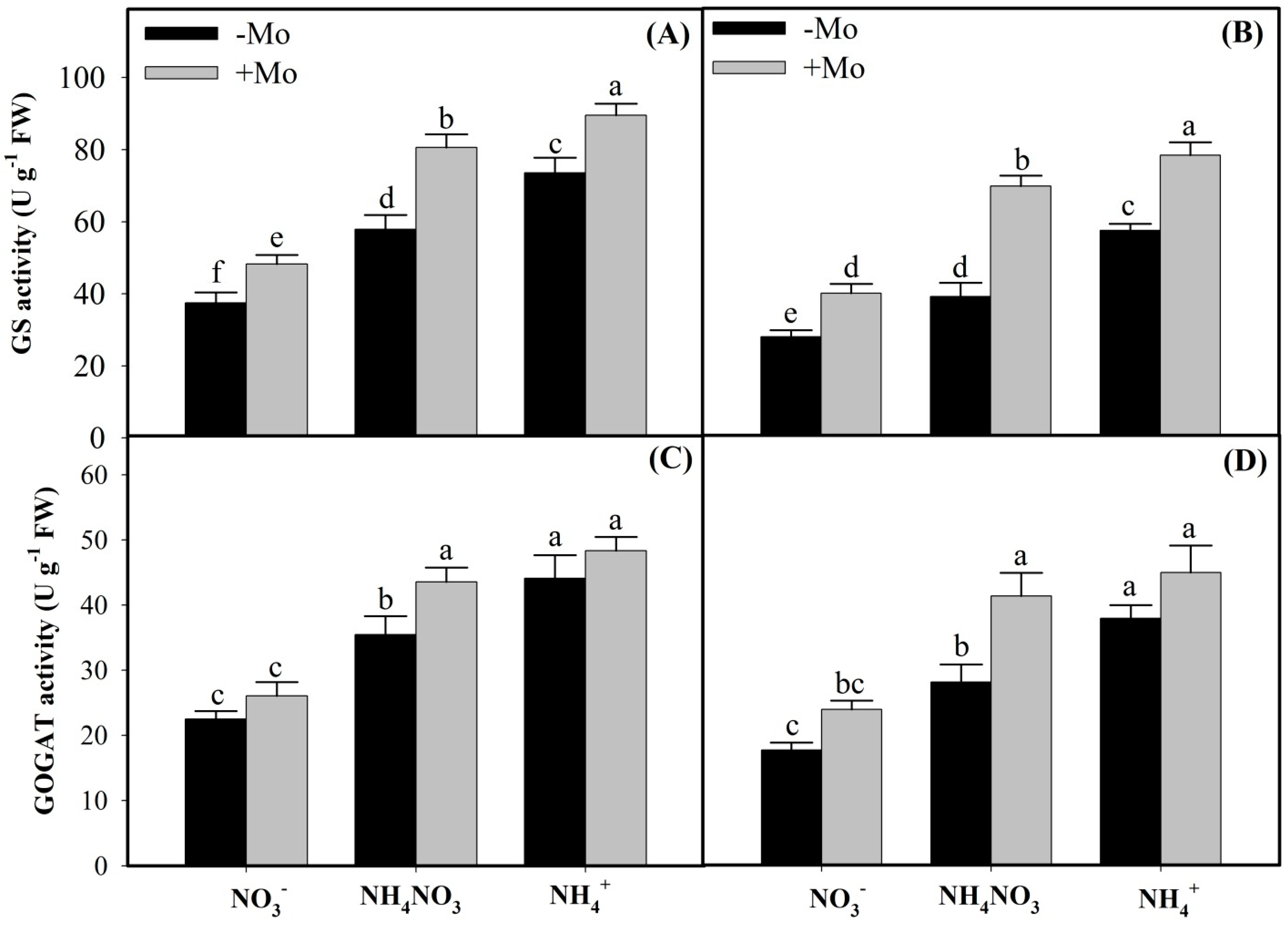
2.1. Effects of Mo Application on N Metabolism Enzymes Activities Under Different N Sources
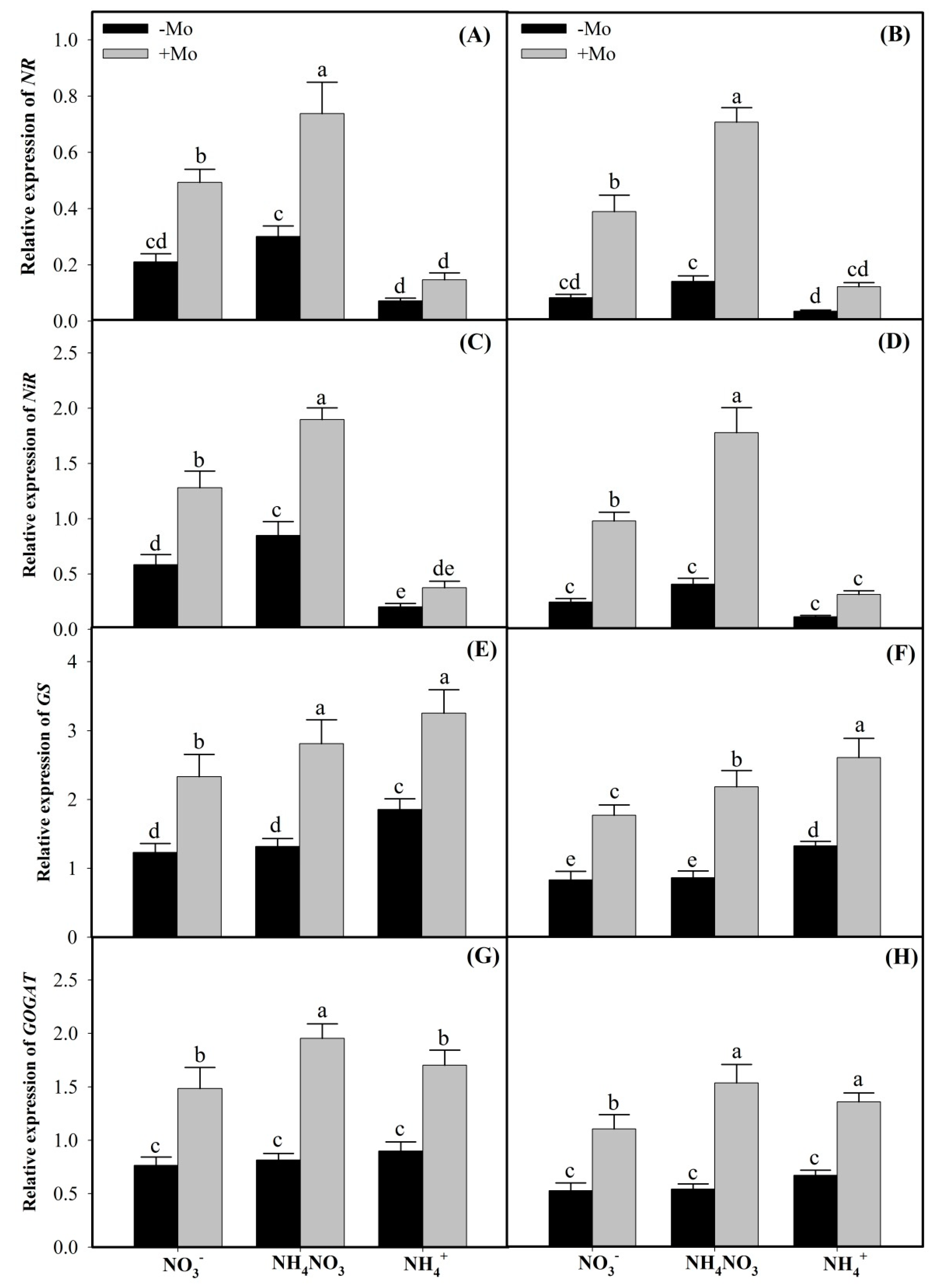
2.2. Mo Supply Regulated the Expressions of NR, NiR, GS, and GOGAT Genes
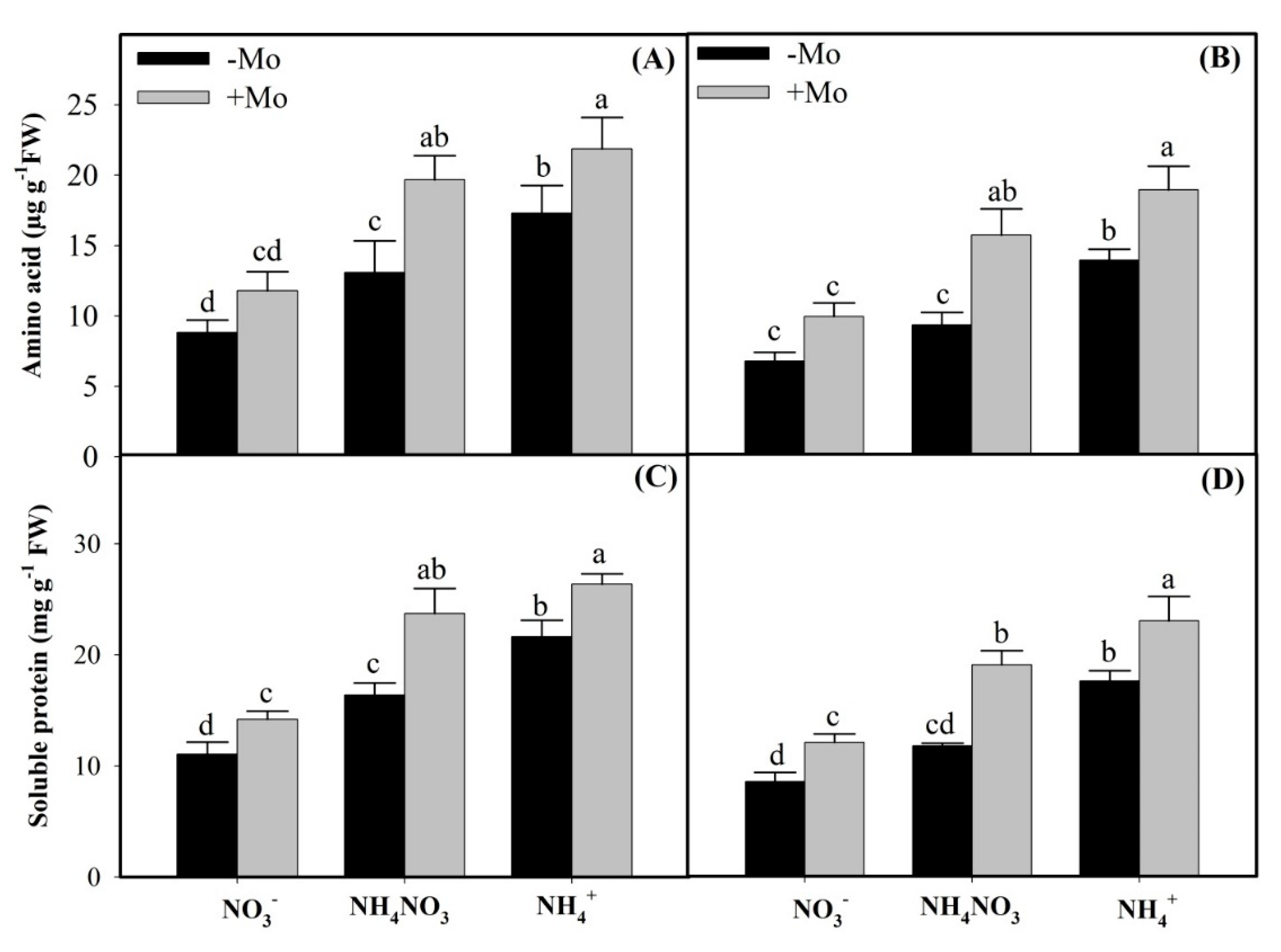
2.3. Effects of Mo Application on Inorganic and Reduced N Accumulation
2.4. Effects of Mo Application on the Elemental Profiling of Winter Wheat Leaves Under Different N Sources
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Analysis of Nitrate Reductase Activity
4.3. Determination of Nitrite Reductase Activity
4.4. Analysis of GS and NADH-GOGAT Activity
4.5. Total RNA Extraction and Quantitative RT-PCR
4.6. Determination of Nitrate, Nitrite, and Ammonium Contents
4.7. Measurement of Amino Acids
4.8. Measurement of Soluble Proteins
4.9. Mineral Elements Assays
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Castaings, L.; Marchive, C.; Meyer, C.; Krapp, A. Nitrogen signalling in arabidopsis: How to obtain insights into a complex signalling network. J. Exp. Bot. 2011, 62, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.M.; Coschigano, K.; Schultz, C.; Melooliveira, R.; Tjaden, G.; Oliveira, I.; Ngai, N.; Hsieh, M.H.; Coruzzi, G. Use of arabidopsis mutants and genes to study amide amino acid biosynthesis. Plant Cell 1995, 7, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Garg, S. Role and hormonal regulation of nitrate reductase activity in higher plants: A review. Plant Sci. Feed 2013, 3, 13–20. [Google Scholar]
- Nemie-Feyissa, D.; Królicka, A.; Førland, N.; Hansen, M.; Heidari, B.; Lillo, C. Post-translational control of nitrate reductase activity responding to light and photosynthesis evolved already in the early vascular plants. J. Plant Physiol. 2013, 170, 662–667. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Travis, R.L.; Rains, D.W. Evidence for substrate induction of a nitrate efflux system in barley roots. Plant Physiol. 1996, 112, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Vanoni, M.; Curti, B. Glutamate synthase: A complex iron-sulfur flavoprotein. Cell. Mol. Life Sci. Cmls 1999, 55, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Németh, E.; Guóth, A.; Bona, L.; Wodala, B.; Pécsváradi, A. Metabolic indicators of drought stress tolerance in wheat: Glutamine synthetase isoenzymes and rubisco. Plant Physiol. Biochem. 2013, 67, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Fidalgo, F.; Teixeira, J.; Aguiar, A.A.; Ferreira, I.M. Influence of the temporal and spatial variation of nitrate reductase, glutamine synthetase and soil composition in the n species content in lettuce (lactuca sativa). Plant Sci. 2014, 219, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Yuan, Y.-Z.; Ou, J.-Q.; Lin, Q.-H.; Zhang, C.-F. Glutamine synthetase and glutamate dehydrogenase contribute differentially to proline accumulation in leaves of wheat (triticum aestivum) seedlings exposed to different salinity. J. Plant Physiol. 2007, 164, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Pajuelo, P.; Pajuelo, E.; Forde, B.G.; Márquez, A.J. Regulation of the expression of ferredoxin-glutamate synthase in barley. Planta 1997, 203, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Turano, F.J.; Muhitch, M.J. Differential accumulation of ferredoxin-and nadh-dependent glutamate synthase activities, peptides, and transcripts in developing soybean seedlings in response to light, nitrogen, and nodulation. Physiol. Plant. 1999, 107, 407–418. [Google Scholar] [CrossRef]
- Forde, B.G. Local and long-range signaling pathways regulating plant responses to nitrate. Annu. Rev. Plant Biol. 2002, 53, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, K.; Li, H.; Song, C.P.; Miao, Y. The glutathione peroxidase gene family ingossypium hirsutum: Genome-wide identification, classification, gene expression and functional analysis. Sci. Rep. 2017, 7, 44743. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.U.; Cheng-Xiao, H.U.; Sun, X.C.; Wang, Y.H. Influences of mo on nitrate reductase, glutamine synthetase and nitrogen accumulation and utilization in mo-efficient and mo-inefficient winter wheat cultivars. Agric. Sci. China 2010, 9, 355–361. [Google Scholar]
- Liu, L.; Xiao, W.; Li, L.; Li, D.-M.; Gao, D.-S.; Zhu, C.-y.; Fu, X.-L. Effect of exogenously applied molybdenum on its absorption and nitrate metabolism in strawberry seedlings. Plant Physiol. Biochem. 2017, 115, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Mendel, R.R.; Schwarz, G. Molybdenum cofactor biosynthesis in plants and humans. Coord. Chem. Rev. 2011, 255, 1145–1158. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Ngaire Brady, J.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef]
- Baxter, I. Ionomics: Studying the social network of mineral nutrients. Curr. Opin. Plant Biol. 2009, 12, 381–386. [Google Scholar] [CrossRef]
- Murgia, I.; Vigani, G. Analysis of arabidopsis thaliana atfer4-1, atfh and atfer4-1/atfh mutants uncovers frataxin and ferritin contributions to leaf ionome homeostasis. Plant Physiol. Biochem. 2015, 94, 65–72. [Google Scholar] [CrossRef]
- Baxter, I.R.; Vitek, O.; Lahner, B.; Muthukumar, B.; Borghi, M.; Morrissey, J.; Guerinot, M.L.; Salt, D.E. The leaf ionome as a multivariable system to detect a plant’s physiological status. Proc. Natl. Acad. Sci. 2008, 105, 12081–12086. [Google Scholar] [CrossRef] [PubMed]
- Na, L.; Li, Z.; Xiangxiang, M.; Ara, N.; Jinghua, Y.; Mingfang, Z. Effect of nitrate/ammonium ratios on growth, root morphology and nutrient elements uptake of watermelon (citrullus lanatus) seedlings. J. Plant Nutr. 2014, 37, 1859–1872. [Google Scholar] [CrossRef]
- Serna, M.; Borras, R.; Legaz, F.; Primo-Millo, E. The influence of nitrogen concentration and ammonium/nitrate ratio on n-uptake, mineral composition and yield of citrus. Plant Soil 1992, 147, 13–23. [Google Scholar] [CrossRef]
- Roosta, H.R.; Schjoerring, J.K. Effects of ammonium toxicity on nitrogen metabolism and elemental profile of cucumber plants. J. Plant Nutr. 2007, 30, 1933–1951. [Google Scholar] [CrossRef]
- Sanchezechaniz, J.; Benitofernández, J.; Minteguiraso, S. Methemoglobinemia and consumption of vegetables in infants. Pediatrics 2001, 107, 1024–1028. [Google Scholar] [CrossRef]
- Wright, R.O.; Lewander, W.J.; Woolf, A.D. Methemoglobinemia: Etiology, pharmacology, and clinical management. Ann. Emerg. Med. 1999, 34, 646. [Google Scholar] [CrossRef]
- Wen, X.; Hu, C.; Sun, X.; Zhao, X.; Tan, Q. Research on the nitrogen transformation in rhizosphere of winter wheat (triticum aestivum) under molybdenum addition. Environ. Sci. Pollut. Res. 2019, 26, 2363–2374. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Li, X.; Chen, S. Suppression of hesa mutation on nitrogenase activity in paenibacillus polymyxa wly78 with the addition of high levels of molybdate or cystine. PeerJ 2019, 7, e6294. [Google Scholar] [CrossRef]
- Afridi, M.M.R.K.; Hewitt, E.J. The inducible formation and stability of nitrate reductase in higher plants: I. Effects of nitrate and molybdenum on enzyme activity in cauliflower (brassica oleracea var. Botrytis). J. Exp. Bot. 1964, 15, 251–271. [Google Scholar] [CrossRef]
- Spencer, D.; Wood, J.G. The role of molybdenum in nitrate reduction in higher plants. Aust. J. Biol. Sci. 1954, 7, 425. [Google Scholar] [CrossRef]
- Kovács, B.; Puskás-Preszner, A.; Huzsvai, L.; Lévai, L.; Bódi, É. Effect of molybdenum treatment on molybdenum concentration and nitrate reduction in maize seedlings. Plant Physiol. Biochem. 2015, 96, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Balotf, S.; Kavoosi, G.; Kholdebarin, B. Nitrate reductase, nitrite reductase, glutamine synthetase, and glutamate synthase expression and activity in response to different nitrogen sources in nitrogen-starved wheat seedlings. Biotechnol. Appl. Biochem. 2016, 63, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Balotf, S.; Islam, S.; Kavoosi, G.; Kholdebarin, B.; Juhasz, A.; Ma, W. How exogenous nitric oxide regulates nitrogen assimilation in wheat seedlings under different nitrogen sources and levels. PLoS ONE 2018, 13, e0190269. [Google Scholar] [CrossRef]
- Xiao, T.; Mi, M.; Wang, C.; Meng, Q.; Chen, Y.; Zheng, L.; Zhang, H.; Hu, Z.; Shen, Z.; Yan, X. A methionine-r-sulfoxide reductase, osmsrb5, is required for rice defense against copper toxicity. Environ. Exp. Bot. 2018, 153. [Google Scholar] [CrossRef]
- Keys, A.J.; Bird, I.F.; Cornelius, M.J.; Lea, P.J.; Wallsgrove, R.M.; Miflin, B.J. Photorespiratory nitrogen cycle. Nature 1978, 275, 741–743. [Google Scholar] [CrossRef]
- Pérez-Delgado, C.M.; García-Calderón, M.; Márquez, A.J.; Betti, M. Correction: Reassimilation of photorespiratory ammonium in lotus japonicus plants deficient in plastidic glutamine synthetase. PLoS ONE 2016, 11, e0156568. [Google Scholar] [CrossRef] [PubMed]
- Valadier, M.H.; Yoshida, A.; Grandjean, O.; Morin, H.; Kronenberger, J.; Boutet, S.; Raballand, A.; Hase, T.; Yoneyama, T.; Suzuki, A. Implication of the glutamine synthetase/glutamate synthase pathway in conditioning the amino acid metabolism in bundle sheath and mesophyll cells of maize leaves. Febs J. 2008, 275, 3193–3206. [Google Scholar] [CrossRef]
- Santi, S.; Locci, G.; Pinton, R.; Cesco, S.; Varanini, Z. Plasma membrane h+-atpase in maize roots induced for no3-uptake. Plant Physiol. 1995, 109, 1277–1283. [Google Scholar] [CrossRef]
- Campbell, W. Structure and function of eukaryotic nad (p) h: Nitrate reductase. Cell. Mol. Life Sci. Cmls 2001, 58, 194–204. [Google Scholar] [CrossRef]
- Yu, M.; Hu, C.-X.; Wang, Y.-H. Molybdenum efficiency in winter wheat cultivars as related to molybdenum uptake and distribution. Plant Soil 2002, 245, 287–293. [Google Scholar] [CrossRef]
- Yu, M.; Hu, C.; Wang, Y. Influences of seed molybdenum and molybdenum application on nitrate reductase activity, shoot dry matter, and grain yields of winter wheat cultivars. J. Plant Nutr. 1999, 22, 1433–1441. [Google Scholar] [CrossRef]
- Hachiya, T.; Watanabe, C.K.; Fujimoto, M.; Ishikawa, T.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H.; Uesono, Y.; Terashima, I.; Noguchi, K. Nitrate addition alleviates ammonium toxicity without lessening ammonium accumulation, organic acid depletion and inorganic cation depletion in arabidopsis thaliana shoots. Plant Cell Physiol. 2012, 53, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Morozkina, E.; Zvyagilskaya, R. Nitrate reductases: Structure, functions, and effect of stress factors. Biochem. (Mosc.) 2007, 72, 1151–1160. [Google Scholar] [CrossRef]
- Peng, L.; Yu, Y. Effect of molybdenum and boron on nitrogen metabolism of soybea. Plant Natrition Fertil. 1999, 5, 347–351. [Google Scholar]
- Calonego, J.C.; Junior, E.U.R.; Barbosa, R.D.; Leite, G.H.P.; Grassi Filho, H. Adubação nitrogenada em cobertura no feijoeiro com suplementação de molibdênio via foliar. Rev. Ciência Agronômica 2010, 41, 334–340. [Google Scholar] [CrossRef]
- Goodall, A.J.; Kumar, P.; Tobin, A.K. Identification and expression analyses of cytosolic glutamine synthetase genes in barley (hordeum vulgare l.). Plant Cell Physiol. 2013, 54, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Kronzucker, H.J.; Britto, D.T.; Davenport, R.J.; Tester, M. Ammonium toxicity and the real cost of transport. Trends Plant Sci. 2001, 6, 335–337. [Google Scholar] [CrossRef]
- Shelden, M.C.; Dong, B.; Guy, L.; Trevaskis, B.; Whelan, J.; Ryan, P.R.; Howitt, S.M.; Udvardi, M.K. Arabidopsis ammonium transporters, atamt1; 1 and atamt1; 2, have different biochemical properties and functional roles. Plant Soil 2001, 231, 151–160. [Google Scholar] [CrossRef]
- Hachiya, T.; Sugiura, D.; Kojima, M.; Sato, S.; Yanagisawa, S.; Sakakibara, H.; Terashima, I.; Noguchi, K. High co2 triggers preferential root growth of arabidopsis thaliana via two distinct systems under low ph and low n stresses. Plant Cell Physiol. 2014, 55, 269–280. [Google Scholar] [CrossRef]
- Lambeck, I.C.; Fischer-Schrader, K.; Niks, D.; Roeper, J.; Chi, J.-C.; Hille, R.; Schwarz, G. Molecular mechanism of 14-3-3 protein-mediated inhibition of plant nitrate reductase. J. Biol. Chem. 2012, 287, 4562–4571. [Google Scholar] [CrossRef]
- Lea, U.S.; Leydecker, M.-T.; Quilleré, I.; Meyer, C.; Lillo, C. Posttranslational regulation of nitrate reductase strongly affects the levels of free amino acids and nitrate, whereas transcriptional regulation has only minor influence. Plant Physiol. 2006, 140, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Miflin, B.J.; Habash, D.Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hu, C.; Tan, Q.; Nie, Z.; Sun, X. Effects of molybdenum on water utilization, antioxidative defense system and osmotic-adjustment ability in winter wheat (triticum aestivum) under drought stress. Plant Physiol. Biochem. 2014, 83, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, Y.; Wei, W. Effect of molybdenum applications on concentrations of free amino acids in winter wheat at different growth stages. J. Plant Nutr. 2002, 25, 1487–1499. [Google Scholar] [CrossRef]
- Engels, C.; Marschner, H. Influence of the form of nitrogen supply on root uptake and translocation of cations in the xylem exudate of maize (zea mays l). J. Exp. Bot. 1993, 44, 1695–1701. [Google Scholar] [CrossRef]
- Helali, S.M.r.; Nebli, H.; Kaddour, R.; Mahmoudi, H.; Lachaâl, M.; Ouerghi, Z. Influence of nitrate—Ammonium ratio on growth and nutrition of arabidopsis thaliana. Plant Soil 2010, 336, 65–74. [Google Scholar] [CrossRef]
- Van Beusichem, M.L.; Kirkby, E.A.; Baas, R. Influence of nitrate and ammonium nutrition on the uptake, assimilation, and distribution of nutrients in ricinus communis. Plant Physiol. 1988, 86, 914–921. [Google Scholar] [CrossRef]
- Vigani, G.; Di Silvestre, D.; Agresta, A.M.; Donnini, S.; Mauri, P.; Gehl, C.; Bittner, F.; Murgia, I. Molybdenum and iron mutually impact their homeostasis in cucumber (cucumis sativus) plants. New Phytol. 2017, 213, 1222–1241. [Google Scholar] [CrossRef]
- Nie, Z.; Hu, C.; Liu, H.; Tan, Q.; Sun, X. Differential expression of molybdenum transport and assimilation genes between two winter wheat cultivars (triticum aestivum). Plant Physiol. Biochem. 2014, 82, 27–33. [Google Scholar] [CrossRef]
- Sun, X.; Hu, C.; Tan, Q.; Liu, J.; Liu, H. Effects of molybdenum on expression of cold-responsive genes in abscisic acid (aba)-dependent and aba-independent pathways in winter wheat under low-temperature stress. Ann. Bot. 2009, 104, 345–356. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Tan, Q.; Xu, S.; Sun, X. Nitric oxide mediates molybdenum-induced antioxidant defense in wheat under drought stress. Front. Plant Sci. 2017, 8, 1085. [Google Scholar] [CrossRef]
- Rao, L.V.M.; Rajasekhar, V.K.; Sopory, S.K.; Guhamukherjee, S. Phytochrome regulation of nitrite reductase—A chloroplast enzyme—In etiolated maize leaves. Plant Cell Physiol. 1981, 22, 577–582. [Google Scholar]
- Rhodes, D.; Rendon, G.A.; Stewart, G.R. The control of glutamine synthetase level in lemna minor L. Planta 1975, 125, 201–211. [Google Scholar] [CrossRef]
- Lin, C.C.; Kao, C.H. Disturbed ammonium assimilation is associated with growth inhibition of roots in rice seedlings caused by nacl. Plant Growth Regul. 1996, 18, 233–238. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time rt–pcr. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, D.; Maroon, M.; Schrader, L.; Youngs, V. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid 1. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Molins-Legua, C.; Meseguer-Lloret, S.; Moliner-Martinez, Y.; Campíns-Falcó, P. A guide for selecting the most appropriate method for ammonium determination in water analysis. Trac Trends Anal. Chem. 2006, 25, 282–290. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Van Slyke, D.D.; Lemish, S. The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J. Biol. Chem. 1943, 150, 251–258. [Google Scholar]
- Bradford, M.M. A rapid method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
| Treatments | 97003 | 97014 | |||||
|---|---|---|---|---|---|---|---|
| NO3− Content (µmol·g−1FW) | NO2− Content (µmol·g−1FW) | NH4+ Content (µmol·g−1FW) | NO3− Content (µmol·g−1FW) | NO2− Content (µmol·g−1FW) | NH4+ Content (µmol·g−1FW) | ||
| NO3− | −Mo | 35.56 ± 5.32 a | 2.22 ± 0.22 c | 7.45 ± 0.57 c | 38.98 ± 4.19 a | 1.80 ± 0.10 b | 6.35 ± 0.82 b |
| +Mo | 28.84 ± 2.78 ab | 2.74 ± 0.32 bc | 8.90 ± 1.06 c | 28.17 ± 4.27 bc | 2.53 ± 0.36 b | 8.19 ± 0.79 b | |
| NH4NO3 | −Mo | 28.04 ± 3.74 ab | 3.30 ± 0.43 b | 10.79 ± 1.22 bc | 33.04 ± 3.25 ab | 2.59 ± 0.21 b | 9.00 ± 0.65 b |
| +Mo | 21.63 ± 1.73 b | 4.52 ± 0.53 a | 13.68 ± 1.38 ab | 21.44 ± 2.15 c | 4.18 ± 0.56 a | 12.85 ± 1.44 a | |
| NH4+ | −Mo | 1.70 ± 0.17 c | 0.34 ± 0.02 d | 13.43 ± 1.07 ab | 1.80 ± 0.22 d | 0.23 ± 0.04 c | 12.94 ± 1.32 a |
| +Mo | 1.50 ± 0.08 c | 0.38 ± 0.04 d | 15.53 ± 1.94 a | 1.48 ± 0.12 d | 0.32 ± 0.04 c | 15.54 ± 1.80 a | |
| Treatments | 97003 | 97014 | |||||
|---|---|---|---|---|---|---|---|
| K (mg·g−1DW) | Mg (mg·g−1DW) | Ca (mg g−1DW) | K (mg·g−1DW) | Mg (mg·g−1DW) | Ca (mg·g−1DW) | ||
| NO3− | −Mo | 37.80 ± 1.62 a | 2.63 ± 0.19 ab | 12.09 ± 1.15 a | 37.31 ± 1.86 a | 2.14 ± 0.19 ab | 10.43 ± 1.49 ab |
| +Mo | 34.30 ± 0.89 b | 3.25 ± 0.55 a | 13.79 ± 1.01 a | 35.49 ± 1.82 a | 2.80 ± 0.23 a | 12.92 ± 1.36 a | |
| NH4NO3 | −Mo | 30.89 ± 0.84 c | 2.24 ± 0.12 bc | 10.49 ± 0.76 a | 30.56 ± 0.64 b | 1.89 ± 0.20 b | 8.08 ± 0.86 bc |
| +Mo | 28.24 ± 1.24 c | 2.87 ± 0.30 ab | 12.05 ± 1.63 a | 26.45 ± 1.39 c | 2.67 ± 0.43 a | 11.37 ± 0.78 a | |
| NH4+ | −Mo | 25.10 ± 0.55 d | 1.32 ± 0.15 d | 4.70 ± 0.23 b | 25.92 ± 0.63 c | 0.88 ± 0.04 c | 4.17 ± 0.29 d |
| +Mo | 22.73 ± 0.74 d | 1.49 ± 0.15 cd | 5.27 ± 0.78 b | 23.71 ± 0.62 c | 1.09 ± 0.14 c | 4.85 ± 0.66 cd | |
| Treatments | 97003 | 97014 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn (µg·g−1DW) | Zn (µg·g−1DW) | Cu (µg·g−1DW) | Fe (µg g−1DW) | Mn (µg·g−1DW) | Zn (µg·g−1DW) | Cu (µg·g−1DW) | Fe (µg·g−1DW) | ||
| NO3− | −Mo | 72.78 ± 7.88 a | 26.51 ± 6.53 b | 7.83 ± 0.51 c | 84.19 ± 3.45 de | 69.28 ± 8.33 a | 30.34 ± 1.37 cd | 6.95 ± 0.53 c | 102.86 ± 22.01 bcd |
| +Mo | 62.88 ± 7.34 ab | 22.39 ± 2.88 b | 8.44 ± 1.25 c | 71.04 ± 9.50 e | 55.53 ± 3.78 ab | 20.97 ± 2.88 d | 7.96 ± 1.09 c | 76.83 ± 6.28 d | |
| NH4NO3 | −Mo | 62.38 ± 10.05 ab | 32.23 ± 3.03 b | 9.89 ± 1.15 bc | 110.07 ± 20.12 bc | 60.64 ± 4.91 b | 34.18 ± 6.08 bc | 8.64 ± 0.84 c | 127.69 ± 15.12 bc |
| +Mo | 50.93 ± 5.45 bc | 26.53 ± 4.33 b | 11.29 ± 1.57 abc | 96.82 ± 9.24 cd | 51.48 ± 7.54 bc | 23.93 ± 1.89 cd | 10.35 ± 1.69 bc | 99.18 ± 10.25 cd | |
| NH4+ | −Mo | 39.58 ± 3.60 cd | 52.35 ± 5.77 a | 15.14 ± 3.24 ab | 150.32 ± 11.47 a | 40.07 ± 4.31 cd | 54.63 ± 7.35 a | 14.58 ± 2.67 ab | 177.30 ± 21.16 a |
| +Mo | 31.10 ± 4.50 d | 44.50 ± 5.78 a | 15.67 ± 1.69 a | 131.83 ± 15.88 ab | 31.06 ± 5.04 d | 41.77 ± 6.30 b | 15.71 ± 1.87 a | 141.69 ± 13.79 ab | |
| Salts | NO3− | NH4:NO3 | NH4+ |
|---|---|---|---|
| Ca(NO3)2.4H2O | 5.00 | 3.75 | 0.00 |
| KNO3 | 5.00 | 0.00 | 0.00 |
| CaCl2 | 0.00 | 1.25 | 5.00 |
| K2SO4 | 0.00 | 2.50 | 2.50 |
| (NH4)2SO4 | 0.00 | 3.75 | 7.50 |
| MgSO4.7H2O | 2.00 | 2.00 | 2.00 |
| KH2PO4 | 1.00 | 1.00 | 1.00 |
| Genes | Strand | Primer Sequence 5′ to 3′ | Annealing Temperature (°C) |
|---|---|---|---|
| NR | Forward | CACCGGCCGCGGCAACTTC | 58 |
| Reverse | CGAGACGGAGATGCACCTGG | ||
| NiR | Forward | CGAGAAGAGGATGCCGAACG | 56 |
| Reverse | CACGCCGAGGTAGTCACG | ||
| GS | Forward | CACACCTCATCTCATCTCATCTC | 52 |
| Reverse | TTCACCGTCCTTGCTTTGC | ||
| GOGAT | Forward | TCATCCAGCCGACCAACACG | 55 |
| Reverse | CCACAATCCATACAACGAGCAGAC | ||
| TaActin | Forward | ACTGGGATGACATGGGGAA | 55 |
| Reverse | ACCGCTGGCATACAAGGAC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.; Sun, X.; Hussain, S.; Ali, U.; Rana, M.S.; Rasul, F.; Saleem, M.H.; Moussa, M.G.; Bhantana, P.; Afzal, J.; et al. Molybdenum-Induced Effects on Nitrogen Metabolism Enzymes and Elemental Profile of Winter Wheat (Triticum aestivum L.) Under Different Nitrogen Sources. Int. J. Mol. Sci. 2019, 20, 3009. https://doi.org/10.3390/ijms20123009
Imran M, Sun X, Hussain S, Ali U, Rana MS, Rasul F, Saleem MH, Moussa MG, Bhantana P, Afzal J, et al. Molybdenum-Induced Effects on Nitrogen Metabolism Enzymes and Elemental Profile of Winter Wheat (Triticum aestivum L.) Under Different Nitrogen Sources. International Journal of Molecular Sciences. 2019; 20(12):3009. https://doi.org/10.3390/ijms20123009
Chicago/Turabian StyleImran, Muhammad, Xuecheng Sun, Saddam Hussain, Usman Ali, Muhammad Shoaib Rana, Fahd Rasul, Muhammad Hamzah Saleem, Mohamed G. Moussa, Parashuram Bhantana, Javaria Afzal, and et al. 2019. "Molybdenum-Induced Effects on Nitrogen Metabolism Enzymes and Elemental Profile of Winter Wheat (Triticum aestivum L.) Under Different Nitrogen Sources" International Journal of Molecular Sciences 20, no. 12: 3009. https://doi.org/10.3390/ijms20123009
APA StyleImran, M., Sun, X., Hussain, S., Ali, U., Rana, M. S., Rasul, F., Saleem, M. H., Moussa, M. G., Bhantana, P., Afzal, J., Elyamine, A. M., & Hu, C. X. (2019). Molybdenum-Induced Effects on Nitrogen Metabolism Enzymes and Elemental Profile of Winter Wheat (Triticum aestivum L.) Under Different Nitrogen Sources. International Journal of Molecular Sciences, 20(12), 3009. https://doi.org/10.3390/ijms20123009










