Cyanate Assimilation by the Alkaliphilic Cyanide-Degrading Bacterium Pseudomonas pseudoalcaligenes CECT5344: Mutational Analysis of the cyn Gene Cluster
Abstract
1. Introduction
2. Results and Discussion
2.1. The cynFABDS Gene Cluster of P. pseudoalcaligenes CECT5344
2.2. Regulation of the Cyanate Assimilation in P. pseudoalcaligenes CECT5344
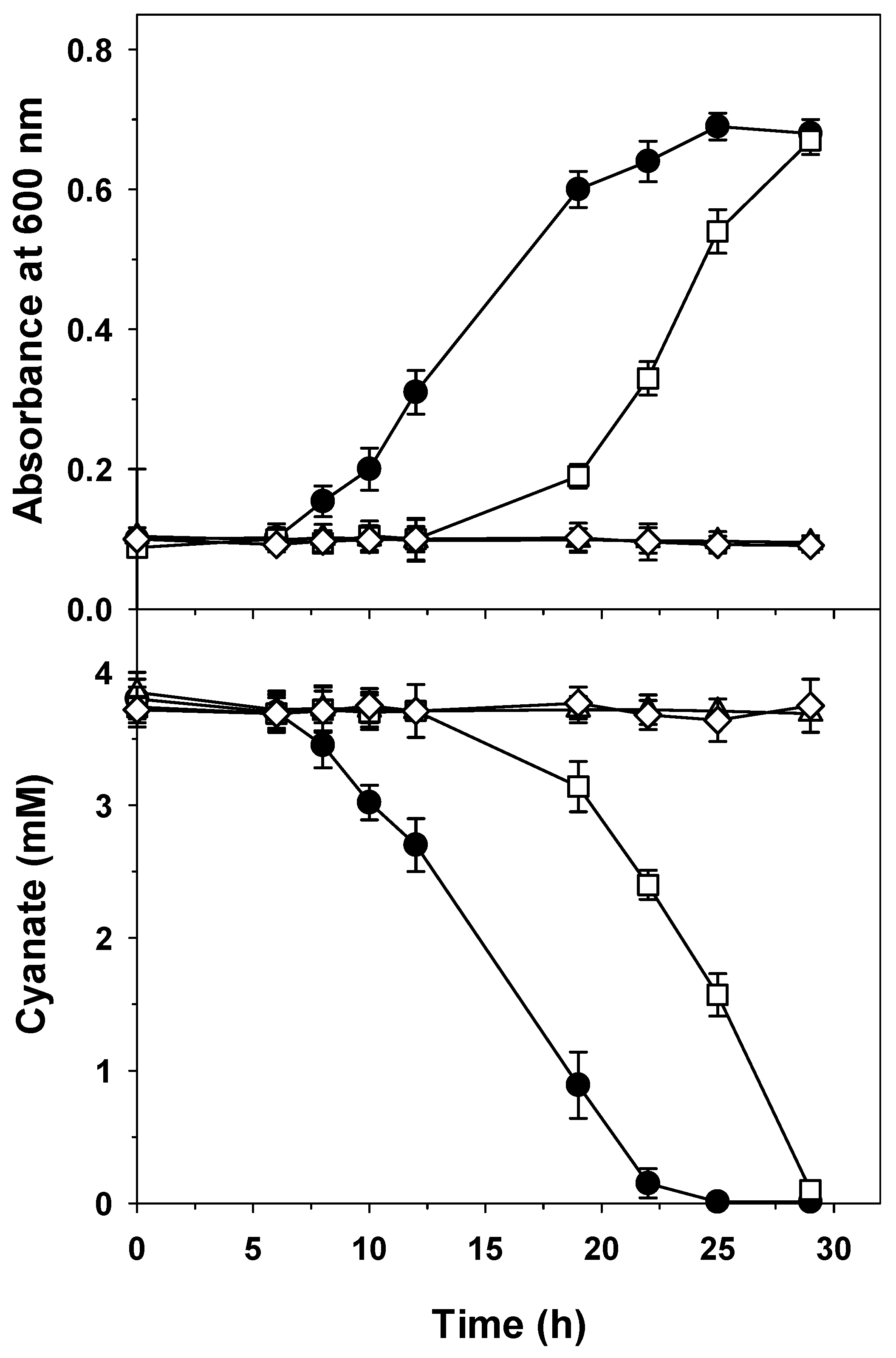
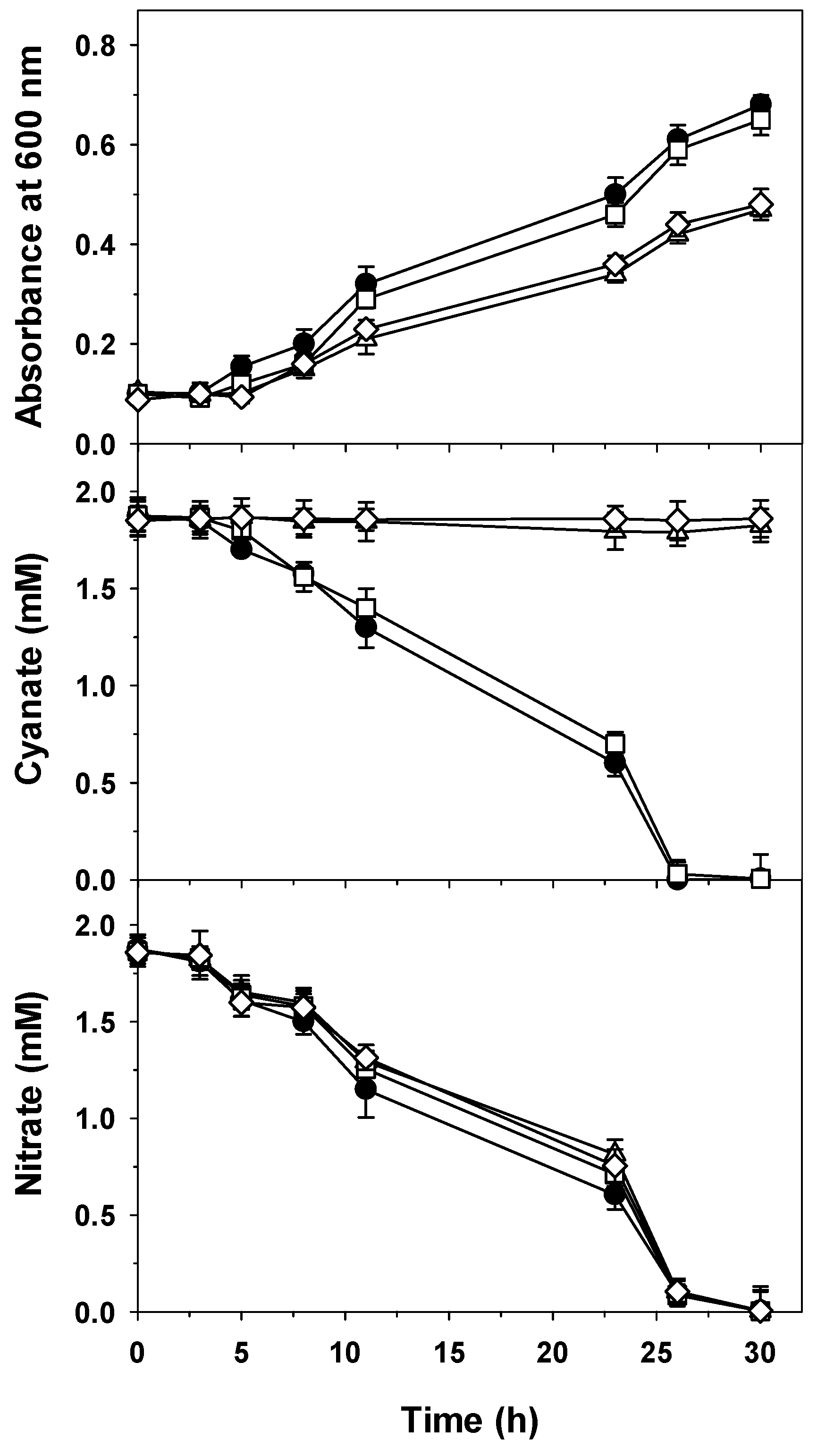
2.3. Mutational Analysis of the cyn Gene Cluster of P. pseudoalcaligenes CECT5344
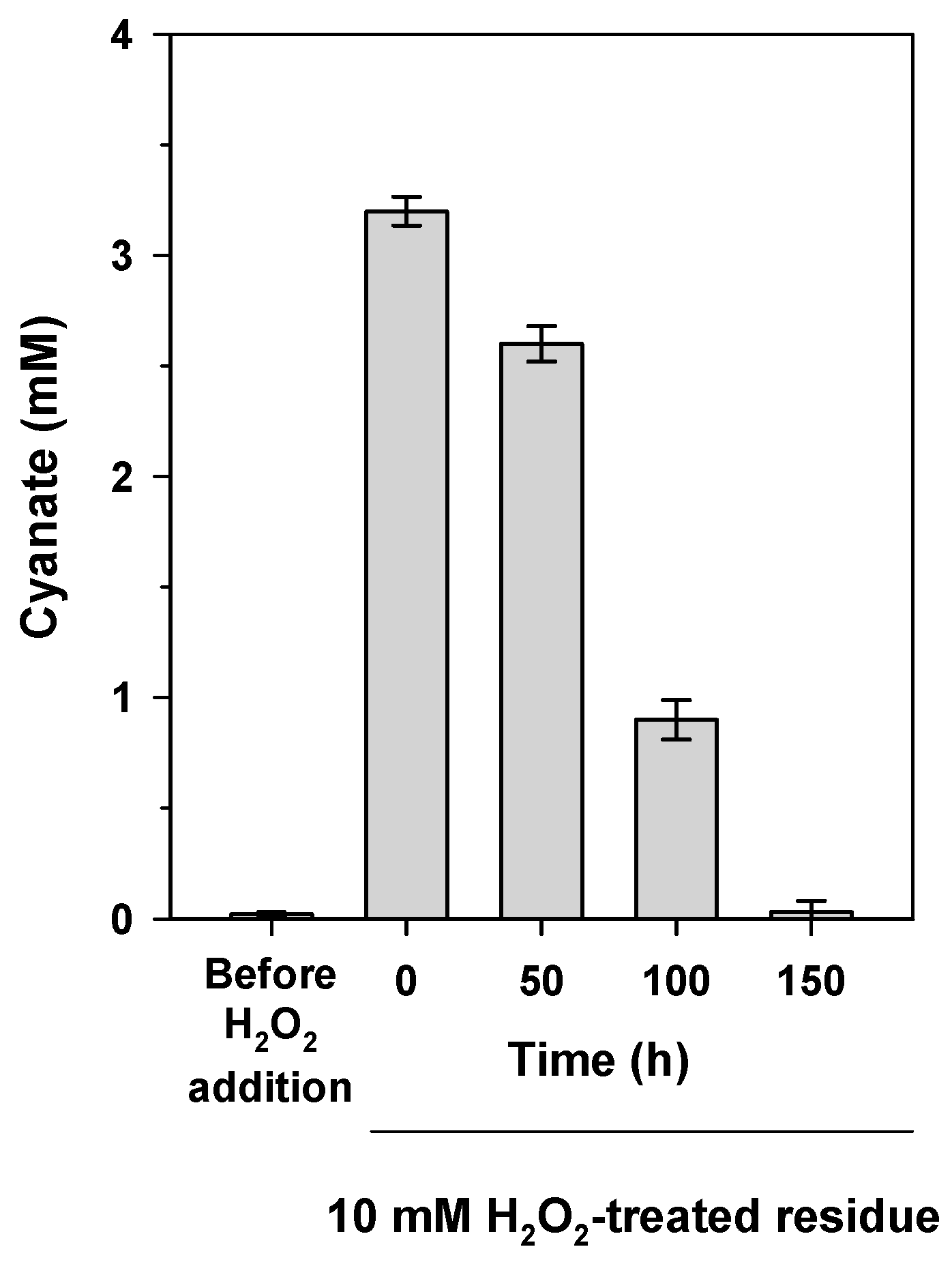
2.4. Biodegradation of Jewelry Wastewater Treated with Hydrogen Peroxide to Oxidize Cyanide to Cyanate
3. Materials and Methods
3.1. Chemicals
3.2. Bacterial Strains, Plasmids, and Growth Conditions
3.3. Analytical Determinations
3.4. Enzyme Activity Assays
3.5. Peroxidation of the Cyanide-Containing Industrial Wastewater
3.6. DNA Manipulations, Sequencing, and Generation of Mutant Strains
3.7. RT-PCR Transcriptional Analysis of the cyn Gene Cluster
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solomonson, L.P. Cyanide as a metabolic inhibitor. In Cyanide in Biology; Vennesland, B., Conn, E.E., Knowles, C.J., Westley, J., Wissing, F., Eds.; Academic Press: New York, NY, USA, 1981; pp. 11–28. [Google Scholar]
- Jünemann, S. Cytochrome bd terminal oxidase. Biochim. Biophys. Acta 1997, 1321, 107–127. [Google Scholar] [CrossRef]
- Ebbs, S. Biological degradation of cyanide compounds. Curr. Opin. Biotechnol. 2004, 15, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Balomajumder, C.; Agarwal, V.K. Enzymatic mechanism and biochemistry for cyanide degradation: a review. J. Hazard. Mater. 2010, 176, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Luque-Almagro, V.M.; Moreno-Vivián, C.; Roldán, M.D. Biodegradation of cyanide wastes from mining and jewellery industries. Curr. Opin. Biotechnol. 2016, 38, 9–13. [Google Scholar] [CrossRef]
- Jaszczak, E.; Polkowska, Z.; Narkowicz, S.; Namiesnik, J. Cyanides in the environment–analysis–problems and challenges. Environ. Sci. Pollut. Res. 2017, 24, 15929–15948. [Google Scholar] [CrossRef] [PubMed]
- Cabello, P.; Luque-Almagro, V.M.; Olaya, A.; Sáez, L.P.; Moreno-Vivián, C.; Roldán, M.D. Assimilation of cyanide and cyano–derivatives by Pseudomonas pseudoalcaligenes CECT5344: From omic approaches to biotechnological applications. FEMS Microbiol. Lett. 2018, 365, fny032. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Cabello, P.; Sáez, L.P.; Olaya-Abril, A.; Moreno-Vivián, C.; Roldán, M.D. Exploring anaerobic environments for cyanide and cyano-derivatives microbial degradation. Appl. Microbiol. Biotechnol. 2018, 102, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Luque-Almagro, V.M.; Huertas, M.J.; Martínez-Luque, M.; Moreno-Vivián, C.; Roldán, M.D.; García-Gil, J.; Castillo, F.; Blasco, R. Bacterial degradation of cyanide and its metal complexes under alkaline conditions. Appl. Environ. Microbiol. 2005, 71, 940–947. [Google Scholar] [CrossRef]
- Huertas, M.J.; Sáez, L.P.; Roldán, M.D.; Luque-Almagro, V.M.; Martínez-Luque, M.; Blasco, R.; Castillo, F.; Moreno-Vivián, C.; García-García, I. Alkaline cyanide degradation by Pseudomonas pseudoalcaligenes CECT5344 in a batch reactor. Influence of pH. J. Hazard. Mater. 2010, 179, 72–78. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Merchán, F.; Blasco, R.; Igeño, M.I.; Martínez-Luque, M.; Moreno-Vivián, C.; Castillo, F.; Roldán, M.D. Cyanide degradation by Pseudomonas pseudoalcaligenes CECT5344 involves a malate:quinone oxidoreductase and an associated cyanide-insensitive electron transfer chain. Microbiology 2011, 157, 739–746. [Google Scholar] [CrossRef]
- Estepa, J.; Luque-Almagro, V.M.; Manso, I.; Escribano, M.P.; Martínez-Luque, M.; Castillo, F.; Moreno-Vivián, C.; Roldán, M.D. The nit1C gene cluster of Pseudomonas pseudoalcaligenes CECT5344 involved in assimilation of nitriles is essential for growth on cyanide. Environ. Microbiol. Rep. 2012, 4, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A. Destruction of cyanide in gold mill effluents: Biological versus chemical treatments. Biotechnol. Adv. 2003, 21, 501–511. [Google Scholar] [CrossRef]
- Akcil, A.; Mudder, T. Microbial destruction of cyanide wastes in gold mining: Process review. Biotechnol. Lett. 2003, 25, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.; Cummings, S.P. The current and future applications of microorganisms in the bioremediation of cyanide contamination. Anton. Leeuw. 2006, 90, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.R.; Gaur, A.; Balomajumder, C. Cyanide in industrial wastewaters and its removal: A review on biotreatment. J. Hazard. Mater. 2009, 163, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Saha, S.; Dhaka, S.; Kurade, M.B.; Kang, C.U.; Baek, S.H.; Jeon, B.H. Remediation of cyanide-contaminated environments through microbes and plants: A review of current knowledge and future perspectives. Geosyst. Eng. 2016, 20, 28–40. [Google Scholar] [CrossRef]
- Ibáñez, M.I.; Cabello, P.; Luque-Almagro, V.M.; Sáez, L.P.; Olaya, A.; Sánchez de Medina, V.; Luque de Castro, M.D.; Moreno-Vivián, C.; Roldán, M.D. Quantitative proteomic analysis of Pseudomonas pseudoalcaligenes CECT5344 in response to industrial cyanide-containing wastewaters using Liquid Chromatography-Mass Spectrometry/Mass Spectrometry (LC-MS/MS). PLoS ONE 2017, 12, e0172908. [Google Scholar] [CrossRef]
- Patil, Y.B.; Paknikar, K.M. Development of a process for biodetoxification of metal cyanides from waste waters. Process Biochem. 2000, 35, 1139–1151. [Google Scholar] [CrossRef]
- Nowakowska, M.; Sterzel, M.; Szczubialka, K. Photosensitized oxidation of cyanide in aqueous solutions of photoactive modified hydroxyethylcellulose. J. Polym. Environ. 2006, 14, 59–64. [Google Scholar] [CrossRef]
- Allen, C.M.; Jones, M.E. Decomposition of carbamoyl phosphate in aqueous solutions. Biochemistry 1984, 3, 1238–1247. [Google Scholar] [CrossRef]
- Guilloton, M.; Karst, F. Isolation and characterization of E. coli mutants lacking inducible cyanase. J. Gen. Microbiol. 1987, 133, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Koshiishi, I.; Mamura, Y.; Imanari, T. Cyanate causes depletion of ascorbate in organisms. Biochim. Biophys. Acta 1997, 1336, 566–574. [Google Scholar] [CrossRef]
- Kraus, L.M.; Kraus, A.P. The search for the uremic toxin: The case for carbamoylation of amino acids and proteins. Wien Klin. Wochenschr. 1998, 110, 521–530. [Google Scholar] [PubMed]
- Walsh, M.A.; Otwinowski, Z.; Perrakis, A.; Anderson, P.M.; Joachimiak, A. Structure of cyanase reveals that a novel dimeric and decameric arrangement of subunits is required for formation of the enzyme active site. Structure 2000, 15, 505–514. [Google Scholar] [CrossRef]
- Anderson, P.M. Purification and properties of the inducible enzyme cyanase. Biochemistry 1980, 19, 2882–2888. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.V.; Anderson, P.M. Bicarbonate is a recycling substrate for cyanase. J. Biol. Chem. 1987, 262, 9021–9025. [Google Scholar] [PubMed]
- Anderson, P.M.; Sung, Y.C.; Fuchs, J.A. The cyanase operon and cyanate metabolism. FEMS Microbiol. Rev. 1990, 7, 247–252. [Google Scholar] [CrossRef]
- Harano, Y.; Suzuki, I.; Maeda, S.; Kaneko, T.; Tabata, S.; Omata, T. Identification and nitrogen regulation of the cyanase gene from the cyanobacteria Synechocystis sp. strain PCC6803 and Synechococcus sp. strain PCC7942. J. Bacteriol. 1997, 179, 5744–5750. [Google Scholar] [CrossRef][Green Version]
- Butryn, A.; Stoehr, G.; Linke-Winnebeck, C.; Hopfner, K.P. Serendipitous crystallization and structure determination of cyanase (CynS) from Serratia proteamaculans. Acta Cryst. 2015, F71, 471–476. [Google Scholar]
- Widner, B.; Fuchsman, C.A.; Chang, B.X.; Rocap, G.; Mulholland, M.R. Utilization of urea and cyanate in waters overlying and within the eastern tropical north Pacific oxygen deficient zone. FEMS Microbiol. Ecol. 2018, 94, fiy138. [Google Scholar] [CrossRef]
- Palatinszky, M.; Herbold, G.; Jehmlich, N.; Pogoda, M.; Han, P.; von Bergen, M.; Lagkouvardos, I.; Karst, S.M.; Galushko, A.; Koch, H.; et al. Cyanate as an energy source for nitrifiers. Nature 2015, 524, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Guilloton, M.; Korte, J.J.; Lamblin, A.F.; Fuchs, J.A.; Anderson, P.M. Carbonic anhydrase in Escherichia coli: a product of the cyn operon. J. Biol. Chem. 1992, 267, 3731–3734. [Google Scholar] [PubMed]
- Sung, Y.C.; Fuchs, J.A. The E. coli K12 cyn operon is positively regulated by a member of the lysR family. J. Bacteriol. 1992, 174, 3645–3650. [Google Scholar] [CrossRef] [PubMed]
- Guilloton, M.B.; Lamblin, A.F.; Kozliak, E.I.; Gerami-Nejad, M.; Tu, C.; Silverman, D.; Anderson, P.M.; Fuchs, J.A. A physiological role for cyanate-induced carbonic anhydrase in Escherichia coli. J. Bacteriol. 1993, 175, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Lamblin, A.F.; Fuchs, J.A. Functional analysis of the Escherichia coli K-12 cyn operon transcriptional regulation. J. Bacteriol. 1994, 176, 6613–6622. [Google Scholar] [CrossRef]
- Kozliak, E.I.; Fuchs, J.A.; Guilloton, M.B.; Anderson, P.M. Role of bicarbonate/CO2 in the inhibition of Escherichia coli growth by cyanate. J. Bacteriol. 1995, 177, 3213–3219. [Google Scholar] [CrossRef]
- Carepo, M.S.P.; Nina de Azevedo, J.S.; Porto, J.I.R.; Bentes-Sousa, A.R.; da Silva Batista, J.; da Silva, A.L.C.; Schneider, M.P.C. Identification of Chromobacterium violaceum genes with potential biotechnological application in environmental detoxification. Genet. Mol. Res. 2004, 3, 181–194. [Google Scholar]
- Espie, G.S.; Jalali, F.; Tong, T.; Zacal, N.J.; So, A.K.C. Involvement of the cynABDS operon and the CO2-concentrating mechanism in the light-dependent transport and metabolism of cyanate by cyanobacteria. J. Bacteriol. 2007, 189, 1013–1024. [Google Scholar] [CrossRef]
- Kamennaya, N.A.; Post, A.F. Characterization of cyanate metabolism in marine Synechococcus and Prochlorococcus spp. Appl. Environ. Microbiol. 2011, 77, 291–301. [Google Scholar] [CrossRef]
- Maeda, S.I.; Omata, T. Nitrite transport activity of the ABC-type cyanate transporter of the cyanobacterium Synechococcus elongatus. J. Bacteriol. 2009, 191, 3265–3272. [Google Scholar] [CrossRef]
- Muñoz-Centeno, M.C.; Paneque, A.; Cejudo, F.J. Cyanate is transported by the nitrate permease in Azotobacter chroococcum. FEMS Microbiol. Lett. 1996, 137, 91–94. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Huertas, M.J.; Sáez, L.P.; Martínez-Luque, M.; Moreno-Vivián, C.; Castillo, F.; Roldán, M.D.; Blasco, R. Characterization of the Pseudomonas pseudoalcaligenes CECT5344 cyanase, an enzyme that is not essential for cyanide assimilation. Appl. Environ. Microbiol. 2008, 74, 6280–6288. [Google Scholar] [CrossRef] [PubMed]
- Luque-Almagro, V.M.; Acera, F.; Igeño, M.I.; Wibberg, D.; Roldán, M.D.; Sáez, L.P.; Hennig, M.; Quesada, A.; Huertas, M.J.; Blom, J.; et al. Draft whole genome sequence of the cyanide-degrading bacterium Pseudomonas pseudoalcaligenes CECT5344. Environ. Microbiol. 2013, 15, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Wibberg, D.; Luque-Almagro, V.M.; Igeño, M.I.; Bremges, A.; Roldán, M.D.; Merchán, F.; Sáez, L.P.; Guijo, M.I.; Macías, D.; Cabello, P.; et al. Complete genome sequence of the cyanide-degrading bacterium Pseudomonas pseudoalcaligenes CECT5344. J. Biotechnol. 2014, 175, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Wibberg, D.; Bremges, A.; Dammann-Kalinowski, T.; Maus, I.; Igeño, M.I.; Vogelsang, R.; König, C.; Luque-Almagro, V.M.; Roldán, M.D.; Sczyrba, A.; et al. Finished genome sequence and methylome of the cyanide-degrading Pseudomonas pseudoalcaligenes strain CECT5344 as resolved by single-molecule real-time sequencing. J. Biotechnol. 2016, 232, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Luque-Almagro, V.M.; Blasco, R.; Fernández-Romero, J.M.; Luque de Castro, M.D. Flow-injection spectrophotometric determination of cyanate in bioremediation processes by use of immobilised inducible cyanase. Anal. Bioanal. Chem. 2003, 377, 1071–1078. [Google Scholar] [CrossRef]
- Quesada, A.; Guijo, M.I.; Merchán, F.; Blázquez, B.; Igeño, M.I.; Blasco, R. Essential role of cytochrome bd-related oxidase in cyanide resistance of Pseudomonas pseudoalcaligenes CECT5344. Appl. Environ. Microbiol. 2007, 73, 5118–5124. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Escribano, M.P.; Manso, I.; Sáez, L.P.; Cabello, P.; Moreno-Vivián, C.; Roldán, M.D. DNA microarray analysis of the cyanotroph Pseudomonas pseudoalcaligenes CECT5344 in response to nitrogen starvation, cyanide and a jewelry wastewater. J. Biotechnol. 2015, 214, 171–181. [Google Scholar] [CrossRef]
- Sambrook, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Reyes, F.; Roldán, M.D.; Klipp, W.; Castillo, F.; Moreno-Vivián, C. Isolation of periplasmic nitrate reductase genes from Rhodobacter sphaeroides DSM158: Structural and functional differences among prokaryotic nitrate reductases. Mol. Microbiol. 1996, 19, 1307–1318. [Google Scholar] [CrossRef]
- Asmus, E.; Garschagen, H. The use of barbituric acid for the photometric determination of cyanide and thiocyanate. Z. Anal. Chem. 1953, 138, 414–422. [Google Scholar] [CrossRef]
- Shakir, F.K.; Audilet, D.; Drake, A.J.; Shakir, K.M.M. A rapid protein determination by modification of the Lowry procedure. Anal. Biochem. 1994, 216, 232–233. [Google Scholar] [CrossRef] [PubMed]
- Olaya-Abril, A.; Luque-Almagro, V.M.; Pérez, M.D.; López, C.M.; Amil, F.; Cabello, P.; Sáez, L.P.; Moreno-Vivián, C.; Roldán, M.D. Putative small RNAs controlling detoxification of industrial cyanide-containing wastewaters by Pseudomonas pseudoalcaligenes CECT5344. PLoS ONE 2019, 14, e0212032. [Google Scholar] [CrossRef] [PubMed]


| Nitrogen Sources | Wild-Type Strain | CynF− Mutant | ||
|---|---|---|---|---|
| Cyanase | Nitrate Reductase | Cyanase | Nitrate Reductase | |
| -N | 176 ± 21 | 114 ± 23 | ND | 201 ± 28 |
| Ammonium | ND | 13 ± 2 | ND | 235 ± 35 |
| Nitrate | 145 ± 18 | 202 ± 32 | ND | 283 ± 30 |
| Glutamate | 208 ± 27 | 86 ± 8 | ND | 241 ± 27 |
| Cyanate | 2677 ± 208 | 124 ± 15 | ND | 272 ± 31 |
| Cyanate + ammonium | ND | 19 ± 5 | ND | 242 ± 33 |
| Cyanate + nitrate | 2210 ± 115 | 194 ± 28 | ND | 290 ± 35 |
| Cyanate + glutamate | 2558 ± 223 | 97 ± 9 | ND | 243 ± 22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez, L.P.; Cabello, P.; Ibáñez, M.I.; Luque-Almagro, V.M.; Roldán, M.D.; Moreno-Vivián, C. Cyanate Assimilation by the Alkaliphilic Cyanide-Degrading Bacterium Pseudomonas pseudoalcaligenes CECT5344: Mutational Analysis of the cyn Gene Cluster. Int. J. Mol. Sci. 2019, 20, 3008. https://doi.org/10.3390/ijms20123008
Sáez LP, Cabello P, Ibáñez MI, Luque-Almagro VM, Roldán MD, Moreno-Vivián C. Cyanate Assimilation by the Alkaliphilic Cyanide-Degrading Bacterium Pseudomonas pseudoalcaligenes CECT5344: Mutational Analysis of the cyn Gene Cluster. International Journal of Molecular Sciences. 2019; 20(12):3008. https://doi.org/10.3390/ijms20123008
Chicago/Turabian StyleSáez, Lara Paloma, Purificación Cabello, María Isabel Ibáñez, Víctor Manuel Luque-Almagro, María Dolores Roldán, and Conrado Moreno-Vivián. 2019. "Cyanate Assimilation by the Alkaliphilic Cyanide-Degrading Bacterium Pseudomonas pseudoalcaligenes CECT5344: Mutational Analysis of the cyn Gene Cluster" International Journal of Molecular Sciences 20, no. 12: 3008. https://doi.org/10.3390/ijms20123008
APA StyleSáez, L. P., Cabello, P., Ibáñez, M. I., Luque-Almagro, V. M., Roldán, M. D., & Moreno-Vivián, C. (2019). Cyanate Assimilation by the Alkaliphilic Cyanide-Degrading Bacterium Pseudomonas pseudoalcaligenes CECT5344: Mutational Analysis of the cyn Gene Cluster. International Journal of Molecular Sciences, 20(12), 3008. https://doi.org/10.3390/ijms20123008






