Integrated Analysis of the Altered lncRNAs and mRNAs Expression in 293T Cells after Ionizing Radiation Exposure
Abstract
1. Introduction
2. Results
2.1. Expression and Classification of lncRNAs in 293T Cells after Exposure to Ionizing Radiation
2.2. Profiles of Differentially Expressed lncRNAs and mRNAs
2.3. Differentially Expressed lncRNA Validation by Quantitative PCR
2.4. Functional Annotation of Differentially Expressed lncRNAs and mRNAs
2.5. KEGG Enrichment Analysis
2.6. lncRNA–miRNA–mRNA Network Analysis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Irradiation of Cells
4.3. RNA Extraction
4.4. High-Throughput Sequencing
4.5. Sequencing Analysis of lncRNA
4.6. Identification and Expression Analysis of lncRNAs and mRNAs
4.7. Quantitative Real-Time PCR
4.8. GO Annotations and KEGG Enrichment
4.9. lncRNA–miRNA–mRNA Network
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; O’Carrigan, B.; Jackson, S.P.; Yap, T.A. Targeting DNA Repair in Cancer: Beyond PARP Inhibitors. Cancer Discov. 2017, 7, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Sandhu, S.K.; Carden, C.P.; De Bono, J.S. Poly(ADP-Ribose) polymerase (PARP) inhibitors: Exploiting a synthetic lethal strategy in the clinic. Ca Cancer J. Clin. 2011, 61, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Horikoshi, N.; Singh, M.; Gupta, A.; Misra, H.S.; Albuquerque, K.; Hunt, C.R.; Pandita, T.K. Chromatin modifications and the DNA damage response to ionizing radiation. Front. Oncol. 2012, 2, 214. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, A.; Elledge, S.J. The DNA Damage Response: Making It Safe to Play with Knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Bartek, J.; Lukas, J. DNA damage checkpoints: From initiation to recovery or adaptation. Curr. Opin. Cell Biol. 2007, 19, 238–245. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Dmitrenko, V.V. HEK293 in cell biology and cancer research: Phenotype, karyotype, tumorigenicity, and stress-induced genome-phenotype evolution. Gene 2015, 569, 182–190. [Google Scholar] [CrossRef]
- Lin, Y.C.; Boone, M.; Meuris, L.; Lemmens, I.; Van Roy, N.; Soete, A.; Reumers, J.; Moisse, M.; Plaisance, S.; Drmanac, R.; et al. Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nat. Commun. 2014, 5, 4767. [Google Scholar] [CrossRef]
- Morris, J.R.; Boutell, C.; Keppler, M.; Densham, R.; Weekes, D.; Alamshah, A.; Butler, L.; Galanty, Y.; Pangon, L.; Kiuchi, T.; et al. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature 2009, 462, 886–890. [Google Scholar] [CrossRef]
- Bass, T.E.; Luzwick, J.W.; Kavanaugh, G.; Carroll, C.; Dungrawala, H.; Glick, G.G.; Feldkamp, M.D.; Putney, R.; Chazin, W.J.; Cortez, D. ETAA1 acts at stalled replication forks to maintain genome integrity. Nat. Cell Biol. 2016, 18, 1185–1195. [Google Scholar] [CrossRef]
- Inano, S.; Sato, K.; Katsuki, Y.; Kobayashi, W.; Tanaka, H.; Nakajima, K.; Nakada, S.; Miyoshi, H.; Knies, K.; Takaori-Kondo, A.; et al. RFWD3-Mediated Ubiquitination Promotes Timely Removal of Both RPA and RAD51 from DNA Damage Sites to Facilitate Homologous Recombination. Mol. Cell 2017, 66, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Chiu, L.Y.; Nehring, R.B.; Bravo Nunez, M.A.; Mei, Q.; Perez, M.; Zhai, Y.; Fitzgerald, D.M.; Pribis, J.P.; Wang, Y.; et al. Bacteria-to-Human Protein Networks Reveal Origins of Endogenous DNA Damage. Cell 2019, 176, 127–143. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Sun, Y.; Yang, M.; Lu, Q.; Wang, J.; Xiao, C.; Wang, Y.; Du, L.; Ji, K.; Xu, C.; et al. Analysis of Circular RNA Expression Profile in HEK 293T Cells Exposed to Ionizing Radiation. Dose-Response A Publ. Int. Hormesis Soc. 2019, 17, 1559325819837795. [Google Scholar] [CrossRef]
- Iyer, M.K.; Niknafs, Y.S.; Rohit, M.; Udit, S.; Anirban, S.; Yasuyuki, H.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Shuang, Z. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef]
- Deniz, E.; Erman, B. Long noncoding RNA (lincRNA), a new paradigm in gene expression control. Funct. Integr. Genom. 2017, 17, 135–143. [Google Scholar] [CrossRef]
- Tiffany, H.; Yulei, W.; Lin, M.F.; Koegel, A.K.; Yojiro, K.; Grant, G.D.; Horlings, H.M.; Nilay, S.; Christopher, U.; Pei, W. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 2011, 43, 621. [Google Scholar]
- Zhang, L.; Zhou, Y.; Huang, T.; Cheng, A.S.; Yu, J.; Kang, W.; To, K.F. The Interplay of lncRNA-H19 and Its Binding Partners in Physiological Process and Gastric Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 450. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2015, 17, 47. [Google Scholar] [CrossRef]
- Luo, J.; Xu, L.; Jiang, Y.; Zhuo, D.; Zhang, S.; Wu, L.; Xu, H.; Huang, Y. Expression profile of long non-coding RNAs in colorectal cancer: A microarray analysis. Oncol. Rep. 2016, 35, 2035–2044. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhu, X.; Chen, Y.; Wei, H.; Chen, Q.; Chi, X.; Qi, B.; Zhang, L.; Zhao, Y.; Gao, G.F. NRAV, a Long Noncoding RNA, Modulates Antiviral Responses through Suppression of Interferon-Stimulated Gene Transcription. Cell Host Microbe 2014, 16, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.H.; Stephens, D.N.; Ho, H.; Chen, J.K.; Salmans, M.L.; Wang, W.; Yu, Z.; Andersen, B. Cofactors of LIM Domains Associate with Estrogen Receptor alpha to Regulate the Expression of Noncoding RNA H19 and Corneal Epithelial Progenitor Cell Function. J. Biol. Chem. 2016, 291, 13271. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Liu, C.; Cui, F.M.; Xu, J.Y.; Tong, J.; Qi, X.F.; Wang, L.L.; Zhu, W. Long intergenic non-coding RNA induced by X-ray irradiation regulates DNA damage response signaling in the human bronchial epithelial BEAS-2B cell line. Oncol. Lett. 2015, 9, 169–176. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; He, Q.; Hu, Z.; Feng, Y.; Fan, L.; Tang, Z.; Yuan, J.; Shan, W.; Li, C.; Hu, X. Long noncoding RNA LINP1 regulates repair of DNA double-strand breaks in triple-negative breast cancer. Nat. Struct. Mol. Biol. 2016, 23, 522. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, Q.; Liu, R.; Chen, Z.; Zhang, X.; Zhou, P.; Wang, Z. lncRNA lnc-RI regulates homologous recombination repair of DNA double-strand breaks by stabilizing RAD51 mRNA as a competitive endogenous RNA. Nucleic Acids Res. 2018, 46, 717. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Lal, A. Long noncoding RNAs in the p53 network. Wiley Interdiscip. Rev. RNA 2017, 8. [Google Scholar] [CrossRef]
- Lee, S.; Kopp, F.; Chang, T.C.; Sataluri, A.; Chen, B.; Sivakumar, S.; Yu, H.; Xie, Y.; Mendell, J.T. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell 2016, 164, 69–80. [Google Scholar] [CrossRef]
- Sharma, V.; Khurana, S.; Kubben, N.; Abdelmohsen, K.; Oberdoerffer, P.; Gorospe, M.; Misteli, T. A BRCA1-interacting lncRNA regulates homologous recombination. EMBO Rep. 2015, 16, 1520–1534. [Google Scholar] [CrossRef]
- Guo, X.; Yang, J.; Liang, B.; Shen, T.; Yan, Y.; Huang, S.; Zhou, J.; Huang, J.; Gu, L.; Su, L. Identification of Novel lncRNA Biomarkers and Construction of lncRNA-Related Networks in Han Chinese Patients with Ischemic Stroke. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 50, 2157–2175. [Google Scholar] [CrossRef]
- Shahrour, M.A.; Nicolae, C.M.; Edvardson, S.; Ashhab, M.; Galvan, A.M.; Constantin, D.; Abu-Libdeh, B.; Moldovan, G.L.; Elpeleg, O. PARP10 deficiency manifests by severe developmental delay and DNA repair defect. Neurogenetics 2016, 17, 227–232. [Google Scholar] [CrossRef]
- Hauer, M.H.; Seeber, A.; Singh, V.; Thierry, R.; Sack, R.; Amitai, A.; Kryzhanovska, M.; Eglinger, J.; Holcman, D.; Owenhughes, T. Histone degradation in response to DNA damage enhances chromatin dynamics and recombination rates. Nat. Struct. Mol. Biol. 2017, 24, 99. [Google Scholar] [CrossRef] [PubMed]
- Arjumand, W.; Asiaf, A.; Ahmad, S.T. Noncoding RNAs in DNA Damage Response: Opportunities for Cancer Therapeutics. Methods Mol. Biol. 2018, 1699, 3. [Google Scholar] [PubMed]
- Dianatpour, A.; Ghafouri-Fard, S. The Role of Long Non Coding RNAs in the Repair of DNA Double Strand Breaks. Int. J. Mol. Cell. Med. 2017, 6, 1–12. [Google Scholar] [PubMed]
- Nie, J.; Peng, C.; Pei, W.; Zhu, W.; Zhang, S.; Cao, H.; Qi, X.; Tong, J.; Jiao, Y. A novel role of long non-coding RNAs in response to X-ray irradiation. Toxicol. In Vitro Int. J. Publ. Assoc. Bibra 2015, 30, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Terradas, M.; Martín, M.; Repullès, J.; Huarte, M.; Genescà, A. Distinct Sets of lncRNAs are Differentially Modulated after Exposure to High and Low Doses of X rays. Radiat. Res. 2016, 186, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Guohui, W.; Xiaoxiao, H.; Yunhua, L.; Cecil, H.; Sood, A.K.; Calin, G.A.; Xinna, Z.; Xiongbin, L. A novel non-coding RNA lncRNA-JADE connects DNA damage signalling to histone H4 acetylation. EMBO J. 2014, 32, 2833–2847. [Google Scholar]
- Min-Xian, Q.; Ye, P.; Cui Hua, L.; Kousuke, H.; Bo-Yu, D.; Dan-Yang, J.; Guang-Fei, W.; Qian-Qian, Z.; Wei, S.; Yadong, Y. Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis. Cell 2013, 153, 1012–1024. [Google Scholar]
- Schwertman, P.; Bekkerjensen, S.; Mailand, N. Regulation of DNA double-strand break repair by ubiquitin and ubiquitin-like modifiers. Nat. Rev. Mol. Cell Biol. 2016, 17, 379–394. [Google Scholar] [CrossRef]
- Karimaian, A.; Majidinia, M.; Baghi, H.B.; Yousefi, B. The crosstalk between Wnt/β-catenin signaling pathway with DNA damage response and oxidative stress: Implications in cancer therapy. DNA Repair 2017, 51, 14–19. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Piyajit, W.; Bijur, G.N.; Zmijewski, J.W.; Ling, S.; Anna, Z.; Xinbin, C.; Johnson, G.V.W.; Jope, R.S. Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage. Proc. Natl. Acad. Sci. USA 2002, 99, 7951–7955. [Google Scholar]
- Tóth, C.; Sükösd, F.; Valicsek, E.; Herpel, E.; Schirmacher, P.; Tiszlavicz, L. Loss of CDX2 gene expression is associated with DNA repair proteins and is a crucial member of the Wnt signaling pathway in liver metastasis of colorectal cancer. Oncol. Lett. 2018, 15, 3586–3593. [Google Scholar] [PubMed]
- Smith, J.; Tho, L.M.; Xu, N.; Gillespie, D.A. Chapter 3–The ATM–Chk2 and ATR–Chk1 Pathways in DNA Damage Signaling and Cancer. Adv. Cancer Res. 2010, 108, 73–112. [Google Scholar] [PubMed]
- Yang, C.; Wu, D.; Gao, L.; Liu, X.; Jin, Y.; Wang, D.; Wang, T.; Li, X. Competing endogenous RNA networks in human cancer: Hypothesis, validation, and perspectives. Oncotarget 2016, 7, 13479–13490. [Google Scholar] [CrossRef]
- Xu, D.; Yu, J.; Gao, G.; Lu, G.; Zhang, Y.; Ma, P. lncRNA DANCR functions as a competing endogenous RNA to regulate RAB1A expression by sponging miR-634 in glioma. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Fang, C.; Qiu, S.; Sun, F.; Li, W.; Wang, Z.; Yue, B.; Wu, X.; Yan, D. Long non-coding RNA HNF1A-AS1 mediated repression of miR-34a/SIRT1/p53 feedback loop promotes the metastatic progression of colon cancer by functioning as a competing endogenous RNA. Cancer Lett. 2017, 410, 50–62. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, H.; Liu, S.; Hu, J.; Zhang, Y.; Jiao, T.; Liu, Y.; Ou, J.; Wang, D.; Yao, L.; et al. lncRNA-HOST2 regulates cell biological behaviors in epithelial ovarian cancer through a mechanism involving microRNA let-7b. Hum. Mol. Genet. 2015, 24, 841–852. [Google Scholar] [CrossRef]
- Choi, K.S.; Choi, H.J.; Lee, J.K.; Im, S.; Zhang, H.; Jeong, Y.; Park, J.A.; Lee, I.K.; Kim, Y.M.; Kwon, Y.G. The endothelial E3 ligase HECW2 promotes endothelial cell junctions by increasing AMOTL1 protein stability via K63-linked ubiquitination. Cell. Signal. 2016, 28, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.E.; Boardman, A.P.; Wang, D.C.; Huttlin, E.L.; Everley, R.A.; Dephoure, N.; Zhou, C.; Koren, I.; Gygi, S.P.; Elledge, S.J. Quantitative Proteomic Atlas of Ubiquitination and Acetylation in the DNA Damage Response. Mol. Cell 2015, 59, 867–881. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Daehwan, K.; Ben, L.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar]


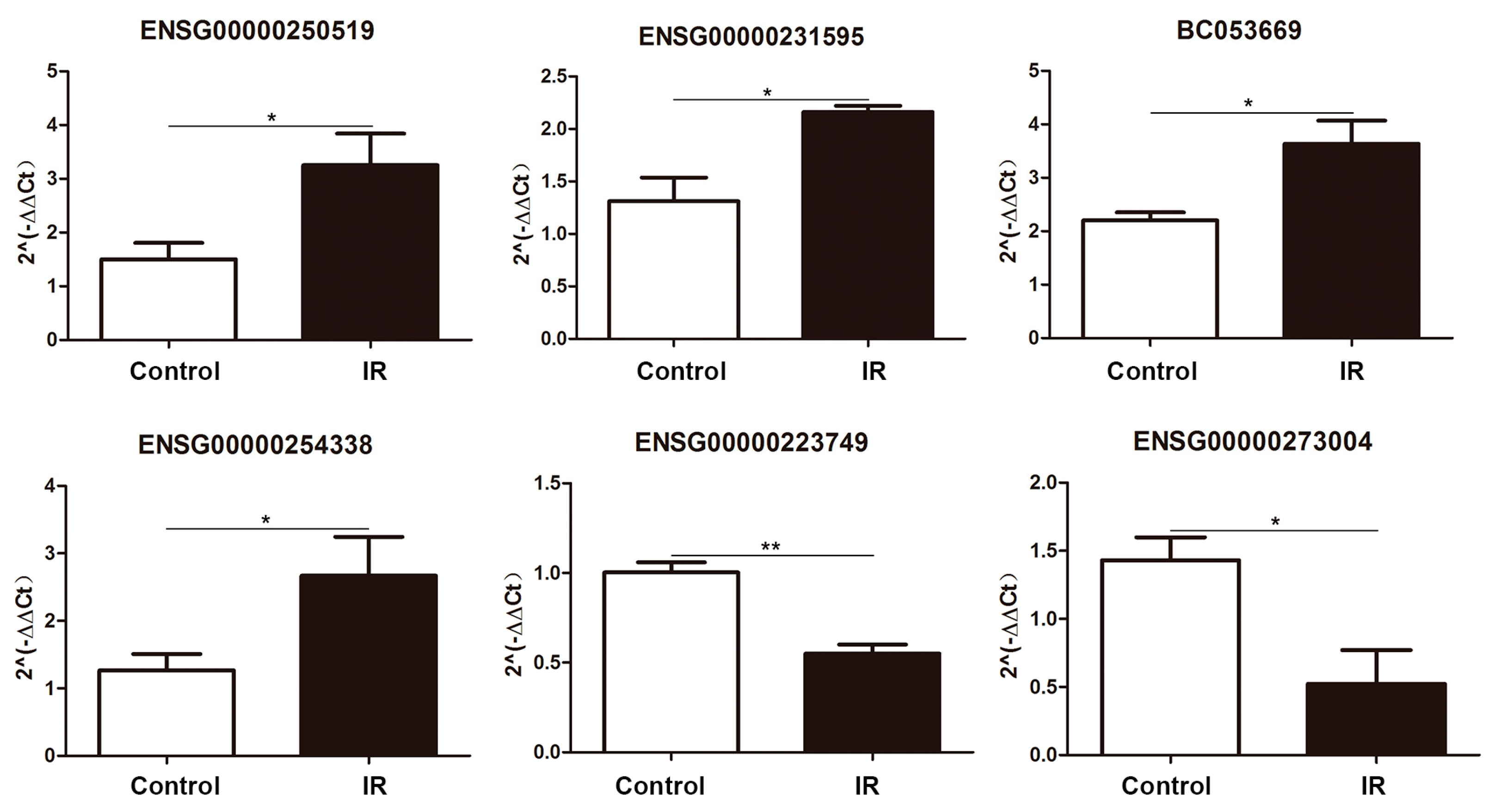
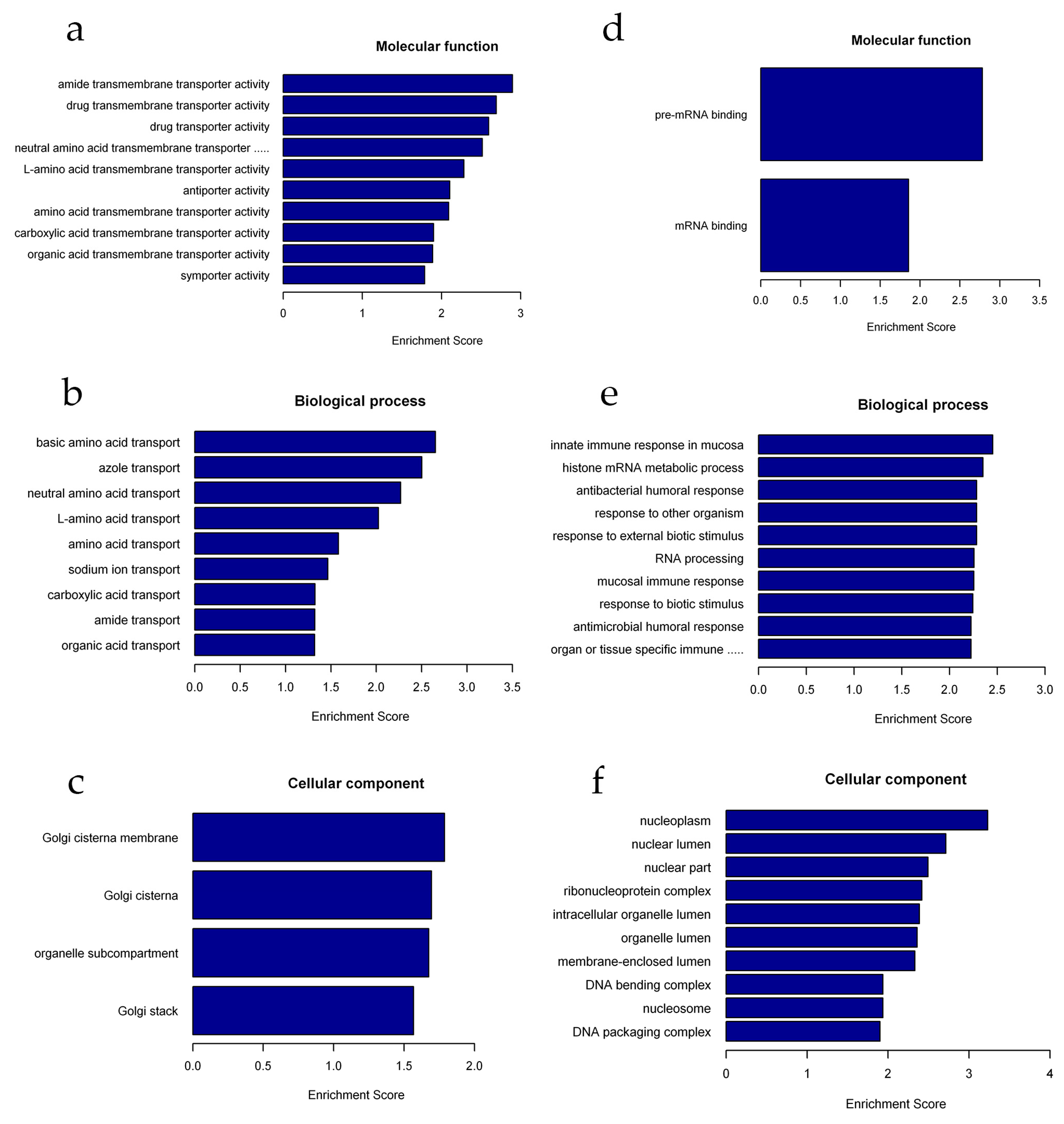
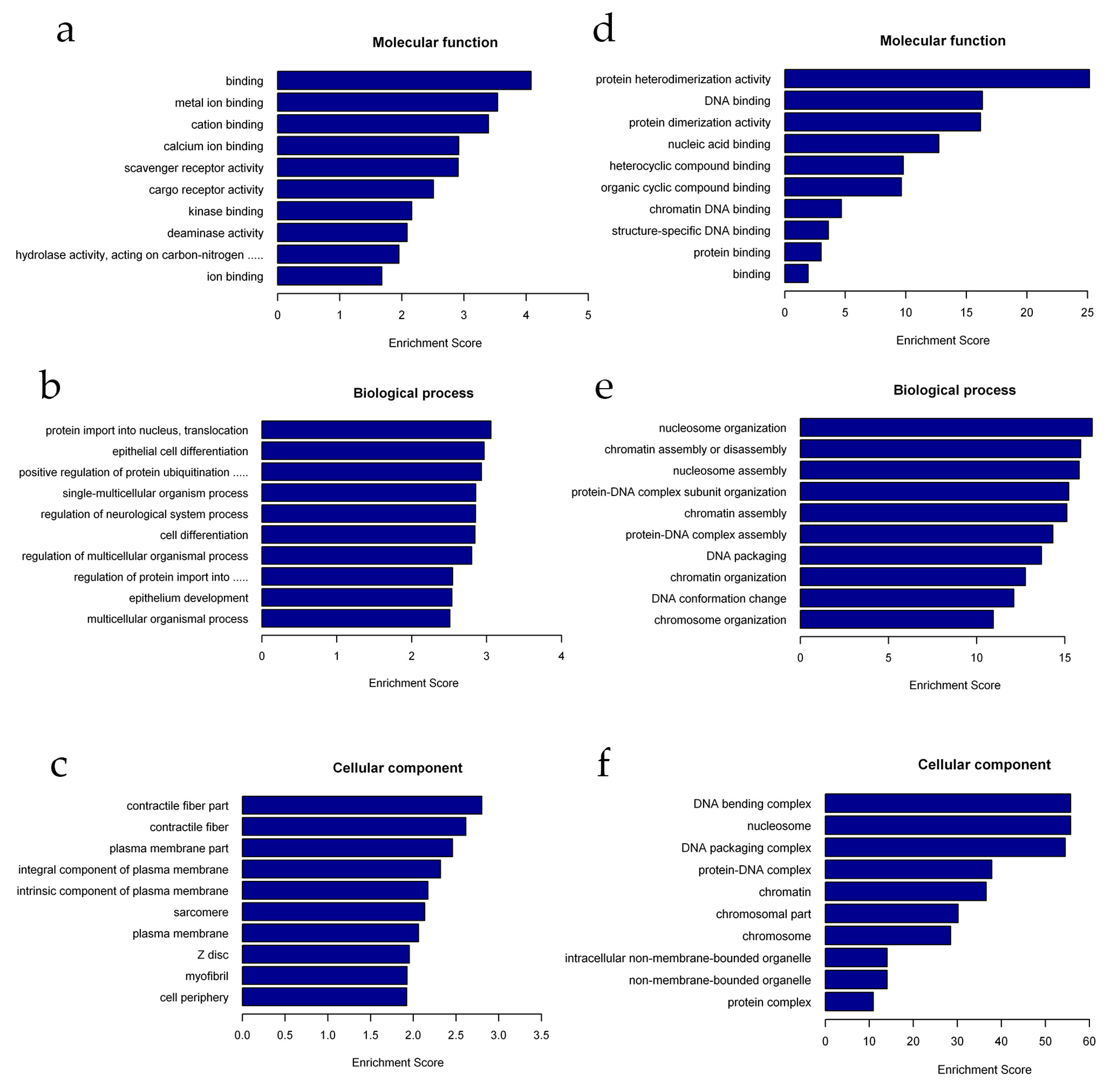


| Gene Name | Primer Sequence (5′–3′) | Amplicon (bp) |
|---|---|---|
| ENSG00000250519 | F: CAATCAGCGAGACTCCGTGG R: CTGTGCTTTCTGGGCTTCTTT | 259 |
| ENSG00000231595 | F: GATTACAGGCACCTACAACAGAAG R: CTTTGGCAGTTTGCTTCACGA | 219 |
| BC053669 | F: GGAATGACACTGCCCGAAC R: TCTTTCAACCTTTCCCTCCAC | 87 |
| ENSG00000254338 | F: GGATGTGATGGTTGGAGTGGA R: CGCTCTGGATTGAGGCTCTT | 89 |
| ENSG00000223749 | F: CAGACAAGAACTAAAGTGGAACCC R: AACGGAAATCAAAAGCAGCA | 97 |
| ENSG00000273004 | F: GTGGAGAGTGTGAGGAAGAAGAGA R: GTGAAAAGTGGAAGTAAACTGGTGT | 173 |
| GAPDH | F: CTCTGATTTGGTCGTATTGGG R: TGGAAGATGGTGATGGGATT | 168 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Sun, Y.; Xiao, C.; Ji, K.; Zhang, M.; He, N.; Wang, J.; Wang, Q.; Sun, Z.; Wang, Y.; et al. Integrated Analysis of the Altered lncRNAs and mRNAs Expression in 293T Cells after Ionizing Radiation Exposure. Int. J. Mol. Sci. 2019, 20, 2968. https://doi.org/10.3390/ijms20122968
Yang M, Sun Y, Xiao C, Ji K, Zhang M, He N, Wang J, Wang Q, Sun Z, Wang Y, et al. Integrated Analysis of the Altered lncRNAs and mRNAs Expression in 293T Cells after Ionizing Radiation Exposure. International Journal of Molecular Sciences. 2019; 20(12):2968. https://doi.org/10.3390/ijms20122968
Chicago/Turabian StyleYang, Mengmeng, Yuxiao Sun, Changyan Xiao, Kaihua Ji, Manman Zhang, Ningning He, Jinhan Wang, Qin Wang, Zhijuan Sun, Yan Wang, and et al. 2019. "Integrated Analysis of the Altered lncRNAs and mRNAs Expression in 293T Cells after Ionizing Radiation Exposure" International Journal of Molecular Sciences 20, no. 12: 2968. https://doi.org/10.3390/ijms20122968
APA StyleYang, M., Sun, Y., Xiao, C., Ji, K., Zhang, M., He, N., Wang, J., Wang, Q., Sun, Z., Wang, Y., Du, L., Liu, Y., Xu, C., & Liu, Q. (2019). Integrated Analysis of the Altered lncRNAs and mRNAs Expression in 293T Cells after Ionizing Radiation Exposure. International Journal of Molecular Sciences, 20(12), 2968. https://doi.org/10.3390/ijms20122968







