Salicylic Acid Signals Plant Defence against Cadmium Toxicity
Abstract
1. Introduction
2. Salicylic Acid (SA) Treatment Methods
2.1. SA Spray
2.2. Presoaking of Seed with SA
2.3. Hydroponic Application
2.4. SA Mutants
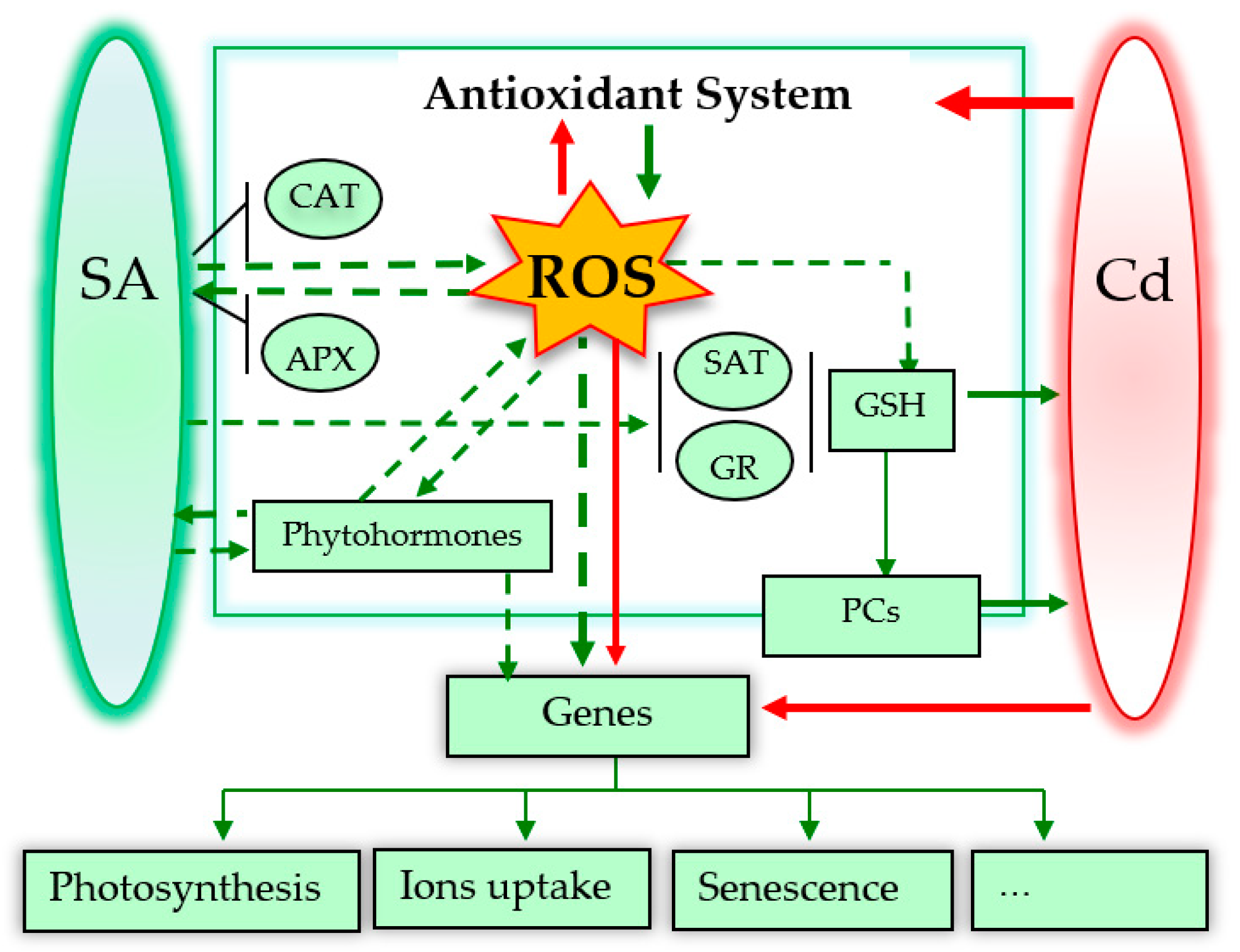
3. Possible Roles of SA in Alleviating Cadmium (Cd) Toxicity
3.1. Plant Growth
3.2. Cd Immobilization in the Cell Wall
3.3. Cd Uptake and Translocation
3.4. Element Uptake
3.5. Photosynthesis
3.6. Reactive Oxygen Species (ROS) and Antioxidant Defence System
3.7. Glutathione and Chelation
3.8. Senescence
4. Future Insights and Conclusions
4.1. SA Homeostasis
4.2. SA-Related Gene Expression
4.2.1. Nonexpressor of Pathogenesis-Related (NPR) Protein
4.2.2. Mitogen-Activated Protein Kinase (MAPK)
4.2.3. ATP-Binding Cassette (ABC) Transporters
4.3. Crosstalk with Other Phytohormones
Funding
Conflicts of Interest
Abbreviations
| AsA | ascorbic acid |
| ABA | abscisic acid |
| ABC transporters | ATP-binding cassette transporters |
| APX | ascorbate peroxidase; |
| Cd | cadmium; |
| CAT | catalase; |
| GR | glutathione reductase |
| GSH | glutathione |
| GSHS | glutathione synthetase |
| HO−1 | haem oxygenase−1 |
| MAPK | mitogen-activated protein kinase |
| NPR1 | Nonexpressor of PR1 |
| NPT | non-protein thiols |
| PAL | phenylalanine ammonia-lyase |
| PCs | phytochelatins |
| POD | peroxidase |
| PPO | polyphenol oxidase |
| PSII | photosystem II |
| ROS | reactive oxygen species |
| SA | salicylic acid |
| SAT | serine acetyltransferase |
| SOD | superoxide dismutase |
References
- Vig, K.; Megharaj, M.; Sethunathan, N.; Naidu, R. Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: A review. Adv. Environ. Res. 2003, 8, 121–135. [Google Scholar] [CrossRef]
- Gong, B.; Nie, W.; Yan, Y.; Gao, Z.; Shi, Q. Unravelling cadmium toxicity and nitric oxide induced tolerance in Cucumis sativus: Insight into regulatory mechanisms using proteomics. J. Hazard. Mater. 2017, 336, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Meng, A.; Li, Q.; Cheng, H. Experimental and thermodynamic investigation on transfer of cadmium influenced by sulfur and chlorine during municipal solid waste (MSW) incineration. J. Hazard. Mater. 2008, 153, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ma, Y.; Zhang, S.; Wei, D.; Zhu, Y. An inventory of trace element inputs to agricultural soils in China. J. Environ. Manag. 2009, 90, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.L. Cadmium and phosphorous fertilizers: The issues and the science. Procedia Eng. 2014, 83, 52–59. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, H.; He, J.; Lyu, D.; Li, H. Integration of cadmium accumulation, subcellular distribution, and physiological responses to understand cadmium tolerance in apple rootstocks. Front. Plant Sci. 2017, 8, 966. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Aarts, M.G.M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- Daud, M.K.; Sun, Y.; Dawood, M.; Hayat, Y.; Variath, M.T.; Wu, Y.X.; Mishkat, U.; Najeeb, U.; Zhu, S. Cadmium-induced functional and ultrastructural alterations in roots of two transgenic cotton cultivars. J. Hazard. Mater. 2009, 161, 463–473. [Google Scholar] [CrossRef]
- Song, Y.; Jin, L.; Wang, X. Cadmium absorption and transportation pathways in plants. Int. J. Phytoremediat. 2017, 19, 133–141. [Google Scholar] [CrossRef]
- Lysenko, E.A.; Klaus, A.A.; Pshybytko, N.L.; Kusnetsov, V.V. Cadmium accumulation in chloroplasts and its impact on chloroplastic processes in barley and maize. Photosynth. Res. 2015, 125, 291–303. [Google Scholar] [CrossRef]
- Hasan, S.A.; Hayat, S.; Ahmad, A. Screening of tomato (Lycopersicon esculentum) cultivars against cadmium through shotgun approach. J. Plant Interact. 2009, 3, 187–201. [Google Scholar] [CrossRef]
- Bertoli, A.C.; Cannata, M.G.; Carvalho, R.; Bastos, A.R.R.; Freitas, M.P.; Dos Santos Augusto, A. Lycopersicon esculentum submitted to Cd-stressful conditions in nutrition solution: Nutrient contents and translocation. Ecotoxicol. Environ. Saf. 2012, 86, 176–181. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A.K. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef]
- Wójcik, M.; D’Haen, V.J.; Tukiendorf, A. Cadmium tolerance in Thlaspi caerulescens II. localization of cadmium in Thlaspi caerulescens. Environ. Exp. Bot. 2005, 53, 163–171. [Google Scholar] [CrossRef]
- Seth, C.S.; Remans, T.; Keunen, E.; Jozefczak, M.; Gielen, H.; Opdenakker, K.; Weyens, N.; Vangronsveld, J.; Cuypers, A. Phytoextraction of toxic metals: A central role for glutathione. Plant Cell Environ. 2012, 35, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Schützendübel, A.; Schwanz, P.; Teichmann, T.; Gross, K.; Langenfeld-Heyser, R.; Godbold, D.L.; Polle, A. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in scots pine roots. Plant Physiol. 2001, 127, 887–898. [Google Scholar] [CrossRef]
- Raskin, I. Role of salicylic acid in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Abreu, M.E.; Munné-Bosch, S. Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 1261–1271. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defense. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Garcion, C.; Lohmann, A.; Lamodiere, E.; Catinot, J.; Buchala, A.; Doermann, P.; Metraux, J.P. Characterization and biological function of the ISOCHORISMATE SYNTHASE2 gene of Arabidopsis. Plant Physiol. 2008, 147, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.A.; Shah, J.; Klessig, D.F. Salicylic acid and disease resistance in plants. Crit. Rev. Plant Sci. 1997, 18, 547–575. [Google Scholar] [CrossRef]
- Kumar, D. Salicylic acid signaling in disease resistance. Plant Sci. 2014, 228, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Kovacs, V.; Szalai, G.; Soos, V.; Ma, X.; Liu, H.; Mei, H.; Janda, T. Salicylic acid and abiotic stress responses in rice. J. Agron. Crop Sci. 2014, 200, 1–11. [Google Scholar] [CrossRef]
- Chini, A.; Grant, J.J.; Seki, M.; Shinozaki, K.; Loake, G.J. Drought tolerance established by enhanced expression of the CC–NBS–LRR gene, ADR1, requires salicylic acid, EDS1 and ABI1. Plant J. 2004, 38, 810–822. [Google Scholar] [CrossRef]
- Janda, T.; Szalai, G.; Tari, I.; Páldi, E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 1999, 208, 175–180. [Google Scholar] [CrossRef]
- Szalai, G.; Janda, T. Effect of salt stress on the salicylic acid synthesis in young maize (Zea mays L.) Plants. J. Agron. Crop Sci. 2009, 195, 165–171. [Google Scholar] [CrossRef]
- Dat, J.F.; Foyer, C.H.; Scott, I.M. Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol. 1998, 118, 1455–1461. [Google Scholar] [CrossRef]
- Horváth, E.; Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Liu, C.F.; Guo, J.L.; Cui, Y.L.; Lü, T.F.; Zhang, X.H.; Shi, G.R. Effects of cadmium and salicylic acid on growth, spectral reflectance and photosynthesis of castor bean seedlings. Plant Soil 2011, 344, 131–141. [Google Scholar] [CrossRef]
- Li, Q.; Wang, G.; Wang, Y.; Yang, D.; Guan, C.; Ji, J. Foliar application of salicylic acid alleviate the cadmium toxicity by modulation the reactive oxygen species in potato. Ecotoxicol. Environ. Saf. 2019, 172, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Jaleel, H.; Sadiq, Y.; Khan, M.M.A.; Shabbir, A. Response of exogenous salicylic acid on cadmium induced photosynthetic damage, antioxidant metabolism and essential oil production in peppermint. Plant Growth Regul. 2018, 86, 273–286. [Google Scholar] [CrossRef]
- Ali, E.; Maodzeka, A.; Hussain, N.; Shamsi, I.H.; Jiang, L. The alleviation of cadmium toxicity in oilseed rape (Brassica napus) by the application of salicylic acid. Plant Growth Regul. 2015, 75, 641–655. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Yang, S.; Chen, Y. Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two melon cultivars (Cucumis melo, L.). Protoplasma 2015, 252, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Ma, L.J.; Bu, N.; Li, Y.Y.; Zhang, L.H. Effects of salicylic acid pre-treatment on cadmium and/or UV-B stress in soybean seedlings. Biol. Plant. 2014, 58, 195–199. [Google Scholar] [CrossRef]
- Raza, S.H.; Shafiq, F. Exploring the role of salicylic acid to attenuate cadmium accumulation in radish (Raphanus sativus). Int. J. Agric. Biol. 2013, 15, 547–552. [Google Scholar]
- Ahmad, P.; Nabi, G.; Ashraf, M. Cadmium-induced oxidative damage in mustard (Brassica juncea (L.) czern. & coss.) plants can be alleviated by salicylic acid. S. Afr. J. Bot. 2011, 77, 36–44. [Google Scholar]
- Roychoudhury, A.; Ghosh, S.; Paul, S.; Mazumdar, S.; Das, G.; Das, S. Pre-treatment of seeds with salicylic acid attenuates cadmium chloride-induced oxidative damages in the seedlings of mungbean (Vigna radiata, L. wilczek). Acta Physiol. Plant. 2016, 38, 11. [Google Scholar] [CrossRef]
- Belkadhi, A.; De Haro, A.; Obregon, S.; Chaïbi, W.; Djebali, W. Positive effects of salicylic acid pretreatment on the composition of flax plastidial membrane lipids under cadmium stress. Environ. Sci. Pollut. Res. 2015, 22, 1457–1467. [Google Scholar] [CrossRef]
- Belkadhi, A.; De Haro, A.; Soengas, P.; Obregόn, S.; Cartea, M.E.; Chaïbi, W.; Djebali, W. Salicylic acid increases tolerance to oxidative stress induced by hydrogen peroxide accumulation in leaves of cadmium-exposed flax (Linum usitatissimum L.). J. Plant Interact. 2014, 9, 647–654. [Google Scholar] [CrossRef]
- Guo, Q.; Meng, L.; Mao, P.C.; Jia, Y.Q.; Shi, Y.J. Role of exogenous salicylic acid in alleviating cadmium-induced toxicity in Kentucky bluegrass. Biochem. Syst. Ecol. 2013, 50, 269–276. [Google Scholar] [CrossRef]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Szalai, G.; Krantev, A.; Yordanova, R.; Popova, L.P.; Janda, T. Influence of salicylic acid on phytochelatin synthesis in Zea mays during Cd stress. Turk. J. Bot. 2013, 37, 708–714. [Google Scholar]
- Belkadhi, A.; De Haro, A.; Soengas, P.; Obregόn, S.; Cartea, M.E.; Djebali, W.; Chaïbi, W. Salicylic acid improves root antioxidant defense system and total antioxidant capacities of flax subjected to cadmium. OMICS J. Integr. Biol. 2013, 17, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Belkadhi, A.; Hediji, H.; Abbes, Z.; Djebali, W.; Chaibi, W. Influence of salicylic acid pre-treatment on cadmium tolerance and its relationship with non-protein thiol production in flax root. Afr. J. Biotechnol. 2012, 11, 9788–9796. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Xia, Y.; Wang, G.; Xu, L.; Shen, Z. Exogenous application of salicylic acid alleviates cadmium toxicity and reduces hydrogen peroxide accumulation in root apoplasts of Phaseolus aureus and Vicia sativa. Plant Cell Rep. 2011, 30, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Belkhadi, A.; Hediji, H.; Abbes, Z.; Nouairi, I.; Barhoumi, Z.; Zarrouk, M.; Chaïbi, W.; Djebali, W. Effects of exogenous salicylic acid pre-treatment on cadmium toxicity and leaf lipid content in Linum usitatissimum, L. Ecotoxicol. Environ. Saf. 2010, 73, 1004–1011. [Google Scholar] [CrossRef]
- Moussa, H.R.; Elgamal, S.M. Effect of salicylic acid pretreatment on cadmium toxicity in wheat. Biol. Plant. 2010, 54, 315–320. [Google Scholar] [CrossRef]
- Dražić, G.; Mihailovic, N. Salicylic acid modulates accumulation of Cd in seedlings of Cd-tolerant and Cd-susceptible soybean genotypes. Arch. Biol. Sci. 2009, 61, 431–439. [Google Scholar] [CrossRef]
- Shi, G.R.; Cai, Q.S.; Liu, Q.Q.; Wu, L. Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. Acta Physiol. Plant. 2009, 31, 969–977. [Google Scholar] [CrossRef]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Patra, H.K. Effect of salicylic acid potentiates cadmium-induced oxidative damage in oryza sativa, l. leaves. Acta Physiol. Plant. 2007, 29, 567–575. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, S.K. Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa L. roots. Bulg. J. Plant Physiol. 2004, 30, 95–110. [Google Scholar]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Yotsova, E.K.; Dobrikova, A.G.; Stefanov, M.A.; Kouzmanova, M.; Apostolova, E.L. Improvement of the rice photosynthetic apparatus defence under cadmium stress modulated by salicylic acid supply to roots. Theor. Exp. Plant Phys. 2018, 30, 57–70. [Google Scholar] [CrossRef]
- Gu, C.S.; Yang, Y.H.; Shao, Y.F.; Wu, K.W.; Liu, Z.L. The effects of exogenous salicylic acid on alleviating cadmium toxicity in nymphaea tetragona georgi. S. Afr. J. Bot. 2018, 114, 267–271. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, T.; Zhang, W.; Su, C.; Yang, Y.; Hu, D.; Xu, Q. Alleviation of cadmium toxicity in Lemna minor by exogenous salicylic acid. Ecotoxicol. Environ. Saf. 2018, 147, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Gondor, O.K.; Pál, M.; Darkó, É.; Janda, T.; Szalai, G. Salicylic acid and sodium salicylate alleviate cadmium toxicity to different extents in maize (Zea mays L.). PLoS ONE 2016, 11, e0160157. [Google Scholar] [CrossRef]
- Singh, I.; Shah, K. Evidences for suppression of cadmium induced oxidative stress in presence of sulphosalicylic acid in rice seedlings. Plant Growth Regul. 2015, 76, 99–110. [Google Scholar] [CrossRef]
- Xu, L.L.; Fan, Z.Y.; Dong, Y.J.; Kong, J.; Bai, X.Y. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of two peanut cultivars under cadmium stress. Biol. Plant. 2015, 59, 171–182. [Google Scholar] [CrossRef]
- Tamás, L.; Mistrík, I.; Alemayehu, A.; Zelinová, V.; Bočová, B.; Huttová, J. Salicylic acid alleviates cadmium-induced stress responses through the inhibition of Cd-induced auxin-mediated reactive oxygen species production in barley root tips. J. Plant Physiol. 2015, 173, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liang, X.; Dong, Y.; Xu, L.; Zhang, X.; Kong, J.; Liu, S. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J. Plant Growth Regul. 2013, 32, 721–731. [Google Scholar] [CrossRef]
- Saidi, I.; Ayouni, M.; Dhieb, A.; Chtourou, Y.; Chaïbi, W.; Djebali, W. Oxidative damages induced by short-term exposure to cadmium in bean plants: Protective role of salicylic acid. S. Afr. J. Bot. 2013, 85, 32–38. [Google Scholar] [CrossRef]
- Noriega, G.; Caggiano, E.; Lecube, M.L.; Santa-Cruz, D.; Batlle, A.; Tomaro, M.; Balestrasse, K.B. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals 2012, 25, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Le, L.; Gao, Z.; Wu, H.; Xie, Y.; Shen, W. Haem oxygenase-1 is involved in salicylic acid-induced alleviation of oxidative stress due to cadmium stress in Medicago sativa. J. Exp. Bot. 2012, 63, 5521–5534. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.Y.; Chen, C.Y.; Huang, W.D.; Chinghuei, K. Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil 2010, 329, 327–337. [Google Scholar] [CrossRef]
- Guo, B.; Liang, Y.; Zhu, Y. Does salicylic acid regulate antioxidant defense system, cell death, cadmium uptake and partitioning to acquire cadmium tolerance in rice? J. Plant Physiol. 2009, 166, 20–31. [Google Scholar] [CrossRef]
- Guo, B.; Liang, Y.; Zhu, Y.; Zhao, F. Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ. Pollut. 2007, 147, 743–749. [Google Scholar] [CrossRef]
- Bai, X.; Dong, Y.; Kong, J.; Xu, L.; Liu, S. Effects of application of salicylic acid alleviates cadmium toxicity in perennial ryegrass. Plant Growth Regul. 2015, 75, 695–706. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Horváth, E.; Janda, T.; Páldi, E. Effect of salicylic acid during heavy metal stress. Acta Biol. Szeged. 2002, 46, 119–120. [Google Scholar]
- Wang, Y.Y.; Wang, Y.; Li, G.Z.; Hao, L. Salicylic acid-altering Arabidopsis plant response to cadmium exposure: Underlying mechanisms affecting antioxidation and photosynthesis-related processes. Ecotoxicol. Environ. Saf. 2019, 169, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, C.; Li, H.; Yi, K.; Ding, N.; Li, N.; Lin, Y.; Fu, Q. Endogenous salicylic acid is required for promoting cadmium tolerance of Arabidopsis by modulating glutathione metabolisms. J. Hazard. Mater. 2016, 316, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Ji, J.; Jia, C.; Guan, W.; Li, X.; Jin, C.; Wang, G. A GSHS-like gene from Lycium chinense maybe regulated by cadmium induced endogenous salicylic acid and overexpression of this gene enhances tolerance to cadmium stress in Arabidopsis. Plant Cell Rep. 2015, 34, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Sun, L.; Ma, C.; Li, L.; Li, G.; Hao, L. Reducing basal salicylic acid enhances Arabidopsis tolerance to lead or cadmium. Plant Soil 2013, 372, 309–318. [Google Scholar] [CrossRef]
- Zawoznik, M.S.; Groppa, M.D.; Tomaro, M.L.; Benavides, M.P. Endogenous salicylic acid potentiates cadmium-induced oxidative stress in Arabidopsis thaliana. Plant Sci. 2007, 173, 190–197. [Google Scholar] [CrossRef]
- Guo, B. Role of salicylic acid in mitigating cadmium toxicity in plants. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 14; pp. 349–374. [Google Scholar]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol. 1997, 115, 137–149. [Google Scholar] [CrossRef]
- Sanità di Toppi, L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Souza, V.L.; Almeida, A.A.F.D.; Lima, S.G.C.; Cascardo, J.; Silva, D.D.C.; Mangabeira, P.A.O.; Gomes, F.P. Morphophysiological responses and programmed cell death induced by cadmium in Genipa Americana L. (Rubiaceae). Biometals 2011, 24, 59–71. [Google Scholar] [CrossRef]
- Qian, H.; Li, J.; Pan, X.; Jiang, H.; Sun, L.; Fu, Z. Photoperiod and temperature influence cadmium’s effects on photosynthesis-related gene transcription in Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2010, 73, 1202–1206. [Google Scholar] [CrossRef]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavaddeur, A.; Forestier, C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002, 32, 539–548. [Google Scholar] [CrossRef]
- Boussama, N.; Ouariti, O.; Suzuki, A.; Ghorbal, M.H. Cd-stress on nitrogen assimilation. J. Plant Physiol. 1998, 155, 310–317. [Google Scholar] [CrossRef]
- Iannone, M.F.; Rosales, E.P.; Groppa, M.D.; Benavides, M.P. Reactive oxygen species formation and cell death in catalase-deficient tobacco leaf disks exposed to cadmium. Protoplasma 2010, 245, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.S.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.; MacKerness, S.A.; Page, T.; John, C.F.; Murphy, A.M.; Carr, J.P.; Buchanan-Wollaston, V. Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J. 2000, 23, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Bowling, S.A.; Clarke, J.D.; Liu, Y.; Klessig, D.F.; Dong, X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 1997, 9, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Rate, D.N.; Cuenca, J.V.; Bowman, G.R.; Guttman, D.S.; Greenberg, J.T. The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses and cell growth. Plant Cell 1999, 11, 1695–1708. [Google Scholar] [CrossRef]
- Rate, D.N.; Greenberg, J.T. The Arabidopsis aberrant growth and death2 mutant shows resistance to Pseudomonas syringae and reveals a role for NPR1 in suppressing hypersensitive cell death. Plant J. 2001, 27, 203–211. [Google Scholar] [CrossRef]
- Šašek, V.; Janda, M.; Delage, E.; Puyaubert, J.; Guivarc’h, A.; López Maseda, E.; Dobrev, P.I.; Caius, J.; Bóka, K.; Valentová, O.; et al. Constitutive salicylic acid accumulation in pi4kIIIβ1β2 Arabidopsis plants stunts rosette but not root growth. New Phytol. 2014, 203, 805–816. [Google Scholar] [CrossRef]
- Janda, M.; Ruelland, E. Magical mystery tour: Salicylic acid signalling. Environ. Exp. Bot. 2015, 114, 117–128. [Google Scholar] [CrossRef]
- Drazic, G.; Mihailovic, N. Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Sci. 2005, 168, 511–517. [Google Scholar] [CrossRef]
- Zhu, X.F.; Lei, G.J.; Jiang, T.; Liu, Y.; Li, G.X.; Zheng, S.J. Cell wall polysaccharides are involved in P-deficiency-induced Cd exclusion in Arabidopsis thaliana. Planta 2012, 236, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tao, Q.; Shohag, M.J.I.; Yang, X.; Sparks, D.L.; Liang, Y. Root cell wall polysaccharides are involved in cadmium hyperaccumulation in Sedum alfredii. Plant Soil 2015, 389, 387–399. [Google Scholar] [CrossRef]
- Parrotta, L.; Guerriero, G.; Sergeant, K.; Cai, G.; Hausman, J.F. Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: Differences in the response mechanisms. Front. Plant Sci. 2015, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.T.; Stein, M.; Hou, B.H.; Vogel, J.P.; Edwards, H.; Somerville, S.C. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 2003, 301, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Napoleão, T.A.; Soares, G.; Vital, C.E.; Bastos, C.; Castro, R.; Loureiro, M.E.; Giordano, A. Methyl jasmonate and salicylic acid are able to modify cell wall but only salicylic acid alters biomass digestibility in the model grass Brachypodium distachyon. Plant Sci. 2017, 263, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liang, Y.C.; Li, Z.J.; Guo, W. Role of salicylic acid in alleviating cadmium toxicity in rice roots. J. Plant Nutr. 2007, 30, 427–439. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Allagulova, C.R.; Maslennikova, D.R.; Klyuchnikova, E.O.; Avalbaev, A.M.; Bezrukova, M.V. Salicylic acid-induced protection against cadmium toxicity in wheat plants. Environ. Exp. Bot. 2016, 122, 19–28. [Google Scholar] [CrossRef]
- Cohen, C.K.; Fox, T.C.; Garvin, D.F.; Kochian, L.V. The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiol. 1998, 116, 1063–1072. [Google Scholar] [CrossRef]
- Clemens, S.; Antosiewicz, D.M.; Ward, J.M.; Schachtman, D.P.; Schroeder, J.I. The plant cdna LCT1 mediates the uptake of calciumand cadmium in yeast. Proc. Natl. Acad. Sci. USA 1998, 95, 12043–12048. [Google Scholar] [CrossRef]
- Fatima, R.N.; Javed, F.; Wahid, A. Salicylic acid modifies growth performance and nutrient status of rice (Oryza sativa) under cadmium stress. Int. J. Agric. Biol. 2014, 16, 1083–1090. [Google Scholar]
- Gordon, L.K.; Minibayeva, F.V.; Rakhmatullina, D.F.; Alyabyev, A.J.; Ogorodnikova, T.I.; Loseva, N.L.; Valitova, Y.N. Heat production of wheat roots induced by the disruption of proton gradient by salicylic acid. Thermochim. Acta 2004, 422, 101–104. [Google Scholar] [CrossRef]
- Harper, J.R.; Balke, N.E. Characterization of the inhibition of k absorption in oat roots by salicylic acid. Plant Physiol. 1981, 68, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Garnier, L.; Simon-Plas, F.; Thuleau, P.; Agnel, J.P.; Blein, J.P.; Ranjeva, R.; Montillet, J.L. Cd affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell Environ. 2006, 29, 1956–1969. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.M.; Chien, P.S.; Huang, J. Distinct signaling pathways for induction of MAP kinase activities by cadmium and copper in rice roots. J. Exp. Bot. 2007, 58, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Deckert, J.; Rucińska-Sobkowiak, R.; Gzyl, J.; Pawlak-Sprada, S.; Abramowski, D.; Jelonek, T.; Gwóźdź, E.A. Nitric oxide implication in cadmium-induced programmed cell death in roots and signaling response of yellowem lupine plants. Plant Physiol. Biochem. 2012, 58, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Klessig, D.F. Salicylic acid is a modulator of tobacco and mammalian catalases. J. Biol. Chem. 1996, 271, 28492–28501. [Google Scholar] [CrossRef]
- Norman, C.; Howell, K.A.; Millar, A.H.; Whelan, J.M.; Day, D.A. Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiol. 2004, 134, 492–501. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Popova, L.P.; Maslenkova, L.T.; Yordanova, R.Y.; Ivanova, A.P.; Krantev, A.P.; Szalai, G.; Janda, T. Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol. Biochem. 2009, 47, 224–231. [Google Scholar] [CrossRef]
- Szalai, G.; Kellős, T.; Galiba, G.; Kocsy, G. Glutathione as an antioxidant and regulatory molecule in plants subjected to abiotic stresses. J. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Chaouch, S.; Queval, G.; Vanderauwera, S.; Mhamdi, A.; Vandorpe, M.; Langlois-Meurinne, M.; Van Breusegem, F.; Saindrenan, P.; Noctor, G. Peroxisomal hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE SYNTHASE 1 in a daylength-related manner. Plant Physiol. 2010, 153, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, S.; Queval, G.; Noctor, G. AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J. 2012, 69, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Mateo, A.; Funck, D.; Muhlenbock, P.; Kular, B.; Mullineaux, P.M.; Karpinski, S. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J. Exp. Bot. 2006, 57, 1795–1807. [Google Scholar] [CrossRef]
- Yi, H.; Sejir, C.; Amna, M.; Guillaume, Q.; Bernd, Z.; Graham, N. Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid. Redox Signal. 2012, 18, 2106–2121. [Google Scholar]
- Freeman, J.L.; Garcia, D.; Kim, D.; Hopf, A.; Salt, D.E. Constitutively elevated salicylic acid signals glutathione-mediated nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Physiol. 2005, 137, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Hager, J.; Chaouch, S.; Queval, G.; Yi, H.; Taconnat, L.; Saindrenan, P.; Gouia, H.; Issakidis-Bourguet, E.; Renou, J.P.; et al. Arabidopsis glutathione reductase1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol. 2010, 153, 1144–1160. [Google Scholar] [CrossRef] [PubMed]
- Michele, R.D.; Vurro, E.; Rigo, C.; Costa, A.; Elviri, L.; Valentin, M.D.; Careri, M.; Zottini, M.; Sanità di Toppi, L.; Lo Schiavo, F. Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol. 2009, 150, 217–228. [Google Scholar] [CrossRef]
- Kovács, V.; Gondor, O.K.; Szalai, G.; Darkó, É.; Majláth, I.; Janda, T.; Pál, M. Synthesis and role of salicylic acid in wheat varieties with different levels of cadmium tolerance. J. Hazard. Mater. 2014, 280, 12–19. [Google Scholar] [CrossRef]
- Pál, M.; Horváth, E.; Janda, T.; Páldi, E.; Szalai, G. Cadmium stimulates the accumulation of salicylic acid and its putative precursors in maize (Zea mays L.) plants. Physiol. Plant. 2005, 125, 356–364. [Google Scholar] [CrossRef]
- Dean, J.V.; Mohammed, L.A.; Fitzpatrick, T. The formation, vacuolar localization, and tonoplast transport of salicylic acid glucose conjugates in tobacco cell suspension cultures. Planta 2005, 221, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Vanacker, H.; Lu, H.; Rate, D.N.; Greenberg, J.T. A role for salicylic acid and npr1 in regulating cell growth in Arabidopsis. Plant J. 2001, 28, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Mohan, R.; Spoel, S.H.; Tada, Y.; Zheng, N.; et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 2012, 486, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite roles of salicylic acid receptors NPR1 and NPR 3/ NPR 4 in transcriptional regulation of plant immunity. Cell 2018, 173, 1454–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Klessig, D.F. MAPK cascades in plant defense signaling. Trends Plant Sci. 2001, 6, 520–527. [Google Scholar] [CrossRef]
- Brodersen, P.; Petersen, M.; Nielsen, H.B.; Zhu, S.; Newman, M.A.; Shokat, K.M.; Rietz, S.; Parker, J.; Mundy, J. Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylene-dependent responses via EDS1 and PAD4. Plant J. 2006, 47, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.W.; Mao, Q.Q.; Luo, B.F.; Lin, X.Y.; Du, S.T. Mutation of mpk6 enhances cadmium tolerance in Arabidopsis plants by alleviating oxidative stress. Plant Soil 2013, 371, 387–396. [Google Scholar] [CrossRef]
- Bovet, L.; Feller, U.; Martinoia, E. Possible involvement of plant ABC transporters in cadmium detoxification: A cDNA sub-microarray approach. Environ. Int. 2005, 31, 263–267. [Google Scholar] [CrossRef]
- Eichhorn, H.; Klinghammer, M.; Becht, P.; Tenhaken, R. Isolation of a novel ABC-transporter gene from soybean induced by salicylic acid. J. Exp. Bot. 2006, 57, 2193–2201. [Google Scholar] [CrossRef]
- Pál, M.; Janda, T.; Szalai, G. Abscisic acid may alter the salicylic acid-related abiotic stress response in maize. J. Agron. Crop Sci. 2011, 197, 368–377. [Google Scholar] [CrossRef]
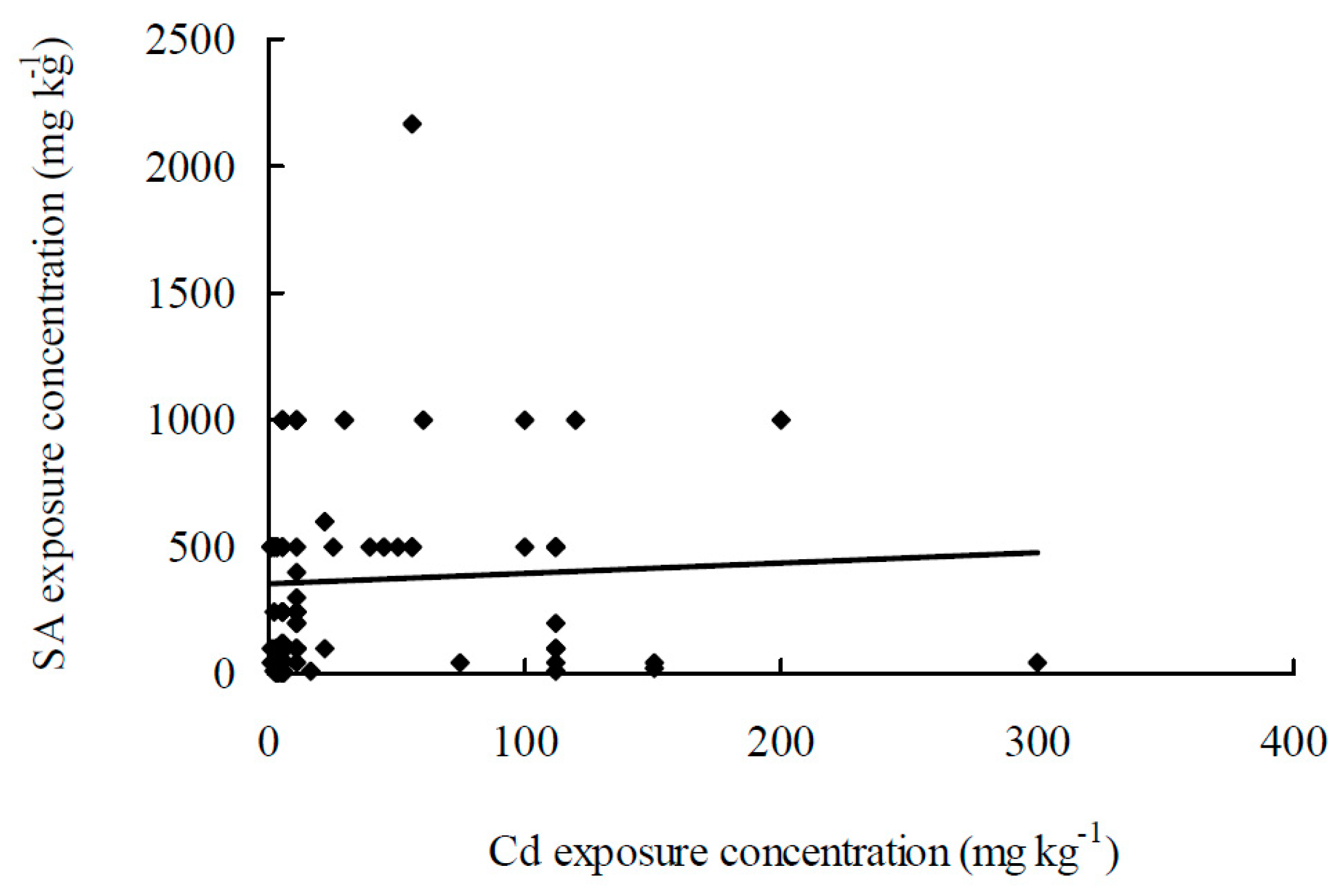
| SA Treatment | Cd Treatment | Timeline | Plant Species | Main Responses * Means Negative or No Effect | Reference | |
|---|---|---|---|---|---|---|
| Spraying | 600 μM, 10 days | 22.5 mg L−1 | Simultaneous | Potato (S. tuberosum L.) | I, II, III, VII | [31] |
| 100 μM, 1 time | 30, 60 and 120 mg kg−1 (pot) | Simultaneous | Peppermint (Mentha piperita) | I, II, III | [32] | |
| 50 μM, 4 times in a 3-day interval | 75, 150, and 300 mg kg−1 | Simultaneous | Oilseed rape (Brassica napus) | II, III, V, VI | [33] | |
| 10, 50, 100, and 200 μM each day treated for 50 mL last 4 days | 44.8 mg kg−1 | Pretreatment | Melon (Cucumis melo L.) | I, II, III | [34] | |
| 500 μM, 1 time | 40 mg kg−1 | Pretreatment | Soybean (Glycine max L. cv. Liaoxing 1) | I, II, III | [35] | |
| 2170 μM 1 time | 56 and 112 mg kg−1 | Simultaneous | Radish (Raphanus sativus) | I, *IV | [36] | |
| 1000 μM for 10 mL, 45 times in a day interval | 100 and 200 mg L−1 | Simultaneous | Indian mustard (Brassica juncea) | I, II, III, IV, V | [37] | |
| Presoaking | 500 μM for 24 h. | 112 mg L−1 for 72 h. | Pretreatment | Mungbean (Vigna radiata L. Wilczek) | I, II | [38] |
| 250 or 1000 μM for 8 h | 5.6 and 11.2 mg L−1 for 10 days | Pretreatment | Flax (Linum usitatissimum L.) | Lipids | [39] | |
| 250 or 1000 μM for 8 h | 5.6 and 11.2 mg L−1 for 10 days | Pretreatment | Flax (Linum usitatissimum L.) | I, II | [40] | |
| 500 μM for 12 h | 0.56, 1.12, and 5.60 mg L−1 for 7 days | Pretreatment | Kentucky bluegrass | I, II, III, *IV, V | [41] | |
| 500 μM for 12 h | 56 and 112 mg kg−1 for 56 days | Pretreatment | Wheat (Triticum aestivum L. cv. Giza 168) | I, II, III, *IV | [42] | |
| 500 μM for 6 h | 1.12, 1.68, and 2.80 mg L−1 for 14 days | Pretreatment | Maize (Zea mays) | VI | [43] | |
| 250 or 1000 μM for 8 h | 5.6 and 11.2 mg L−1 for 10 days | Pretreatment | Flax (cv. Viking) | I, II | [44] | |
| 250 or 1000 μM for 8 h | 5.6 and 11.2 mg L−1 for 10 days | Pretreatment | Flax (Linum usitatissimum L.) | I, *IV, VI | [45] | |
| 100 μM for 12 h | 5.6 and 11.2 mg L−1 for 6 days | Pretreatment | Legume (Phaseolus aureus and Vicia sativa) | I, II | [46] | |
| 250 and 500 μM for 12 h | 5.6 mg L−1 for 12 days | Pretreatment | Bean (R. communis cv. Zibi 5) | I, III, IV | [30] | |
| 250 and 1000 μM for 12 h | 5.6 and 11.2 mg L−1 for 12 days | Pretreatment | Flax (Linum usitatissimum L.) | I, II, III, IV, V | [47] | |
| 500 μM for 20 h | 11.2, 44.8 and 112 mg kg−1 for 30 days | Pretreatment | Wheat (Triticum aestivum L.) | I, II, III | [48] | |
| 100 μM for 3 h | 3, 5, and 7 mg kg−1 for 3 days | Pretreatment | Soybean (Balkan, L608) | II, III, *IV | [49] | |
| 500 μM for 6 h | 25, 50, and 100 mg kg−1 | Pretreatment | Hemp (Cannabis sativa L.) | I, II, III, *IV | [50] | |
| 500 μM for 6 h | 1.12, 1.68, and 2.80 mg L−1 for 14 days | Pretreatment | Maize (Zea mays L., hybrid Norma) | I, II, III, IV | [51] | |
| 100 μM for 16 h | 11.2 and 112 mg L−1 for 1 day | Pretreatment | Rice (cv: Longai) | I, II | [52] | |
| 100 μM for 1, 3, 6 h | 3 and 5 mg L−1 for 7 days | Pretreatment | Alfalfa (Medicago sativa L. cv. Evropa) | I, IV, V | [49] | |
| 100 μM for 8 h | 1.12, 11.2, and 112 mg L−1 for 1 day | Pretreatment | Rice (cv: Longai) | I, II, *IV | [53] | |
| 500 μM for 6 h | 2.8 mg L−1 for 12 days | Pretreatment | Barley (Hordeum vulgare cv Gerbel) | I, II, III, IV, V, VI, VII | [54] | |
| Hydroponic application | 10 μM for 15 days | 16.8 mg L−1 for 15 days | Simultaneous | Rice (Oryza sativa L. Galileo)) | I, II, III | [55] |
| 20 μM for 1 day | 150 mg L−1 for 9 days | Pretreatment | Nymphaea tetragona Georgi | II, III, *IV,V | [56] | |
| 50 μM for 7 days | 1.12 mg L−1 for 7 days | Simultaneously | Lemna minor | II, III, IV, V | [57] | |
| 50 μM for 1 day | 11.2 mg L−1 for 8 h | Pretreatment | Wheat (Triticum aestivum L.) | I, *IV, Hormones | [33] | |
| 500 μM for 24 h | 56 mg L−1 for 1 day | Pretreatment | Maize (Zea mays L., hybrid Norma) | II, III, *IV, VI | [58] | |
| 100, 200, 300 and 400 μM for 14 days | 11.2 mg L−1 for 14 days | Simultaneous | Ryegrass (Lolium perenne L.) | I, II, III, *IV, V | [50] | |
| 50 μM for 10 days | 5.6 mg L−1 for 10 days | Simultaneous | Rice (Oryza sativa cv. HUR3022) | I, II, III | [59] | |
| 100 μM for 14 days | 22.4 mg L−1 for 14 days | Simultaneously | Peanut (Arachis hypogaea L.) | I, II, III, *IV, V | [60] | |
| 250 and 500 μM for 10 mins | 1.68 mg L−1 for 3 and 6 h | Post-treatment | Barley (Hordeum vulgare L.) cv. Slaven | I, II, Auxin | [61] | |
| 200 μM for 14 days | 11.2 mg L−1 for 14 days | Simultaneously | Ryegrass (Lolium perenne L.) | I, II, III, VI | [62] | |
| 10, 50 and 100 μM for 7 days | 2.24 mg L−1 for 3 days | Pretreatment | Bean (Phaseolus vulgaris) | I, II, III, IV, V | [63] | |
| 60, 120, 250 and 500 mM | 5.6 mg L−1 for 5 days | Pretreatment | Soybean (Glycine max L., A6445RG) | II, III, *IV, V, VI, VII (HO−1) | [64] | |
| 1, 10, and 100 μM for 72 h | 5.6 mg L−1 for 1 day | Pretreatment | Alfalfa (Medicago sativa L. cv Zhongmu No.1) | I, II, *IV, VI, VII (HO−1) | [65] | |
| 3000 μM for 3 h | 560 mg L−1 for 1 day | Pretreatment | Rice (Oryza sativa L., cv. Taichung Native 1) | II, IV | [66] | |
| 10 μM for 72 h | 5.6 mg L−1 for 6 days | Pretreatment | Rice (O. sativa cv Jiahua 1) | I, II, IV | [67] | |
| 10 μM for 72 h | 5.6 mg L−1 for 6 days | Pretreatment | Rice (O. sativa cv Jiahua 1) | I, II | [68] | |
| 10 μM for 24 h | 5.6 mg L−1 for 6 days | Pretreatment | Rice (O. sativa cv Jiahua 1) | I, II, IV | [65] | |
| 1, 10, and 100 μM | 3 and 6 mg L−1 for 3 days | Simultaneous | Soybean (Glycinemax L. cv SG1) | *I, *IV, IV | [54] | |
| 500 μM for 24 h | 2.8 mg L−1 for 10 days | Pretreatment | Barley leaves (Hordeum vulgare cv Gerbel) | I, II, III, IV, V, VI, VII | [69] | |
| 500 μM for 24 h | 56 mg L−1 for 1 day | Pretreatment and simultaneously | Maize (Zea mays L., hybrid Norma) | *I, *II, *III, *VI | [70] | |
| SA mutants | Up and down-regulating endogenesis SA | 5.6 mg L−1 for 7 days | - | NahG,snc1 | I, II, III, IV, VII | [71] |
| Down-regulating endogenesis SA | 0.56 mg L−1 for 12 days | - | Sid2 | I, II, III, IV, V, VI, VII | [72] | |
| SA accumulation | 16.8 mg L−1 for 28 days | - | Lycium chinense | II, III, IV, VII(LcGSHS) | [73] | |
| Up and down-regulating endogenesis SA | 5.6, 11.2, and 16.8 mg L−1 for 7days | - | Accumulating mutant snc1, npr1−1, Reducing mutant nahG, snc1/nahG | *I, *II, *III | [74] | |
| Down-regulating endogenesis SA | 56 mg L−1 for 5 days | - | NahG | *II, *III, *VII (CAT1) | [75] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Liu, C.; Liang, Y.; Li, N.; Fu, Q. Salicylic Acid Signals Plant Defence against Cadmium Toxicity. Int. J. Mol. Sci. 2019, 20, 2960. https://doi.org/10.3390/ijms20122960
Guo B, Liu C, Liang Y, Li N, Fu Q. Salicylic Acid Signals Plant Defence against Cadmium Toxicity. International Journal of Molecular Sciences. 2019; 20(12):2960. https://doi.org/10.3390/ijms20122960
Chicago/Turabian StyleGuo, Bin, Chen Liu, Yongchao Liang, Ningyu Li, and Qinglin Fu. 2019. "Salicylic Acid Signals Plant Defence against Cadmium Toxicity" International Journal of Molecular Sciences 20, no. 12: 2960. https://doi.org/10.3390/ijms20122960
APA StyleGuo, B., Liu, C., Liang, Y., Li, N., & Fu, Q. (2019). Salicylic Acid Signals Plant Defence against Cadmium Toxicity. International Journal of Molecular Sciences, 20(12), 2960. https://doi.org/10.3390/ijms20122960






