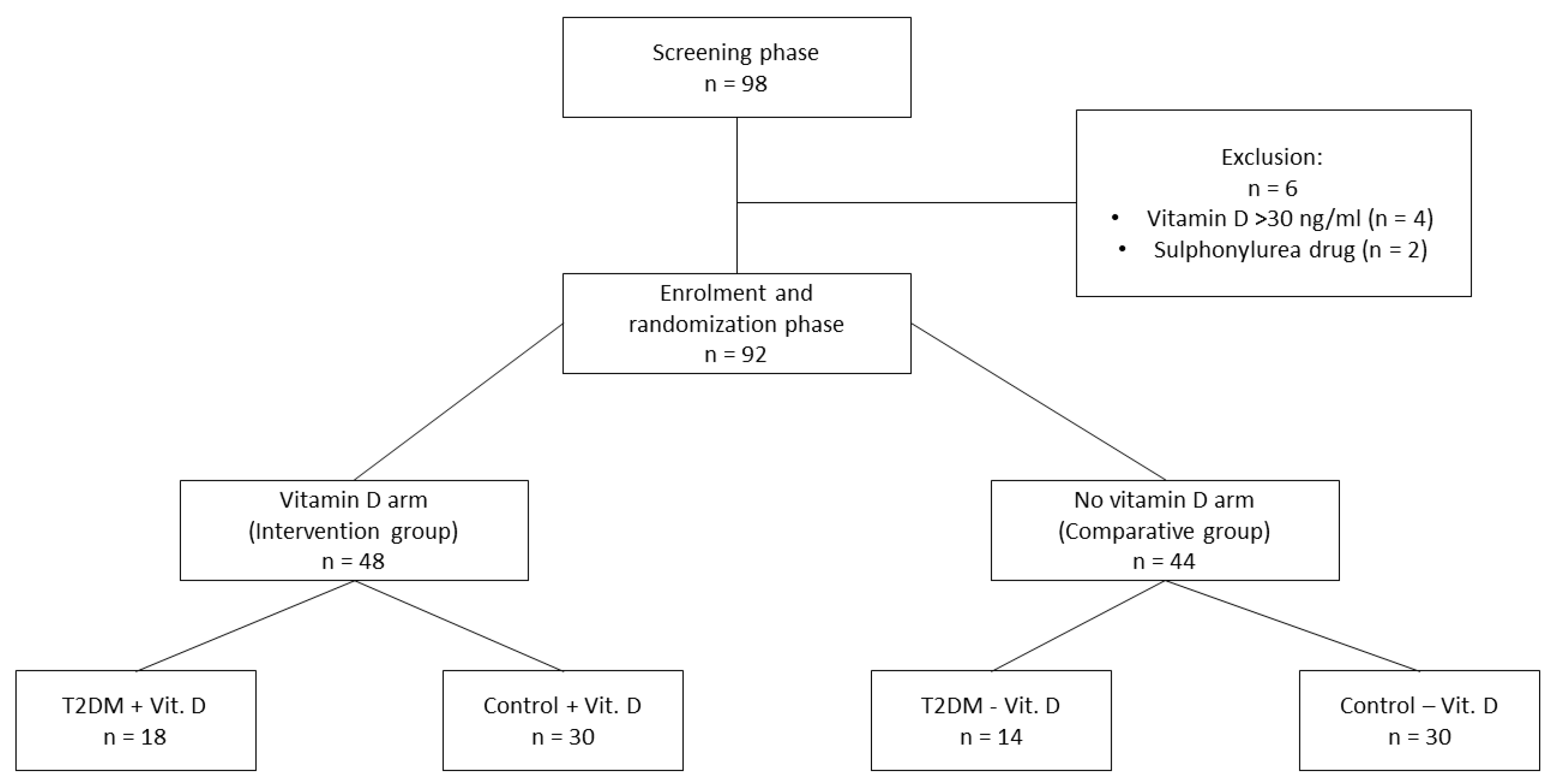
1. Introduction
Vitamin D is a fat-soluble steroid hormone, made of a sterol ring, that together with calcitonin and parathyroid hormone plays a role as a main regulator of calcium and phosphate metabolism. The discovery that vitamin D receptors (VDR) are present not only in bone cell and kidney tubes, but also in the majority of human cells, was a breakthrough. As a result, growing evidence has reported about its extra-skeletal functions. Vitamin D was found to be involved in maintenance of glucose homeostasis through numerous mechanisms connected with the insulin signaling pathway. Vitamin D takes part in sustaining of normal level of Ca
2+ and reactive oxygen species (ROS) both in pancreatic beta cells and insulin responsive cells. It is well recognized that augmented oxidative stress and vitamin D deficiency are associated with insulin resistance (IR)-related diseases, including type 2 diabetes (T2DM) [
1,
2,
3,
4].
A large number of studies support improving T2DM control, decreasing of insulin resistance, obesity, and metabolic syndrome with vitamin D adequacy. It was also found that vitamin D supplementation improved glycemic control and attenuated insulin resistance in prediabetics or individuals at high risk of diabetes development [
5]. Beyond the impact on the glycemic parameters, observational studies showed that high level of vitamin D is associated with a favorable lipid profile, while low levels of vitamin D are connected to an atherogenic lipid profile [
6]. Vitamin D deficiency and insufficiency is a global health issue afflicting more than one billion children and adults worldwide [
7]. In Poland, 90% of healthy adults have vitamin D concentrations below 30 ng/mL. An epidemiological study conducted in 2016 revealed that mean vitamin D serum concentration in the Polish population was 18.0 ± 9.6 ng/mL; 65.8% of the patients had a level below 20 ng/mL, 24.1% had suboptimal level of 20 to 30 ng/mL and only 9.1% demonstrated the optimal level of 30 to 50 ng/mL [
8].
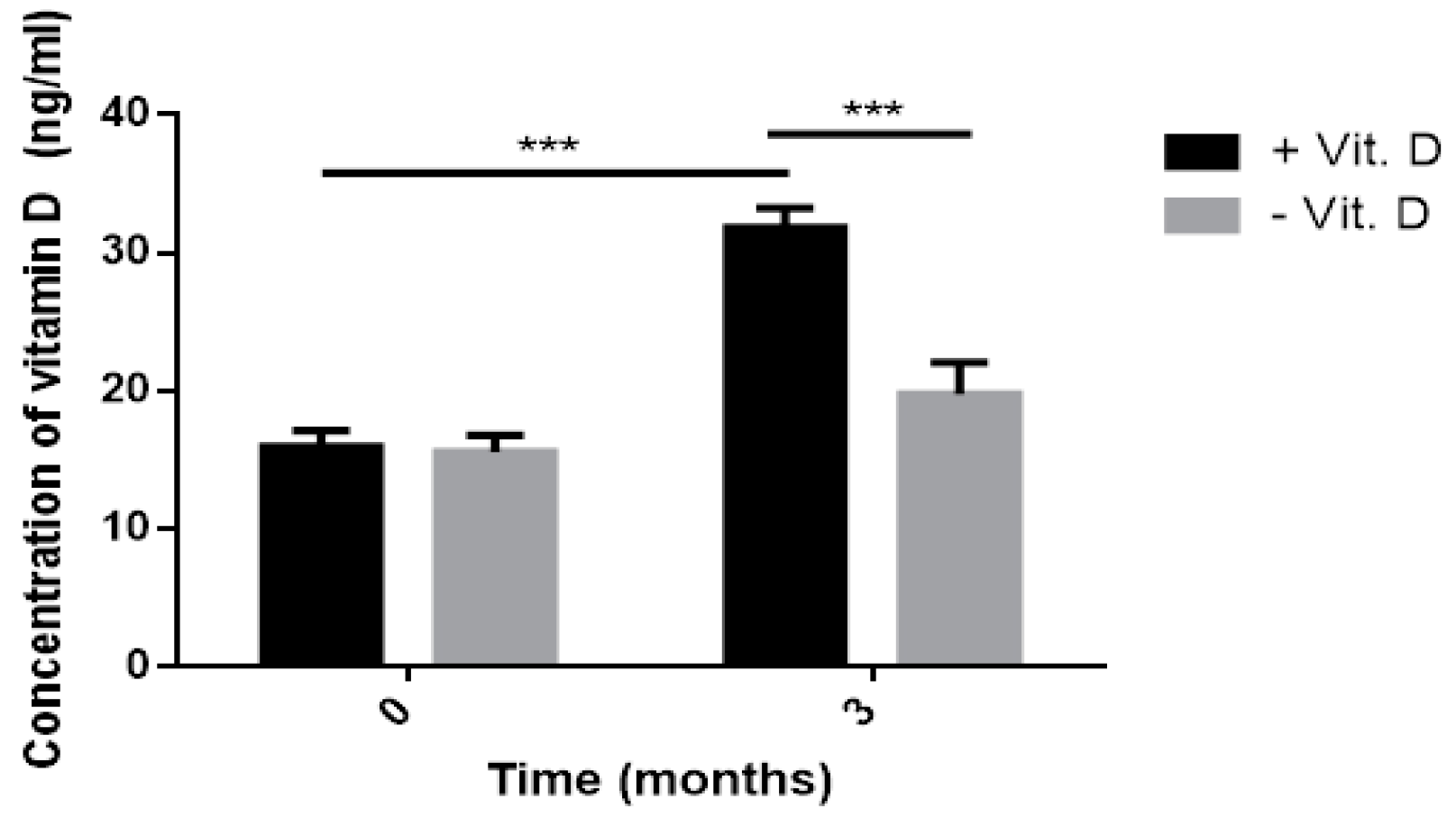
Therefore, the aim of our study was to evaluate whether three-month vitamin D supplementation is enough to increase its level to physiological concentration and to affect the level of endogenous and oxidative DNA damage being considered as a marker of oxidative stress. Moreover, we determined the effect of vitamin D on body mass index (BMI), metabolic parameters and IR indicators.
3. Discussion
The primary function of vitamin D is the regulation of bone metabolism and calcium-phosphate homeostasis. Also, studies have reported that vitamin D may play an important role in the maintenance of pancreatic beta cell function [
1]. It is achieved by the activation of the vitamin D receptor (VDR) and regulation of insulin secretion via the calcium channel located in pancreatic beta cells. A deficiency of vitamin D was found to be be associated with the development of various diseases, including IR-dependent diseases such as T2DM, obesity, dyslipidemia, cardiovascular diseases (CVDs) [
9]. Growing body of evidence shows that oxidative stress plays an important role in the pathogenesis of IR-dependent disorders. It suggests that vitamin D can reduce deleterious effect of oxidative stress [
10]. Thus, in this study we hypothesized that supplementation of vitamin D can reduce the level of oxidative DNA damage.
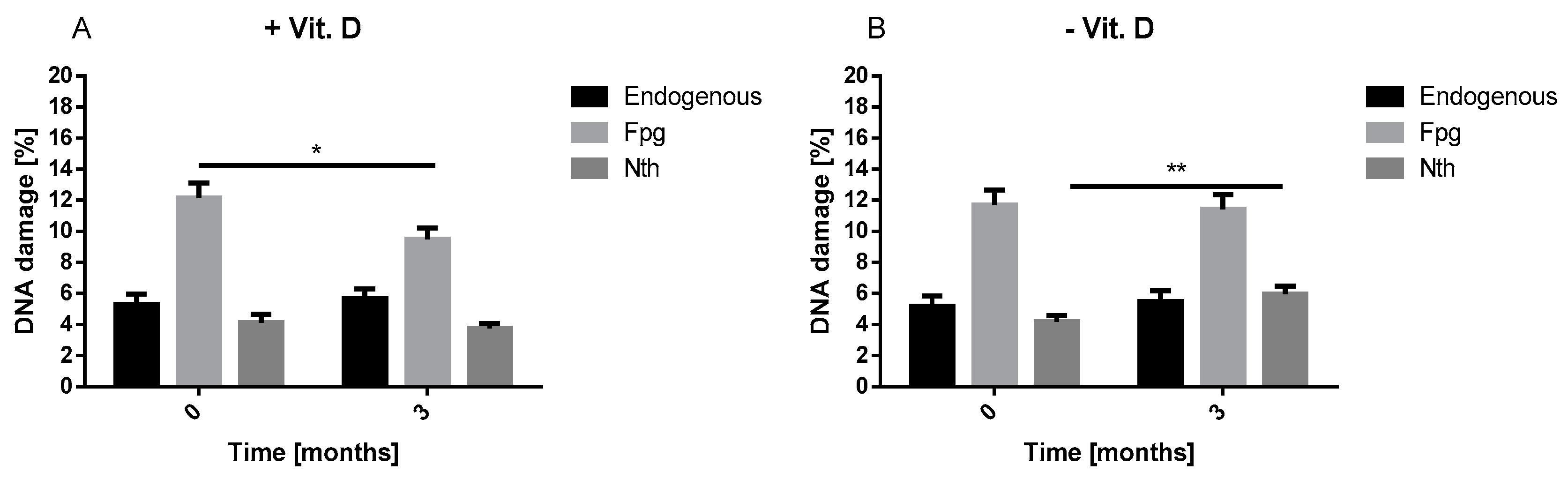
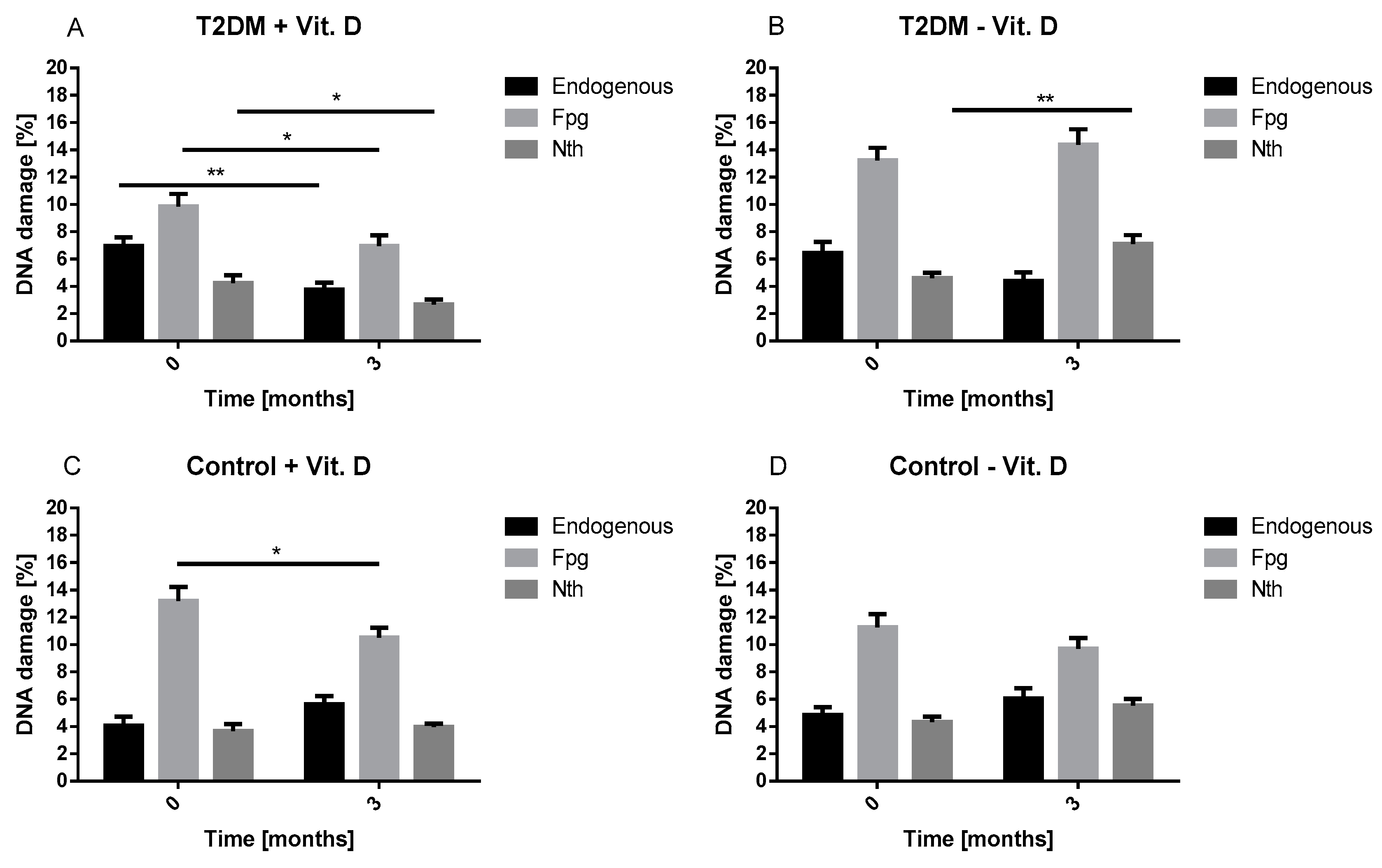
Our result showed that, vitamin D decreased oxidative DNA damage. Our results are in line with Fedriko et al. and Lan et al. [
11,
12]. We demonstrated that vitamin D supplementation decreased the level of endogenous and oxidative DNA damage in lymphocytes of T2DM patients after three months. These results confirm that vitamin D may reduce the adverse effects of oxidative stress in patients with diabetes. It is well known that diabetes via chronic hyperglycemia is associated with an increased level of oxidative damage, including DNA damage [
13]. What is more, high percentage of HbA1c detected in T2DM patients reflects high glucose level during the last 2–3 months. Thus, high percentage of HbA1c corresponds to poor glycemic control, hyperglycemia and related oxidative stress. Both the increased DNA damage and HbA1c percentage in our study at baseline suggest that patients with T2DM were poorly controlled and developed oxidative DNA damage associated with oxidative stress. Additionally, we observed that the increase in vitamin D concentration was associated with a reduction in HbA1c percentage in T2DM patients. This observation supports the beneficial effects of vitamin D on the metabolic control of diabetes which can be seen as a decrease in insulin resistance. These findings suggest that vitamin D may contribute to better diabetes alignment by reducing oxidative stress. In practice, this effect may translate into a delay in the occurrence of diabetes complications, including micro- and macroangiopathies.
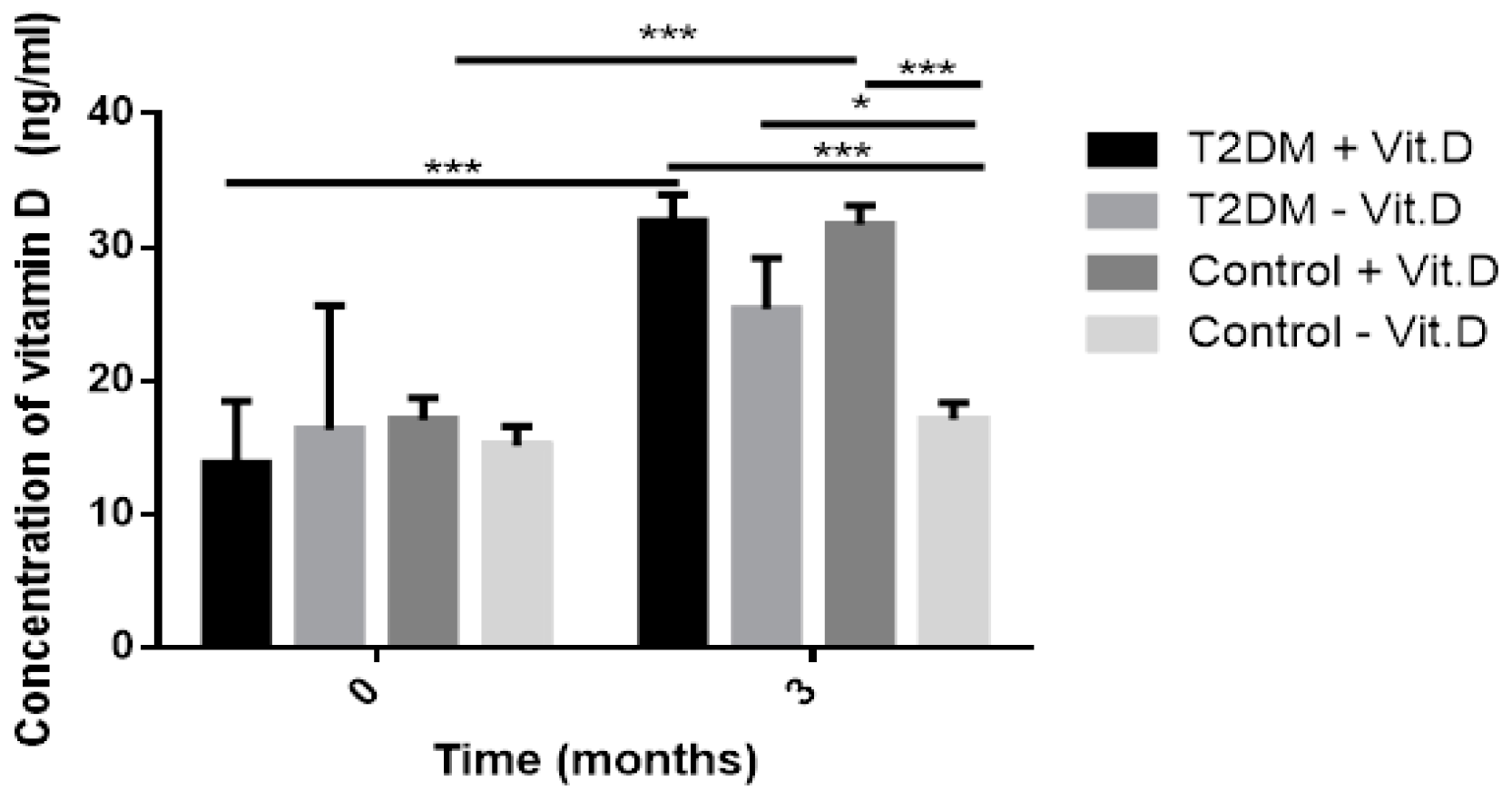
In this study lipid profile, glucose homeostasis parameters (FPG, HbA1c), BMI, IR markers (HOMA-IR, TG/HDL ratio) and the level of endogenous and oxidative DNA damage were measured at baseline and after three-month supplementation with 2000 IU vitamin D/day. We found a significant effect of three-month vitamin D supplementation on HDL level. The increase in HDL was noted in the intervention group, but its growth was particularly visible in the T2DM group (T2DM + Vit. D). This effect of vitamin D may be especially important for patients with T2DM who frequently suffer from obesity-related dyslipidemia. It is primarily characterized by increased levels of plasma free fatty acids and TG, decreased levels of HDL and abnormal low-density lipoprotein (LDL) composition. Additional benefit can be achieved by patients who are treated with statins, since these drugs do not affect HDL level significantly. The advantageous effect of vitamin D on HDL was also confirmed by the correlation analysis that showed a significant positive correlation between vitamin D and HDL at baseline in the intervention group. It suggests that vitamin D deficiency is correlated with the low level of HDL cholesterol. This observation stays in accordance with the study by Jiang et al., who detected vitamin D deficiency correlated with low HDL level in 3788 Chinese adults aged 35–74 [
14]. When, we performed the correlation analysis after three-month vitamin D supplementation, the relationship between vitamin D and HDL was not observed. It supports that vitamin D has a beneficial impact on HDL level. This desired effect of vitamin D on HDL was also confirmed in the meta-analysis by Mirrhosseini et al. [
15].
In turn, our results concerning the impact of vitamin D on other metabolic parameters are ambiguous. We did not find any significant effect of three-month vitamin D supplementation on TC, LDL and TG in the intervention group. On the one hand, our results are consistent with previous studies showing that one-year supplementation with 20,000 or 40,000 IU/week vitamin D did not improve serum lipids in Caucasian subjects [
16]. Grimnes et al. showed that six months of 40,000 IU/week vitamin D treatment did not exert a beneficial effect on lipid profile [
17]. On the other hand, the results of the present study are in contrast to the findings of Qin et al. [
18] who observed metabolic changes, including improvement of TC and TG levels evoked by vitamin D supplementation. It should be emphasized that this study had a longer follow-up period compared to our study, although the dose of vitamin D was the same. Similarly, Barzegari et al. achieved a significant reduction of TC, LDL and TG level after vitamin D supplementation. The study was conducted on 25 patients deficient in vitamin D with T2DM, who received this vitamin for eight weeks at a dose of 50,000 IU/week [
10]. In the face of ambiguous results regarding the effect of vitamin D on lipid profile, especially in metabolic disorders, further research is needed.
In our study, we also evaluated the effect of three-month vitamin D supplementation on glucose homeostasis parameters (FPG, HbA1c). We found a significant decrease of FPG in the comparative group, T2DM + Vit. D group and Control − Vit. D group. The reduction of FPG in T2DM group with vitamin D supplementation (T2DM + Vit. D) stays in accordance with meta-analysis by Mirrhosseini et al. [
5]. They found that vitamin D supplementation caused a significant decrease of FPG in diabetes patients. The significant decrease in FPG in control group without supplementation (Control − Vit. D) might be caused by the influence of other factors. It is worth noting that this group was characterized by a high level of FPG at baseline suggesting the presence of patients with prediabetes. The changes of lifestyle such as moderate physical activity and healthy diet were recommended for these participants. As a result of these changes, we observed a decrease in HOMA-IR and a trend toward decrease in TG/HDL ratio and BMI in the Control − Vit. D group. There was no significant diminish in HbA1c percentage in the intervention group, but in T2DM patients (T2DM + Vit. D) we found a trend toward decrease of HbA1c percentage (0.46 %). It is generally accepted that lower HbA1c, results in lower risk of diabetes-related complications [
19]. This desired effect in T2DM patients (T2DM + Vit. D) was also confirmed by the correlation analysis. It showed a significant negative relationship between vitamin D and HbA1c percentage after three-month vitamin D supplementation. However, other studies on the effect of vitamin D on HbA1c are inconclusive. Al-Sofiani et al. demonstrated that HbA1c did not change considerably after intervention of 5000 IU/day of vitamin D for 12 weeks [
20]. Likewise, Sadyia et al. also reported no significant change in HbA1c percentage after six months of supplementation with vitamin D of Emirati population [
21]. The study conducted by Tabesh et al. [
22] stays in agreement with our results. It demonstrated a decrease in HbA1c percentage in volunteers using 50,000 IU/week of vitamin D in combination with 1000 mg/day of calcium for six months. Upreti et al. found that six-month vitamin D supplementation was associated with a decrease in HbA1c percentage in patients with T2DM [
23]. Altogether, the findings suggest that vitamin D may exert a favorable effect on diabetes control.
Due to fact that IR is one of the major hallmark of T2DM [
24], we analyzed the effect of vitamin D on IR markers, including HOMA-IR and TG/HDL ratio. McLaughin et al. were first to report the potential utility of the TG/HDL ratio to detect IR in Caucasian population [
25]. We found that three-month vitamin D supplementation significantly reduced TG/HDL ratio and HOMA-IR in the intervention group. The decrease in HOMA-IR was also observed in the comparative group. Analyzing groups with and without T2DM, we noticed a statistically significant decrease of both IR indicators in groups supplemented with vitamin D (T2DM + Vit. D and Control + Vit. D). This is in line with findings by El Haji et al. who observed significant decrease in HOMA-IR in old adults supplemented with 4000 IU/day of vitamin D for 6 months [
26]. Similarly, Mirrosheini et al. showed that vitamin D diminished HOMA-IR [
27]. Taken together, these findings strongly support that vitamin D is effective in IR reducing. It should be also noted, that we observed a significant inverse correlation between vitamin D and BMI in patients without T2DM (Control + Vit. D group) and a trend toward this correlation in the T2DM + Vit. D group. This may suggest that higher vitamin D concentration may facilitate the reduction of weight and maintenance of proper BMI. This result stays in line with observation of Cassity et al. who reported that after monthly supplementation with 4000 IU/day of vitamin D BMI value was diminished [
28].
The study was limited by a relatively small research group, especially T2DM subgroups. As a result, the analysis adjusted to gender was not performed. The strength of the study is the analysis of metabolic and anthropometric parameters. Our results enhance to extend the supplementation period to the whole autumn and winter season, especially in the group of patients with T2DM, who may benefit more from the supplementation with vitamin D.