Murine Models of Acute Pancreatitis: A Critical Appraisal of Clinical Relevance
Abstract
1. Introduction
2. Applicability of Murine Models to Assess the Severity of Acute Pancreatitis
3. Murine Models and the Etiology of Acute Pancreatitis
4. Murine Models: A Practical Overview
4.1. Hormone-Induced or Hyperstimulation Acute Pancreatitis Model
4.2. Alcohol-Induced Acute Pancreatitis Model
4.3. Gene Knockout Acute Pancreatitis Model
4.4. Nutrient-Induced Acute Pancreatitis Model
4.5. Closed Duodenal Loop Acute Pancreatitis Model
4.6. Biliopancreatic Duct Injection Acute Pancreatitis Model
4.7. Vascular-Induced Acute Pancreatitis Model
4.8. Ischemia/Reperfusion Acute Pancreatitis Model
4.9. Duct Ligation Acute Pancreatitis Model
5. Clinical Relevance of the Models and Future Directions
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Garg, S.K.; Sarvepalli, S.; Campbell, J.P.; Obaitan, I.; Singh, D.; Bazerbachi, F.; Singh, R.; Sanaka, M.R. Incidence, Admission Rates, and Predictors, and Economic Burden of Adult Emergency Visits for Acute Pancreatitis. J. Clin. Gastroenterol. 2019, 53, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.; Frakes, C.; Arora, Z.; Chahal, P. Mechanisms and Management of Acute Pancreatitis. Gastroenterol. Res. Pract. 2018, 2018, 6218798. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.K.; Ma, Q.; Sha, H.; Palikhe, M. Acute pancreatitis: A literature review. Med Sci. Monit. 2009, 15, RA147–RA156. [Google Scholar] [PubMed]
- Ikeura, T.; Horibe, M.; Sanui, M.; Sasaki, M.; Kuwagata, Y.; Nishi, K.; Kariya, S.; Sawano, H.; Goto, T.; Hamada, T. Validation of the efficacy of the prognostic factor score in the Japanese severity criteria for severe acute pancreatitis: A large multicenter study. United Eur. Gastroenterol. J. 2017, 5, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Frossard, J.L.; Steer, M.L.; Pastor, C.M. Acute pancreatitis. Lancet 2008, 371, 143–152. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.P.; Mourad, M.M.; Bramhall, S.R. Acute pancreatitis: Current perspectives on diagnosis and management. J. Inflamm. Res. 2018, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, S.; Park, W.; Habtezion, A. Pharmacologic therapy for acute pancreatitis. World J. Gastroenterol. 2014, 20, 16868. [Google Scholar] [CrossRef]
- Gukovskaya, A.S.; Pandol, S.J.; Gukovsky, I. New insights into the pathways initiating and driving pancreatitis. Curr. Opin. Gastroenterol. 2016, 32, 429–435. [Google Scholar] [CrossRef]
- Steer, M.L. Search for the trigger mechanism of pancreatitis. Gastroenterology 1984, 86, 764–766. [Google Scholar]
- Bernard, C. Lecons de Physiologie Experimentale Appliquee a la Medecine, Faites au College de France par M. Claude Bernard: Cours du Semestre d’ete 1855; Bailliere: Paris, France, 1856; Volume 2. [Google Scholar]
- Gorelick, F.S.; Lerch, M.M. Do animal models of acute pancreatitis reproduce human disease? Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Su, K.H.; Cuthbertson, C.; Christophi, C. Review of experimental animal models of acute pancreatitis. HPB 2006, 8, 264–286. [Google Scholar] [CrossRef] [PubMed]
- Samanta, J.; Singh, S.; Arora, S.; Muktesh, G.; Aggarwal, A.; Dhaka, N.; Sinha, S.K.; Gupta, V.; Sharma, V.; Kochhar, R. Cytokine profile in prediction of acute lung injury in patients with acute pancreatitis. Pancreatology 2018, 18, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-L.; Lv, Z.-Y.; Yang, H.-W.; Liu, Y.; Long, F.-W.; Zhou, B.; Sun, X.-F.; Peng, Z.-H.; Zhou, Z.-G.; Li, Y. Effects of tocilizumab on experimental severe acute pancreatitis and associated acute lung injury. Crit. Care Med. 2016, 44, e664–e677. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, X.; Chen, W.; Yang, N.; Gao, L.; Mao, W.; Yang, J.; Yang, Q.; Dong, J.; Tong, Z. Protective effects of HTD4010, a Reg3α/PAP-derived peptide, in mouse model of acute pancreatitis via toll-like receptor 4 pathway. Biochem. Biophys. Res. Commun. 2019, 512, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Sikha, M.S.; Ramesh, K.; Venkatesh, P.; Godugu, C. Modulation of cerulein-induced pancreatic inflammation by hydroalcoholic extract of curry leaf (Murraya koenigii). Phytother. Res. 2019, 33, 1510–1525. [Google Scholar] [CrossRef]
- Yu, W.-Q.; Zhang, S.-Y.; Fu, S.-Q.; Fu, Q.-H.; Lu, W.-N.; Zhang, J.; Liang, Z.-Y.; Zhang, Y.; Liang, T.-B. Dexamethasone protects the glycocalyx on the kidney microvascular endothelium during severe acute pancreatitis. J. Zhejiang Univ. Sci. B 2019, 20, 355–362. [Google Scholar] [CrossRef]
- Soyalp, M.; Yalcin, M.; Oter, V.; Ozgonul, A. Investigation of procalcitonin, IL-6, oxidative stress index (OSI) plasma and tissue levels in experimental mild and severe pancreatitis in rats. Bratisl. Lek. Listy 2017, 118, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Rattner, D.W.; Lewandrowski, K.; Compton, C.C.; Mandavilli, U.; Knoefel, W.T.; Warshaw, A.L. A better model of acute pancreatitis for evaluating therapy. Ann. Surg. 1992, 215, 44–56. [Google Scholar] [CrossRef]
- Ding, S.-P.; Li, J.-C.; Jin, C. A mouse model of severe acute pancreatitis induced with caerulein and lipopolysaccharide. World J. Gastroenterol. 2003, 9, 584–589. [Google Scholar] [CrossRef]
- Klopfleisch, R. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology-a systematic review. BMC Vet. Res. 2013, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, X.; Zhang, R.; Liang, S.; Kang, X.; Zhang, X.; Lou, Q.; Xiong, K.; Yang, J.; Si, L. Rectal Indomethacin and Spraying of Duodenal Papilla with Epinephrine Increases Risk of Pancreatitis Following Endoscopic Retrograde Cholangiopancreatography. Clin. Gastroenterol. Hepatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lerch, M.; Hoppe-Seyler, P.; Gerok, W. Origin and development of exocrine pancreatic insufficiency in experimental renal failure. Gut 1994, 35, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Li, J.; Fan, X.; Liu, Q.; Lian, J. The effect of selective serotonin re-uptake inhibitors on risk of type II diabetes mellitus and acute pancreatitis: A meta-analysis. Biosci. Rep. 2018, 38, BSR20180967. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.G.; Pendharkar, S.A.; Cervantes, A.; Cho, J.; Miranda-Soberanis, V.; Petrov, M.S. Abdominal obesity and insulin resistance after an episode of acute pancreatitis. Dig. Liver Dis. 2018, 50, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.G. How does cigarette smoking cause acute pancreatitis? Pancreatology 2016, 16, 157–163. [Google Scholar] [CrossRef]
- Schneider, L.; Jabrailova, B.; Soliman, H.; Hofer, S.; Strobel, O.; Hackert, T.; Büchler, M.W.; Werner, J. Pharmacological cholinergic stimulation as a therapeutic tool in experimental necrotizing pancreatitis. Pancreas 2014, 43, 41–46. [Google Scholar] [CrossRef]
- Martins, F.d.O.; Gomes, B.C.; Rodrigues, A.S.; Rueff, J. Genetic susceptibility in acute pancreatitis: Genotyping of GSTM1, GSTT1, GSTP1, CASP7, CASP8, CASP9, CASP10, LTA, TNFRSF1B, and TP53 gene variants. Pancreas 2017, 46, 71–76. [Google Scholar] [CrossRef]
- Khaoula, Y.; Mokni, J.; Feten, A.; Ameni, B.; Chedly, M. Blunt abdominal trauma causing acute pancreatitis: Presentation of the case study. Pan Afr. Med J. 2018, 30, 126. [Google Scholar]
- Vujasinovic, M.; Valente, R.; Maier, P.; von Beckerath, V.; Haas, S.L.; Arnelo, U.; Del Chiaro, M.; Kartalis, N.; Pozzi-Mucelli, R.M.; Fernandez-Moro, C. Diagnosis, treatment and long-term outcome of autoimmune pancreatitis in Sweden. Pancreatology 2018, 18, 900–904. [Google Scholar] [CrossRef]
- Zhang, G.; Feng, J.; Xu, Q.; Huang, H. Double filtration plasmapheresis in treatment of hyperlipidemic acute pancreatitis. Zhejiang Da Xue Xue Bao Yi Xue Ban 2008, 37, 93–96. [Google Scholar] [PubMed]
- Dąbrowski, A.; Konturek, S.J.; Konturek, J.W.; Gabryelewicz, A. Role of oxidative stress in the pathogenesis of caerulein-induced acute pancreatitis. Eur. J. Pharmacol. 1999, 377, 1–11. [Google Scholar] [CrossRef]
- Lampel, M.; Kern, H.F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch. A 1977, 373, 97–117. [Google Scholar] [CrossRef]
- Niederau, C.; Ferrell, L.D.; Grendell, J.H. Caerulein-Induced Acute Necrotizing Pancreatitis in Mice; Protective Effects of Proglumide Benzotript, and Secretin. Gastroenterology 1985, 88, 1192–1204. [Google Scholar] [CrossRef]
- Wisner, J.; Green, D.; Ferrell, L.; Renner, I. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut 1988, 29, 1516–1523. [Google Scholar] [CrossRef]
- Hartwig, W.; Schimmel, E.; Hackert, T.; Fortunato, F.; Bergmann, F.; Baczako, A.; Strobel, O.; Büchler, M.W.; Werner, J. A novel animal model of severe pancreatitis in mice and its differences to the rat. Surgery 2008, 144, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Clemons, A.P.; Holstein, D.M.; Galli, A.; Saunders, C. Cerulein-induced acute pancreatitis in the rat is significantly ameliorated by treatment with MEK1/2 inhibitors U0126 and PD98059. Pancreas 2002, 25, 251–259. [Google Scholar] [CrossRef]
- Niederau, C.; Ude, K.; Niederau, M.; Lüthen, R.; Strohmeyer, G.; Ferrell, L.D.; Grendell, J.H. Effects of the seleno-organic substance Ebselen in two different models of acute pancreatitis. Pancreas 1991, 6, 282–290. [Google Scholar] [CrossRef]
- Siveke, J.T.; Lubeseder-Martellato, C.; Lee, M.; Mazur, P.K.; Nakhai, H.; Radtke, F.; Schmid, R.M. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology 2008, 134, 544–555.e543. [Google Scholar] [CrossRef]
- Xing, M.; Ni, J.B.; Wan, R.; Tang, M.C.; Hu, Y.L.; Yu, G.; Yin, G.J.; Chen, C.Y.; Fan, Y.T.; Xiao, W.Q. Tetraspanin CD 9 is involved in pancreatic damage during caerulein-induced acute pancreatitis in mice. J. Dig. Dis. 2015, 16, 43–51. [Google Scholar] [CrossRef]
- Jia, R.; Ma, J.; Meng, W.; Wang, N. Dihydromyricetin inhibits caerulin-induced TRAF3-p38 signaling activation and acute pancreatitis response. Biochem. Biophys. Res. Commun. 2018, 503, 1696–1702. [Google Scholar] [CrossRef]
- Venglovecz, V.; Pallagi, P.; Kemény, L.; Balázs, A.; Balla, Z.; Becskeházi, E.; Gál, E.; Zvara, Á.; Puskás, L.; Katalin, B. The Importance of Aquaporin 1 in Pancreatitis and Its Relation to the CFTR Cl-Channel. Front. Physiol. 2018, 9, 854. [Google Scholar] [CrossRef]
- Gao, L.; Lu, G.T.; Lu, Y.Y.; Xiao, W.M.; Mao, W.J.; Tong, Z.H.; Yang, N.; Li, B.Q.; Yang, Q.; Ding, Y.B.; et al. Diabetes aggravates acute pancreatitis possibly via activation of NLRP3 inflammasome in db/db mice. Am. J. Transl. Res. 2018, 10, 2015–2025. [Google Scholar]
- Zhang, L.; Zhang, J.; Shea, K.; Xu, L.; Tobin, G.; Knapton, A.; Sharron, S.; Rouse, R. Autophagy in pancreatic acinar cells in caerulein-treated mice: Immunolocalization of related proteins and their potential as markers of pancreatitis. Toxicol. Pathol. 2014, 42, 435–457. [Google Scholar] [CrossRef]
- Sledzinski, M.; Borkowska, A.; Sielicka-Dudzin, A.; Halon, M.; Wozniak, M.; Spodnik, J.H.; Antosiewicz, A.H.; Antosiewicz, J. Cerulein-Induced Acute Pancreatitis Is Associated With c-Jun NH (2)-Terminal Kinase 1–Dependent Ferritin Degradation and Iron-Dependent Free Radicals Formation. Pancreas 2013, 42, 1070–1077. [Google Scholar] [CrossRef]
- García-Hernández, V.; Sarmiento, N.; Sánchez-Bernal, C.; Matellán, L.; Calvo, J.J.; Sanchez-Yaguee, J. Modulation in the expression of SHP-1, SHP-2 and PTP1B due to the inhibition of MAPKs, cAMP and neutrophils early on in the development of cerulein-induced acute pancreatitis in rats. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 192–201. [Google Scholar] [CrossRef]
- Liu, R.; Qi, H.; Wang, J.; Wang, Y.; Cui, L.; Wen, Y.; Yin, C. Angiotensin-converting enzyme (ACE and ACE2) imbalance correlates with the severity of cerulein-induced acute pancreatitis in mice. Exp. Physiol. 2014, 99, 651–663. [Google Scholar] [CrossRef]
- Ou, X.; Cheng, Z.; Liu, T.; Tang, Z.; Huang, W.; Szatmary, P.; Zheng, S.; Sutton, R.; Toh, C.H.; Zhang, N. Circulating histone levels reflect disease severity in animal models of acute pancreatitis. Pancreas 2015, 44, 1089–1095. [Google Scholar] [CrossRef]
- Szentkereszty, Z.; Kotan, R.; Kiss, F.; Klarik, Z.; Posan, J.; Furka, I.; Sapy, P.; Miko, I.; Peto, K.; Nemeth, N. Effects of various drugs (flunixin, pentoxifylline, enoxaparin) modulating micro-rheological changes in cerulein-induced acute pancreatitis in the rat. Clin. Hemorheol. Microcirc. 2014, 57, 303–314. [Google Scholar]
- Cao, J.; Liu, Q. Protective effects of sivelestat in a caerulein-induced rat acute pancreatitis model. Inflammation 2013, 36, 1348–1356. [Google Scholar] [CrossRef]
- Bae, G.-S.; Heo, K.-H.; Park, K.-C.; Choi, S.B.; Jo, I.-J.; Seo, S.-H.; Kim, D.-G.; Shin, J.-Y.; Kang, D.-G.; Lee, H.-S. Apamin attenuated cerulein-induced acute pancreatitis by inhibition of JNK pathway in mice. Dig. Dis. Sci. 2013, 58, 2908–2917. [Google Scholar] [CrossRef]
- Huang, W.; Cash, N.; Wen, L.; Szatmary, P.; Mukherjee, R.; Armstrong, J.; Chvanov, M.; Tepikin, A.V.; Murphy, M.P.; Sutton, R. Effects of the mitochondria-targeted antioxidant mitoquinone in murine acute pancreatitis. Mediat. Inflamm. 2015, 2015, 901780. [Google Scholar] [CrossRef]
- Huang, W.; Cane, M.C.; Mukherjee, R.; Szatmary, P.; Zhang, X.; Elliott, V.; Ouyang, Y.; Chvanov, M.; Latawiec, D.; Wen, L. Caffeine protects against experimental acute pancreatitis by inhibition of inositol 1, 4, 5-trisphosphate receptor-mediated Ca2+ release. Gut 2017, 66, 301–313. [Google Scholar] [CrossRef]
- Schick, V.; Scheiber, J.A.; Mooren, F.C.; Turi, S.; Ceyhan, G.O.; Schnekenburger, J.; Sendler, M.; Schwaiger, T.; Omercevic, A.; van den Brandt, C. Effect of magnesium supplementation and depletion on the onset and course of acute experimental pancreatitis. Gut 2014, 63, 1469–1480. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhang, Y.C.; Cheng, J.S.; Ni, Q.; Li, P.J.; Wang, S.W.; Han, W.; Zhang, Y.L. BML-111, a lipoxin receptor agonist, ameliorates ‘two-hit’-induced acute pancreatitis-associated lung injury in mice by the upregulation of heme oxygenase-1. Artif. Cells Nanomed. Biotechnol. 2014, 42, 110–120. [Google Scholar] [CrossRef]
- Weng, T.-I.; Wu, H.-Y.; Chen, B.-L.; Jhuang, J.-Y.; Huang, K.-H.; Chiang, C.-K.; Liu, S.-H. C/EBP homologous protein deficiency aggravates acute pancreatitis and associated lung injury. World J. Gastroenterol. 2013, 19, 7097–7105. [Google Scholar] [CrossRef]
- Marciniak, A.; Walczyna, B.; Rajtar, G.; Marciniak, S.; Wojtak, A.; Lasiecka, K. Tempol, a membrane-permeable radical scavenger, exhibits anti-inflammatory and cardioprotective effects in the cerulein-induced pancreatitis rat model. Oxidative Med. Cell. Longev. 2016, 2016, 4139851. [Google Scholar] [CrossRef]
- Bartholomew, C. Acute scorpion pancreatitis in Trinidad. Br. Med. J. 1970, 1, 666–668. [Google Scholar] [CrossRef]
- Marsh, W.H.; Vukov, G.A.; Conradi, E.C. Acute pancreatitis after cutaneous exposure to an organophosphate insecticide. Am. J. Gastroenterol. 1988, 83, 1158–1160. [Google Scholar]
- Steer, M.L.; Meldolesi, J. The cell biology of experimental pancreatitis. N. Engl. J. Med. 1987, 316, 144–150. [Google Scholar]
- Steer, M.L. Frank Brooks memorial Lecture: The early intraacinar cell events which occur during acute pancreatitis. Pancreas 1998, 17, 31–37. [Google Scholar] [CrossRef]
- Norberg, K.J.; Nania, S.; Li, X.; Gao, H.; Szatmary, P.; Segersvärd, R.; Haas, S.; Wagman, A.; Arnelo, U.; Sutton, R. RCAN1 is a marker of oxidative stress, induced in acute pancreatitis. Pancreatology 2018, 18, 734–741. [Google Scholar] [CrossRef]
- García-Hernández, V.; Sánchez-Bernal, C.; Schvartz, D.; Calvo, J.J.; Sanchez, J.-C.; Sánchez-Yagüe, J. A tandem mass tag (TMT) proteomic analysis during the early phase of experimental pancreatitis reveals new insights in the disease pathogenesis. J. Proteom. 2018, 181, 190–200. [Google Scholar] [CrossRef]
- Tang, M.; Hu, G.; Zhao, Y.; Su, M.; Wang, Y.; Jia, W.; Qiu, Y.; Liu, G.; Wang, X. A serum metabolomic investigation on lipoprotein lipase-deficient mice with hyperlipidemic pancreatitis using gas chromatography/mass spectrometry. Biomed. Rep. 2013, 1, 469–473. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, Y.; Xu, G.; Tao, R.; Yuan, W.; Huang, Z.; Zhang, D. TRAM1 protects AR42J cells from caerulein-induced acute pancreatitis through ER stress-apoptosis pathway. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 530–536. [Google Scholar] [CrossRef]
- Terao, K.; Wake, H.; Adachi, N.; Liu, K.; Teshigawara, K.; Takahashi, H.; Mori, S.; Nishibori, M. Histidine-Rich Glycoprotein Suppresses Hyperinflammatory Responses of Lung in a Severe Acute Pancreatitis Mouse Model. Pancreas 2018, 47, 1156–1164. [Google Scholar] [CrossRef]
- Sun, K.; He, S.-B.; Qu, J.-G.; Dang, S.-C.; Chen, J.-X.; Gong, A.-H.; Xie, R.; Zhang, J.-X. IRF5 regulates lung macrophages M2 polarization during severe acute pancreatitis in vitro. World J. Gastroenterol. 2016, 22, 9368. [Google Scholar] [CrossRef]
- Sun, J.; Fu, J.; Zhong, Y.; Li, L.; Chen, C.; Wang, X.; Wang, L.; Hou, Y.; Wang, H.; Zhao, R. NRF2 mitigates acute alcohol-induced hepatic and pancreatic injury in mice. Food Chem. Toxicol. 2018, 121, 495–503. [Google Scholar] [CrossRef]
- Huang, W.; Booth, D.M.; Cane, M.C.; Chvanov, M.; Javed, M.A.; Elliott, V.L.; Armstrong, J.A.; Dingsdale, H.; Cash, N.; Li, Y. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis. Gut 2014, 63, 1313–1324. [Google Scholar] [CrossRef]
- Schneider, L.; Dieckmann, R.; Hackert, T.; Gebhard, M.-M.; Werner, J. Acute alcohol-induced pancreatic injury is similar with intravenous and intragastric routes of alcohol administration. Pancreas 2014, 43, 69–74. [Google Scholar] [CrossRef]
- Kiziler, A.R.; Aydemir, B.; Gulyasar, T.; Unal, E.; Gunes, P. Relationships among iron, protein oxidation and lipid peroxidation levels in rats with alcohol-induced acute pancreatitis. Biol. Trace Elem. Res. 2008, 124, 135–143. [Google Scholar] [CrossRef]
- Kui, B.; Balla, Z.; Vasas, B.; Végh, E.T.; Pallagi, P.; Kormányos, E.S.; Venglovecz, V.; Iványi, B.; Takács, T.; Hegyi, P. New insights into the methodology of L-arginine-induced acute pancreatitis. PLoS ONE 2015, 10, e0117588. [Google Scholar] [CrossRef]
- Tashiro, M.; Schäfer, C.; Yao, H.; Ernst, S.; Williams, J. Arginine induced acute pancreatitis alters the actin cytoskeleton and increases heat shock protein expression in rat pancreatic acinar cells. Gut 2001, 49, 241–250. [Google Scholar] [CrossRef]
- Le, T.; Eisses, J.F.; Lemon, K.L.; Ozolek, J.A.; Pociask, D.A.; Orabi, A.I.; Husain, S.Z. Intra-ductal infusion of taurocholate followed by distal common bile duct ligation leads to a severe, necrotic model of pancreatitis in mice. Pancreas 2015, 44, 493–499. [Google Scholar]
- Zhao, Q.; Zhang, H.; Huang, J.; Yu, H.; Li, J.; Che, Q.; Sun, Y.; Jin, Y.; Wu, J. Melatonin attenuates the inflammatory response via inhibiting the C/EBP homologous protein-mediated pathway in taurocholate-induced acute pancreatitis. Int. J. Mol. Med. 2018, 42, 3513–3521. [Google Scholar] [CrossRef]
- Aho, H.; Koskensalo, S.-L.; Nevalainen, T. Experimental pancreatitis in the rat: Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand. J. Gastroenterol. 1980, 15, 411–416. [Google Scholar] [CrossRef]
- Hua, J.; He, Z.-G.; Qian, D.-H.; Lin, S.-P.; Gong, J.; Meng, H.-B.; Yang, T.-S.; Sun, W.; Xu, B.; Zhou, B. Angiopoietin-1 gene-modified human mesenchymal stem cells promote angiogenesis and reduce acute pancreatitis in rats. Int. J. Clin. Exp. Pathol. 2014, 7, 3580–3595. [Google Scholar]
- Kim, H.-W.; Song, W.-J.; Li, Q.; Han, S.-M.; Jeon, K.-O.; Park, S.-C.; Ryu, M.-O.; Chae, H.-K.; Kyeong, K.; Youn, H.-Y. Canine adipose tissue-derived mesenchymal stem cells ameliorate severe acute pancreatitis by regulating T cells in rats. J. Vet. Sci. 2016, 17, 539–548. [Google Scholar] [CrossRef]
- Uysal, B.; Yasar, M.; Ersoz, N.; Coskun, O.; Kilic, A.; Cayc, T.; Kurt, B.; Oter, S.; Korkmaz, A.; Guven, A. Efficacy of hyperbaric oxygen therapy and medical ozone therapy in experimental acute necrotizing pancreatitis. Pancreas 2010, 39, 9–15. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Dlugosz, J.; Jurkowska, G. The effect of antecedent acute ethanol ingestion on the pancreas ultrastructure in taurocholate pancreatitis in rats. Exp. Mol. Pathol. 1998, 65, 64–77. [Google Scholar] [CrossRef]
- Pandol, S.J.; Periskic, S.; Gukovsky, I.; Zaninovic, V.; Jung, Y.; Zong, Y.; Solomon, T.E.; Gukovskaya, A.S.; Tsukamoto, H. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology 1999, 117, 706–716. [Google Scholar] [CrossRef]
- Foitzik, T.; Lewandrowski, K.B.; Fernández-del Castillo, C.; Rattner, D.W.; Klar, E.; Warshaw, A.L. Exocrine hyperstimulation but not pancreatic duct obstruction increases the susceptibility to alcohol-related pancreatic injury. Arch. Surg. 1994, 129, 1081–1085. [Google Scholar] [CrossRef]
- Vonlaufen, A. Modeling alcoholic pancreatitis by ethanol feeding and lipopolysaccharide (LPS) challenge. Pancreapedia Exocrine Pancreas Knowl. Base 2011. [Google Scholar] [CrossRef]
- Javed, M.A.; Wen, L.; Awais, M.; Latawiec, D.; Huang, W.; Chvanov, M.; Schaller, S.; Bordet, T.; Michaud, M.; Pruss, R. TRO40303 Ameliorates Alcohol-Induced Pancreatitis Through Reduction of Fatty Acid Ethyl Ester–Induced Mitochondrial Injury and Necrotic Cell Death. Pancreas 2018, 47, 18–24. [Google Scholar] [CrossRef]
- Schneider, A.; Whitcomb, D.C.; Singer, M.V. Animal models in alcoholic pancreatitis–what can we learn? Pancreatology 2002, 2, 189–203. [Google Scholar] [CrossRef]
- Siech, M.; Weber, H.; Letko, G.; Dummler, W.; Schoenberg, M.; Beger, H. Similar morphological and intracellular biochemical changes in alcoholic acute pancreatitis and ischemic acute pancreatitis in rats. Pancreas 1997, 14, 32–38. [Google Scholar] [CrossRef]
- Hall, B.; Limaye, A.; Kulkarni, A.B. Overview: Generation of gene knockout mice. Curr. Protoc. Cell Biol. 2009, 44, 19.12.1–19.12.17. [Google Scholar]
- Capecchi, M.R. Targeted gene replacement. Sci. Am. 1994, 270, 52–59. [Google Scholar] [CrossRef]
- Tesson, L.; Cozzi, J.; Menoret, S.; Remy, S.; Usal, C.; Fraichard, A.; Anegon, I. Transgenic modifications of the rat genome. Transgenic Res. 2005, 14, 531–546. [Google Scholar] [CrossRef]
- Zan, Y.; Haag, J.D.; Chen, K.-S.; Shepel, L.A.; Wigington, D.; Wang, Y.-R.; Hu, R.; Lopez-Guajardo, C.C.; Brose, H.L.; Porter, K.I. Production of knockout rats using ENU mutagenesis and a yeast-based screening assay. Nat. Biotechnol. 2003, 21, 645–651. [Google Scholar] [CrossRef]
- Tao, L.; Lin, X.; Tan, S.; Lei, Y.; Liu, H.; Guo, Y.; Zheng, F.; Wu, B. β-Arrestin1 alleviates acute pancreatitis via repression of NF-κBp65 activation. J. Gastroenterol. Hepatol. 2019, 34, 284–292. [Google Scholar] [CrossRef]
- Norkina, O.; Graf, R.; Appenzeller, P.; De Lisle, R.C. Caerulein-induced acute pancreatitis in mice that constitutively overexpress Reg/PAP genes. BMC Gastroenterol. 2006, 6, 16. [Google Scholar] [CrossRef]
- Jancsó, Z.; Hegyi, E.; Sahin-Tóth, M. Chymotrypsin Reduces the Severity of Secretagogue-Induced Pancreatitis in Mice. Gastroenterology 2018, 155, 1017–1021. [Google Scholar] [CrossRef]
- Yu, J.; Ni, L.; Zhang, X.; Zhang, J.; Abdel-Razek, O.; Wang, G. Surfactant Protein D Dampens Lung Injury by Suppressing NLRP3 Inflammasome Activation and NF-κB Signaling in Acute Pancreatitis. Shock 2019, 51, 557–568. [Google Scholar] [CrossRef]
- Zhao, Q.; Wei, Y.; Pandol, S.J.; Li, L.; Habtezion, A. STING Signaling Promotes Inflammation in Experimental Acute Pancreatitis. Gastroenterology 2018, 154, 1822–1835.e2. [Google Scholar] [CrossRef]
- Farber, E.; Popper, H. Production of acute pancreatitis with ethionine and its prevention by methionine. Proc. Soc. Exp. Biol. Med. 1950, 74, 838–844. [Google Scholar] [CrossRef]
- Lombardi, B.; Rao, N.K. Acute hemorrhagic pancreatic necrosis in mice. Influence of the age and sex of the animals and of dietary ethionine, choline, methionine, and adenine sulfate. Am. J. Pathol. 1975, 81, 87. [Google Scholar]
- Kui, B.; Balla, Z.; Végh, E.T.; Pallagi, P.; Venglovecz, V.; Iványi, B.; Takács, T.; Hegyi, P.; Rakonczay, Z., Jr. Recent advances in the investigation of pancreatic inflammation induced by large doses of basic amino acids in rodents. Lab. Investig. 2014, 94, 138–149. [Google Scholar] [CrossRef]
- Gilliland, L.; Steer, M. Effects of ethionine on digestive enzyme synthesis and discharge by mouse pancreas. Am. J. Physiol.-Gastrointest. Liver Physiol. 1980, 239, G418–G426. [Google Scholar] [CrossRef]
- Akita, S.; Kubota, K.; Kobayashi, A.; Misawa, R.; Shimizu, A.; Nakata, T.; Yokoyama, T.; Takahashi, M.; Miyagawa, S. Role of bone marrow cells in the development of pancreatic fibrosis in a rat model of pancreatitis induced by a choline-deficient/ethionine-supplemented diet. Biochem. Biophys. Res. Commun. 2012, 420, 743–749. [Google Scholar] [CrossRef]
- Nagao, S.; Taguchi, K.; Sakai, H.; Yamasaki, K.; Watanabe, H.; Otagiri, M.; Maruyama, T. Carbon monoxide-bound hemoglobin vesicles ameliorate multiorgan injuries induced by severe acute pancreatitis in mice by their anti-inflammatory and antioxidant properties. Int. J. Nanomed. 2016, 11, 5611–5620. [Google Scholar] [CrossRef]
- Mizunuma, T.; Kawamura, S.; Kishino, Y. Effects of injecting excess arginine on rat pancreas. J. Nutr. 1984, 114, 467–471. [Google Scholar] [CrossRef]
- Ou, X.; Hua, Y.; Liao, X.; Gong, C.; Kang, Y. Cognitive impairments induced by severe acute pancreatitis are attenuated by berberine treatment in rats. Mol. Med. Rep. 2018, 18, 3437–3444. [Google Scholar] [CrossRef]
- Tani, S.; Itoh, H.; Okabayashi, Y.; Nakamura, T.; Fujii, M.; Fujisawa, T.; Koide, M.; Otsuki, M. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig. Dis. Sci. 1990, 35, 367–374. [Google Scholar] [CrossRef]
- Uçmak, F.; Ekin, N.; İbiloğlu, İ.; Arslan, S.; Kaplan, İ.; Şenateş, E. Prophylactic Administration of Silybin Ameliorates L-Arginine-Induced Acute Pancreatitis. Med Sci. Monit. Int. Med J. Exp. Clin. Res. 2016, 22, 3641–3646. [Google Scholar] [CrossRef]
- Saka, M.; Tuzun, A.; Ates, Y.; Bagci, S.; Karaeren, N.; Dagalp, K. Acute pancreatitis possibly due to arginine use: A case report. Turk. J. Gastroenterol. 2004, 15, 56–58. [Google Scholar]
- Binet, Q.; Dufour, I.; Agneessens, E.; Debongnie, J.-C.; Aouattah, T.; Covas, A.; Coche, J.-C.; De Koninck, X. The second case of a young man with l-arginine-induced acute pancreatitis. Clin. J. Gastroenterol. 2018, 11, 424–427. [Google Scholar] [CrossRef]
- Seidel, H. Bemerkungen zu meiner Methode der experimentellen Erzeugung der akuten hämorrhagischen Pankreatitis. Zentralbl Chir 1910, 37, 1601–1604. [Google Scholar]
- Dickson, A.; Foulis, A.; Imrie, C. Histology and bacteriology of closed duodenal loop models of experimental acute pancreatitis in the rat. Digestion 1986, 34, 15–21. [Google Scholar] [CrossRef]
- Adler, G. Experimental models and concepts in acute pancreatitis. Exocrine Pancreas Biol. Pathobiol. Dis. 1986, 407–421. [Google Scholar]
- Nevalainen, T.; Seppä, A. Acute pancreatitis caused by closed duodenal loop in the rat. Scand. J. Gastroenterol. 1975, 10, 521–527. [Google Scholar]
- Chetty, U.; Gilmour, H.; Taylor, T. Experimental acute pancreatitis in the rat—A new model. Gut 1980, 21, 115–117. [Google Scholar] [CrossRef][Green Version]
- Orda, R.; Hadas, N.; Orda, S.; Wiznitzer, T. Experimental acute pancreatitis: Inducement by taurocholate sodium-trypsin injection into a temporarily closed duodenal loop in the rat. Arch. Surg. 1980, 115, 327–329. [Google Scholar] [CrossRef]
- Deitch, E.A.; Sittig, K.; Li, M.; Berg, R.; Specian, R.D. Obstructive jaundice promotes bacterial translocation from the gut. Am. J. Surg. 1990, 159, 79–84. [Google Scholar] [CrossRef]
- Nieuwenhuijs, V.B.; van Dijk, J.E.; Gooszen, H.G.; Akkermans, L.M. Obstructive jaundice, bacterial translocation and interdigestive small-bowel motility in rats. Digestion 2000, 62, 255–261. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takada, T.; Yasuda, H. A new experimental pancreatitis by incomplete closed duodenal loop: The influence of pancreatic microcirculation on the development and progression of induced severe pancreatitis in rats. Pancreas 2004, 28, e112–e119. [Google Scholar] [CrossRef]
- Savu, A.; Savu, B.; Luca, C.; Mihaila, D.; Toma, O.; Crauciuc, E. Experimental models of acute pancreatitis-closed duodenal loop mode. Analele Stiintifice ale Universitatii” Al. I. Cuza” Din Iasi. 2009, 10, 83–88. [Google Scholar]
- Reber, H.A.; Roberts, C.; Way, L.W. The pancreatic duct mucosal barrier. Am. J. Surg. 1979, 137, 128–134. [Google Scholar] [CrossRef]
- Cen, Y.; Liu, C.; Li, X.; Yan, Z.; Kuang, M.; Su, Y.; Pan, X.; Qin, R.; Liu, X.; Zheng, J. Artesunate ameliorates severe acute pancreatitis (SAP) in rats by inhibiting expression of pro-inflammatory cytokines and Toll-like receptor 4. Int. Immunopharmacol. 2016, 38, 252–260. [Google Scholar] [CrossRef]
- Aho, H.; Nevalainen, T.; Aho, A. Experimental pancreatitis in the rat. Development of pancreatic necrosis, ischemia and edema after intraductal sodium taurocholate injection. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 1983, 15, 28–36. [Google Scholar]
- Unal, E.; Atalay, S.; Tolan, H.K.; Yuksekdag, S.; Yucel, M.; Acar, A.; Basak, F.; Gunes, P.; Bas, G. Biliopancreatic duct injection of ethanol as an experimental model of acute and chronic pancreatitis in rats. Int. J. Clin. Exp. Med. 2015, 8, 304–310. [Google Scholar]
- Lu, F.; Wang, F.; Chen, Z.; Huang, H. Effect of mesenchymal stem cells on small intestinal injury in a rat model of acute necrotizing pancreatitis. Stem Cell Res. Ther. 2017, 8, 12. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Peng, J.-S.; Li, C.-J.; Yang, Z.-L.; Xiang, J.; Song, H.; Wu, X.-B.; Chen, J.-R.; Diao, D.-C. A simple taurocholate-induced model of severe acute pancreatitis in rats. World J. Gastroenterol. 2009, 15, 5732–5739. [Google Scholar] [CrossRef]
- Zhu, R.; Zhao, Y.; Li, X.; Bai, T.; Wang, S.; Wang, W.; Sun, Y. Effects of penehyclidine hydrochloride on severe acute pancreatitis-associated acute lung injury in rats. Biomed. Pharmacother. 2018, 97, 1689–1693. [Google Scholar] [CrossRef]
- Shi, C.; Hou, C.; Zhu, X.; Huang, D.; Peng, Y.; Tu, M.; Li, Q.; Miao, Y. SRT1720 ameliorates sodium taurocholate-induced severe acute pancreatitis in rats by suppressing NF-κB signalling. Biomed. Pharmacother. 2018, 108, 50–57. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, Y.; Sun, Z.; Gao, Z.; Wang, J.; Zhang, D. Autophagy strengthens intestinal mucosal barrier by attenuating oxidative stress in severe acute pancreatitis. Dig. Dis. Sci. 2018, 63, 910–919. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, H.; Huang, C.; Fan, J.; Mei, Q.; Lu, Y.; Lou, L.; Wang, X.; Zeng, Y. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition. Pancreatology 2018, 18, 742–752. [Google Scholar]
- Zhang, Y.-M.; Zhu, L.; Zhao, X.-L.; Chen, H.; Kang, H.-X.; Zhao, J.-L.; Wan, M.-H.; Li, J.; Zhu, L.; Tang, W.-F. Optimal timing for the oral administration of Da-Cheng-Qi decoction based on the pharmacokinetic and pharmacodynamic targeting of the pancreas in rats with acute pancreatitis. World J. Gastroenterol. 2017, 23, 7098–7109. [Google Scholar] [CrossRef]
- Yan, L.; Li, Q.F.; Rong, Y.T.; Chen, Y.H.; Huang, Z.H.; Wang, Z.Z.; Peng, J. The protective effects of rutaecarpine on acute pancreatitis. Oncol. Lett. 2018, 15, 3121–3126. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Li, Z.-J.; Zhang, J. Inflammatory mediators and microcirculatory disturbance in acute pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 351–357. [Google Scholar]
- Pfeffer, R.B.; Lazzarini-Robertson, A.; Safadi, D.; Mixter, G.; Secoy, C.F.; Hinton, J.W. Gradations of pancreatitis, edematous, through hemorrhagic, experimentally produced by controlled injection of microspheres into blood vessels in dogs. Surgery 1962, 51, 764–769. [Google Scholar]
- Lasson, Å.; Ohlsson, K. Consumptive coagulopathy, fibrinolysis and protease antiprotease interactions during acute human pancreatitis. Thromb. Res. 1986, 41, 167–183. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Fan, L.; Zhao, Q.; Wang, D.; Cheng, S.; Zhang, A.; Qin, Y.; Zhang, B. Effect of vascular bradykinin on pancreatic microcirculation and hemorheology in rats with severe acute pancreatitis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2646–2650. [Google Scholar]
- Spormann, H.; Sokolowski, A.; Birkigt, H.; Letko, G. Contribution of pancreatic edema and short-term ischemia to experimental acute pancreatitis in the rat. I. Procedure and pathomorphological investigations. Zeitschrift fur Experimentelle Chirurgie Transplantation und Kunstliche Organe Organ der Sektion Experimentelle Chirurgie der Gesellschaft fur Chirurgie der DDR 1986, 19, 323–330. [Google Scholar]
- Sjövall, S.; Holmin, T.; Evander, A.; Stenram, U. Splenic and gastro-duodenal vein occlusion—Influence on the pancreatic gland and on the outcome of experimental pancreatitis. Int. J. Pancreatol. 1988, 3, 143–149. [Google Scholar]
- Hoffmann, T.; Leiderer, R.; Waldner, H.; Arbogast, S.; Messmer, K. Ischemia reperfusion of the pancreas: A new in vivo model for acute pancreatitis in rats. Res. Exp. Med. 1995, 195, 125–144. [Google Scholar] [CrossRef]
- Dembinski, A.; Warzecha, Z.; Ceranowicz, P.; Stachura, J.; Tomaszewska, R.; Konturek, S.; Sendur, R.; Dembinski, M.; Pawlik, W. Pancreatic damage and regeneration in the course of ischemia-reperfusion induced pancreatitis in rats. J. Physiol. Pharmacol. 2001, 52, 221–235. [Google Scholar]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kuśnierz-Cabala, B.; Tomaszewska, R. Obestatin accelerates the recovery in the course of ischemia/reperfusion-induced acute pancreatitis in rats. PLoS ONE 2015, 10, e0134380. [Google Scholar] [CrossRef]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kusnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Therapeutic effect of ghrelin in the course of ischemia/reperfusion-induced acute pancreatitis. Curr. Pharm. Des. 2015, 21, 2284–2290. [Google Scholar] [CrossRef]
- Schanaider, A.; de Carvalho, T.P.; de Oliveira Coelho, S.; Renteria, J.M.; Eleuthério, E.C.A.; Castelo-Branco, M.T.L.; Madi, K.; Baetas-da-Cruz, W.; de Souza, H.S.P. Ischemia–reperfusion rat model of acute pancreatitis: Protein carbonyl as a putative early biomarker of pancreatic injury. Clin. Exp. Med. 2015, 15, 311–320. [Google Scholar] [CrossRef]
- Churg, A.; Richter, W. Early changes in the exocrine pancreas of the dog and rat after ligation of the pancreatic duct. A light and electron microscopic study. Am. J. Pathol. 1971, 63, 521–546. [Google Scholar]
- Buchwalow, I.; Schnekenburger, J.; Atiakshin, D.; Samoilova, V.; Wolf, E.; Boecker, W.; Tiemann, K. Oxidative stress and NO generation in the rat pancreatitis induced by pancreatic duct ligation. Acta Histochem. 2017, 119, 252–256. [Google Scholar] [CrossRef]
- Baxter, J.; Jenkins, S.; Day, D.; Roberts, N.; Cowell, D.; Mackie, C.; Shields, R. Effects of somatostatin and a long-acting somatostatin analogue on the prevention and treatment of experimentally induced acute pancreatitis in the rat. Br. J. Surg. 1985, 72, 382–385. [Google Scholar] [CrossRef]
- Buscail, L.; Sénégas-Balas, F.; Balas, D.; Bouisson, M.; Bertrand, C.; Ribet, A. Protective effect of misoprostol, a synthetic prostaglandin E1 analog, on experimental pancreatitis induced by pancreatic duct ligation in rat. Pancreas 1989, 4, 715–723. [Google Scholar] [CrossRef]
- Ohshio, G.; Saluja, A.; Steer, M. Effects of short-term pancreatic duct obstruction in rats. Gastroenterology 1991, 100, 196–202. [Google Scholar] [CrossRef]
- Cohen, D.B.; Magnotti, L.J.; Lu, Q.; Xu, D.Z.; Berezina, T.L.; Zaets, S.B.; Alvarez, C.; Machiedo, G.; Deitch, E.A. Pancreatic duct ligation reduces lung injury following trauma and hemorrhagic shock. Ann. Surg. 2004, 240, 885–891. [Google Scholar] [CrossRef]
- De-Madaria, E.; Martínez, J.F.; Aparicio, J.R.; Lluís, F. Aggressive Fluid Resuscitation in Acute Pancreatitis: In Aqua Sanitas? Am. J. Gastroenterol. 2017, 112, 1617–1618. [Google Scholar] [CrossRef]
- De-Madaria, E.; Herrera-Marante, I.; González-Camacho, V.; Bonjoch, L.; Quesada-Vázquez, N.; Almenta-Saavedra, I.; Miralles-Maciá, C.; Acevedo-Piedra, N.G.; Roger-Ibáñez, M.; Sánchez-Marin, C. Fluid resuscitation with lactated Ringer’s solution vs normal saline in acute pancreatitis: A triple-blind, randomized, controlled trial. United Eur. Gastroenterol. J. 2018, 6, 63–72. [Google Scholar] [CrossRef]
- Singh, V.P. High on drugs: Lessons from opiates in pancreatitis. Gut 2018, 67, 600–602. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, S.; Tan, D.; Ma, A.; Yang, Y.; Xu, J. A meta-analysis of early oral refeeding and quickly increased diet for patients with mild acute pancreatitis. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2019, 25, 14–19. [Google Scholar]
- Van Brunschot, S.; van Grinsven, J.; van Santvoort, H.C.; Bakker, O.J.; Besselink, M.G.; Boermeester, M.A.; Bollen, T.L.; Bosscha, K.; Bouwense, S.A.; Bruno, M.J. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: A multicentre randomised trial. Lancet 2018, 391, 51–58. [Google Scholar] [CrossRef]
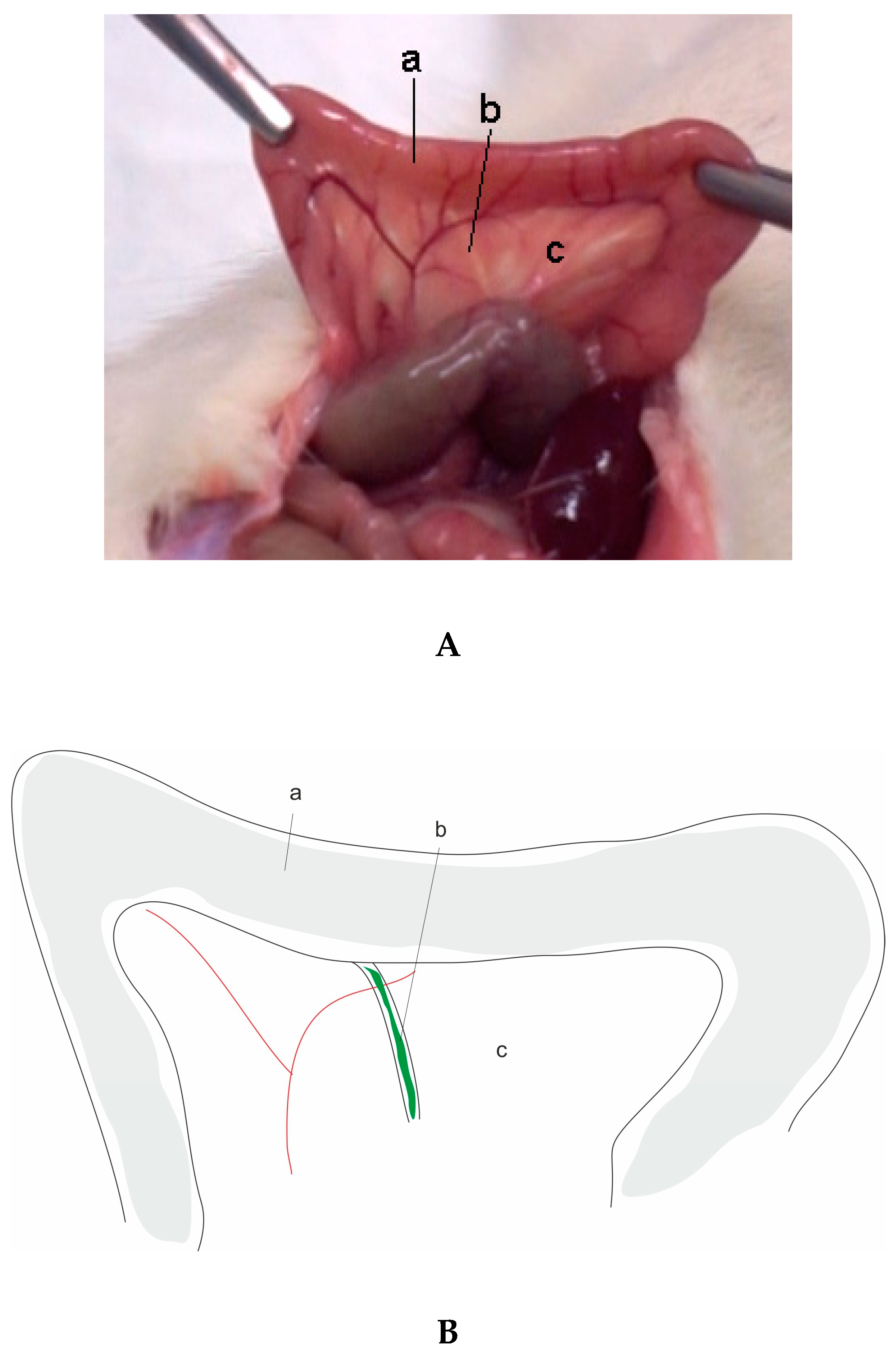
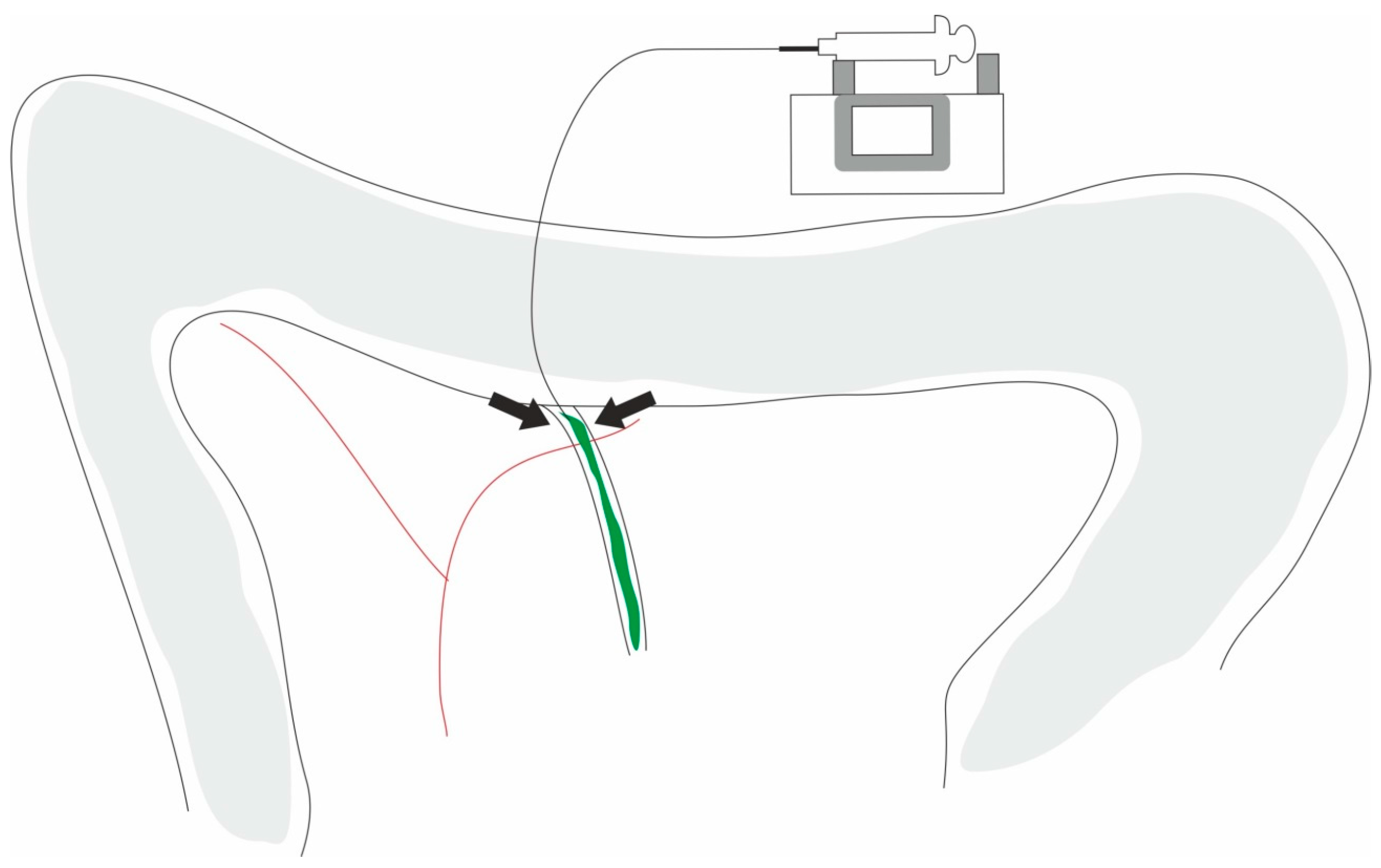
| AP Classification (According Revised Atlanta Classification [6]) | Animals | Models |
|---|---|---|
Mild acute pancreatitis
| Rats | Hormone-induced model |
Moderately severe acute pancreatitis
| - | |
Severe acute pancreatitis
| Mice | Hormone-induced model |
| Mice and Rats | Closed duodenal loop model * | |
| Mice | Alcohol-induced model * | |
| Mice and Rats | Nutrient-induced model | |
| Mice and Rats | Biliopancreatic duct injection model | |
| Mice and Rats | Vascular-induced model | |
| Mice and Rats | Ischemia/Reperfusion model * | |
| Mice and Rats | Duct ligation model * |
| Animals | Models | |
|---|---|---|
| Etiology | Mice and rats | Biliary pancreatitis Biliopancreatic duct injection model Duct ligation model Alcoholic pancreatitis Alcohol-induced model |
| Factors | Mice and rats | Bacterial translocation Closed duodenal loop model Duct ligation model Microcirculation impairment Vascular-induced model Ischemia/Reperfusion model Cholinergic agents Hormone-induced model Diets Nutrient-induced model Genetic Gene knockout model Trauma Ischemia/Reperfusion model Closed duodenal loop model |
| AP Model | Animals | Protocols | References | Clinical Relevance | |
|---|---|---|---|---|---|
| Administration Route | Doses | ||||
| caerulein | mice | Intravenous | 6 h continuous infusion of 100 µg/kg/h | [37] |
|
| subcutaneous | 7 h of injections at 50 µg/kg | [39] | |||
| intraperitoneal | 8 h of injections of 10 µg/mL, 0.2 mL/mouse) over two consecutive days | [40] | |||
| 7 h of injections at 50 µg/kg | [44,45] | ||||
| 50 µg/kg every two hours for five rounds | [42] | ||||
| 10 h of injections at 50 µg/kg | [41,43] | ||||
| rats | intravenous | 5 µg/kg/h for periods up to 24 h | [34] | ||
| 3–h continuous infusion of 7.5 µg/kg/h (7.5 µg/kg/h × 3 h) | [36] | ||||
| subcutaneous | 5 µg/kg/h for 3 h (hourly injection) | [38] | |||
| Four injections of 20 µg/kg/h hourly | [47,51] | ||||
| Injection of 10 µg/kg | [50] | ||||
| intraperitoneal | Two injections of 40 µg/kg at hourly intervals | [46] | |||
| alcohol | mice | oral or intragastric | Single intragastric dose of ethanol (6.0 g/kg BW) in NRF2-KO mice | [69] |
|
| intraperitoneal | Two intraperitoneal injections of ethanol (1.32 g/kg BW) and palmitoleic acid (1.5 mg/kg BW), separated by one hour | [70] | |||
| rats | intravenous | Bolus of 2 g/kg BW followed by continuous IV alcohol application of 0.365 g/kg BW/h with an additional 3 mL/kg BW saline solution | [71] | ||
| oral or intragastric | Intragastric bolus of ethanol 2.3 g/kg BW followed by the continuous infusion of 0.365 g/kg BW/h IV | [71] | |||
| intraductal | Injection of 48% ethyl alcohol in a volume of 1 cm3 into the common biliary duct | [72] | |||
| L-arginine | rats | intraperitoneal | 2-h injections of 8% | [73] |
|
| 250–500 mg/100 g BW | [74] | ||||
| Duct infusion-induced model | mice | sodium taurocholate | 10 µL/min for 5 min of 2.5–5% | [75] |
|
| rats | sodium taurocholate | 5–10 mM with caerulein intravenous 5 µg/kg/h for 6 h | [76,77] | ||
| 1 mL/kg of 3% injected over a 60-second period | [78,79,80] | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Vaz, P.; Abrantes, A.M.; Castelo-Branco, M.; Gouveia, A.; Botelho, M.F.; Tralhão, J.G. Murine Models of Acute Pancreatitis: A Critical Appraisal of Clinical Relevance. Int. J. Mol. Sci. 2019, 20, 2794. https://doi.org/10.3390/ijms20112794
Silva-Vaz P, Abrantes AM, Castelo-Branco M, Gouveia A, Botelho MF, Tralhão JG. Murine Models of Acute Pancreatitis: A Critical Appraisal of Clinical Relevance. International Journal of Molecular Sciences. 2019; 20(11):2794. https://doi.org/10.3390/ijms20112794
Chicago/Turabian StyleSilva-Vaz, Pedro, Ana Margarida Abrantes, Miguel Castelo-Branco, António Gouveia, Maria Filomena Botelho, and José Guilherme Tralhão. 2019. "Murine Models of Acute Pancreatitis: A Critical Appraisal of Clinical Relevance" International Journal of Molecular Sciences 20, no. 11: 2794. https://doi.org/10.3390/ijms20112794
APA StyleSilva-Vaz, P., Abrantes, A. M., Castelo-Branco, M., Gouveia, A., Botelho, M. F., & Tralhão, J. G. (2019). Murine Models of Acute Pancreatitis: A Critical Appraisal of Clinical Relevance. International Journal of Molecular Sciences, 20(11), 2794. https://doi.org/10.3390/ijms20112794








